Abstract
Streptococcus pneumoniae usually colonizes the nasopharynx of humans asymptomatically but occasionally translocates from this niche to the lungs, the brain, and the blood, causing potentially fatal infections. Spread to other host tissues requires a significant morphological change and the expression of virulence factors, such as capsular polysaccharide, and virulence proteins, such as pneumolysin (Ply), PspA, and CbpA. Modulation of the expression of pneumococcal virulence genes by heat shock and by heat shock proteins ClpL and ClpP, as well as the attenuation of virulence of a clpP mutant in a murine intraperitoneal infection model, was demonstrated previously. In this study, we further investigated the underlying mechanism of virulence attenuation by the clpP mutation. The half-lives of the mRNAs of ply and of the first gene of the serotype 2 capsule synthesis locus [cps(2)A] in the clpP mutant were more than twofold longer than those of the parent after heat shock, suggesting that the mRNA species were regulated posttranscriptionally by ClpP. In addition, the clpP mutant was defective in colonization of the nasopharynx and survival in the lungs of mice after intranasal challenge. The mutant was also killed faster than the parent in the murine macrophage RAW264.7 cell line, indicating that ClpP is required for colonization and intracellular survival in the host. Furthermore, fractionation studies demonstrated that ClpP was translocated into the cell wall after heat shock, and immunization of mice with ClpP elicited a protective immune response against fatal systemic challenge with S. pneumoniae D39, making ClpP a potential vaccine candidate for pneumococcal disease.
Streptococcus pneumoniae (pneumococci) causes a variety of potentially life-threatening infections, such as pneumonia, bacteremia, and meningitis (37). It is carried asymptomatically in the nasopharynx of healthy individuals, and this niche serves as a major reservoir for pneumococcal infections. A change in the environmental niche of the host, such as penetration of pneumococci from the nasopharynx into the bloodstream, can provoke dramatic morphological changes as well as changes in gene expression. For instance, it has been demonstrated that pneumococci in the nasopharynx are predominantly of the transparent colony phenotype and tend to express less capsule and more choline-binding protein A (CbpA) than those in the bloodstream. On the other hand, pneumococci in the bloodstream are predominantly of the opaque colony morphology and tend to produce more capsule and less CbpA than those in the nasopharynx (12, 34). Furthermore, S. pneumoniae may encounter heat stress after penetration from the nasal mucosa (30 to 34°C) (18) into the blood and/or meninges (37°C) during the pathogenic process. Such changes in temperature may serve as a key trigger for a rapid, transient increase in the synthesis of a highly conserved set of proteins referred to as heat shock proteins (HSPs) (22). The induction of HSPs by elevated temperatures or by exposure to ethanol, oxidative stresses, or heavy metals serves to protect bacteria against such adverse effects, thereby increasing their survival rate (22). Therefore, a thorough understanding of the heat shock response could provide useful information on the adaptation of pneumococci to the hostile environment encountered in the host.
The protein profiles of the heat shock response in pneumococci after exposure of the cells to several stresses were previously examined. Pulse-labeling of proteins with [35S]methionine revealed that a temperature shift from 30 to 37°C in vitro, similar to that encountered by S. pneumoniae after translocation from the nasal mucosa to the lungs, triggered the induction of DnaK and GroEL (6). The persistence of ClpL, DnaK, and GroEL upon return to 30°C indicated that HSPs do not appear to be actively degraded upon return to normal culture conditions (15). Moreover, ClpL contains two ATP-binding regions and was found to function as a chaperone and to modulate virulence gene expression (15). A clpP mutant of S. pneumoniae was recently shown to be sensitive to high temperatures, H2O2, and puromycin and was significantly attenuated for virulence in mice (15, 28). The specific roles of other heat shock genes, such as clpC, clpE, and clpX, have not been fully clarified (4, 5, 7, 32).
Recently, it was shown that heat shock induced the expression of pneumolysin (Ply) and modulated the expression of other virulence factors in wild-type pneumococci. A mutation in clpP resulted in an increase in ply mRNA expression but not in the level and hemolytic activity of Ply after heat shock (15). In this study, we investigated the underlying mechanism by which ClpP attenuates virulence and assessed whether immunization with ClpP could protect mice against challenge with virulent pneumococci.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and transformation.
The bacterial strains and plasmid vectors used in this study, along with the new recombinants generated in this study, are presented in Table 1. S. pneumoniae CP1200, a derivative of Rx1, was grown in Casitone-tryptone-based medium (6). S. pneumoniae D39 (type 2) was grown in Todd-Hewitt medium with yeast extract (THY). For selection of pneumococcal transformants, erythromycin was added to the growth medium at a concentration of 0.2 μg/ml. Escherichia coli strains were grown in Luria-Bertani broth or on Luria-Bertani agar. Plasmids were introduced into E. coli by transformation as described by Hanahan (10). For selection of E. coli transformants, kanamycin (30 μg/ml) was added to the growth medium.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BL21(DE3) | gal (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1) | Novagen |
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) | Gibco BRL |
| XL1-Blue | relA1 lac [F′ proAB laclqZΔM15 Tn10 (Tetr)] | Stratagene |
| S. pneumoniae | ||
| CP1200 | Nonencapsulated derivative of Rx1; malM511 str-1 | Choi et al. (6) |
| HYK2 | CP1200 ΔclpP::ermB | Kwon et al. (15) |
| D39 | Encapsulated; type 2 | Avery et al. (1) |
| HYK302 | D39 ΔclpP::ermB | Kwon et al. (15) |
| Plasmids | ||
| pET30(a) | 5.4 kb; Apr | Novagen |
| pKHY002 | 6.0 kb; histidine-tagged clpP in pET30(a) | This study |
Cell culture.
