Highlights
-
•
Glucosinolates, isothiocyanates and amino acids increase significantly over time.
-
•
Glucoraphanin is not significantly affected by harvesting and processing.
-
•
Sulforaphane significantly increases after processing in E. sativa cultivars.
-
•
Bacterial load of leaves is correlated with glucosinolate and amino acid abundance.
-
•
Commercial processing may increase the nutritional value of E. sativa to consumers.
Keywords: Eruca sativa, Diplotaxis tenuifolia, Brassicaceae, Liquid chromatography mass spectrometry, Capillary electrophoresis, Gas chromatography mass spectrometry, Sugars, Processing
Abstract
Five cultivars of Eruca sativa and a commercial variety of Diplotaxis tenuifolia were grown in the UK (summer) and subjected to commercial growth, harvesting and processing, with subsequent shelf life storage. Glucosinolates (GSL), isothiocyanates (ITC), amino acids (AA), free sugars, and bacterial loads were analysed throughout the supply chain to determine the effects on phytochemical compositions.
Bacterial load of leaves increased significantly over time and peaked during shelf life storage. Significant correlations were observed with GSL and AA concentrations, suggesting a previously unknown relationship between plants and endemic leaf bacteria.
GSLs, ITCs and AAs increased significantly after processing and during shelf life. The supply chain did not significantly affect glucoraphanin concentrations, and its ITC sulforaphane significantly increased during shelf life in E. sativa cultivars. We hypothesise that commercial processing may increase the nutritional value of the crop, and have added health benefits for the consumer.
1. Introduction
The majority of rocket (Eruca sativa & Diplotaxis tenuifolia) consumed in the UK is imported from Italy (Bell, Spadafora, Müller, Wagstaff, & Rogers, 2016). In 2015 sales of bagged rocket salad in the UK increased 3.9% on the previous year (Dr. Lorraine Shaw, Bakkavor, Spalding, UK; personal communication, 2016) and this trend is expected to continue in future.
Leaves are typically harvested by machine from long, linear beds in open fields, polytunnels or glasshouses. Time from sowing to harvest can be between 20 and 40 days depending on the growing region and species (Bell, Oruna-Concha, & Wagstaff, 2015), and has been reported to extend up to 99 days in winter months (Hall, Jobling, & Rogers, 2012). Growing methods vary according to region and grower preference. Produce for the bagged salad market is generally processed in the same way; after harvesting, leaves are vacuum chilled and stored under cool-chain conditions (<5 °C) until processing. This may be at the site of harvest, a nearby facility, or after transport to the country where it will be sold and consumed. Leaves enter a ‘low care’ environment, and are typically washed in chlorinated water (Rico, Martín-Diana, Barat, & Barry-Ryan, 2007) with mechanically induced water turbulence to remove detritus. Leaves are spin-dried in a high care environment to remove excess water, and then passed into a ‘high care’ environment, where it is weighed and bagged. Products use micro or laser perforated bags that contain modified or unmodified atmosphere to preserve and prolong self life (Hall, Jobling, & Rogers, 2013). Bags are shipped through a cold-chain to supermarkets and other vendors who store them in open-fronted chiller cabinets (Hall et al., 2013). Shelf life of rocket has been reported to range from seven to 14 days depending on environmental conditions (Martínez-Sánchez, Allende, Cortes-Galera, & Gil, 2008).
The stressful nature of the supply chain on leafy produce has led to questions regarding how nutritional value is affected (Verkerk et al., 2009). It is known that adverse storage conditions post harvest have a negative impact upon the appearance and odour of leaves (Lokke, Seefeldt, & Edelenbos, 2012). Cutting and processing material also makes it more perishable during storage (Watada, Ko, & Minott, 1996), and temperature is the predominant means by which degradation is controlled (Lokke et al., 2012). There has been little research into how nutritional traits are affected by the industrial supply chain in leafy salads. Studies have covered parts of the supply chain for different Brassicaceae, such as effects of cutting and washing (Martínez-Sánchez et al., 2008), post harvest storage (Bell et al., 2016), and packaging treatments (Rangkadilok et al., 2002).
In this study, a commercial supply chain was utilised to assess phytochemical profiles of rocket salads across multiple time points – immature leaves, harvest, processing, and throughout shelf life. Building upon previous phytochemical, sensory and consumer analyses (Bell et al., 2015, Bell et al., 2016, Bell et al., 2017), six underutilised germplasm accessions and one commercial variety were tested for glucosinolate (GSL), isothiocyanate (ITC), free amino acid (AA), and free sugar concentrations. The aim of our work is to inform the breeding selections and practices of industrial collaborators to create new, sensorially and nutritively enhanced varieties of rocket. The accessions used throughout have been shown to vary significantly in phytochemical composition under controlled environmental conditions, but it is unknown how these might change under industrial circumstances.
Rocket is well known for accumulating GSL compounds, which are hydrolysed by myrosinase enzymes into ITCs, nitriles, and other degradation products (Bell & Wagstaff, 2014). ITCs such as erucin (4-(methylthio)-butyl-ITC) and sulforaphane (4-(methylsulfinyl)-butyl-ITC) are both present in rocket species, and their potential anticarcinogenic properties are well studied in the literature (Traka et al., 2013). Other ITCs present in rocket are not well understood. The GSLs DMB (dimeric 4-mercaptobutyl-GSL), glucosativin (4-mercaptobutyl-GSL), diglucothiobeinin (4-(β-d-glucopyranosyldisulfanyl)-butyl-GSL), and their respective myrosinase degradation products are poorly understood in terms of abundance and anti-cancer properties. As demonstrated in Bell et al. (2017) some of the volatile derivatives of the GSL-myrosinase reaction, infer significant associations with sensory attributes such as bitterness and pungency. Some GSLs such as glucoerucin and glucoraphanin have no significant sensory properties associated with them.
In Bell et al. (2017), total AA concentration was negatively correlated with the perceptions of bitterness and pungency, leading to the hypothesis that certain AAs contribute to sensory qualities of the crop (Solms, 1969). The way AAs respond to commercial processing may therefore impact upon sensory traits, and are an important indicator of senescence and tissue breakdown (Buchanan-Wollaston et al., 2003). Free sugars may also impact sensory attributes by masking bitter and pungent sensations, though it is unknown how they are affected by processing in rocket.
