Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) secretes a 20-kDa metalloprotease toxin termed B. fragilis toxin (BFT). ETBF disease in animals is associated with an acute inflammatory response in the intestinal mucosa, and lethal hemorrhagic colitis may occur in rabbits. In this study, we confirmed recent reports (J. M. Kim, Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho, Clin. Exp. Immunol. 123:421-427, 2001; L. Sanfilippo, C. K. Li, R. Seth, T. J. Balwin, M. J. Menozzi, and Y. R. Mahida, Clin. Exp. Immunol. 119:456-463, 2000) that purified BFT stimulates interleukin-8 (IL-8) secretion by human intestinal epithelial cells (HT29/C1 cells) and demonstrate that stimulation of IL-8 production is dependent on biologically active BFT and independent of serum. Induction of IL-8 mRNA expression occurs rapidly and ceases by 6 h after BFT treatment, whereas IL-8 secretion continues to increase for at least 18 h. Our data suggest that BFT-stimulated IL-8 secretion involves tyrosine kinase-dependent activation of nuclear factor-κB (NF-κB) as well as activation of the mitogen-activated protein kinases (MAPKs), p38 and extracellular signal-related kinase. Simultaneous activation of NF-κB and MAPKs appears necessary for secretion of IL-8 by HT29/C1 cells treated with BFT.
Bacteroides fragilis is a normal intestinal commensal and is identified in the colonic flora of up to 80% of children and adults (21). A subset of B. fragilis termed enterotoxigenic Bacteroides fragilis (ETBF) is associated with acute, self-limited diarrheal diseases in children, adults, and livestock (reviewed in reference 39). In addition, and consistent with data on other enteric pathogens, a sizeable proportion (4 to 20%) of control populations without diarrhea may be colonized, apparently asymptomatically, with ETBF strains (39). The pathogenicity of ETBF is ascribed to a heat-labile ∼20-kDa metalloprotease toxin (B. fragilis toxin [BFT], also called fragilysin) (23, 30). Our previous studies have shown that BFT rapidly (by 1 min) cleaves E-cadherin, an intercellular adhesion protein forming the zonula adherens of intestinal epithelial cells, and that cleavage of E-cadherin correlates with the onset of morphologic changes in the cells (occurring by 10 min after BFT treatment of HT29/C1 cells) (47). Consistent with this biological activity, BFT increases the permeability of intestinal epithelial cell monolayers and human colonic mucosa studied in vitro (23, 29, 35, 45). BFT also stimulates secretion in ligated intestinal segments of lambs, rats, rabbits, and calves, and secretion is also associated with changes in intestinal epithelial cell morphology (26, 30, 39).
Recent studies have demonstrated that BFT induces the expression of interleukin-8 (IL-8) in human intestinal epithelial cells (HT29, T84, and Caco-2) (15, 37). A small study has also suggested a significant association between detection of the bft gene in stool specimens of inflammatory bowel disease patients and the presence of active inflammatory bowel disease (33). Of note, enhanced synthesis of IL-8 has been shown in the mucosa from patients with active ulcerative colitis and Crohn's disease (1, 20). These data suggest the hypothesis that colonization with ETBF may promote acute or chronic intestinal inflammation in humans. Animal studies have demonstrated the presence of acute ileal and colonic inflammation in ETBF disease; in rabbits, severe inflammation with intestinal hemorrhage results (14, 24, 25, 27, 30, 40). These data suggest that intestinal inflammation may also contribute to the secretory response to BFT. However, the pathogenesis of ETBF-induced human intestinal disease is poorly understood. Neither intestinal histology nor studies of an intestinal inflammatory response are currently available for human ETBF disease or colonization. The aim of this study was to further evaluate the kinetics of IL-8 induction stimulated by BFT in intestinal epithelial cells and to investigate the intracellular signaling events yielding increased IL-8 levels following treatment of intestinal epithelial cells with BFT.
MATERIALS AND METHODS
Cell lines and cell culture.
HT29/C1 cells (cloned HT29 cell, obtained from Daniel Louvard, Institute Pasteur, Paris, France) derived from a human colon carcinoma were grown subconfluently on 24-well plates or as polarized monolayers as previously described (4). The cells were grown in Dulbecco's minimum essential medium (DMEM) containing streptomycin (0.1 mg/ml), penicillin (0.1 mg/ml), and 10% fetal bovine serum (FBS; HyClone, Logan, Utah). For detection of phosphorylated proteins, HT29/C1 cell lysates were prepared in 1% sodium dodecyl sulfate buffer containing 1 mM sodium orthovanadate (Sigma, St. Louis, Mo.) and protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, Ind.). All culture media and reagents were purchased from GIBCO BRL Life Technologies (Rockville, Md.) unless otherwise stated.
BFT purification and inhibitors/agonists.
BFT was purified from the culture supernatants of B. fragilis strain 086-5443-2-2 as previously described (43, 46). Cultured cells were washed once with Hanks' balanced salt solution before being treated with purified BFT at the specified concentrations in DMEM with or without 2% serum. The inhibitors utilized include the mitogen-activated protein kinase (MAPK) inhibitors SB203580 (p38 inhibitor; Calbiochem, San Diego, Calif.) and U126 (extracellular signal-related kinase [ERK] inhibitor; Calbiochem) and the tyrosine kinase inhibitors genistein (broad-spectrum tyrosine kinase inhibitor; Sigma), PP2 (selective Src-family tyrosine kinase inhibitor; Calbiochem), and tyrphostin AG1478 (selective epidermal growth factor receptor [EGFr] tyrosine kinase inhibitor; Calbiochem). The inhibitors were incubated with the cells for 30 min before BFT treatment or, for genistein, at intervals after BFT treatment (see Results). Phorbol myristate acetate (PMA) was obtained from Sigma.
Immunoblot analysis.
Immunoblotting was performed as described by Sambrook et al. (36). p38, phospho-p38, ERK, and phospho-ERK MAPK antibodies were obtained from Cell Signaling Technology, Inc. (Beverly, Mass.); anti-NF-κB p65 and anti-IκBα antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was a gift from M. Sirover (Temple University, Philadelphia, Pa.). Following the primary antibody incubation, blots were incubated with a 1:10,000 dilution of horseradish perioxidase-coupled secondary antibodies and developed using Supersignal Chemiluminescent Substrate (Pierce, Rockford, Ill.). Protein concentrations were measured using the bicinchoninic protein assay (Pierce).