Human lung epithelial carcinoma A549 and murine macrophage RAW264.7 cell lines were obtained from the American Type Culture Collection and cultured at 37°C in the presence of 95% air-5% CO2. The A549 cells were cultured in Dulbecco modified Eagle medium with 4.5 g of glucose/liter, 10% fetal bovine serum (Gibco BRL, Gaithersburg, Md.), 100 U of penicillin G/ml, and 100 μg of streptomycin/ml. For culturing of RAW264.7 cells, RPMI 1640 culture medium (Gibco BRL) supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin G/ml, 100 μg of streptomycin/ml, and 0.2% NaHCO3 was used as the basic medium, and fetal bovine serum was added at a concentration of 10%.
Antisera, gel electrophoresis, and immunoblotting.
Sera against PspA and PdB (the toxoid derivative of Ply) were prepared essentially as described previously (23). Bacteria for immunoblotting were grown in THY to an A600 of 0.3 and prepared as described previously (15). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (15% polyacrylamide gel) was carried out as described by Laemmli (16). The proteins were electroblotted onto nitrocellulose membranes and then reacted with a 1:5,000 dilution of polyclonal mouse sera raised against PspA and PdB. The secondary antibody was a 1:2,000 dilution of goat anti-mouse immunoglobulin G conjugated to alkaline phosphatase (Bio-Rad).
Pneumolysin assay.
Hemolytic activity was determined as previously described (19) with a minor modification. Briefly, pneumococci grown in THY to early log to mid-log phase (A600, 0.05 to 0.1) were harvested by centrifugation at 3,900 × g for 10 min at 4°C and resuspended in phosphate-buffered saline (PBS). Sodium deoxycholate was added to a final concentration of 0.1%, and the samples were incubated at 37°C for 10 min. After centrifugation of the samples, the supernatant was withdrawn and serially diluted. Hemolytic activity was determined by incubation with an equal volume of 1.5% washed human red blood cells (containing 0.001% 2-mercaptoethanol [Merck]) for 30 min at 37°C in 96-well microtiter plates. The hemolytic titer was determined as the reciprocal of the estimated dilution at which 50% of erythrocytes were lysed at A540.
RNA techniques.
Aliquots of 1.5 ml of culture suspension were collected at intervals for extraction of total RNA. For measurement of mRNA half-lives, rifampin (100 μg/ml) was added. Total RNA was extracted by the hot acid-phenol method as described previously (24). Levels of mRNAs for cps(2)A and ply were quantified by one-step real-time reverse transcription (RT)-PCR by use of an Access RT-PCR system (Promega Biotech catalog no. A1250). The specific and internal control (16S rRNA) primers for these reactions were described elsewhere (24). RT-PCR preparations, cycling conditions, and analyses of data were essentially as described previously (15). All reactions were carried out by use of a Rotor-Gene 2000 real-time cycler (Corbett Research, Mortlake, New South Wales, Australia). Analysis of mRNA half-lives was performed with the SigmaPlot curve fitter program (nonlinear least-squares fitting to the sum of exponential functions). Two models were proposed, monophasic decay or biphasic decay. The model which fitted the data with the minimum deviation in each case was considered the more valid model.
Cloning, expression, and purification of ClpP in E. coli.
The clpP open reading frame was PCR amplified with forward and reverse primers (5′-CGA ATT CAT GAT TCC TGT AGT TAT-3′ and 5′-CGA GCT CTT AGT TCA ATG AAT TGT TG-3′, which incorporate EcoRI and SacI sites, respectively) from CP1200 DNA. The PCR fragment was digested with the same enzymes and cloned into the corresponding restriction sites in pET30(a) (Novagen) to generate plasmid pET30(a)-clpP. Expression in E. coli BL21(DE3) was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) exposure for 3 h. The cells were harvested by centrifugation at 6,000 × g for 10 min and then resuspended in lysis buffer (50 mM sodium phosphate [pH 8.0], 2 M NaCl, 40 mM imidazole) to which the protease inhibitor phenylmethylsulfonyl fluoride (PMSF) had been added to a final concentration of 1 mM. The cells were lysed in a French pressure cell (SLM Aminco, Inc.) at 12,000 lb/in2, and the lysates were centrifuged at 100,000 × g for 1 h. The resultant supernatant, containing the His6-tagged protein, was loaded into a nickel-nitrilotriacetic acid column (Probond; Invitrogen), which was then washed with 10 column volumes of 10 mM sodium phosphate-20 mM imidazole-1 M NaCl (pH 6.0). Nickel-bound His6-tagged protein was eluted with a 30-ml gradient of 0 to 500 mM imidazole in 10 mM sodium phosphate buffer (pH 6.0) and dialyzed against 10 mM sodium phosphate buffer (pH 7.0). The protein was judged to be >95% pure by SDS-PAGE and staining with Coomassie brilliant blue R-250 (data not shown).
Isolation and localization of subcellular fractions.
Exponentially growing cells were collected by centrifugation, and sucrose-induced protoplast formation was performed as described by Vijayakumar and Morrison (35). Briefly, cells were converted to protoplasts by incubation at 30°C for 1 h with 1 M sucrose buffer (1 M sucrose, 100 mM Tris-HCl [pH 7.6], 2 mM MgCl2, 1 mM PMSF). Centrifugation at 13,000 × g for 20 min separated the cell wall fraction (supernatant) from the protoplasts (pellet). The protoplasts were subjected to osmotic lysis by dilution with 19 volumes of hypotonic buffer (100 mM Tris-HCl [pH 7.6], 1 mM PMSF, 1 mM EDTA). Lysates were centrifuged initially at 5,000 × g for 5 min to remove unlysed cells and then at 50,000 × g for 30 min to obtain the cytoplasmic fraction (supernatant) and the membrane fraction (pellet).
Determination of MDH activity.
The enzymatic activity of malate dehydrogenase (MDH) was determined by monitoring the rate of reduction of the A340 of 0.2 mM NADH at 25°C (A340, 6.22 mM−1 cm−1) in 0.15 M potassium phosphate (pH 7.6) containing 0.5 mM oxalacetate. After the sample was added, the reaction mixture was incubated at 25°C for 1 min 40 s, and the A340 was determined. The addition of the substrate was used to start the reaction. Initial slopes for the rate of oxidation of NADH from the first 1 min 40 s of the reaction were used to calculate MDH activity.
