Abstract
Anogenital distance (AGD) at birth is regarded as a useful measurement that reflects the prenatal androgenic status in rodents. However, the impact of xenoantiandrogens on human development is largely unknown. The aim of this study was to evaluate the potential antiandrogenic impact of prenatal DDT metabolites (p,p′-DDE and p,p′-DDT) exposure on infant AGD, using a non-age–dependent anal position index (API). As part of an ongoing perinatal cohort study on the effects of organochlorine pesticides in children’s neurodevelopment, we conducted a cross-sectional study in 71 infants (37 males and 34 females). Maternal serum levels of DDT metabolites (p,p′-DDE and p,p′-DDT) before and during each trimester of pregnancy were determined by electron capture gas–liquid chromatography. During postnatal home visits at 3, 6, and 12 or 18 months of age, the children’s weight and API were evaluated. Multiple lineal regression models were used to estimate the potential endocrine disruptor activity of prenatal p,p′-DDE exposure. Boys had significantly higher API values than girls (0.6 versus 0.5; P < 0.001). Only among boys, a doubling increase of maternal p,p′-DDE serum levels during the first trimester of pregnancy, were associated with a significant reduction of API (β = −0.02; P = 0.02). No effect of p,p′-DDT on AGD was observed. Evidence of the effect of prenatal p,p′-DDE on external genital differentiation is scarce and not consistent in the literature. Further studies are needed to confirm a hormonal disruptive effect on the development of external genitalia, due not only to p,p′-DDE but also due to other antiandrogenic persistent compounds.
Keywords: first trimester; endocrine disrupters; pregnancy; p,p′-DDE; anogenital distance; male infants; Mexico
Introduction
The distance from the anus to the onset of the penis is androgen dependent.1 Male rats have twice the content of androgens and a greater anogenital distance (AGD) than females, as male infants have a twofold greater AGD than female infants.2–4 Androgen exposure among female rodents increases the AGD and decreases the number of nipples.5,6 Very little information exists in relation to the effect of hormonal disruptors on human development; only one recent epidemiological study reported a negative association between phthalate exposure during pregnancy and AGD,7 in contrast to another study that was not able to detect a relationship between the DDT maternal exposure and AGD.8
The environmental exposure to the metabolite of DDT, 1,1-dichloro-2,2-bis (p-chlorophenyl)ethylene (DDE), during the critical period for sex differentiation in humans (the first 14 weeks of gestation) is a concern.9 DDE is a potent antiandrogenic compound that inhibits androgen-induced androgen-receptor (AR) transcriptional activity.10 DDT metabolites, and mainly DDE, are ubiquitous substances that remain in the environment and are found in biological samples from pregnant women even in countries where its use was banned many years ago.8,11–15
We report the evaluation of AGD in male and female infants, using a non-age–dependent measurement, the anal position index (API), in relation to the DDE maternal levels, before and during each trimester of pregnancy in a perinatal cohort study that is ongoing in a region in Morelos, Mexico, where there is a malaria endemic and where DDT was extensively used.
Materials and Methods
As part of an ongoing cohort study, we conducted a cross-sectional assessment of maternal DDT metabolites (before and during pregnancy) and AGD of the first children that were born in this cohort, by evaluating the effects of organochlorine maternal exposure on the neurodevelopment of their children. Details of the cohort assembling and follow-up have been published elsewhere.15
Briefly, the cohort study began in 2001 and included women of reproductive age, who were residents in four municipalities in the state of Morelos, Mexico, and attended premarital talks required by law. The women were followed until they became pregnant after which three individualized home visits were scheduled for their first, second, and third trimester of pregnancy, respectively. During each of the three individual home visits, we administered a questionnaire concerning the progress of the pregnancy and made anthropometric measurements (maternal weight and height). Blood samples were provided before and during each trimester of pregnancy. We obtained a re-consent form signature from each mother. This study was approved by the National Institute of Public Health’s Ethics Committee.
Postnatal, ongoing follow-up began at 1 month of age. A total of 71 children (37 males and 34 females) were selected for this report, after 12 exclusions (6 nonsingleton births, 5 births before 36 gestational weeks, and 1 child with cerebral atrophy). Anthropometric data (weight, height, and cephalic circumference) were obtained during each postnatal visit. The AGD, anococcyx (AC), and perineal (PA) distances were measured during the postnatal home visits between 3 to 18 months of the children’s age. Maternal serum levels of DDT metabolites (p,p′-DDE and p,p′-DDT) before and during each trimester of pregnancy were available for this study.