IL-8 ELISA.
Cell culture medium was harvested from cells treated with BFT, BFT plus inhibitors, or control conditions for the times indicated in Results and frozen at −70°C until assayed for IL-8 by capture enzyme-linked immunosorbent assay (ELISA). Samples were run in duplicate on an enhanced protein binding ELISA plate (Corning Costar, Corning, N.Y.) and analyzed in reference to a 31.25- to 2,000-pg/ml standard curve of IL-8. Plates were coated with the primary anti-IL-8 capture monoclonal antibody (2 μg/ml) (PharMingen, San Diego, Calif.) overnight at 4°C, washed twice with phosphate-buffered saline (PBS)-Tween 20 (0.5%, vol/vol), and then blocked with PBS-10% FBS for 2 h at 25°C. The plates were washed to remove blocking buffer, and then the standards and samples were diluted in PBS-FBS, added to the plates coated with primary antibody, and incubated overnight at 4°C. The secondary anti-IL-8 biotinylated monoclonal antibody (PharMingen) was diluted in PBS-FBS (1 μg/ml) and added to washed plates, which were incubated for 45 min at 25°C. Avidin-peroxidase (Sigma) was added to the plates at a 1:400 (vol/vol) in PBS-FBS, the mixture was incubated at 25°C for 30 min, and the substrate o-phenylenediamine in citrate-phosphate buffer (0.4 mg/ml) was added to all wells. After 30 min, the absorbance at 405 nm was determined (SpectroMar 200; Molecular Devices).
Immunofluorescent staining.
HT29/C1 monolayers were fixed with 4% paraformaldehyde (Sigma), immunostained with a polyclonal rabbit antibody to NF-κB (C20; Santa Cruz), and incubated with Cy-5-labeled anti-rabbit immunoglobulin G. Cell nuclei were stained with Hoechst (Molecular Probes, Eugene, Oreg.). After the samples were washed to remove excess fluorescent dye, the signal was observed by single- or dual-channel confocal microscopy (LSM410; Zeiss).
RNA extraction and reverse transcription-PCR (RT-PCR).
Total cellular RNA was isolated using Trizol reagent (GIBCO BRL Life Technology, Rockville, Md.) as specified by the manufacturer. Total RNA (3 μg) was used for first-strand cDNA synthesis, using SuperScript II (GIBCO BRL Life Technology), at 42°C for 50 min. After RNA digestion, the IL-8 cDNA was amplified using primers 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ and 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ (13). To analyze the PCR products semiquantitatively, the cDNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in the same reaction tube by using the upstream and downstream primers 5′-TCCTGCACCACCAACTGCTTAG-3′ and 5′-TGCTTCACCACCTTCTTGATATC-3′, respectively, or the classic 18S PCR primer pair (Ambion, Austin, Tex.). PCR products were electrophoresed in a 2% agarose gel, and the bands were visualized by ethidium bromide staining.
Electrophoretic mobility shift assay (EMSA).
HT29/C1 cells were treated with toxin buffer (120 mM NaCl in 0.05 M Tris [pH 7.5], negative control), BFT (100 ng/ml for 1 or 3 h) or PMA (1 μg/ml for 1 h; positive control). Nuclear protein extraction was performed using NE-PER nuclear extraction reagent (Pierce, Rockford, Ill.) as specified by the manufacturer. Nuclear proteins (5 μg) in a buffer containing 20 mM Tris (pH 7.5), 0.5 μM EDTA, 1 μM dithiothreitol, 125 μg of bovine serum albumin per ml, 10% glycerol, and 1 μg of poly(dI-dC) were mixed with 0.5 ng of 32P-labeled double-stranded DNA probe (3) (kindly provided by W. Cao, Johns Hopkins University School of Medicine) corresponding to the promoter binding region of the NF-κB protein subunits. After a 30-min incubation at room temperature, the samples were separated on 5% polyacrylamide gel and exposed to X-ray film. The autoradiograph was analyzed using ImageQuant (Molecular Dynamics).
Statistical analysis.
Statistical analysis was performed using GraphPad InStat (GraphPad Software, San Diego, Calif.). Data were analyzed by Student's paired or unpaired t test. Standard deviations are shown unless otherwise indicated. A P value of ≤0.05 was considered statistically significant.
RESULTS
BFT biological activity, but not serum, is required for IL-8 induction.
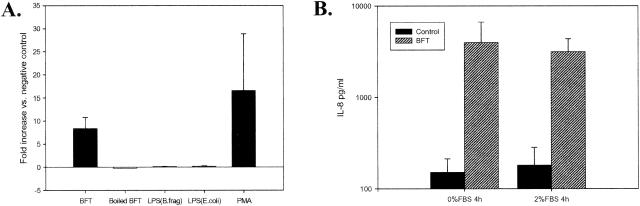
IL-8 secretion by human intestinal epithelial cells in vitro (HT29, Caco-2, and T84) in response to BFT treatment was recently reported by Kim et al. (15) and Sanfilippo et al. (37). However, the specificity of IL-8 induction by BFT was not addressed in these reports. In our experiments, subconfluent HT29/C1 cells, a cloned HT29 cell line, were treated with BFT (100 ng/ml), boiled BFT (to destroy the biological activity of BFT), and purified lipopolysaccharide (LPS) (5 μg/ml) from strains of B. fragilis and Escherichia coli for 18 h in DMEM with 2% FBS. HT29/C1 cells treated with PMA (1 μg/ml), a protein kinase C activator, or BFT buffer were used as positive and negative controls, respectively. BFT and PMA stimulated significant increases in IL-8 secretion compared to the negative control (Fig. 1A; P < 0.01 for each condition). Neither B. fragilis LPS nor E. coli LPS stimulated IL-8 secretion. Similarly, no IL-8 was secreted by HT29/C1 cells treated with boiled BFT, indicating that biologically active BFT is required to stimulate IL-8 secretion by HT29/C1 cells.
FIG. 1.