Another important aspect of rocket in the supply chain is the presence of bacteria (which are naturally present on leaves). Usually these are non-pathogenic strains and do not pose a health concern for humans, but can contribute to spoilage and shorten shelf life (Lokke et al., 2012). It has been known for over 20 years that chlorinated or chemically treated water does not erradicate bacterial populations from leaves, but does have a role to play in ensuring sanitation of recirculated water in processing facilities. Strict field technical control protocols are followed to prevent contamination with pathogenic strains (Dr. Lorraine Shaw, Bakkavor, Spalding, UK; personal communication, 2016), however native leaf bacteria reside within cells and crevaces on the leaf surface, making it impossible to fully remove them from fresh-cut produce (Watada et al., 1996). ITCs are known to have antibacterial effects (Vig, Rampal, Thind, & Arora, 2009) but this relationship has not been studied in the context of the commercial supply chain. Free sugars may also provide a food source for bacteria, and we question how natural populations respond to concentrations within leaves during commercial processing and shelf life.
With the aforementioned aspects in mind (Verkerk et al., 2009), we hypothesised that GSL and ITC content would decline significantly over time due to a combination of GSL hydrolysis and leaching into wash water. We theorised that this would lead to a reduction in the nutritive and health beneficial properties of leaves. We also hypothesised that with a decrease in potentially anti-microbial compounds (ITCs) bacterial populations would increase and peak during shelf-life. The results presented in this paper show however that these hypotheses could be rejected, and that processing of rocket leaves may add nutritional value to the crop.
2. Materials & methods
2.1. Plant material
The five non-commercial accessions used in this paper (E. sativa) were originally sourced from European germplasm collections. See Bell et al. (2015) for information regarding the supplying institutes. Due to the small amounts of seed given, each cultivar was individually bulked by open pollination in separate glasshouse compartments at Elsoms Seeds Ltd. (Spalding, UK) in the spring/summer of 2014. The amount of seed produced for each cultivar weighed >500 g. The commercial variety Torino (Diplotaxis tenuifolia) used as a comparator to gene bank-sourced cultivars.
2.2. Growing & industrial supply chain conditions
Plants were grown in an open field at a Bakkavor supplier, (Dorchester, England) from the 3rd to the 25th of July 2014. Cultivars were sown using a tractor mounted air drill in parallel beds measuring approximately 50 m in length. Torino was sown as a guard crop surrounding the trial beds, and crop protection and irrigation of the trial was as per standard commercial practice.
Plants were harvested on the morning of 25th of July 2014 (22 days old) by machine. Due to the slower growth of Torino, plants drilled on the same date as the E. sativa cultivars were not harvested. Leaves were loaded into crates, which were placed into a waiting trailer. From harvest (H) onwards, five temperature data loggers (Tinytag Transit 2, −40 to +70 °C sensitivity range; Gemini Data Loggers Ltd., Chichester, UK) were added to crates and set to record one data point every five minutes for the remainder of the trial. See Fig. S1 for a temperature-time plot of averaged data. The temperature on the day of harvest was unusually hot for UK summer time, and the recorded average was 34.8 °C.
A tractor-trailer loaded with samples was driven approximately one mile to a storage facility. Crates were unloaded into a vacuum cooler, which removed field heat from the produce. Samples were stored in a 4 °C cold store, in the dark, for two days; the average temperature for this period was 4.9 °C. Samples were transported on the third day after harvest to a Bakkavor processing site via temperature-controlled lorry. Produce was stored in a 4 °C environment for the remainder of that day, but temperatures ranged between 2.2 °C and 8.6 °C during this time.
The following day, samples were processed using a commercial wash line with mild water chlorination. Each cultivar was entered into the line separately with a five-minute gap between to prevent mixing. Leaves were spin-dried, before being transferred by conveyor belt to be bagged in unmodified atmosphere, micro-perforated bags. Produce was stored overnight under controlled conditions; temperatures averaged 5.1 °C in the processing environment. The day after, samples were transported via courier in a temperature-controlled vehicle to the University of Reading (UoR), but temperatures as high as 14.3 °C were recorded during this time, representing a potential breach in the cold-chain (Fig. S1). The temperature upon arrival at UoR was 21.7 °C.
2.3. Shelf-life storage conditions
Samples were then stored in the dark continuously, for nine days in a controlled temperature storage room set to 4 °C. Temperatures varied, reaching an average low of 3.9 °C, and an average high of 6.4 °C. Storage conditions represent typical refrigeration temperatures used for storing rocket salad, although a range between 0 °C and 4 °C is considered optimal within the literature (Dekker, Verkerk, & Jongen, 2000).
2.4. Sample collection
Leaf samples were taken at ten time points (n = 3), spanning the previously described supply chain, with each bagged sample treated as one replicate. Bacterial count samples were taken separately, with each replicate weighing ∼30 g (n = 3).
The first samples were taken 12 days after sowing and included all E. sativa samples but not Torino because of the disparity in growth stages. These samples were designated ‘preharvest’ (PH) and represent produce at an immature growth stage. Both leaf and cotyledon were sampled and taken from random points along the complete length of each bed to avoid any potential bias from localised field effects. At harvest (H) Torino was again not sampled, as it was not of marketable leaf size. Samples were taken from multiple crates of harvested material spanning the length of the trial to again avoid bias. For both PH and H, samples were placed immediately into Ziploc freezer bags and frozen on dry ice in polystyrene containers to prevent phytochemical changes during transit. Samples were transported by car to UoR, taking approximately two hours. Upon arrival samples were placed into a −80 °C freezer.
Sampling at delivery to the Bakkavor processing site was designated ‘post transport’ (PT). It was at this time that the commercial variety Torino was first sampled. Samples were taken from a crop from the same producer and harvested on the same day, but had been sown approximately seven days before the E. sativa cultivars.
The following day, two time point samples were taken and designated ‘pre-wash’ (PR) and ‘post-wash’ (PW), and were again taken from random crates to avoid bias, and frozen on dry ice. Transit time from the Bakkavor processing site to UoR was approximately one hour.
Upon arrival at UoR the following day, transported bagged samples were taken and placed directly into a −80 °C freezer. This time point was designated ‘day 0′ of shelf life (D0). Subsequent samples were taken at ‘day 2′ (D2), ‘day 5′ (D5), ‘day 7′ (D7; commercial display-until date, DUD), and ‘day 9′ (D9; DUD + 2) in an identical fashion.
All samples were lyophilized in batches for three days. Dried tissue was milled using a Mini Cutting Mill (Thomas Scientific, Swedesboro, NJ, USA) into fine powder. Samples were stored in a cool, dry, dark place until analyses began. All time points were examined by analytical methods (see following sections), with the exception of pH and H time points for Torino (as explained previously), and D9 for bacterial counts. Due to the time consuming nature of extraction, only time points PT and D7 were analysed for GSL hydrolysis products in each cultivar.