Anogenital Measurement
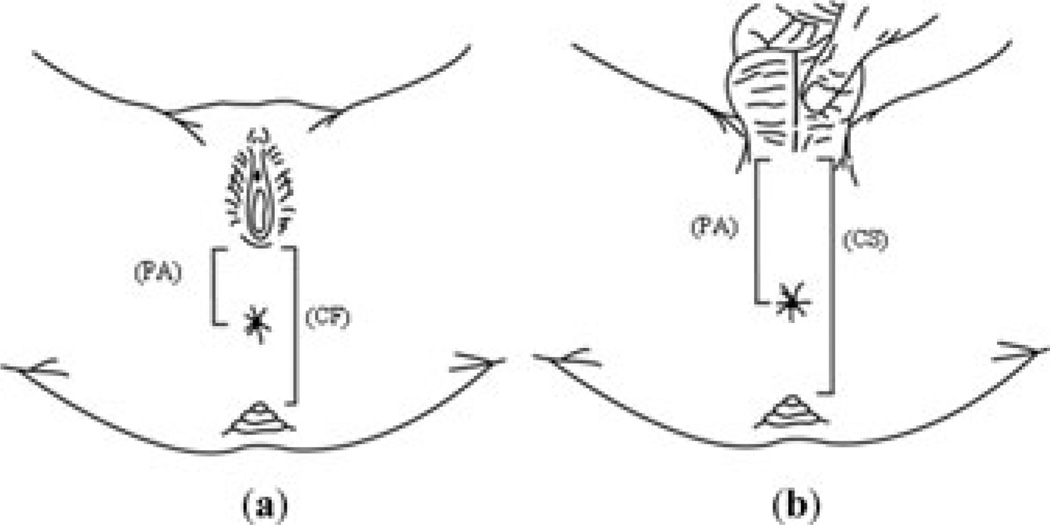
The AGD, AC, and PA distances were measured in the children studied once, either at 3, 6, 12, or 18 months of age. One trained medical student, who was unaware of the maternal organochlorine serum levels, performed all measurements. The infants were held in the lithotomy position and two paper strips with dull edges were placed along the longitudinal axis of the perineum and were marked at the center of the anus, the tip of the coccyx and at the first fold of the scrotum (scrotal–PA junction) for boys or vaginal fourchette for girls. The tape was placed on a table and we measured two separate distances: (1) the AC distance, measured between the center of the anus and the tip of the coccyx, and (2) the PA distance, measured between the center of the anus and the scrotal–PA junction for boys or the vaginal fourchette for girls. We used AC and PA distances to calculate the coccyx–scrotum (CS) or coccyx–fourchette (CF) distances for boys or girls, respectively (Fig. 1). The API was defined as the ratio of the PA to the CS distances for boys, and the ratio of the PA to the CF distances for girls. The API has been used previously and validated in clinical studies to evaluate normal anal implantation in relation to other pelvic structures and to diagnose anteriorly displaced anus.16
Figure 1.
Anogenital measurement according to sex of child. (a) The perineal (PA) distance, measured between the center of the anus and the scrotal–PA junction for boys or the vaginal–fourchette for girls. (b) The coccyx–scrotum (CS) or coccyx–fourchette (CF) distances for boys or girls, respectively.
Serum DDT Metabolites
Blood samples (7 mL) were taken from participating women at basal enrollment and during each trimester of pregnancy. After centrifugation, the serum obtained was stored at −70°C in glass vials (prewashed with pesticide-grade hexane) that were covered with a Teflon cap, until analyses. The measurements of, p,p′-DDE and p,p′-DDT, were carried out by means of electron capture gas–liquid chromatography (Model 3400; Varian, Inc., Palo Alto, CA), in agreement with the protocol recommended by the U.S. Environmental Protection Agency (1980).17 DDT metabolite concentrations were reported in lipid (ng/g) and wet basis (ng/mL; ppb). The detection limits in wet and lipid basis were 0.05 ng/mL and 0.685 ng/g for DDE, and 0.0045 ng/mL and 0.012 ng/g for p,p′-DDT. The total lipids in serum were determined using a colorimetric method kit (Randox Laboratories Ltd., Antrim, United Kingdom).