Adhesion and invasion assays.
Invasion of human lung A549 cells by pneumococci was performed by a modification of the antibiotic protection assay described previously (31). A549 cells were grown to confluence in 24-well tissue culture plates and washed three times with PBS (pH 7.2), after which 1 ml of culture medium (without antibiotics) was added per well. Exponential-phase cultures of R-type CP1200 and its isogenic clpP mutant derivatives (A550, 0.3; 108 CFU/ml) were pelleted by centrifugation, washed once with PBS, and resuspended in Dulbecco modified Eagle medium. Monolayers were infected with 107 bacteria (bacterium/cell ratio, 10:1), and initial contact of the bacteria with the cell monolayers was aided by centrifugation at 800 × g for 10 min at 4°C followed by 2 h of incubation at 37°C. Fresh medium containing 10 μg of penicillin/ml and 200 μg of gentamicin/ml was added to each well, and this treatment was confirmed to be sufficient to kill all of the extracellular bacteria. After an additional 1 h of incubation, the monolayers were washed three times with PBS, and the cells were detached from the plates by treatment with 100 μl of 0.25% trypsin-0.02% EDTA and then lysed by the addition of 400 μl of Triton X-100 (0.025% in H2O). Appropriate dilutions were plated on blood agar to determine the numbers of viable bacteria.
To determine the total numbers of adherent and intracellular bacteria, infected monolayers were washed as described above and then trypsinized, lysed, and plated quantitatively without antibiotic treatment. All samples were assayed in triplicate, and each assay was repeated at least three times.
Survival in RAW264.7 cells.
Cell monolayers were infected with 107 CFU of pneumococci (bacterium/cell ratio, 10:1) in RPMI 1640 culture medium without antibiotics and then incubated for 2 h at 37°C. The cells were washed three times with PBS, and fresh medium containing 10 μg of penicillin/ml and 200 μg of gentamicin/ml was added to kill the extracellular bacteria (time zero of the assay). To enumerate intracellular pneumococci at different times after infection, the supernatants were removed, and the cells were washed three times with PBS and then lysed with Triton X-100 as described above. Serial dilutions of the lysate from each well were plated on blood agar. The number of CFU was determined after 24 h of incubation at 37°C. Three independent assays were carried out (in triplicate) for each bacterial strain. Statistical analysis was performed by use of paired or unpaired Student's t tests.
Colonization studies.
Before challenge, bacteria were cultured at 37°C overnight on blood agar (supplemented with erythromycin where appropriate) and then grown in THY for approximately 4 h at 37°C to give ca. 4 × 107 CFU/ml (A600, 0.1). Each bacterial culture then was adjusted in THY to ca. 109 CFU/ml, and 10 μl (ca. 107 CFU) of cells was inoculated into the nares of 5-week-old CD1 mice. At 1, 2, and 4 days postinfection, four mice from each group were sacrificed randomly to estimate the carriage of each strain.
Nasopharyngeal, blood, and lung samples were serially diluted as appropriate in sterile PBS and plated in duplicate on blood agar containing the appropriate antibiotic(s). Plates were incubated for approximately 16 h at 37°C in an atmosphere of 95% air-5% CO2, after which colonies were counted and averaged between replicates.
Immunization of mice and analysis of sera.
Mice were immunized intraperitoneally as described previously (23). Four groups of 5- to 6-week-old female CBA/N mice (12 per group) were immunized intraperitoneally with either AlPO4 alone, genetically modified Ply toxoid (PdB) plus AlPO4, PspA plus AlPO4, or ClpP plus AlPO4. Each mouse received three doses of 10 μg of each protein antigen at 12- to 14-day intervals, and sera were collected from the mice by retro-orbital bleeding 1 week after the third immunization. The sera were pooled on a group-by-group basis and assayed for Ply-, PspA-, or ClpP-specific antibodies by an enzyme-linked immunosorbent assay. The sera were also subjected to Western immunoblot analysis with whole-cell lysates of S. pneumoniae D39 or purified Ply, PspA, or ClpP as the antigen.
Challenge.
Intraperitoneal challenge of immunized mice with a highly virulent capsular type 2 strain (D39) was carried out 2 weeks after the last immunization. Before challenge, the bacteria were grown at 37°C overnight on blood agar and then inoculated into serum broth, consisting of 10% (vol/vol) horse serum in meat extract broth. They were then grown statically for 3 h at 37°C to give approximately 108 CFU/ml, and the inoculum was adjusted to 7.5 × 105 CFU per challenge dose. Serotype-specific capsule production was confirmed by the Quellung reaction with antisera obtained from Statens Serum Institut, Copenhagen, Denmark. After challenge, the mice were monitored every 4 h initially for 7 days and then daily up to 21 days, and the survival time for each mouse was recorded. Differences between the median survival times of groups were analyzed by the Mann-Whitney U test (one-tailed).
RESULTS
Determination of mRNA half-lives.
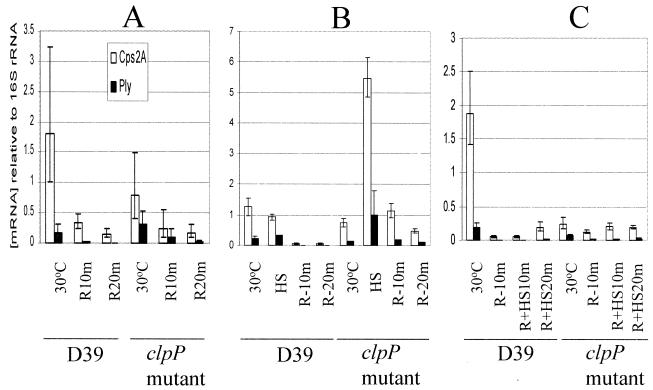
It was recently demonstrated that in a clpP mutant of S. pneumoniae, there was an increase in ply mRNA expression with no concomitant increase in Ply protein expression or Ply hemolytic activity after heat shock. However, in the wild type, the ply mRNA level, the level of protein, and hemolytic activity increased significantly after heat shock (15). This inconsistency could be attributed to an instability of ply mRNA at high temperatures in the clpP mutant. Therefore, the decay kinetics of ply mRNA after heat shock were investigated. To study the stability of the mRNA at 30°C, de novo mRNA synthesis was blocked by the addition of rifampin, and the decay was monitored by real-time RT-PCR with cps(2)A- and ply-specific primers (Fig. 1A). A comparison of the degradation kinetics demonstrated that at 30°C, the half-lives of ply mRNA in the wild type and the clpP mutant were 2.75 and 5.8 min, respectively; these data indicate that the half-life of ply in the mutant was 2.1-fold higher than that in the wild type at 30°C (Table 2). However, the degradation of cps(2)A mRNA in the clpP mutant was only 1.31-fold higher than that in the parent at 30°C (Fig. 1A and Table 2). These data suggest that ClpP protease could be responsible for the degradation of cps(2)A and ply mRNAs at 30°C in some unknown way.