2.5. Bacterial counting
2.5.1. General
Total plate count (TPC) of the plant materials was determined at nine different processing points (PH – D7) for the cultivars of E. sativa. Samples of Torino began at time point PT.
2.5.2. Preparation of nutrient agar for TPC
11.75 g of standard plate count agar (APHA; Oxoid Ltd., Basingstoke, UK) was diluted in 500 ml of distilled water and stirred until boiling, giving a final concentration of 2.4% (w/v). Agar was sterilised (15 min at 121 °C) and subsequently kept in a 50 °C water bath to maintain molten state.
2.5.3. Preparation of maximum recovery diluent for sample preparation & enumeration
For sample preparation, 9.5 g of maximum recovery diluent (MRD; Sigma, Gillingham, UK) was diluted in 1 L of distilled water (0.95% w/v) and stirred until completely dissolved. 90 ml of MRD was poured into 100 ml bottles and then sterilised (15 min at 121 °C). The mixture was left to cool, or was kept in a 4 °C cold room for longer-term storage. For enumeration, MRD was prepared in an identical fashion. 9 ml of MRD was then transferred to a bottle, sterilised and cooled, as above.
2.5.4. Total plate count
10 g of rocket leaves was added to the 90 ml preparation of MRD and placed in a stomacher (400 Circulator; Seward, Worthing, UK) and shaken for 120 s (230 rpm) to create a 10−1 dilution (w/v). 1 ml of the homogenised inoculum was sampled and serially diluted into the 9 ml MRD preparation to obtain 10−2, 10−3, 10−4, 10−5, 10−6, and 10−7. 1 ml of each respective solution was added to 15 ml nutrient agar (45–50 °C) in petri dishes, using the pour plate technique. Plates were swirled to mix evenly. Inoculated plates were allowed to cool at room temperature (∼22 °C) until the liquid solidified, and incubated at 30 °C in inverted condition. After 72 ± 3 h, the number of colonies per plate were counted using a colony counter. Bacterial numbers for each sample were estimated in colony forming units (cfu·g−1).
2.6. Glucosinolate extraction & analysis
Glucosinolates were extracted and analysed according to the protocol in Bell et al. (2015) with the following alterations: Extracts were filtered with 0.22 μm Arcrodisc syringe filters with Supor membrane (hydrophilic polyethersulfone; VWR, Lutterworth, UK) after extraction. Analysis was performed using an Agilent 1200 Series LC system (Agilent, Stockport, UK) equipped with a variable wavelength detector (GSLs quantified at 229 nm), and coupled with a Bruker HCT ion trap (Bruker, Coventry, UK). A Gemini 3 μm C18 110 Å (150 × 4.6 mm) column was utilised (with Security Guard column, C18; 4 mm × 3 mm; Phenomenex, Macclesfield, UK), and separation was optimised for use with the Bell et al. (2015) isocratic gradient, at a flow rate of 0.4 ml/min. A six point sinigrin hydrate calibration curve was prepared (r2 = 0.977, y = 7.763; Jin et al., 2009). Compounds were identified using literature ion data and characteristic ion fragments (Table 1). Quantification was performed using Bruker Daltonics HyStar software (Bruker) with relative response factors (Clarke, 2010).
Table 1.
Identification of intact glucosinolates by LC-MS/MS, and glucosinolate hydrolysis products by GC–MS in Eruca sativa and Diplotaxis tenuifolia by comparison to literature ion data.
| Common name | Chemical name | [M−H]−m/z | MS2 (Base ion in bold) | References |
|---|---|---|---|---|
| Glucosinolates | ||||
| Glucoerucin | 4-(Methylthio)-butyl-GSL | 420 | 340, 275, 259, 242, 195 | Rochfort, Trenerry, Imsic, Panozzo, and Jones (2008) |
| Glucoraphanin | 4-(Methylsulfinyl)-butyl-GSL | 436 | 372, 259 | |
| Glucoiberverin | 3-(Methylthio)-propyl-GSL | 406 | 275, 259, 227 | |
| 4-Hydroxyglucobrassicin | 4-Hydroxy-3-indolylmethyl-GSL | 463 | 383, 285 | |
| Epi/progoitrin | (R,S)-2-Hydroxy-3-butenyl-GSL | 388 | 332, 259, 136 | |
| Diglucothiobeinin | 4-(b-d-Glucopyranosyldisulfanyl)-butyl-GSL | 600 | 521 | Lelario, Bianco, Bufo, and Cataldi (2012) |
| Glucosativin | 4-Mercaptobutyl-GSL | 406 | 326, 275, 259, 228, 145 | |
| DMB | Dimeric 4-mercaptobutyl-GSL | 811 | 731, 469, 405 | |
| Common name | Chemical name | MS spectra m/z (Base ion in bold) | References | |
| Glucosinolate hydrolysis products | ||||
| – | 4-Isothiocyanato-1-butene | 113 | 85, 72 | Arora et al., 2014, Guo et al., 2014 |
| Sativin | 4-Mercaptobutyl-ITC | 147 | 114, 87, 72, 60 | Cerny, Taube, and Battaglia (1996) |
| – | Bis (4-isothiocyanatobutyl) disulfide | 292 | 146, 114, 87, 72, 55 | |
| Erucin | 4-(Methylthio)-butyl-ITC | 161 | 115, 72, 61 | Arora et al. (2014) |
| Erucin nitrile | 1-Cyano-4-(methylthio)-butane | 129 | 114, 82, 61 | Vaughn and Berhow (2005) |
| Sulforaphane nitrile | 5-(Methylsulfinyl)-pentane-nitrile | 145 | 82, 64, 55, 41 | Chiang, Pusateri, and Leitz (1998) |
| Sulforaphane | 4-(Methylsulfinyl)-butyl-ITC | 177 | 160, 114, 72, 55 | Chiang et al., 1998, Arora et al., 2014 |
2.7. Free amino acid & free sugar analysis
Free sugars and free AAs were extracted and analysed using the protocols and instrumentation presented in Bell et al. (2017).