For internal quality control, each serum sample was fortified with Aldrin, and the average percentage recovery was 98.15 ± 8.8. For every 10 samples, we analyzed one bovine serum sample with known quantities of Aldrin, HCB, DDE, and DDD; the mean recovery rates were 100.0, 100.9, 103.4, and 104.1%, respectively. Additionally, in each batch we duplicated the analysis of a randomly selected sample; the coefficients of variation of the duplicate analyses were 4.37% for DDE and 0.77% for DDT.
Statistical Analysis
Selected infant characteristics were compared according to the sex of the child using Student’s t-test or χ2 tests, as appropriate. For comparison purposes with previous studies, the PA for males and females (anoscrotal and anofourchette, respectively) with (PA/W) and without (PA) weight adjustment were included in the analysis. Maternal levels of DDT metabolites before and during the pregnancy were not normally distributed, and their median differences were compared with the Kruskal-Wallis-test, depending on the child’s gender.
Lineal regression models were used to evaluate the association between DDT metabolites and genital parameters. Logarithm base 2 of p′p-DDT and p′p-DDE levels were entered in the models. Potential confounding variables included: birth weight (kg), age (months), and weight (kg) of a child at the time of anogenital evaluation, gestational age (weeks), pregnancy-induced hypertension (yes/no), tobacco use during pregnancy (yes/no), and alcohol consumption during pregnancy (yes/no). The final models only included those variables that modified the coefficients of interest by more than 10%. Model fitness included the evaluation of residual normality distributions and homoskedasticity, through Shapiro-Wilks and Shapiro-Francia tests and graphs (histograms, normal quartile, predicted versus standardized residuals). All analyses were conducted using STATA 9.2 (STATA, College Station, TX).
Results
The study population comprised a slightly higher proportion of boys compared to girls (52.1% versus 47.9%). Boys were significantly taller and heavier at birth and at the time of evaluation than girls (Table 1). Data not included in the table showed that 90.1% of the children were a product of a first pregnancy and 56.3% were born vaginally; only 5.6% of the mothers reported having pregnancy-induced hypertension, 4.2% of the mothers used tobacco, and 11.3% consumed alcohol during pregnancy.
TABLE 1.
Selected Characteristics by the Sex of the Child
| Characteristic | Boys n = 37 | Girls n = 34 | P-valueb,c |
|---|---|---|---|
| Gestational age (weeks) | |||
| Mean (SD) | 39.1 (1.6) | 39.2 (1.5) | 0.93 |
| Birth weight (kg) | |||
| Mean (SD) | 3.4 (0.4) | 3.0 (0.4) | <0.001 |
| Height (cm)a | |||
| Mean (SD) | 50.8 (1.8) | 49.4 (2.4) | 0.03 |
| Breast-feeding (%) | |||
| 0–12 weeks | 32.4 | 26.5 | 0.58 |
| >12 weeks | 67.6 | 73.5 | |
| Weight at evaluation (kg)d | |||
| Mean (SD) | 5.6 (1.4) | 4.8 (1.5) | <0.001 |
| Anogenital parameters | |||
| Anogenital distance (PA) (mm) | |||
| Mean (SD) | 42 (8.0) | 23 (6.0) | <0.001 |
| Anogenital distance (PA/W) (mm/kg) | |||
| Mean (SD) | 6.0 (13) | 3.6 (1.1) | <0.001 |
| Anal position index (API) | |||
| Mean (SD) | 0.6 (0.07) | 0.5 (0.07) | <0.001 |
Height: boys n = 34; girls n = 34.
t-Test for gestational age, birth weight, height, API.
χ2 test for breast-feeding.
Adjusted for age at evaluation.