BFT specifically stimulates IL-8 secretion by HT29/C1 cells that is not serum dependent. (A) Subconfluent HT29/C1 cells were treated with BFT (100 ng/ml, for 18 h), boiled BFT, B. fragilis LPS (5 μg/ml), E. coli LPS (5 μg/ml), or PMA (1 μg/ml) in DMEM with 2% FBS. Secreted IL-8 was measured in the culture medium as described in Materials and Methods. P < 0.01, BFT versus untreated control HT29/C1 cells (n = 8 experiments); P < 0.01, PMA versus untreated control HT29/C1 cells (n = 3 experiments). (B) HT29/C1 cells were treated with BFT (100 ng/ml, for 4 h) or BFT buffer alone, and the level of IL-8 was measured in the culture medium as described in Materials and Methods. P ≤ 0.05 or 0.01, BFT versus control in the presence or absence of 2% FBS, respectively (N = 3 to 7 experiments per condition).
In some systems, stimulation of IL-8 production is serum dependent. For example, LPS-stimulated IL-8 production requires CD14 and/or LPS binding protein, which are present in serum (16, 34). Therefore, we tested if BFT induction of IL-8 secretion by HT29/C1 cells was serum dependent. HT29/C1 cells were treated with BFT (100 ng/ml) or BFT buffer in the presence or absence of serum for 4 h, and the level of IL-8 in the culture medium was measured. IL-8 production was significantly stimulated by BFT in both the absence of serum (P ≤ 0.01, n = 7) and the presence of 2% serum (P < 0.05, n = 3) (Fig. 1B).
Time course of BFT induction of IL-8 production.
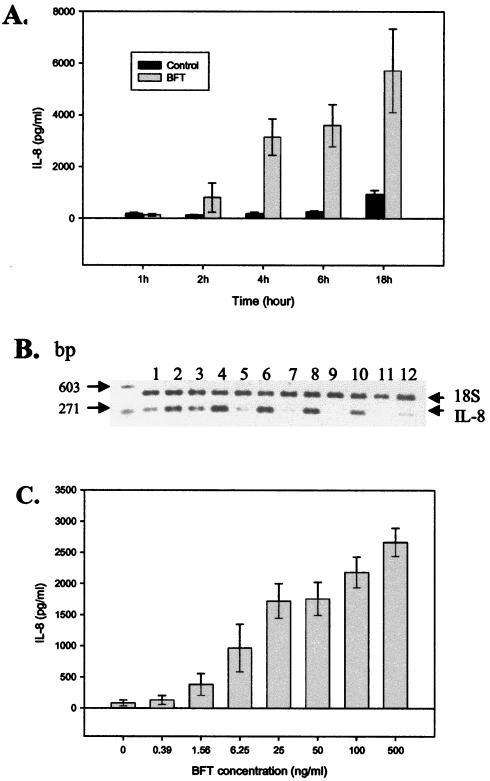
IL-8 secretion by BFT-treated HT29/C1 cells was first detected at 2 h and a significant increase in the amount of IL-8 was detected at 4, 6, and 18 h after treatment with BFT (100 ng/ml; P < 0.01 for each time point, n = 3) (Fig. 2A). Analysis of IL-8 mRNA by semiquantitative RT-PCR revealed increased expression from 1 to 6 h after BFT stimulation of HT29/C1 cells (Fig. 2B). Although the level of IL-8 mRNA decreased 6 h after BFT treatment of HT29/C1 cells, as shown by RT-PCR analysis, IL-8 concentrations in cell culture supernatants did not plateau until 18 to 24 h after BFT treatment (Fig. 2A and data not shown). Figure 2C shows the concentration dependence of BFT-stimulated IL-8 secretion. A significant increase in IL-8 secretion by HT29/C1 cells was stimulated by 1.56 ng of BFT per ml (7.8 × 10−11 M), and the response plateaued at 100 ng of BFT per ml (5 × 10−9 M).
FIG. 2.
Time course and concentration dependence of BFT-induced IL-8 production by HT29/C1 cells. (A) Subconfluent HT29/C1 cells were treated with BFT (100 ng/ml), and the level of secreted IL-8 was measured in the culture medium (DMEM with 2% FBS) at the indicated times, as described in Materials and Methods. P < 0.01 at 4, 6, and 18 h, BFT versus untreated control HT29/C1 cells (n = 3 experiments). (B) The time course of IL-8 expression was assessed by semiquantitative RT-PCR after BFT (100 ng/ml) treatment of subconfluent HT29/C1 cells. Results for untreated control cells are given in lanes 1 (1 h), 3 (2 h), 5 (3 h), 7 (4 h), 9 (6 h), and 11 (18 h). Results for BFT-treated cells are given in lanes 2 (1 h), 4 (2 h), 6 (3 h), 8 (4 h), 10 (6 h), and 12 (18 h). Synthesis of B. fragilis 18S rRNA was used as an internal control. (C) The concentration dependence of BFT-induced IL-8 production was assessed by treating HT29/C1 cells with various concentrations of BFT for 4 h. Cell supernatants were then assessed for IL-8 by ELISA. BFT at 1.56 ng/ml significantly stimulated IL-8 secretion compared to the control (P < 0.02), with a maximal effect at 100 ng/ml. n = 3 to 4 experiments per BFT concentration.
NF-κB, tyrosine kinase, and MAPK signaling pathways are involved in BFT-stimulated IL-8 secretion.
Activation of the transcriptional regulator, NF-κB, plays a key role in IL-8 expression (12, 41). In most cells, NF-κB is found in the cytoplasm and is associated with several inhibitory molecules termed IκBs, whose major isoforms are IκBα and IκBβ. NF-κB activation by a stimulus is typically initiated when IκBα is phosphorylated and then ubiquinated and degraded by proteasome proteolysis. Free NF-κB then translocates to the nucleus, inducing the transcription of genes possessing κB binding sites (19). Using EMSA and reporter assay methods, Kim et al. (15) demonstrated that NF-κB was activated by BFT in uncloned HT29 cells and that activation of an IL-8 reporter by BFT was inhibited by IκBα and IκB kinase superrepressors. Using EMSA, we confirmed that NF-κB is activated in HT29/C1 cells by 3 h, but not 1 h, of treatment with BFT (100 ng/ml) and that this activation was similar to that observed when HT29/C1 cells were treated with the positive control, PMA (1 μg/ml, 1 h) (data not shown). The specificity of the detected binding was demonstrated by inhibition of the binding of the labeled DNA probe by a 100-fold excess of unlabeled DNA probe corresponding to the NF-κB promoter binding region. Analysis of the time course of IκBα degradation in HT29/C1 cell lysates by Western blotting after the cells were treated for up to 3 h with BFT (100 ng/ml) revealed that degradation of IκBα occurred at approximately 2 h of BFT treatment (data not shown).