2.8. Glucosinolate hydrolysis product extraction & analysis
Samples were extracted and run in a random sequence to avoid bias (as for all other analyses). 0.5 g of lyophilized rocket powder was mixed with 10 ml of deionized water. Tubes were incubated for three hours at 30 °C in a temperature-controlled room. The mixture was subsequently centrifuged for ten minutes (4600 rpm) and supernatant collected. This last step was repeated twice more and supernatants were combined and filtered (0.45 μm syringe filters, Sartorius Minisart cellulose acetate, surfactant free membrane; Sartorius, Epsom, UK) into glass centrifuge tubes. An equal volume of dichloromethane (DCM) was added, vortexed and centrifuged (3500 rpm) for ten minutes. The organic phase was collected using glass Pasteur pipettes and transferred into a new glass centrifuge tube. Sample was salted with sodium sulphate (2 g; Sigma) and filtered using Whatman Grade 1 filter paper into a round-bottomed flask. Filtrate was dried using a rotary evaporator (37 °C) and re-dissolved in 1 ml of DCM. This volume was filtered again with a 0.22 μm filter (VWR) into glass GC–MS vials for analysis.
GC–MS was performed on an Agilent 7693/5975 GC–MS with autosampler (Agilent, Manchester, UK). Sample was injected onto a HP-5MS 15 mm wax plus column (0.25 μm film thickness, 0.25 mm I.D.; Agilent). Injection temperature was 250 °C in split mode (1:20); oven temperature was programmed from 40 to 320 °C at a rate of 5 °C/min until 250 °C. Carrier gas was helium, with a flow rate of 1.1 ml/min and a pressure of 7.1 psi. Mass spectra were obtained by electron ionization at 70 eV, and mass scan from 35 to 500 amu. 1 μl of sample was injected, and separation occurred within a 42 min run. Compounds were identified using literature ion data (Table 1) and quantified based on integrated peak areas of an external standard calibration curve of sulforaphane (Sigma). Standards for the other ITCs and nitrile compounds detected were unobtainable. Five concentrations of sulforaphane were prepared from a stock of 5 mg·ml−1 in DCM: 0, 0.175, 0.25, 0.375, and 0.5 mg·ml−1 (r2 = 0.947; y = 4E + 08). Data analysis was performed using ChemStation for GC–MS (Agilent).
2.9. Statistical analysis
Results from three biological replicates of each sample (n = 3) at each time point, for all compounds analysed were averaged. All statistical analyses were performed using XL Stat (Addinsoft, Paris, France).
ANOVA followed by Tukey’s HSD test was used to conduct multiple pairwise comparisons and determine significant differences (P < 0.05) between cultivars at each respective time point (i.e. SR2 vs. SR5 at H), and between time points for each cultivar (i.e. H vs. D7 for SR6).
Averaged data were entered into Principal Component Analysis (PCA) with correlation matrix (Pearson, n-1) in two separate tests. The first test extracted principal components (PCs) using GSL, AA, sugar and bacterial count data, with time point regressed as a supplementary, qualitative variable. The second test extracted PCs using data from time points PT and D7 (for each of the aforementioned analyses) with the addition of GSL hydrolysis product data. Significance thresholds of P < 0.05, 0.01, and 0.001 were applied to each respective analysis.
3. Results & discussion
3.1. Bacterial counts & phytochemical composition of rocket extracts within the commercial supply chain
3.1.1. Bacterial counts
Bacterial count data for each time point and cultivar are presented in Fig. 1. The general trend in the data matched our hypothesis that bacterial populations would increase during shelf life, which is in agreement with Martinez-Sanchez, Marin, Llorach, Ferreres, and Gil (2006). With the exception of SR12 and Torino, all other cultivar TPC numbers peaked on D7 (DUD); and with the exception of SR12 and SR14, these values were significantly higher than PR levels. Torino had significantly greater numbers of bacteria present from PW through to D7; possibly due to the difference in leaf morphology of D. tenuifolia.
Fig. 1.
Total plate count (TPC) numbers of bacteria from rocket salad leaves (cfu·g−1) at each respective time point during the commercial supply chain (a) and shelf life (b) periods which are both part of the same ANOVA with Tukey’s HSD pairwise comparison tests. Error bars represent standard errors of the mean TPC. Letters a, b, c: bars not sharing a common letter differ significantly (P < 0.05) between accessions for each individual time point. Letters w, x, y, z: bars not sharing a common letter differ significantly (P < 0.05) across time points for each individual accession. Abbreviations: PH, preharvest (12 days old); H, harvest (22 days old); PT, post transport; PR, pre-wash; PW, post wash; D0, day 0 shelf life; D2, day 2 shelf life; D5, day 5 shelf life; D7, day 7 shelf life (display until date).
The breaches in the cool-chain, combined with high summer field temperatures, likely contributed to the high bacterial counts. Previous data presented under pseudo-commercial conditions for rocket (D. tenuifolia; Spadafora et al., 2016) showed that produce stored above 10 °C for 14 days (∼4.0 cfu·g−1 fw) has significantly more bacteria on the leaves than those stored at 5 °C and 0 °C. The samples in this experiment were stored for only nine days, and bacterial counts were highest on D2 of shelf life, and much higher in abundance (Torino; Fig. 1). Conversely, the cultivar SR14 saw no significant changes in bacterial load throughout the entire supply chain. This indicates that there may be a genotypic component imparted by each cultivar on the endemic leaf bacteria that determines their proliferation. Fig. S1 demonstrates that temperatures breached the 10 °C threshold twice after harvest, but without independent data it is difficult to determine if this is the absolute cause for the high bacterial numbers seen for the other cultivars in the subsequent days.
Bacteria continued to propagate during shelf life on all accessions, possibly due to the unmodified air and high relative humidity within bags (Watada et al., 1996). The aforementioned factors likely allowed the natural bacterial populations present within/on leaves to proliferate. Non-pathogenic field bacteria are likely to be resistant to extremes of temperature due to the variable climate of the UK, and so are likely to grow even under cold-chain conditions. Further experimentation is needed on commercial produce in order to properly elucidate these effects.
3.1.2. Glucosinolates
GSL concentrations for each cultivar are presented in Fig. 2, and LC-MS/MS ion data used for identification are presented in Table 1. At each respective time point, total GSL concentrations between cultivars did not differ significantly.
Fig. 2.
Glucosinolate (GSL) concentrations within each cultivar at each time point (mg·g−1·dw). Letters a, b, c: bars not sharing a common letter differ significantly (P < 0.05) between time points for each individual accession. Letters x, y, z: bars not sharing a common letter differ significantly (P < 0.05) across accessions for each time point. Letters above bars refer to differences in total GSL concentration; letters within/beside bars refer to differences in individual compounds. An absence of letters above/within bars indicates no significant differences were observed for the total GSL concentration/individual compounds. Abbreviations: PH, preharvest (12 days old); H, harvest (22 days old); PT, post transport; PR, pre-wash; PW, post wash; D0, day zero shelf life; D2, day two shelf life; D5, day five shelf life; D7, day seven shelf life (display until date); D9, day nine shelf life.