All anogenital parameters were significantly higher for boys compared to girls (Table 1). The lowest difference was found with the API; the boys/girls ratio of each anogenital parameter ranged from 1.3 for the API to 1.8 for PA (data not included in Table 1). According to the cutoff point for the API suggested by Reisner et al. (0.46 for boys and 0.34 for girls), only one boy had an API value lower than of 0.42.16
Quantifiable levels of p′p-DDE were found in 100% of the maternal serum samples before and during the pregnancy (Table 2). The proportion of samples above the detection limit for p′p-DDT ranged from 10.2% in the third trimester to 31.7% in the first trimester (data not included in the table), and the DDT/DDE ratio tended to be zero. No significant differences of maternal median DDT metabolites levels were found between boys and girls at the basal, first, second, and third trimester of pregnancy.
TABLE 2.
Distribution of DDT Metabolites according to the Sex of the Child and Trimester of Pregnancy
| Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|
| DDT metabolites | Base line (n = 25) |
1st Trimester (n = 31) |
2nd Trimester (n = 29) |
3rd Trimester (n = 22) |
Base line (n = 24) |
1st Trimester (n = 29) |
2nd Trimester (n = 24) |
3rd Trimester (n = 27) |
| p,p′-DDE | ||||||||
| Wet basis (ng/mL) | ||||||||
| 10th Percentile | 1.7 | 2.1 | 1.1 | 3.4 | 2.1 | 1.1 | 7.0 | 4.3 |
| Median | 10.4 | 8.8 | 8.2 | 15.5 | 11.2 | 6.6 | 7.1 | 10.2 |
| 90th Percentile | 33.7 | 35.2 | 31.6 | 36.3 | 21.2 | 20.1 | 28.2 | 48.7 |
| Lipid basis (ng/g) | ||||||||
| 10th Percentile | 209.8 | 216.3 | 154.4 | 249.0 | 292.6 | 216.6 | 97.0 | 326.1 |
| Median | 2456.6 | 1714.8 | 1276.5 | 1274.2 | 1688.2 | 1407.9 | 1083.0 | 1040.1 |
| 90th Percentile | 5887.5 | 8864.2 | 5209.8 | 3688.3 | 3015.2 | 4354.5 | 4442.1 | 4273.6 |
| p,p′-DDT | ||||||||
| Wet basis (ng/L) | ||||||||
| 10th Percentile | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Median | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| 90th Percentile | 500 | 400 | 100 | 4.5 | 110 | 140 | 200 | 340 |
| Lipid basis (ng/kg) | ||||||||
| 10th Percentile | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 |
| Median | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 |
| 90th Percentile | 7700 | 10100 | 25 | 12.3 | 9300 | 26800 | 1700 | 12700 |