MAPKs regulate cellular responses to growth factors and stress stimuli and transmit signals from the cell surface to the nucleus via three distinct but related pathways dependent on ERK, p38 and JNK (c-Jun N-terminal kinase) (9, 44). All three signaling cascades have been implicated in the control of cytokine, including IL-8, transcription (8, 9, 44). To evaluate whether a MAPK pathway was involved in BFT-stimulated IL-8 production, specific p38 and ERK MAPK inhibitors (SB203580 and U126, respectively) were tested (18). Compared to HT29/C1 cells stimulated by BFT (100 ng/ml for 4 h) without inhibitors, SB203580 (10 μM) and U126 (10 μM) both inhibited approximately 80% of BFT-stimulated IL-8 secretion (Table 1). Treatment of HT29/C1 cells with both inhibitors extinguished IL-8 induction by BFT (data not shown). Neither SB203580 nor U126 inhibited the HT29/C1 cell morphologic changes stimulated by BFT (data not shown).
TABLE 1.
Amount of IL-8 secreted after treatment of HT29/C1 cells with inhibitorsa
| Inhibitorb | Amt of secreted IL-8 (pg/ml) (mean ± SD)c |
|---|---|
| Control | 130.60 ± 61.82 |
| BFT | 1973.60 ± 170.68 |
| Genistein | 45.00 ± 53.40 |
| Genistein + BFT | 184.75 ± 100.16 |
| SB203580 | 53.50 ± 9.19 |
| SB203580 + BFT | 359.90 ± 20.51 |
| U126 | 37.25 ± 54.96 |
| U126 + BFT | 386.25 ± 210.97 |
| PP2 | 75.33 ± 56.59 |
| PP2 + BFT | 533.33 ± 296.34 |
| AG1478 | 86.00 ± 71.04 |
| AG1478 + BFT | 710.00 ± 239.89 |
HT29/C1 cells were treated with BFT (100 ng/ml) for 4 h.
Inhibitor concentrations were as follows: genistein 100 μM; SB203580, 10 μM; U126, 10 μM; PP2, 5 μM; and AG1478, 10 μM.
P < 0.001, BFT versus control or BFT versus BFT plus each inhibitor tested.
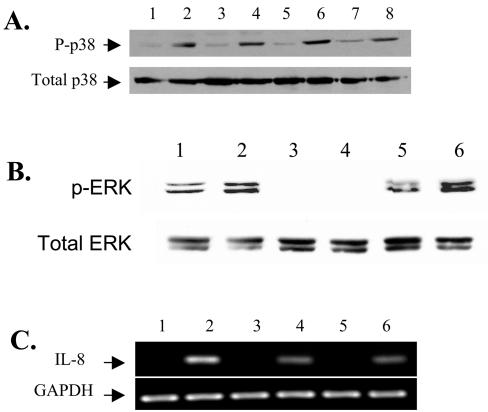
To confirm the activation of the p38 kinase in HT29/C1 cells stimulated by BFT, the active phosphorylated form of p38 kinase was examined by Western blotting in cells treated with BFT for 30 min to 3 h. These data revealed that p38 kinase is activated by 30 min after BFT stimulation and that p38 activation persists for at least 4 h (Fig. 3A and data not shown). Based on these data, the active phosphorylated form of ERK was assessed at 1 h by Western blotting, which revealed that BFT treatment of HT29/C1 cells also induced the phosphorylation of ERK (Fig. 3B, lanes 1 and 2). In addition, the ERK inhibitor, U126, inhibited both basal and BFT-augmented ERK phosphorylation (lanes 3 and 4). To assess whether p38 kinase and/or ERK regulates IL-8 transcription, the amount of IL-8 mRNA in cells treated with BFT (100 ng/ml, 4 h) in the presence and absence of SB203580 (10 μM) or U126 (10 μM) was measured semiquantitatively by RT-PCR. IL-8 transcription was inhibited similarly in HT29/C1 cells treated with BFT and SB203580 or with BFT and U126 compared to cells treated with BFT alone (Fig. 3C). These results suggest that BFT acts through both p38 kinase and the ERK pathways to stimulate IL-8 transcription.
FIG. 3.
BFT activates p38 and ERK MAPK. (A) Subconfluent HT29/C1 cells were treated with BFT (100 ng/ml) for various periods and assessed for phosphorylated p38 kinase by Western blotting. Results for untreated control cells are in lanes 1 (30 min), 3 (1 h), 5 (2 h), and 7 (3 h). Results for BFT-treated cells are in lanes 2 (30 min), 4 (1 h), 6 (2 h), and 8 (3 h). Total p38 protein served as an internal control for protein loading. (B) Subconfluent HT29/C1 cells were treated with BFT for 1 h and assessed for phosphorylated ERK kinase by Western blotting. Lanes: 1, control; 2, BFT (100 ng/ml); 3, U126 (10 μM) alone; 4, U126 plus BFT; 5, genistein (100 μM) alone; 6, Genistein plus BFT. Total ERK served as an internal control for protein loading. (C) IL-8 expression was assessed by semiquantitative RT-PCR after BFT treatment of subconfluent HT29/C1 cells for 4 h in the presence or absence of p38 (SB203580) or ERK (U126) MAPK inhibitors. Lanes: 1, untreated control cells; 2, BFT (100 ng/ml); 3, SB203580 (10 μM) alone; 4, SB203580 plus BFT; 5, U126 (10 μM) alone; 6, U126 plus BFT. Synthesis of GAPDH mRNA was used as an internal benchmark control.