The highest total GSL concentration was in Torino on D7 (11.5 mg·g−1 dw) and the lowest in SR12 at PR (1.0 mg·g−1 dw). The trend for GSL concentrations was to increase over time from H, before lowering at D9 (Fig. 2), which was contrary to our hypothesis. We hypothesise that the increases are due to a stress response, which is initiated by harvesting and culminates in the synthesis of secondary metabolites during shelf-life.
SR5 had significantly higher GSL accumulation at D7 (9.7 mg·g−1 dw) compared with PH (1.9 mg·g−1 dw) and H (2.0 mg·g−1 dw) and this was also seen in SR6: H = 1.3 mg·g−1 dw, PW = 7.7 mg·g−1 dw, D0 = 9.3 mg·g−1 dw, D5 = 8.4 mg·g−1 dw; and in SR12: H = 1.8 mg·g−1 dw, D2 = 8.1 mg·g−1 dw. These GSL concentrations at PH (12 days old) and H (22 days old) are low compared to controlled environment (Bell et al., 2015), as no cultivar contained >3.6 mg·g−1 dw (SR14).
The low concentrations at PH are likely due to the low dry matter content at this immature stage of growth. Work conducted in A. thaliana (Brown, Tokuhisa, Reichelt, & Gershenzon, 2003) has indicated that dry matter and leaf number are related to total GSL concentration. One would therefore have expected GSLs in rocket to increase over this 10 day gap in growth and sampling (PH to H) but no significant differences in the concentrations were observed between the two time points. This implies that concentrations measured in the H samples were possibly reduced due to the damage induced by harvesting and the high ambient field temperature (Fig. S1). Further study is needed to ascertain the true effects of harvesting on GSL concentrations of commercial rocket.
When the data at H are compared to controlled environment conditions (Bell et al., 2015), concentrations are 48.5% lower for SR2, 82.6% lower for SR5, 87.0% lower for SR6, 78.6% lower for SR12, and 52.0% lower for SR14. Despite this difference in observed GSL abundances, only SR6 and SR12 failed to recover during shelf life and exceed concentrations previously reported (Bell et al., 2015).
When looking at the respective GSLs over time for each cultivar, it is apparent that concentrations are highly dynamic. Concentrations of glucosativin varied significantly between time points and most of the changes occurring in total GSL concentration are because of the increases/decreases of this GSL.
DMB was also observed at each respective time point, though no significant differences were seen until D9 (SR5; 2.4 mg·g−1 dw). The propensity for certain rocket accessions and varieties to accumulate DMB and glucosativin in differing ratios has been documented by Bell et al. (2015), though few studies have acknowledged that it is an independent GSL and is naturally present in rocket leaves. This was originally proposed by Cataldi, Rubino, Lelario, and Bufo (2007), and our study lends further support to the hypothesis that both monomeric glucosativin and DMB should be identified and quantified separately.
Glucoerucin and glucoraphanin did not show any significant difference across either time points or cultivars. The lack of any significant changes in glucoraphanin concentration is in agreement with a previous study in broccoli florets (Winkler, Faragher, Franz, Imsic, & Jones, 2007). This GSL seems to be far more stable than others in rocket, such as glucosativin (Fig. 2).
Several other GSLs were also observed. These were: diglucothiobeinin, glucoiberverin, 4-hydroxyglucobrassicin, and epi/progoitrin. Diglucothiobeinin had significant changes over the course of the trial in both SR5 (Fig. 2b) and Torino (Fig. 2f). No significances were observed for glucoiberverin, which was transient between time points. 4-hydroxyglucobrassicin was only detected in SR5 and SR6 in small amounts (<0.3 mg·g−1 dw), though this GSL may infer important sensory attributes as suggested in Bell et al. (2017), where it was correlated with pungent sensations.
3.1.3. Glucosinolate hydrolysis products
ITC and nitrile concentrations are presented in Fig. 3, and GC–MS ion data used for identifications are presented in Table 1. All concentrations are expressed as equivalents of sulforaphane.
Fig. 3.
Glucosinolate (GSL) hydrolysis product concentrations at time points PT and D7 (μg·g−1·dw; sulforaphane equivalents). Letters a, b: bars not sharing a common letter differ significantly (P < 0.05) within each respective time; point PT (post transport) and D7 (day seven shelf life; display until date). Asterisks indicate significantly higher concentrations between each time point for each respective compound (within/to the side of bars) and total amounts (above bars). ∗ = P < 0.05; ∗∗ = P < 0.01.
Total GSL hydrolysis products were predominantly composed of the ITCs sativin and sulforaphane (derived from glucosativin and glucoraphanin, respectively). The nitriles of erucin and sulforaphane were also observed, as well as a sativin degradation product, bis(4-isothiocyanatobutyl)-disulfide. Total concentrations varied significantly for SR2, SR12, and SR14 between the two time points analysed. Total hydrolysis products were significantly higher in Torino at PT (0.4 mg·g−1 dw) than the E. sativa cultivars, but by D7 there were no significant differences between the cultivars. The decline in sulforaphane concentration between PT and D7 was significant in Torino, measuring 0.3 mg·g−1 dw (PT) and falling to 0.1 mg·g−1 dw (D7). This suggests that although glucoraphanin concentration remains stable over time, this may not translate into consistent ITC formation. All of the E. sativa cultivars saw significant increases in sulforaphane between PT and D7, and SR14 saw significantly higher concentrations than any of the other cultivars (0.4 mg·g−1 dw). This is contrary to the reductions seen in headspace ITC concentrations (Bell et al., 2016), indicating this method of analysis may not be reflective of abundance within leaves, or of GSL concentration as has been suggested by Spadafora et al. (2016).
Sulforaphane (derived from glucoraphanin) was the most abundant ITC detected, which does not mirror the total GSL composition of rocket salad. The observations were variable for sativin, and did not generally exceed those for sulforaphane. Significant differences were only observed for SR12 between each time point, despite the obvious large differences seen in the other cultivars (Fig. 3). We conclude that the variability of sativin is due to degradation during analysis, as has been demonstrated for other ITCs (Arora et al., 2014).