| DDT:DDE | 0.001 | 0.001 | 0.001 | 0.0003 | 0.001 | 0.001 | 0.001 | 0.0004 |
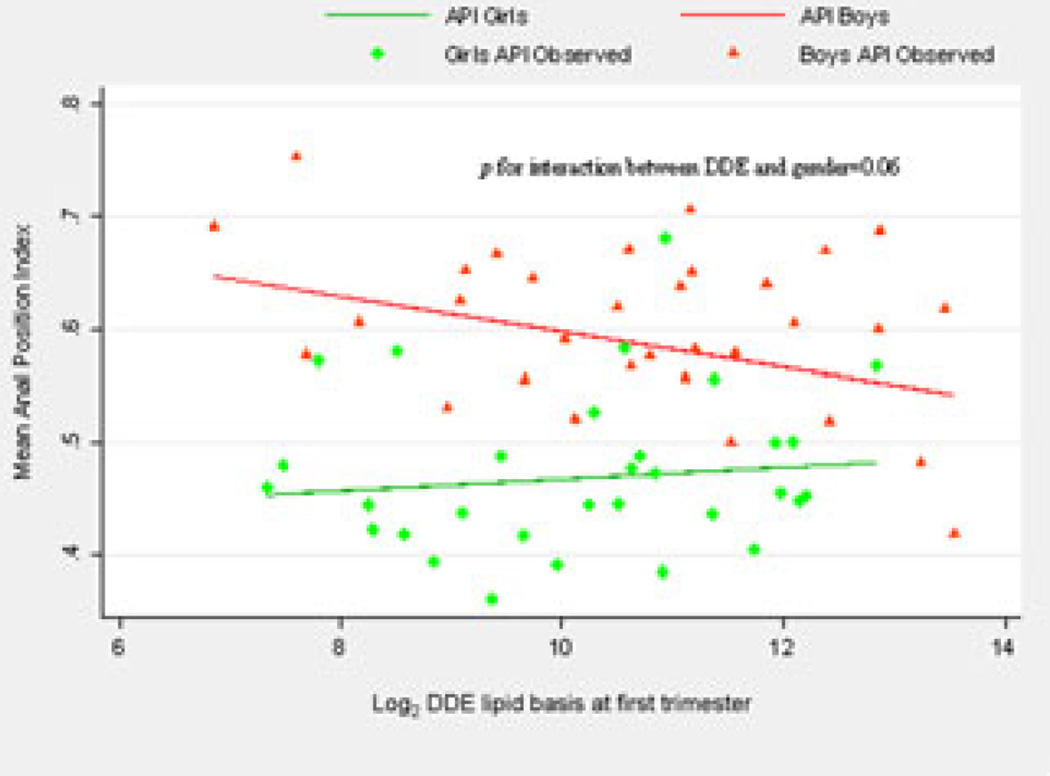
A significant reduction of API among boys (β = −0.02; P = 0.02), but not among girls, was found for a doubling increase of maternal DDE serum levels only during the first trimester of pregnancy, after adjusting by weight at birth as well as age at evaluation. No other significant associations were found between the rest of anogenital parameters and DDE (Table 3) nor DDT levels, respectively (data not shown). Gender significantly modified (Fig. 2) the effect of p′p-DDE on the API (P for interaction = 0.06.)
TABLE 3.
Effects of Maternal p,p′-DDE Serum Levels on Anogenital Parameters according to the Sex of the Child and Trimester of Pregnancy
| Boys | Girls | |||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P-value | Coefficient | 95% CI | P-value | |
| Anal position index (API) | ||||||
| p,p′-DDE (ng/g) | ||||||
| Base linea | −0.01 | −0.03, 0.01 | 0.34 | 0.004 | −0.02, 0.02 | 0.91 |
| 1st Trimesterb | −0.02 | −0.03, −0.003 | 0.02 | 0.003 | −0.01, 0.02 | 0.70 |
| 2nd Trimesterc | −0.01 | −0.02, 0.004 | 0.20 | 0.003 | −0.01, 0.02 | 0.82 |
| 3rd Trimesterd | −0.01 | −0.02, 0.01 | 0.43 | −0.01 | −0.03, 0.002 | 0.09 |
| Anogenital distance (PA) (mm)e | ||||||
| p,p′-DDE (ng/g) | ||||||
| Base linea | −0.02 | −0.23, 0.20 | 0.87 | 0.11 | −0.08, 0.34 | 0.35 |
| 1st Trimesterb | −0.08 | −0.22, 0.06 | 0.27 | 0.14 | −0.05, 0.30 | 0.10 |
| 2nd Trimesterc | −0.06 | −0.20, 0.07 | 0.36 | 0.11 | −0.05, 0.27 | 0.15 |
| 3rd Trimesterd | −0.12 | −0.26, 0.02 | 0.10 | 0.05 | −0.08, 0.18 | 0.44 |
| Anogenital distance/weight (PA/W) (mm/kg) | ||||||
| p,p′-DDE (ng/g) | ||||||
| Base linea | −0.02 | −0.29, 0.33 | 0.90 | 0.15 | −0.18, 0.48 | 0.36 |
| 1st Trimesterb | −0.15 | −0.37, 0.06 | 0.16 | 0.18 | −0.07, 0.43 | 0.15 |
| 2nd Trimesterc | −0.03 | −0.23, 0.17 | 0.75 | 0.09 | −0.12, 0.31 | 0.40 |
| 3rd Trimesterd | −0.10 | −0.31, 0.11 | 0.33 | 0.08 | −0.11, 0.29 | 0.40 |
Adjusted by weight and height at birth, age at evaluation.
Adjusted by birth weight, age at evaluation.
Adjusted by age at evaluation.
Adjusted by age at evaluation, birth weight.
All models included weight and age at evaluation.
Figure 2.
p,p′-DDE effect on the anal position index (API) according to the sex of the child.