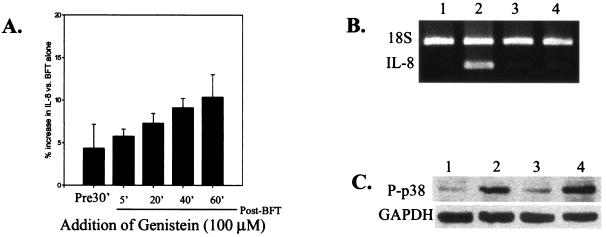
To investigate a role for tyrosine phosphorylation in BFT-stimulated IL-8 production, the effect of genistein on IL-8 secretion stimulated by BFT was evaluated. Genistein is a broad-spectrum tyrosine kinase inhibitor that blocks both receptor and nonreceptor protein tyrosine kinases (6, 7). When HT29/C1 cells were incubated with genistein (100 μM) for 30 min prior to the addition of BFT (100 ng/ml, for 4 h), IL-8 secretion and transcription were nearly completely blocked (Table 1; Fig. 4A and B). Of note, genistein treatment of HT29/C1 cells prior to the addition of BFT transiently (for approximately 30 min) delayed the onset of HT29/C1 cell morphology changes and E-cadherin cleavage in response to BFT (data not shown). Genistein added after BFT (5 to 60 min after BFT addition) also significantly inhibited IL-8 secretion (Fig. 4A; N = 3 or 4, P ≤ 0.01 at each time point). However, addition of genistein after BFT did not modify the time course of HT29/C1 morphologic changes in response to BFT compared to the response to BFT alone (data not shown). Because both genistein and MAPK inhibitors blocked IL-8 transcription, we used Western blotting to evaluate whether genistein (100 μM, for 4 h) inhibited the activation of p38 or ERK kinases. Figures 3B (lanes 5 and 6) and 4C reveal that genistein did not inhibit the activation of ERK and p38 kinases, respectively, suggesting that MAPK activation in BFT-treated cells is not tyrosine kinase dependent.
FIG. 4.
The tyrosine kinase inhibitor, genistein, inhibits BFT-induced IL-8 secretion. (A) Subconfluent HT29/C1 cells treated with BFT (100 ng/ml, for 4 h) in serum-free DMEM. Genistein (100 μM) was added either 30 min before or at different times (5 to 60 min) after BFT. Data are presented as the percentage of IL-8 secreted compared to that in BFT-treated cells in the absence of genistein (n = 3 to 4 experiments per time point). P ≤ 0.01, BFT alone versus genistein plus BFT at any time point. (B) IL-8 expression was assessed by semiquantitative RT-PCR after BFT treatment (100 ng/ml, for 4 h) of subconfluent HT29/C1 cells in the presence or absence of genistein (100 μM). Genistein was added 30 min prior to treatment of the cells with BFT. Lanes: 1, control HT29/C1 cells; 2, BFT treatment only; 3, genistein treatment only; 4, BFT plus genistein. Synthesis of B. fragilis 18S rRNA was used as an internal control. (C) The activation of p38 MAPK was analyzed by Western blotting, as described in Materials and Methods, in the presence or absence of BFT (100 ng/ml, for 4 hs) or genistein (100 μM). Genistein was added 30 min prior to treatment of the cells with BFT. Lanes: 1, control HT29/C1 cells; 2, BFT treatment only; 3, genistein treatment only; 4, BFT plus genistein. GAPDH was used as a benchmark control for protein loading.
To further examine the role of tyrosine kinase activation in BFT-stimulated IL-8 production, the effect of PP2 (5 μM), a selective inhibitor of Src-family nonreceptor protein tyrosine kinases, and tyrphostin AG1478 (10 μM), an EGFr tyrosine kinase-specific inhibitor, were studied (6). PP2 and AG1478 significantly blocked the secretion of IL-8 by BFT-treated HT29/C1 cells by approximately 73 and 64%, respectively (P < 0.001 for each) (Table 1). In additional experiments, the effect of simultaneous treatment with both PP2 and AG1478 on BFT-induced IL-8 production by HT29/C1 cells was shown to be nonadditive (data not shown). Unlike genistein, neither PP2 nor AG1478 inhibited BFT-induced HT29/C1 cell morphologic changes.
Relationship of NF-κB, tyrosine kinase(s), and MAPK to BFT-stimulated IL-8 production.
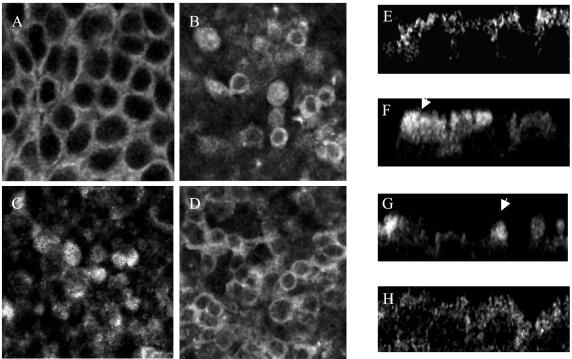
To evaluate the interrelationship of the NF-κB, tyrosine kinase, and MAPK pathways activated in response to BFT treatment of HT29/C1 cells, NF-κB nuclear translocation in HT29/C1 monolayers was evaluated by confocal microscopy after BFT treatment in the presence and absence of tyrosine kinase and MAPK inhibitors. Figure 5A and E shows that NF-κB is located mainly at the cell borders and in the cytoplasm of untreated control HT29/C1 cells. After BFT treatment (100 ng/ml, for 3 h), redistribution of NF-κB was observed (Fig. 5B and F) in some, but not all, cells. NF-κB that redistributed after BFT treatment coalesced in a supranuclear position (arrows), with a modest speckled pattern of intranuclear NF-κB accumulation in some HT29/C1 cells. This pattern of NF-κB cellular redistribution differed from that observed in HT29/C1 cells treated with PMA (1 μg/ml) for 1 h. In PMA-treated cells, NF-κB translocated predominantly to the nuclei, where a diffuse pattern of fluorescence was detected (data not shown). In HT29/C1 cells treated with BFT in the presence of SB203580 (Fig. 5C and G) or U126 (data not shown), the pattern of NF-κB distribution was similar to that in cells treated only with BFT. Simultaneous treatment with SB203580 and U126 also resulted in a pattern of NF-κB redistribution similar to that in cells treated only with BFT (data not shown). Thus, MAPK inhibitors did not alter NF-κB redistribution in HT29/C1 cells after BFT treatment. In contrast, when HT29/C1 cells were treated with BFT in the presence of genistein (Fig. 5D and H), NF-κB redistribution did not occur in the majority of the BFT-treated cells, resulting in an NF-κB immunofluorescence pattern most similar to that in untreated control HT29/C1 cells. However, pretreatment of HT29/C1 cells with PP2 or AG1478 did not inhibit NF-κB redistribution, yielding a NF-κB immunofluorescent pattern similar to that in cells treated with BFT alone (data not shown).
FIG. 5.