FIG. 1.
Detection of relative mRNA stabilities of cps(2)A and ply by real-time RT-PCR. (A) To determine the mRNA half-lives at 30°C, S. pneumoniae strains were grown at 30°C, and then rifampin was added. Aliquots for RNA extraction were withdrawn before the addition of rifampin and at 10 and 20 min after rifampin was added (R10m and R20m, respectively). (B) To determine the mRNA half-lives after heat shock, S. pneumoniae strains were grown at 30°C and then heat shocked (HS) at 42°C. After 10 min at 42°C, rifampin was added. Aliquots for RNA extraction were withdrawn before and after heat shock and at 10 and 20 min after the addition of rifampin at 42°C. (C) To determine the effect of heat shock on the mRNA half-lives, S. pneumoniae strains grown at 30°C were treated with rifampin for 10 min and then heat shocked at 42°C. Aliquots for RNA extraction were withdrawn before and at 10 min after the addition of rifampin at 30°C (R-10m) and then at 10 and 20 min after heat shock (R+HS10m and R+HS20m, respectively). Between RNA extracts, levels of individual mRNA species were corrected by reference to that obtained for the internal 16S rRNA control. Data points represent means and standard deviations of quadruplicate samples from each RNA extract.
TABLE 2.
Effect of heat shock on half-lives of cps(2)A and ply mRNAsa
| Temp (°C) | Half-life (min) of mRNA for:
|
|||
|---|---|---|---|---|
| D39
|
ClpP mutant
|
|||
| cps(2)A | ply | cps(2)A | ply | |
| 30 | 3.8 ± 1.23 | 2.75 ± 0.16 | 5.0 ± 0.62 | 5.8 ± 2.71 |
| 42 | 2.0 ± 0.45b | 1.75 ± 0.33b | 4.1 ± 0.55b,c | 3.75 ± 0.79d |
For measuring mRNA half-lives, rifampin (100 μg/ml) was added, and 1.5-ml aliquots of culture suspension were collected at 10-min intervals. Total RNA was extracted from these aliquots by the hot acid-phenol method. Subsequently, mRNA levels were determined by real-time RT-PCR. The mRNA half-lives were analyzed by nonlinear least-squares fitting to the sum of exponentials functions. All experiments were carried out in quadruplicate, and values are reported as means and standard deviations.
Significantly different (P < 0.05) from the half-life at 30°C.
Significantly different (P < 0.001) from the half-life of D39 cps(2)A mRNA at 42°C.
Significantly different (P < 0.05) from the half-life of D39 ply mRNA at 42°C.
To study the stabilities of the transcripts after heat shock, S. pneumoniae cells were first grown at 30°C and then shifted to 42°C. Total RNA was prepared immediately before the addition of rifampin at 42°C and at 10 and 20 min thereafter. After heat shock, the level of ply mRNA in the clpP mutant increased 7.5-fold (Fig. 1B), but the level of Ply protein did not increase (data not shown), corroborating previous results (15). The data for the decay kinetics showed that the half-lives of the ply transcript in the parent and the clpP mutant were 1.75 and 3.75 min, respectively, indicating that ply mRNA in the clpP mutant was degraded 2.14-fold more slowly than that in the parent after heat shock (P < 0.05). Furthermore, the half-lives of the cps(2)A transcript in the parent and the clpP mutant were 2.0 and 4.1 min, respectively, indicating that cps(2)A mRNA in the clpP mutant was degraded 2.05-fold more slowly than that in the parent after heat shock (P < 0.001) (Fig. 1B and Table 2).
Given that after heat shock, the half-lives of the ply and cps(2)A mRNA transcripts became shorter than those at 30°C in both the parent and the clpP mutant (the P value was <0.05, except for the ply mRNA in the clpP mutant), it is possible that the mRNA species are either liable to faster degradation at 42°C than is 16S rRNA or are subject to faster decay by some unknown mechanism(s). Therefore, we investigated whether heat shock itself could affect the half-life of ply mRNA. Cells cultured at 30°C were treated with rifampin and then heat shocked, and the decay kinetics of the mRNA were determined by RT-PCR analysis. Under these conditions, the levels of both cps(2)A and ply transcripts were stable or increased slightly over 20 min in the parent and the clpP mutant (Fig. 1C), a result which was in contrast to the scenario observed at 30°C (Fig. 1A). These results demonstrated that the transcripts synthesized at 30°C were indeed stabilized by heat shock at 42°C (Fig. 1C) but did not indicate that the transcripts synthesized at 42°C were stabilized by heat shock. The fact that the half-lives of the ply and cps(2)A mRNA transcripts after heat shock became shorter than those at 30°C in both the parent and the clpP mutant (Fig. 1B and Table 2) could partly explain the discrepancy between high RNA transcript levels and low protein levels.
The hemolytic activity of Ply is not activated by ClpP.