Discussion
Our results showed a significant reduction in the male AGD due to the prenatal DDE exposure, specifically during the first trimester of pregnancy. To our knowledge, this is the first study to evaluate body burden levels of DDT metabolites before and during pregnancy that was able to identify the critical window of the hormonal disrupting effect of DDE on sex development (AGD). These findings are in good agreement with experimental studies, in particular with one that shows that the critical time frame for androgen action in the sexual development of rats is during a very early stage of gestation, as well as with recent epidemiological results showing a negative association between hormonal disrupting phthalate exposure and AGD in male children.7,10,18,19
The critical period of external genital differentiation is the first trimester of gestation, when a normal male fetus reaches high fetal androgen levels that result in full masculinization of the external genitalia. To achieve those morphological changes, external genitalia targeted tissue cells must reduce testosterone to the more potent, slower dissociating androgen, 5α-dihydrotestosterone (DHT), by the intracellular enzyme 5α-reductase.9 DHT is bound to AR and undergoes conformational changes and is imported to nuclei, where it dimerizes. It then binds to androgen response element DNA regulatory sequences within intron regions or flanking androgen responsive genes, resulting in transcriptional activation. During sex differentiation and development, androgen-induced gene products bring about androgen-dependent cell functions critical to sex differentiation of developing tissues.9 Probably the most important mechanisms for the putative androgen deficiency by p′p-DDE prenatal exposure are a reduction of transcriptional activity by blocking AR and/or a reduction in the bioavailability of testosterone due to an increase in the levels of sex hormone binding globulin.10,20,21
In contrast, among girls normal external genital development occurs as a consequence of the absence of androgens, which explains the lack of effect of exposure on anogenital female distance due to p′p-DDE observed in this study.9 Moreover, the lack of association with the levels of p′p-DDT and the three anogenital parameters strengthen the validity of our epidemiological results, since this specific metabolite does not exhibit antiandrogenic activity.
The AGD in infants has been heterogeneously assessed. At least two groups of measurement approaches are identified: those that directly account for an anthropometric reference value and those that do not.4,7,8,16,22–25 In a recent epidemiological study, a better fitness between prenatal phthalate exposure and AGD was reported when a model including AGD to weight instead of AGD and weight of the baby as independent variables was used.7 Thus, the direct adjustment of AGD with an anthropometric parameter should be a better estimator of the normal position of the anus. In this study, we used the API, which is a widely used index to diagnose displacement of the anus in infants.16,22–24 This index considers the distance to the coccyx as an anatomical reference and does not significantly change with age nor with the ethnic group.22
Recently, some authors in a larger study were not able to detect an association between p′p-DDE and AGD, which is the same observation we report in this study regarding the AGD distance (without considering the anthropometric parameter).8 Unfortunately, those authors did not include a non-age–dependent measurement of AGD; such is the case of the API, thus, our results are not strictly comparable with theirs.
In the view of the incipient evidence about the impact of prenatal p′p-DDE exposure on AGD, and the consistent results about its antiandrogenic effects on testicular function and/or the regulation of reproductive hormones, further studies are needed to confirm a hormonal disruptive effect on the development of external genitalia, not only due to p′p-DDE but also due to other antiandrogenic-persistent compounds.20,21
Footnotes
Conflicts of Interest
The authors declare no Conflicts of interest.