BFT induces IL-8 secretion that is dependent on activation of MAPKs, tyrosine kinase(s), and NF-κB. Confocal analysis of the location of NF-κB in HT29/C1 monolayers in the presence or absence of BFT treatment and/or inhibitors was performed. (A to D) are xy plane confocal sections; (E to H) z plane confocal sections. (A and E). Untreated control HT29/C1 cells. (B and F) BFT-treated (100 ng/ml, for 3 hs) HT29/C1 cells. Note the coalescence of NF-κB in a supranuclear position (arrow) of some, but not all, cells. (C and G). HT29/C1 cells treated with the p38 inhibitor, SB203580 (10 μM), for 30 min prior to BFT treatment (100 ng/ml, for 3 h). Note that the redistribution of NF-κB (arrow) is similar to that in cells treated only with BFT. (D and H) HT29/C1 cells treated with the tyrosine kinase inhibitor, genistein (100 μM), for 30 min prior to BFT treatment (100 ng/ml, for 3 h). Note that the distribution of NF-κB is similar to that in control cells for the majority of BFT-treated cells. The observed differences from control cells are secondary to the cell shape changes stimulated by BFT, which are associated, in some cells, with loss of the basal location of the HT29/C1 cell nuclei (4), resulting in a less uniform pattern on xy confocal sections compared to control cells. Treatment of HT29/C1 cells with any of the inhibitors in the absence of BFT did not alter the cellular location of NF-κB compared to that in control HT29/C1 cells (data not shown). The data shown are representative of three experiments.
DISCUSSION
IL-8 is a major chemotactic and activating peptide for neutrophils, and increased IL-8 expression is found in both acute and chronic inflammation (12). Both purified BFT and/or ETBF infections have been demonstrated to stimulate acute mucosal inflammation in ligated ileal loops in lambs, rabbits, and rats (14, 24, 25, 27, 30, 40). However, the mechanism by which BFT induces inflammation is unknown. Recent studies by Kim et al. (15) and Sanfilippo et al. (37) revealed that BFT induces IL-8 secretion from epithelial cell lines including HT29, T84, and Caco-2, suggesting that IL-8 induction is one mechanism by which BFT stimulates inflammation. Our study further characterizes IL-8 induction by BFT in HT29/C1 cells, a cloned human colonic carcinoma epithelial cell line. Our data reveal that IL-8 is induced in intestinal epithelial cells in vitro in a concentration-dependent but serum-independent manner by biologically active BFT but not by purified E. coli or B. fragilis LPS. Additional data (A. A. Franco, C. Polyak, J. P. Castillo, and C. L. Sears, submitted for publication) indicate that a single amino acid substitution in the zinc binding metalloprotease motif of BFT yields biologically inactive BFT that is also incapable of stimulating IL-8 secretion by HT29/C1 cells.
Activation of NF-κB is essential for up-regulation of IL-8 gene transcription in epithelial cells and other cell lines, although maximal IL-8 transcriptional activation usually requires more than one transcriptional activator (22, 41). Consistent with these data, mutation of the NF-κB binding site in the promoter region of the IL-8 gene abolishes IL-8 expression (22). IL-8 up-regulation has also been linked to MAPK as well as calcium- and tyrosine kinase-dependent pathways (10, 42, 44). The precise interactions of signal transduction mechanisms, such as NF-κB and MAPKs, contributing to the regulation of IL-8 gene expression in response to cellular stimuli remain under active investigation (5, 9, 17, 38). Depending on the cell type studied, both MAPK-dependent activation of NF-κB and apparently direct activation of cytokine gene transcription by MAPKs have been reported (5, 17, 38).
Our studies begin to examine the signaling pathways that BFT triggers to stimulate IL-8 expression and secretion by HT29/C1 cells. We confirmed the results of Kim et al (15) indicating that BFT activates NF-κB. The degradation of IκBα after BFT treatment of HT29/C1 cells, expected to occur prior to NF-κB activation and initiation of IL-8 transcription, as well as the activation of NF-κB, was relatively delayed compared to, for example, the rapid activation of NF-κB activation by LPS in dendritic cells (2). In addition, NF-κB redistribution after BFT treatment of HT29/C1 cells revealed a predominantly supranuclear localization, in contrast to a diffuse nuclear pattern after NF-κB activation by PMA. These results mimic the pattern of NF-κB activation previously detected in uncloned HT29 cells treated with IL-1β (11). Our data suggest that both ERK and p38 MAPKs act to activate IL-8 expression and secretion in BFT-treated HT29/C1 cells. Although inhibition of both MAPKs abolished BFT-initiated IL-8 secretion, the cellular redistribution of NF-κB (consistent with NF-κB activation) was not altered. These results suggest that NF-κB activation by BFT is independent of MAPK activation and that MAPK activation may act at the transcriptional level, probably in concert with NF-κB, to induce IL-8 gene expression in response to BFT.
Genistein, a broad-spectrum tyrosine kinase inhibitor, also nearly ablated IL-8 production induced by BFT, even as late as 60 min after treatment of HT29/C1 cells with BFT. Genistein also inhibited NF-κB cellular redistribution in the majority of BFT-treated cells, suggesting that tyrosine phosphorylation may regulate BFT-initiated NF-κB activation and IL-8 expression. Genistein did not block the phosphorylation of p38 or ERK MAPKs, further suggesting that MAPK activation alone by BFT is insufficient to stimulate IL-8 transcription. Since genistein inhibited NF-κB redistribution, but not p38 or ERK activation, in BFT-treated HT29/C1 cells, it is also unlikely that BFT stimulates MAPK-dependent NF-κB activation. Of note, genistein added to HT29/C1 cells prior to BFT not only blocked IL-8 production by HT29/C1 cells but also transiently (for approximately 30 min) delayed the onset of HT29/C1 cell morphology changes and E-cadherin cleavage in response to BFT. In contrast, genistein added after BFT blocked BFT-induced IL-8 secretion without modifying the morphologic response to BFT. Based on these data, we postulate that BFT activates an early tyrosine kinase-dependent signal transduction step prior to the onset of E-cadherin cleavage (detected as early as 1 min after treatment of HT29/C1 cells with BFT and linked to BFT-induced cell morphology changes [47]) whereas an additional distinct tyrosine kinase(s) may be involved in the regulation of BFT-induced IL-8 transcription.