The destabilizing function of ClpP could be responsible for the low stability of the ply processing product and that of the ply primary transcript. Even though the ply mRNA was stabilized by heat shock, there was no corresponding increase in the level of the Ply protein or its hemolytic activity. This discrepancy could be due to the activation of Ply directly by ClpP protease so that hemolytic activity could be increased in the parent but not in the clpP mutant. To test this hypothesis, S. pneumoniae cells were cultured at 30°C and then heat shocked at 42°C for 20 min. The cells were lysed with 0.1% sodium deoxycholate by incubation at 37°C for 10 min. Subsequently, cell lysates were incubated further at 37°C for 20, 40, and 60 min, and the hemolytic activity of Ply was determined. The hemolytic activities in both the wild type and the clpP mutant decreased over the period at 37°C, and the hemolytic titer of the clpP mutant was not significantly different from that of the parent; these data suggested that ClpP protease is not required for activation of the hemolytic activity of Ply (data not shown).
ClpP is translocated to the cell wall after heat shock.
For Bacillus subtilis, immunogold labeling with antibodies revealed that ClpC and ClpX ATPases were detected at the cell envelope as well as inside the cells (14), suggesting that Hsp100, ClpC, and ClpX proteins are associated closely with protein aggregates during heat shock treatment. To localize ClpP in S. pneumoniae, fractionation of subcellular proteins was initially attempted with encapsulated strains. However, after incubation of the cells in 1 M sucrose buffer, centrifugation did not separate the cell wall from protoplasts due to the presence of a thick capsule (data not shown). Therefore, unencapsulated strains were used for localization experiments. Since the fractionation method may yield partial lysis or leakage of cytoplasmic contents during or after heat shock, MDH was used as an internal cytoplasmic marker. MDH activity in the cell wall was 11.8% of the total MDH activity at 30°C. In addition, MDH activity in the cell wall was 9.3% even after heat shock, suggesting that heat shock does not cause lysis or leakage of the cell membrane.
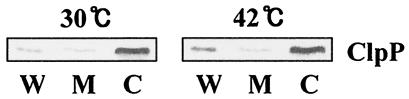
Exponentially growing S. pneumoniae CP1200 cells were exposed to 42°C, and cellular proteins were separated into cell wall, membrane, and cytoplasmic fractions by sucrose-induced protoplast formation followed by lysis in hypotonic buffer (see Materials and Methods). Subsequently, subcellular fractions were subjected to immunoblot analysis with polyclonal anti-ClpP serum. At 30°C, ClpP was detected predominantly in the cytoplasmic fraction (82.4% of the total ClpP); a smaller amount was detected in the cell wall fraction (11.8% of the total ClpP). However, after heat shock, the proportion of ClpP in the cell wall increased to 25.6%, whereas that in the cytosol decreased to 68.4%, although there was an increase in the absolute amount of ClpP in both fractions (Fig. 2). These results suggested that a significant amount of ClpP may be translocated to the cell wall after heat shock.
FIG. 2.
Translocation of ClpP after heat shock. Exponentially growing S. pneumoniae at 30°C was heat shocked at 42°C for 30 min. Cells were collected by centrifugation, and the proteins were fractionated and subjected to SDS-PAGE. Subsequently, ClpP was visualized by immunoblot analysis with polyclonal ClpP-specific antiserum. W, cell wall; M, membrane; C, cytoplasm.
Failure of the clpP mutant to colonize the nasopharynx or invade the lungs.
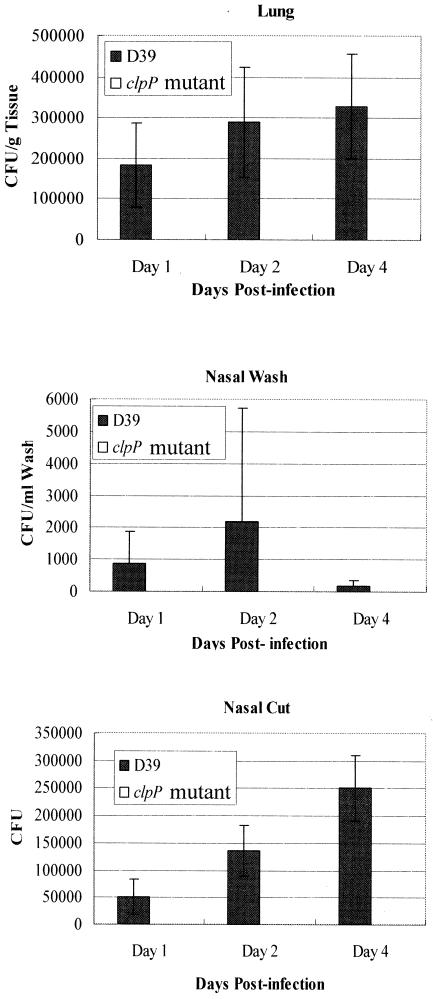
It was shown previously that the clpP mutant was attenuated for virulence in a murine septicemia model (15, 28). Moreover, the clpP mutant failed to colonize the lungs of mice at significant levels after intratracheal challenge, and no mortality was recorded throughout the 48-h infection (28). However, invasion and dissemination of S. pneumoniae are usually accomplished from its natural niche, the nasopharynx. Intranasal challenge of mice with a highly virulent capsular type 2 strain (D39) and an isogenic clpP mutant (HYK302) revealed that the clpP mutant was unable to colonize the nasopharynx or the lungs at all throughout the 4-day period (Fig. 3).
FIG. 3.
Bacterial recovery from the nasopharynx of CD1 mice after intranasal challenge with D39 and its isogenic clpP derivative over a 4-day period. The values are means and standard errors of the means (n = 5) for each time point.
Given that S. pneumoniae ClpC has been shown to be involved in adherence (4) and that mutations in clpC (29), clpE (20), and clpP (26) in Listeria and Yersinia resulted in a decrease in adherence to host epithelial cells, we investigated the involvement of ClpP in adherence to host epithelial cells. Since the presence of a polysaccharide capsule significantly attenuates the adherence of pneumococci to the surface of host cells (31), unencapsulated (rough) strains were used. Rough clpP deletion mutant HYK2 was not significantly different from the isogenic parent with regard to adherence to and invasion of A549 cells (data not shown). The difference in adherence to the nasopharynx may be due to a difference in target receptors, such as glycolipids or glycoproteins present in the nasopharynx (2).