References
- 1.Kalloo NB, Gearhart JP, Barrack ER. Sexually dimorphic expression of estrogen receptors, but not of androgen receptors in human fetal external genitalia. J. Clin. Endocrinol. Metab. 1993;77:692–698. doi: 10.1210/jcem.77.3.8370691. [DOI] [PubMed] [Google Scholar]
- 2.Baum MJ, Woutersen PJ, Slob AK. Sex difference in whole-body androgen content in rats on fetal days 18 and 19 without evidence that androgen passes from males to females. Biol. Reprod. 1991;44:747–751. doi: 10.1095/biolreprod44.5.747. [DOI] [PubMed] [Google Scholar]
- 3.Marty MS, Chapin RE, Parks LG, Thorsrud BA. Development and maturation of the male reproductive system. Birth Defects Res. Part B. Dev. Reprod. Toxicol. 2003;68:125–136. doi: 10.1002/bdrb.10015. [DOI] [PubMed] [Google Scholar]
- 4.Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, et al. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ. Health. 2004;3:3–8. doi: 10.1186/1476-069X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss AK, Lambright CS, Ostby JS, et al. Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol. Sci. 2007;96:335–345. doi: 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- 6.Wolf CJ, LeBlanc GA, Gray LE., Jr Interactive effects of vinclozolin and testosterone propionate on pregnancy and sexual differentiation of the male and female SD rat. Toxicol. Sci. 2004;78:135–143. doi: 10.1093/toxsci/kfh018. [DOI] [PubMed] [Google Scholar]
- 7.Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longnecker MP, Gladen BC, Cupul-Uicab LA, et al. In Utero Exposure to the Antiandrogen 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) in Relation to Anogenital Distance in Male Newborns from Chiapas, Mexico. Am. J. Epidemiol. 2007;165:1015–1022. doi: 10.1093/aje/kwk109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. 6th. Baltimore, MD: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 10.Kelce WR, Stone CR, Laws SC, et al. Persistent DDT Metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;75:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Carrillo L, Torres-Arreola L, Torres-Sanchez L, et al. Is DDT se a public health problem in Mexico? Environ. Health Perspect. 1996;104:584–588. doi: 10.1289/ehp.104-1469381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koepke R, Warner M, Pet M, et al. Serum DDT and DDE levels in pregnant women of Chiapas, Mexico. Arch. Environ. Health. 2004;59:559–565. doi: 10.1080/00039890409603434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358:110–114. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- 14.Torres-Arreola L, Berkowitz G, Torres-Sánchez L, et al. Preterm birth in relation to maternal organochlorine serum levels. Ann. Epidem. 2003;13:158–162. doi: 10.1016/s1047-2797(02)00424-6. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Sánchez L, Rothenberg SJ, Schnaas L, et al. In utero p,p′-DDE exposure and infant neurodevelopment: a perinatal cohort in México. Environ. Health Perspect. 2007;115:435–439. doi: 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisner SH, Sivan Y, Nitzan M, Merlob P. Determination of anterior displacement if the anus in the newborn infants and children. Pediatrics. 1984;73:216–217. [PubMed] [Google Scholar]
- 17.U.S. Environmental Protection Agency. Manual of Analytical Methods for the Analysis of Pesticides in Humans and Environmental Samples. Washington, DC: U.S. EPA; 1980. [Google Scholar]
- 18.You L, Casanova M, Archibeque-Engle S, et al. Impaired male sexual development in perinatal Sprague-Dawley and Long-Evans hooded rats exposed in utero and lactationally to p,p′-DDE. Toxicol. Sci. 1998;45:162–173. doi: 10.1093/toxsci/45.2.162. [DOI] [PubMed] [Google Scholar]
- 19.Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148:3185–3195. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- 20.Ayotte P, Giroux S, Dewailly E, et al. DDT spraying for malaria control and reproductive function in Mexican men. Epidemiology. 2001;12:366–367. doi: 10.1097/00001648-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 21.De Jager C, Farias P, Barraza-Villarreal A, et al. Reduced seminal parameters associated with environmental DDT exposure and p,p′-DDE concentrations in men in Chiapas, Mexico: a cross-sectional study. J. Androl. 2006;27:16–27. doi: 10.2164/jandrol.05121. [DOI] [PubMed] [Google Scholar]
- 22.Bar-Maor JA, Eitan A. Determination of the normal position of the anus (with reference to idiopathic constipation) J. Pediatr. Gastroenterol. Nutr. 1987;6:559–561. doi: 10.1097/00005176-198707000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Davari HA, Nazem M. The anal position index: a simple method to define the normal position of the anus in the neonate. J. Res. Med. Sci. 2004;6:45–49. doi: 10.1080/08035250500449882. [DOI] [PubMed] [Google Scholar]
- 24.Genc A, Taneli C, Tansug N, et al. Evaluation of the location of the anus by a modified technique in the neonate. J. Pediatr. Surg. 2002;37:80–82. doi: 10.1053/jpsu.2002.29432. [DOI] [PubMed] [Google Scholar]
- 25.Rizk DEE, Tomas L. Relationship between the length of the perineum and the position of the anus and vaginal delivery in primigravidae. Int. Urogynecol. J. 2000;11:79–83. doi: 10.1007/s001920050074. [DOI] [PubMed] [Google Scholar]