Of interest, both specific receptor (EGFr) and nonreceptor (Src kinase) tyrosine kinase inhibitors also significantly inhibited BFT-induced IL-8 production by HT29/C1 cells. However, in contrast to the ablation of BFT-induced IL-8 secretion by simultaneous inhibition of p38 and ERK, the EGFr and Src kinase inhibitors tested were nonadditive in inhibiting BFT-induced IL-8 secretion. In addition, in contrast to genistein, neither inhibitor blocked NF-κβ activation when examined by confocal microscopy or inhibited HT29/C1 cell morphologic changes in response to BFT treatment. The nonadditive inhibition of BFT-stimulated IL-8 secretion suggests that EGFr and Src kinases probably act serially or serve redundant functions in the IL-8 signal transduction pathway stimulated by BFT. Consistent with this interpretation, it has been proposed, using similar experimental approaches, that EGFr transactivation by protease-activated receptor 2 agonists triggers Src, contributing to subsequent cell proliferation (6). Intriguingly, BFT also stimulates HT29/C1 cell proliferation (48), suggesting overlap between the signal transduction pathways yielding IL-8 production and cellular proliferation in response to BFT. The more limited activities of the EGFr and Src kinase inhibitors than of genistein is also consistent with our postulate (cited above) that the biological activity of BFT is mediated by distinct early and late tyrosine kinase-dependent mechanisms. Together, our data suggest that BFT-induced IL-8 transcriptional regulation is complex, involving NF-κB, p38, and ERK MAPKs and receptor and nonreceptor tyrosine kinases. Additional studies of the timing and sequence of the signal transduction mechanisms activated by BFT are necessary before the interrelationships of these signaling proteins are understood.
Cleavage of E-cadherin in response to BFT treatment of intestinal epithelial cells results in the loss of intercellular adhesion and a complete disruption of at least some tight junctions (4, 47). Our preliminary data (S. Wu and C. L. Sears, unpublished data) suggest that BFT specifically binds to a host cell receptor that is not E-cadherin. We propose a model in which ETBF colonizes the luminal surface of the ileum and/or colon, releasing BFT, which stimulates cleavage of E-cadherin after binding to its intestinal epithelial cell receptor. The biological activity of BFT has been detected in the stools of ETBF-infected humans and animals (28, 31, 32). However, it is not yet known how much BFT is secreted by ETBF in vivo or whether BFT directly cleaves E-cadherin or if E-cadherin cleavage occurs via activation of a host cell protease. Our data herein suggest that an early tyrosine kinase-dependent signal transduction step precedes E-cadherin cleavage in BFT-treated intestinal epithelial cells. Cleavage of E-cadherin is associated with loss of the integrity of the apical F-actin ring of intestinal epithelial cells, resulting in diminished epithelial cell resistance and cell morphology changes (4, 35). BFT also triggers the activation of multiple signal transduction mechanisms including β-catenin signaling, stimulating intestinal epithelial cell proliferation (48), and NF-κB, tyrosine kinase(s), and MAPKs, leading to IL-8 secretion. However, our data demonstrating a dissociation between the timing of the cellular morphologic changes (an early event) stimulated by BFT and IL-8 secretion (a delayed event) suggest that E-cadherin cleavage may not be essential to activation of all of these signal transduction mechanisms (Fig. 4A). Polarized secretion of IL-8 by BFT-treated intestinal epithelial cells (15; Wu and Sears, unpublished) probably results in an IL-8 concentration gradient in the lamina propia predicted to chemotactically attract neutrophils, yielding a mucosal inflammatory response that contributes to the pathogenesis of ETBF infection. Further studies to investigate the interrelationships of the signal transduction pathways stimulated by BFT and their linkage to E-cadherin cleavage will provide further insights into the mechanism of action of BFT and the pathogenesis of ETBF infection.
Acknowledgments
We thank Dwight Derr for his assistance with tissue culture and Kathy Strauss for her assistance with the IL-8 ELISAs.
This work was supported by National Institutes of Health award RO1 DK45496 to C.L.S.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Banks, C., A. Bateman, R. Payne, P. Johnson, and N. Sheron. 2003. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J. Pathol. 199:28-35. [DOI] [PubMed] [Google Scholar]
- 2.Blaecke, A., Y. Delneste, N. Herbault, P. Jeannin, J. Y. Bonnefoy, A. Beck, and J. P. Aubry. 2002. Measurement of nuclear factor-kappa B translocation on lipopolysaccharide-activated human dendritic cells by confocal microscopy and flow cytometry. Cytometry 48:71-79. [DOI] [PubMed] [Google Scholar]
- 3.Cao, W., C. Bao, and C. J. Lowenstein. 2003. Inducible nitric oxide synthase expression inhibition by adenovirus E1A. Proc. Natl. Acad. Sci. USA 100:7773-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers, F. G., S. S. Koshy, R. F. Saidi, D. P. Clark, R. D. Moore, and C. L. Sears. 1997. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 cells) in vitro. Infect. Immun. 65:3561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, R., A. Larkin, A. M. Mingo, D. J. Thuerauf, C. Andrews, P. M. McDonough, and C. C. Glembotski. 2000. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 275:23814-23824. [DOI] [PubMed] [Google Scholar]
- 6.Darmoul, D., V. Gratio, H. Devaud, and M. Laburthe. 2004. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J. Biol. Chem. 279:20927-20934. [DOI] [PubMed] [Google Scholar]
- 7.Du, X. L., Z. Gao, C. P. Lau, S. W. Chiu, H. F. Tse, C. M. Baumgarten, and G. R. Li. 2004. Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J. Gen. Physiol 123:427-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English, J., G. Pearson, J. Wilsbacher, J. Swantek, M. Karandikar, S. Xu, and M. H. Cobb. 1999. New insights into the control of MAP kinase pathways. Exp. Cell Res. 253:255-270. [DOI] [PubMed] [Google Scholar]
- 9.Hazzalin, C. A., and L. C. Mahadevan. 2002. MAPK-regulated transcription: a continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 3:30-40. [DOI] [PubMed] [Google Scholar]
- 10.Jefferson, K. K., M. F. Smith, Jr., and D. A. Bobak. 1999. Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J. Immunol. 163:5183-5191. [PubMed] [Google Scholar]
- 11.Jobin, C., S. Haskill, L. Mayer, A. Panja, and R. B. Sartor. 1997. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J. Immunol. 158:226-234. [PubMed] [Google Scholar]
- 12.Jobin, C., and R. B. Sartor. 2000. The I kappa B/NF-kappa B system: a key determinant of mucosal inflammation and protection. Am. J. Physiol. Ser. C. 278:C451-C462. [DOI] [PubMed] [Google Scholar]