Reduced survival of the clpP mutant in murine macrophage RAW264.7 cells.
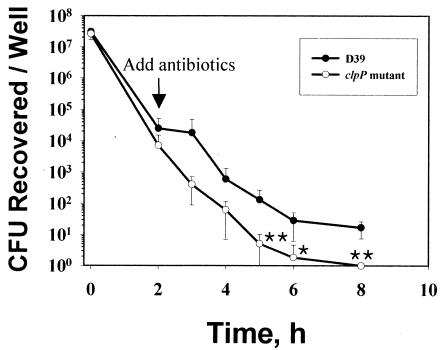
Alveolar macrophages are the primary elements in host defense against invasion by S. pneumoniae (13). Since it was observed in this study that the clpP mutant was defective in colonization of the nasopharynx compared to the parent, it was reasoned that this finding might be due to rapid clearance of the pneumoccoci in addition to the slower growth of the mutant. Therefore, the survival of the clpP mutant in murine macrophage RAW264.7 cells was examined (Fig. 4). The viability of the clpP mutant in RAW264.7 cells was significantly lower than that of the parent after 5 h (P < 0.01), 6 h (P < 0.05), and 8 h (P < 0.01) of infection (Fig. 4). The number of viable cells at 2 h after the addition of antibiotic would be expected to be approximately half the number present when the antibiotic was added. However, the actual number of CFU was much smaller, indicating that this result is not due to a growth defect of the clpP mutant but rather is due to a stress-sensitive phenotype or susceptibility of the clpP mutant to macrophages. These data suggested that ClpP is required for intracellular survival in RAW264.7 cells.
FIG. 4.
Survival of the clpP mutant in macrophage cells. RAW264.7 cell monolayers were infected with ca. 107 CFU of pneumococci (bacterium/cell ratio, 10:1) in RPMI 1640 culture medium. Samples were taken at different times after infection to quantify intracellular pneumococci after gentamicin treatment. Three independent assays were carried out in triplicate for each bacterial strain. Error bars indicate standard deviations. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, for comparisons with parent strain D39.
Immune response to ClpP protects mice from fatal pneumococcal challenge.
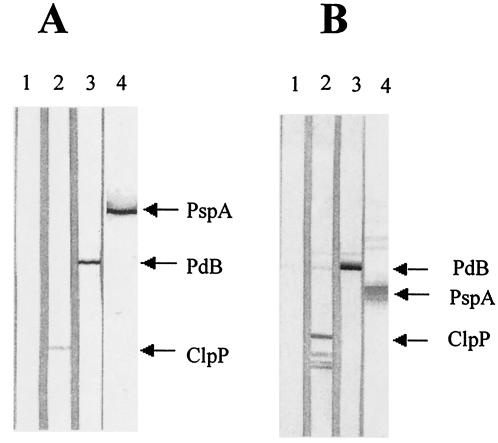
HSPs serve as antigens in some pathogens (3, 8, 11, 22) and serve to protect against infectious diseases (38). Since ClpP is translocated into the cell wall after heat shock, its ability to elicit protection against fatal pneumococcal challenge was evaluated. Mice immunized with ClpP exhibited a strong, specific antibody response to the antigen; the enzyme-linked immunosorbent assay titer (mean and standard deviation) of pooled sera from mice immunized with purified ClpP was 8,400 ± 2,250. This titer was similar to those obtained from mice immunized with purified PdB (8,000 ± 600) and PspA (8,300 ± 2,400). Mice immunized with alum (AlPO4) adjuvant alone produced a titer of 100, which was the limit of detection. Western immunoblot analysis of the sera against whole-cell lysates of D39 also demonstrated the specificity of the antibody responses to each of the antigens (Fig. 5A). The sera also reacted specifically to each of the purified proteins (Fig. 5B). However, for purified ClpP, some degradation products of the ClpP protein were present (Fig. 5B).
FIG. 5.
Western immunoblot analysis of whole-cell lysates of S. pneumoniae D39 (A) and purified preparations of PdB (53 kDa), PspA fragment (43 kDa), and ClpP (21 kDa) (B) showing specificities of antibody responses to protein antigens. The proteins were separated by SDS-PAGE and then electroblotted onto nitrocellulose. They were then reacted with sera from groups of mice immunized with the proteins. Nitrocellulose membrane strips were reacted with sera from mice immunized with AlPO4 adjuvant (lane 1), PdB plus AlPO4 (lane 2), PspA plus AlPO4 (lane 3), and ClpP plus AlPO4 (lane 4).
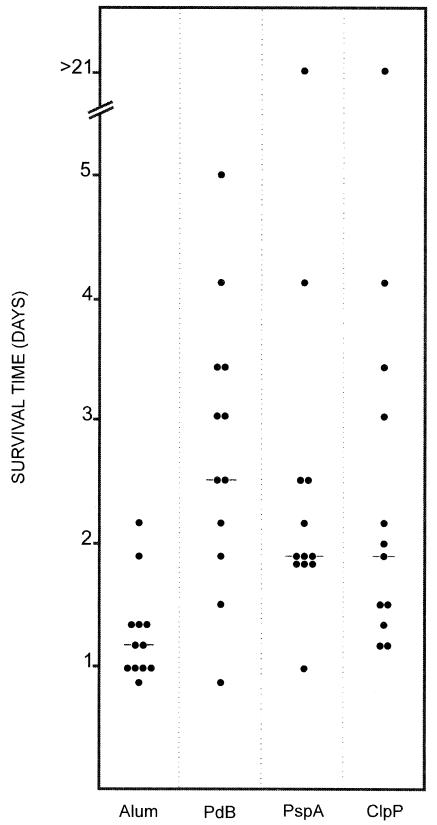
In the active immunization-challenge experiment, mice were challenged with ca. 7 × 105 CFU of D39. In this experiment, the median survival time for mice that received ClpP was approximately 2 days (Fig. 6). This survival time was significantly longer than that for mice that received the alum adjuvant alone (P < 0.01). Similarly, the median survival time for mice that received PspA (2 days) was significantly longer than that for mice that received the alum adjuvant alone (P < 0.001). For mice that received PdB (Ply toxoid), the median survival time was approximately 2.5 days, and this survival time was also significantly longer than that for mice that received the alum adjuvant alone (P < 0.001). However, when the median survival time for mice that received ClpP was compared to that obtained with either PdB or PspA, no significant differences were obtained.