Another anomaly observed in our data are the low concentrations of erucin. Erucin increased significantly from PT to D7 in SR2, SR5 and SR12, and declined significantly in Torino. The highest concentration was only 5.7 μg·g−1 dw however (SR12; D7). A study comparing ITC extraction methods in E. sativa seeds (Arora et al., 2014) showed that erucin recovery was dependent on the homogenisation time and GC–MS injection temperature, which may account for the low concentrations observed here. It may be that the extraction method has a significant impact on determining the abundances of ITCs, as well as their inherent volatility.
4-isothiocyanato-1-butene was observed, and has been quoted in the literature as a breakdown product of gluconapin (Guo, Yang, Wang, Guo, & Gu, 2014). No gluconapin was observed in the samples, and we hypothesise that this compound may be a breakdown product of either sulforaphane or erucin (Arora et al., 2014). Concentrations were significantly higher in SR14 (11.6 μg·g−1 dw) on D7 than any other cultivar, and SR14 also has high concentrations of both erucin and sulforaphane.
Another point of note is the low amount of nitrile compounds detected in leaves. This may depend greatly upon the acidity of hydrolysis conditions (Bell & Wagstaff, 2014), however nitrile formation over ITC in broccoli has been shown to account for a substantial reduction in potential health promoting properties (Matusheski & Jeffery, 2001). Our data infer that the prevalence of ITC formation in rocket may have important implications for health benefits to the consumer. ITCs do survive commercial processing, and increase significantly post harvest. This suggests that consumers are able to ingest ITCs (particularly sulforaphane) from rocket salad bags, and that processing actually enhances this property of leaves.
In a hypothetical scenario where SR14 contains 1.97 μmol·g−1 dw (0.35 mg·g−1 dw) of sulforaphane, a commercial 50 g bag would therefore contain approximately 9.85 μmol, assuming 0.2 μmol·g−1 fresh weight with 10% dry matter. Cooked broccoli contains ∼5.8 μmol·g−1 dw after boiling for four minutes, ∼2.0 μmol·g−1 dw after eight minutes, and ∼1.2 μmol·g−1 dw after 12 min (Ghawi, Methven, & Niranjan, 2013). This means that weight for weight, SR14 contains almost as much sulforaphane as a typical broccoli cultivar after cooking for eight minutes. Rocket requires no cooking in order to be eaten, and the present study data suggest that consuming rocket after commercial processing could be an effective way for consumers to enhance their intake of sulforaphane. Clinical studies testing the direct and indirect effects of sulforaphane consumption are few, and the concentrations needed to elicit health beneficial effects in humans are ambiguous within the literature. Nevertheless, the weight of consensus suggests that increased consumption of sulforaphane in the diet has important long-term health benefits (Traka et al., 2013).
3.1.4. Amino acids
Free AA concentrations are presented in Fig. 4 and 18 compounds were detected and quantified. Significant differences between cultivars and time points are presented in Table S1.
Fig. 4.
Amino acid concentrations for each cultivar at each time point (μg·g−1·dw). Letters above bars refer to total concentration. Letters a, b, c, d: bars not sharing a common letter differ significantly (P < 0.05) between time points for each individual accession. Letters x, y, z: bars not sharing a common letter differ significantly (P < 0.05) across accessions for each time point. Significant differences for each individual AA are presented in Table S1. Abbreviations: PH, preharvest (12 days old); H, harvest (22 days old); PT, post transport; PR, pre-wash; PW, post wash; D0, day zero shelf life; D2, day two shelf life; D5, day five shelf life; D7, day seven shelf life (display until date); D9, day nine shelf life.
There were numerous significant differences between the abundances of respective AAs of Torino (Fig. 4f) and the E. sativa cultivars (Fig. 4a-e). Torino had significantly higher concentrations of valine, threonine, asparagine, aspartic acid, and phenylalanine, and significantly lower concentrations of proline. The increases seen in proline in E. sativa, and asparagine in D. tenuifolia, is possible evidence of stress signalling and response within tissues (Okumoto, Funck, Trovato, & Forlani, 2016). This change may also impact upon sensory attributes, though in what manner is difficult to predict, as no sensory study has previously compared D. tenuifolia with E. sativa by examining AAs. Little is known about the specific influence of AAs in other Brassicaceae species, but it is thought that glycine and alanine influence sweetness, valine and leucine create bitterness, and aspartic acid creates sourness (Park et al., 2014).
There is a significant trend for AA concentrations to increase throughout shelf life, and a substantial proportion of this is due to elevations of glutamine, which peaked in accessions between D0 and D9. Increases in free glutamine are associated with leaf senescence and are a result of protein breakdown, enzymatic conversion, and nitrogen transport (Buchanan-Wollaston et al., 2003). This has the possibility to impact bitter and pungent notes as glutamine is known to infer sweetness (Nelson et al., 2002). Concentrations observed in Bell et al. (2017) did not exceed 90.8 μg·g−1 dw in freshly harvested leaves. In this study they reached as high as 1.4 mg·g−1 dw in Torino (D5; Fig. 4f) – over 15 times higher. For the consumer, this may have a significant impact in the pleasurability and acceptance of leaves, especially if it masks other attributes such as pungency. Further study is needed on consumer preference and sensory properties during shelf-life, and between different species of rocket.
Total AA concentrations were highest in SR3 (0.7 mg·g−1 dw; characterised as a ‘mild’ accession) and lowest in SR5 (0.4 mg·g−1 dw; characterised as having hot, pungent and bitter attributes, Bell et al., 2017). In this study, total AA concentrations were substantially higher overall, being highest in Torino (Fig. 4f) on D5 (2.7 mg·g−1 dw) and lowest in SR5 (Fig. 4b) at H (0.2 mg·g−1 dw). Thus, depending on the time point at which the produce is hypothetically consumed, AA concentration may have a greater or lesser effect on perceived pungency and bitterness.
In Bell et al. (2017), alanine was determined to influence rocket sensory properties. In this study, alanine varied significantly across time points for SR5 (Fig. 4b), SR14 (Fig. 4e) and Torino (Fig. 4f). SR14 displayed a trend for alanine concentrations to decline post D0, and this is even more pronounced in Torino. As free alanine can confer sweetness, this may indicate a loss in some sweet taste attributes during shelf-life (Solms, 1969). Previously, the highest concentration observed was 65.1 μg·g−1 dw in SR2 (Bell et al., 2017), whereas in this study, alanine was highest in SR14 (61.6 μg·g−1 dw; D0) and was not detected at all in Torino on D2, D5, D7 and D9. This may infer a stronger perception of pungent and bitter tastes in Torino but more sensory studies are needed to properly ascertain the sensory differences between species/genotypes.