- 13.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka- Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay, B. A., R. Rahman, D. A. Sack, M. A. K. Azad, K. A. Chowdhury, and R. B. Sack. 1990. Bacteroides fragilis as a potential cause of human diarrheal disease in Bangladesh, p. 269-276. In R. B. Sack and Y. Zinnaka (ed.), Advances in research on cholera and related diarrheas, vol. 7. KTK Scientific Publishers, Tokyo, Japan. [Google Scholar]
- 15.Kim, J. M., Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho. 2001. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-kappa B plays a major role in the regulation of IL-8 expression. Clin. Exp. Immunol. 123:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirschning, C., A. Unbehaun, N. Lamping, D. Pfeil, F. Herrmann, and R. R. Schumann. 1997. Control of transcriptional activation of the lipopolysaccharide binding protein (LBP) gene by proinflammatory cytokines. Cytokines Cell Mol. Ther. 3:59-62. [PubMed] [Google Scholar]
- 17.Krause, A., H. Holtmann, S. Eickemeier, R. Winzen, M. Szamel, K. Resch, J. Saklatvala, and M. Kracht. 1998. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 273:23681-23689. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, J. R. Heys, S. W. Landvatter, et al. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 19.Mahida, Y. R., and S. Johal. 2001. NF-kappa B may determine whether epithelial cell-microbial interactions in the intestine are hostile or friendly. Clin. Exp. Immunol. 123:347-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormack, G., D. Moriarty, D. P. O'Donoghue, P. A. McCormick, K. Sheahan, and A. W. Baird. 2001. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm. Res. 50:491-495. [DOI] [PubMed] [Google Scholar]
- 21.Moore, W. E. C., and L. V. Holdeman. 1974. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl. Microbiol. 27:961-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 23.Mundy, L. M., and C. L. Sears. 1996. Detection of toxin production by Bacteroides fragilis: assay development and screening of extraintestinal clinical isolates. Clin. Infect. Dis. 23:269-276. [DOI] [PubMed] [Google Scholar]
- 24.Myers, L. L., D. S. Shoop, and J. E. Collins. 1990. Rabbit model to evaluate enterovirulence of Bacteroides fragilis. J. Clin. Microbiol. 28:1658-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers, L. L., D. S. Shoop, J. E. Collins, and W. C. Bradbury. 1989. Diarrheal disease caused by enterotoxigenic Bacteroides fragilis in infant rabbits. J. Clin. Microbiol. 27:2025-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, L. L., D. S. Shoop, B. D. Firehammer, and M. M. Border. 1985. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in calves. J. Infect. Dis. 152:1344-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers, L. L., D. S. Shoop, L. L. Stackhouse, F. S. Newman, R. J. Flaherty, G. W. Letson, and R. B. Sack. 1987. Isolation of enterotoxigenic Bacteroides fragilis from humans with diarrhea. J. Clin. Microbiol. 25:2330-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers, L. L. and C. S. Weikel. 1992. Enterotoxin as a virulence factor in Bacteroides fragilis-associated diarrhoeal disease, p. 90-100. In B. I. Duerden, J. S. Brazier, S. V. Seddon, and W. G. Wade (eds.), Medical and environmental aspects of anaerobes. Wrightson Biomedical Publishing Ltd., Petersfield, United Kingdom.
- 29.Obiso, R. J., Jr., A. O. Azghani, and T. D. Wilkins. 1997. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect. Immun. 65:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obiso, R. J., Jr., D. M. Lyerly, R. L. Van Tassell, and T. D. Wilkins. 1995. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 63:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantosti, A., M. G. Menozzi, A. Frate, L. Sanfilippo, F. D'Ambrosio, and M. Malpeli. 1997. Detection of enterotoxigenic Bacteroides fragilis and its toxin in stool samples from adults and children in Italy. Clin. Infect. Dis. 24:12-16. [DOI] [PubMed] [Google Scholar]
- 32.Pantosti, A., C. Piersimoni, and G. Perissi. 1994. Detection of Bacteroides fragilis enterotoxin in the feces of a child with diarrhea. Clin. Infect. Dis. 19:809-810. [DOI] [PubMed] [Google Scholar]
- 33.Prindiville, T. P., R. A. Sheikh, S. H. Cohen, Y. J. Tang, M. C. Cantrell, and J. Silva, Jr. 2000. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 6:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugin, J., C. C. Schurer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riegler, M., M. Lotz, C. L. Sears, C. Pothoulakis, I. Castagliuolo, CC. Wang, R. Sedivy, T. Sogukoglu, E. Cosentini, G. Bischof, W. Feil, B. Teleky, G. Hamilton, J. T. LaMont, and E. Wenzl. 1999. Bacteroides fragilis toxin-2 damages human colonic mucosa in vitro. Gut 44:504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Sanfilippo, L., C. K. Li, R. Seth, T. J. Balwin, M. G. Menozzi, and Y. R. Mahida. 2000. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-beta) by human colonic epithelial cells. Clin. Exp. Immunol. 119:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC- activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Ser. G 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 39.Sears, C. L. 2001. The toxins of Bacteroides fragilis. Toxicon 39:1737-1746. [DOI] [PubMed] [Google Scholar]
- 40.Sears, C. L., L. L. Myers, A. Lazenby, and R. L. Van Tassell. 1995. Enterotoxigenic Bacteroides fragilis. Clin. Infect. Dis. 20(Suppl. 2):S142-S148. [DOI] [PubMed] [Google Scholar]
- 41.Thanos, D., and T. Maniatis. 1995. NF-kappa B: a lesson in family values. Cell 80:529-532. [DOI] [PubMed] [Google Scholar]
- 42.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1992. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect. Immun. 60:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warny, M., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weikel, C. S., F. D. Grieco, J. Reuben, L. L. Myers, and R. B. Sack. 1992. Human colonic epithelial cells, HT29/C1, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect. Immun. 60:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu, S., L. A. Dreyfus, A. O. Tzianabos, C. Hayashi, and C. L. Sears. 2002. Diversity of the metalloprotease toxin produced by enterotoxigenic Bacteroides fragilis. Infect. Immun. 70:2463-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu, S., K.-C. Lim, J. Huang, R. F. Saidi, and C. L. Sears. 1998. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 95:14979-14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, S., P. J. Morin, D. Maouyo, and C. L. Sears. 2003. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 124:392-400. [DOI] [PubMed] [Google Scholar]