FIG. 6.
Survival times for mice after intraperitoneal challenge. Groups of 12 CBA/N mice were immunized with the indicated antigens and challenged 2 weeks after the third immunization with approximately 7.5 × 105 CFU of capsular type 2 strain D39. Each datum point represents one mouse. A horizontal line denotes the median survival time for the group.
DISCUSSION
The aim of this study was to evaluate the role of ClpP, an HSP, in the pathogenesis of pneumococcal disease. Previously, Kwon et al. demonstrated that there was an increase in ply mRNA expression in a clpP mutant after heat shock, although there was no increase in the expression of Ply or its hemolytic activity (15). Since ClpP is known to be an ATP-dependent serine protease and does not bind DNA, it is unlikely that the clpP mutation affects ply gene expression directly. Thus, the increase in ply mRNA expression in the clpP mutant after heat shock could be due to an increase in the transcript level or an increase in ply mRNA stability. After heat shock, the ply transcript level in the clpP mutant was much higher than that in the D39 parent. In addition, the amount of cps(2)A mRNA in the clpP mutant increased after heat shock. These results corroborate previous findings that after heat shock, cps(2)A and ply transcript levels are increased in the clpP mutant (15).
In a previous report, the expression of ail was repressed in wild-type Yersinia enterocolitica, but in the respective clpP mutant, the expression of ail mRNA at 28°C was increased almost 20-fold, indicating that ClpP protease degrades a repressor of ail transcription. In contrast, the stability of ail mRNA did not increase in the clpP mutant (26). Also, in Salmonella enterica, ClpP was found to be a transcriptional repressor of the flagellar regulon (33). Recently, proteomic analysis revealed that ClpX, a substrate donor for ClpP protease, recognized transcriptional regulators such as the lexA (LexA repressor), rpoS (sigma S), rseA (negative regulator of sigma E), and rsd (regulator of sigma D) genes (9). Furthermore, an ATP-dependent Lon protease degraded a positive transcriptional regulator of capsule synthesis, RcsA, and induced the expression of capsular polysaccharide (colanic acid) synthesis genes in E. coli (30). Therefore, it is possible that S. pneumoniae ClpP acts as a transcriptional regulator of the ply and cps(2)A genes in some unknown way.
At 30°C, ply and cps(2)A mRNAs had longer half-lives in the clpP mutant than in the D39 parent. Furthermore, after heat shock, the half-lives of both cps(2)A and ply mRNAs in the clpP mutant were more than twofold longer than those in the wild type, indicating clearly that the half-lives of the mRNA species were increased in the absence of ClpP at 30 and 42°C. The hemolytic activity of Ply was not increased further by incubation of cell lysates at 37°C, indicating that ClpP is not directly responsible for activation of the hemolytic activity of Ply. We conclude from these findings that cps(2)A and ply mRNAs are subject to degradation in a ClpP-dependent fashion at the posttranscriptional level, but the specific mechanism by which this process occurs is unclear. This is the first demonstration that ClpP could affect mRNA stability.
Microarray analysis of the S. pneumoniae clpP mutant during exponential growth demonstrated the induction of genes that classically comprise the bacterial heat shock regulon (28). In contrast, the transcription of clpX and ftsH (ATP-dependent protease) was not induced even by heat shock (28). However, the levels of virulence factors after heat shock have not been determined. In this study, we demonstrated for the first time that ply transcript levels are much higher in the clpP mutant than in the wild type at both 30 and 42°C, whereas Ply protein levels are lower in the mutant than in the wild type (15). This paradox could be due to (i) rapid degradation of the ply mRNA after heat shock, (ii) decreased translation of the ply mRNA, and/or (iii) posttranslational modification of the ply transcript. Further studies are needed to distinguish among these possibilities.
In some pathogens, HSPs are present on the surface of cells and may mediate adherence to host cells (25, 27). In S. pneumoniae (4) and Listeria monocytogenes (21), ClpC was shown to be required for adherence and expression of virulence factors; S. pneumoniae clpC mutants displayed a deficiency in adherence to human type II alveolar cells and did not express Ply or the choline-binding protein CbpA, CbpE, CbpF, or CbpJ, suggesting that ClpC exerts a pleiotropic effect on adherence (4). In this study, we demonstrated that adherence and invasion of the clpP mutant of S. pneumoniae to host cells were not affected (data not shown).
Molecular chaperones of the Hsp70 and Hsp100 family have been shown to be associated with the translocation complex and to interact with translocation precursors (17, 36). Recently, B. subtilis ClpC and ClpX ATPases were detected at the cell envelope and in the cytoplasm (14). In those studies, translocation of the Clp protein after heat shock or by other stresses was not demonstrated. In this study, however, fractionation of S. pneumoniae revealed a substantial increase in the amount of ClpP in the cell wall after heat shock. Thus, ClpP is the first Clp protein shown to be mobilized into the cell wall fraction after heat shock; in this location it may interact with host cells or otherwise act by degrading pneumococcal proteins destined for transport or translocation.
Bacterial HSPs are induced during infection and mediate adherence and invasion in addition to playing a role in the proper folding of intracellular proteins (4, 21, 25). In this study, we demonstrated that immunization of mice with purified pneumococcal ClpP elicited protective immunity against systemic challenge with D39 to a level comparable to that achieved with the well-characterized pneumococcal protein vaccine candidates PspA and detoxified Ply (PdB). The fact that strong, antigen-specific antibody responses were generated in immunized mice prior to challenge suggests that the protection could, at least in part, be antibody mediated.