Leucine showed the opposite trend, and is known to have bitter taste properties (Solms, 1969). The change in abundance of this AA may have implications for bitter perception also. The compound increased in the E. sativa cultivars potentially making them more bitter, but declined in Torino from PT. In Bell et al. (2017), leucine concentrations were low, only reaching 4.0 μg·g−1 dw (SR6). Here, leucine was also highest in SR6 (D2; Fig. 4c), but measured 24.7 μg·g−1 dw. This coupled with the losses of alanine may increase bitterness during shelf life for E. sativa cultivars, although it is unknown if this would significantly enhance the bitterness caused by ITCs, or be mitigated by the large increases in glutamine concentrations.
Methionine is conspicuous by its absence in or results. No concentrations were detected in any of the samples tested, and the analysis by Bell et al. (2017) similarly found no concentrations. This is puzzling, as methionine is the predominant precursor AA to aliphatic GSLs. Graser, Schneider, Oldham, and Gershenzon (2000) observed that methionine is involved in the synthesis of glucoerucin in rocket. The lack of any detectable free methionine in this study suggests that it is perhaps stored in another form; possibly as one of the precursor molecules postulated by Graser et al. (2000). This may explain some of the dynamic fluctuations seen in GSL concentrations between time points, facilitating rapid synthesis, as seen during shelf-life. The disparity between aliphatic GSL and free methionine concentration has yet to be addressed within the literature.
3.1.5. Sugars
Concentrations of free sugars are presented in Fig. S2. Few significant differences were observed overall, though Torino showed a trend to accumulate lower amounts than the E. sativa cultivars. SR2 contained significantly more fructose (46.1 mg·g−1 dw) and total sugar (141.9 mg·g−1 dw) at H than SR5 (7.2 mg·g−1 dw and 52.7 mg·g−1 dw, respectively). A significant difference in total sugar was also observed at PW between SR2 (161.1 mg·g−1 dw) and Torino (32.4 mg·g−1 dw).
During shelf life significant differences became more numerous between samples at each time point. On D0, SR14 had significantly higher glucose (118.8 mg·g−1 dw) and total free sugars (143.1 mg·g−1 dw) than Torino (14.9 mg·g−1 dw; 28.5 mg·g−1 dw). By D7 Torino contained significantly less fructose (2.6 mg·g−1 dw), glucose (16.6 mg·g−1 dw) and total free sugar (32.4 mg·g−1 dw) than both SR12 (20.0 mg·g−1 dw, 131.7 mg·g−1 dw, and 164.7 mg·g−1 dw, respectively) and SR2 (32.6 mg·g−1 dw, 111.0 mg·g−1 dw, and 156.6 mg·g−1 dw, respectively).
Bell et al. (2017) observed that high free sugar concentrations in and of themselves do not correspond to milder taste, and that the ratio between sugars and GSLs/ITCs is the more significant attribute in determining pungency and bitterness. As GSLs and ITCs increase over time, and sugars are stable, this is likely to have a large impact on how leaves taste.
3.2. Principal component analyses
3.2.1. General
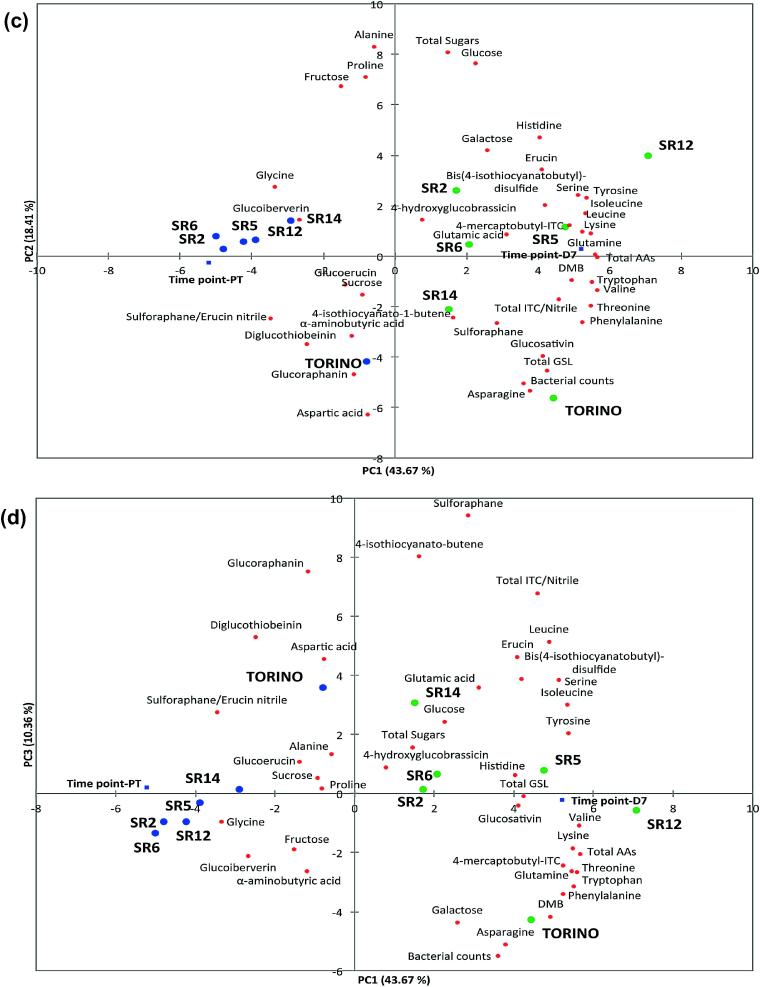
Fig. 5 displays the PCA for all phytochemical and time point data of each cultivar. Fig. 5a (loadings) and b (scores) are plotted with GSL, sugar, and AA data for all time points. Fig. 5c and d are plotted with these same data for time points PT and D7, with the addition of GSL hydrolysis product data.
Fig. 5.
PCA loadings (a) and scores (b) plot for glucosinolate, sugar, amino acid and time point data for the five cultivars tested (PC1 vs. PC2; 51.5% variation explained). PCA biplots (c: PC1 vs. PC2, 62.1% variation explained; d: PC1 vs. PC3, 54.0% variation explained) for all phytochemical data, including ITCs and nitriles, at time points PT and D7. Plots a, c, d: red = active variables, blue = supplementary variables. Plot b: see inset for score plot key. Plots c, d: blue circles = time point PT, green circles = time point D7. Abbreviations: PH, preharvest (12 days old); H, harvest (22 days old); PT, post transport; PR, pre-wash; PW, post wash; D0, day zero shelf life; D2, day two shelf life; D5, day five shelf life; D7, day seven shelf life (display until date); D9, day nine shelf life.