Acknowledgments
This work was supported by grants from the Korea Science and Engineering Foundation, the National Health and Medical Research Council of Australia, and the Meningitis Research Foundation (Bristol, United Kingdom). H.-Y.K was supported in part by the Brain Korea 21 Project in 2003.
Editor: V. J. DiRita
REFERENCES
- 1.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachey, E. H., C. S. Giampapa, and S. N. Abraham. 1988. Bacterial adherence. Adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am. Rev. Respir. Dis. 138:S45-S48. [DOI] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., and F. Heffron. 1990. Induction of Salmonella stress proteins upon infection of macrophages. Science 248:730-732. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier, E., R. Novak, and E. Tuomanen. 2000. Regulation of growth inhibition at high temperature, autolysis, transformation and adherence in Streptococcus pneumoniae by clpC. Mol. Microbiol. 37:717-726. [DOI] [PubMed] [Google Scholar]
- 5.Chastanet, A., M. Prudhomme, J. P. Claverys, and T. Msadek. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183:7295-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, I. H., J. H. Shim, S. W. Kim, S. N. Kim, S. N. Pyo, and D. K. Rhee. 1999. Limited stress response in Streptococcus pneumoniae. Microbiol. Immunol. 43:807-812. [DOI] [PubMed] [Google Scholar]
- 7.Derre, I., G. Rapoport, K. Devine, M. Rose, and T. Msadek. 1999. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol. Microbiol. 32:581-593. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez, R. C., S. M. Logan, S. H. Lee, and P. S. Hoffman. 1996. Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlate with virulence. Infect. Immun. 64:1968-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. M., S. B. Neher, Y. I. Kim, R. T. Sauer, and T. A. Baker. 2003. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11:671-683. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann, S. H. E., and B. Schoel. 1994. Heat shock proteins as antigens in immunity against infection and self, p. 495-532. In R. I. Morimoto, A. Tissieres, and C. Georgopoulos (ed.), Biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of CPS and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 13.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 14.Kruger, E., E. Witt, S. Ohlmeier, R. Hanschke, and M. Hecker. 2000. The clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins. J. Bacteriol. 182:3259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon, H. Y., S. W. Kim, M. H. Choi, A. D. Ogunniyi, J. C. Paton, S. H. Park, S. N. Pyo, and D. K. Rhee. 2003. Effect of heat shock and mutations in ClpL and ClpP on virulence gene expression in Streptococcus pneumoniae. Infect. Immun. 71:3757-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lim, J. H., F. Martin, B. Guiard, N. Pfanner, and W. Voos. 2001. The mitochondrial Hsp70-dependent import system actively unfolds preproteins and shortens the lag phase of translocation. EMBO J. 20:941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindemann, J., R. Leiacker, G. Rettinger, and T. Keck. 2002. Nasal mucosal temperature during respiration. Clin. Otolaryngol. 27:135-139. [DOI] [PubMed] [Google Scholar]
- 19.Lock, R. A., Q. Y. Zhang, A. M. Berry, and J. C. Paton. 1996. Sequence variation in the Streptococcus pneumoniae pneumolysin gene affecting haemolytic activity and electrophoretic mobility of the toxin. Microb. Pathog. 21:71-83. [DOI] [PubMed] [Google Scholar]
- 20.Nair, S., C. Frehel, L. Nguyen, V. Escuyer, and P. Berche. 1999. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol. Microbiol. 31:185-196. [DOI] [PubMed] [Google Scholar]
- 21.Nair, S., E. Milohanic, and P. Berche. 2000. ClpC ATPase is required for cell adhesion and invasion of Listeria monocytogenes. Infect. Immun. 68:7061-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidhardt, F. C., and R. A. VanBogelen. 1987. Heat shock response, p. 1334-1345. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 23.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 25.Parsons, L. M., R. J. Limberger, and M. Shayegani. 1997. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect. Immun. 65:2413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pederson, K. J., S. Carlson, and D. E. Pierson. 1997. The ClpP protein, a subunit of the Clp protease, modulates ail gene expression in Yersinia enterocolitica. Mol. Microbiol. 26:99-107. [DOI] [PubMed] [Google Scholar]
- 27.Raulston, J. E., C. H. Davis, D. H. Schmiel, M. W. Morgan, and P. B. Wyrick. 1993. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J. Biol. Chem. 268:23139-23147. [PubMed] [Google Scholar]
- 28.Robertson, G. T., W. L. Ng, J. Foley, R. Gilmour, and M. E. Winkler. 2002. Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J. Bacteriol. 184:3508-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouquette, C., C. de Chastellier, S. Nair, and P. Berche. 1998. The ClpC ATPase of Listeria monocytogenes is a general stress protein required for virulence and promoting early bacterial escape from the phagosome of macrophages. Mol. Microbiol. 27:1235-1245. [DOI] [PubMed] [Google Scholar]
- 30.Stout, V., A. Torres-Cabassa, M. R. Maurizi, D. Gutnick, and S. Gottesman. 1991. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 173:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple. H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 33.Tomoyasu, T., T. Ohkishi, Y. Ukyo, A. Tokumitsu, A. Takaya, M. Suzuki, K. Sekiya, H. Matsui, K. Kutsukake, and T. Yamamoto. 2002. The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuomanen, E. 1999. Molecular and cellular biology of pneumococcal infection. Curr. Opin. Microbiol. 2:35-39. [DOI] [PubMed] [Google Scholar]
- 35.Vijayakumar, M. N., and D. A. Morrison. 1986. Localization of competence-induced proteins in Streptococcus pneumoniae. J. Bacteriol. 165:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voisine, C., E. A. Craig, N. Zufall, O. von Ahsen, N. Pfanner, and W. Voos. 1999. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97:565-574. [DOI] [PubMed] [Google Scholar]
- 37.Willett, H. P. 1992. Streptococcus pneumoniae, p. 432-442. In W. K. Joklik, H. P. Willet, D. B. Amos, and C. M. Wilfert (ed.), Zinsser microbiology. Prentice-Hall International, London, United Kingdom.
- 38.Zugel, U., and S. H. Kaufmann. 1999. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 12:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]