From the data used to generate Fig. 5a and b, 31 principal components (PCs) were extracted, with the first eight having Eigenvalues >1.0. Of these only PC1 and PC2 contained >10.0% of the explained variability (51.5% cumulatively) and as such were selected for presentation. PC1 separates for bacterial counts, the major GSL compounds of rocket (total, glucosativin, DMB), and amino acid concentrations. PC2 separates for sugar and proline concentration.
In the analysis presented in Fig. 5c and d, 11 PCs were extracted. PCs 1–8 had Eigenvalues >1.0, but only PCs 1–3 explained >10.0% of the variation (72.4% cumulatively). PC1 vs. PC2 and PC1 vs. PC3 were selected for presentation as biplots. PC1 separates for glucosativin/DMB and the associated ITC hydrolysis products, as well as bacterial counts and amino acid concentrations. PC2 separates for sugars, alanine, glycine and proline concentrations, and PC3 separates for glucoraphanin, sulforaphane and 4-isothiocyanato-1-butene. Loadings values can be found in Table S2 and correlation matrices in Table S3 for each of the PCA analyses.
3.2.2. Bacterial counts
The most unexpected result from this study was the significant correlation between bacterial counts present on leaves and phytochemical composition. Our hypothesis was that higher GSLs/ITCs would reduce bacterial load, but the exact opposite was observed (Fig. 5). Significant correlations were recorded with glucosativin, DMB and total GSL concentration (Fig. 5a; Table S3). It has been previously hypothesised (Schreiner, Krumbein, & Ruppel, 2009) that under nutrient limited conditions some bacterial strains may use GSLs as a source of carbon. In our study nutrients were not limited, and were abundant in leaves due to the high free sugar concentrations. Bacterial counts were in fact inversely correlated with total sugars and fructose. No significant correlations were observed between bacterial counts and GSL hydrolysis products, indicating that any ITCs formed throughout the supply chain and shelf life have no discernable anti-microbial effect on endemic bacteria.
We hypothesise microbes on rocket leaves are highly adapted to that environment, and have evolved a tolerance or for high ITC concentrations, or a way to circumvent the GSL-myrosinase system. It has been documented that soil bacteria (Citrobacter spp.) possess a glucoside hydrolase family 3 (GH3) β-glucosidase enzyme, which may potentially aid them in the scavenging of glucose from GSLs (Albaser et al., 2016). The same may be true of bacteria that live on leaves, but very little research has been conducted in this area. Adaptation by insects to the GSL-myrosinase system is well documented (Alan & Renwick, 2002), but how bacteria have adapted is poorly understood.
Positive correlations were observed for some AAs and bacterial numbers, whereas others displayed a negative association. Positive correlations (Table S3) were seen for valine, isoleucine, threonine, asparagine, phenylalanine, glutamine, lysine, histidine, tyrosine, tryptophan, and total AA concentration. Negative correlations were with alanine and proline. Coupled with the trends seen for GSLs as a potential carbon source, we hypothesise that bacteria may similarly utilise free amino acids as a nutrient source.
3.2.3. Glucosinolates & hydrolysis products
Aside from the aforementioned correlations with bacterial counts (Fig. 5; Table S3), several other significant correlations are present in the results. Total GSLs were significantly correlated with numerous AAs and total AA concentration. This association may be reflective of the underlying degradation of proteins due to senescence (Buchanan-Wollaston et al., 2003) and up-regulation of secondary defense compounds (Jin et al., 2009).
GSLs and respective hydrolysis products correlated significantly (Fig. 5c & d) as was expected. Total GSL concentration correlated with both sativin and total ITC/nitrile concentration. Glucoraphanin correlated significantly with both sulforaphane and 4-isothiocyanato-1-butene, further supporting the hypothesis that the latter ITC is a degradation product of the former.
3.2.4. Sugars & amino acids
The free sugars fructose, glucose and galactose shared significant correlations with alanine (Fig. 5a; Table S3), and fructose and glucose with proline. It is interesting to note that these AAs are known to have sweet tastes (Solms, 1969), which could potentially influence the sensory properties of leaves. They were also negatively correlated with bacterial growth, perhaps indicating a relationship between sugar/AA content and bacterial load of leaves, though in what respect is presently unclear.
3.2.5. Time points
Several of the studied phytochemical components correlated significantly with specific time points (Fig. 5; Table S3). Many of these have important implications for rocket breeding and commercial supply chain management.
In the PCA (Fig. 5b) the profile of D7 samples separate along PC1, and are indicative of significant phytochemical changes by this point of the trial. Time point PH and H form a distinct and tight cluster to the lower left of the plot on the PC1 axis, blending with PT and PW before separating towards the top right, loosely according to shelf life time point. Torino is very distinct, separating away to the bottom right. This is due to the high bacterial load of these samples (PC2), as well as low sugar, and high GSL/AA concentration during shelf life (Fig. 5a). This pattern is similar in Fig. 5c and d where E. sativa PT samples are tightly clustered, with D7 separating along the PC1 axis in a more dispersed fashion to the right. The two Torino time points are isolated however, associated again with high bacterial counts, asparagine, aspartic acid, and high total GSL concentration. The E. sativa cultivars trend towards higher total sugars and sweet AAs (PC2).
PH was distinct in several aspects from the subsequent time points (Fig. 5a). Glucosativin and total GSL concentrations were typically low, and were negatively correlated. There were also several AAs significantly correlated with PH, and not with any subsequent time point. Glycine, alanine, α-aminobutyric acid and glutamic acid are all higher in abundance, whereas others, such as proline, leucine and tryptophan were negatively correlated with PH.
At H, significant negative correlations can be seen with glucoraphanin, glucosativin and total GSL concentration; this is perhaps indicative of GSL depletion during the harvest procedure. Numerous AAs and total AA concentrations were also low and negatively correlated (Fig. 5a).
D7 is perhaps the key time point within the trial, as total GSL, glucosativin and DMB concentrations were all significantly correlated (Fig. 5c & d; Table S3). Total ITC/nitriles, sativin and erucin were also significantly correlated, demonstrating that all cultivars displayed the ability to re-synthesize GSLs/ITCs to a high level during shelf life. Total AAs and glutamine shared a strong correlation with this time point.
There seems to be juxtaposition between the point of highest nutritional content (GSLs/ITCs) and the high degree of tissue and protein breakdown evidenced by the increases in free AAs. This may lead to visual and aroma changes that consumers will reject, and may dissuade them from consuming leaves that contain the highest ITC concentrations.
4. Conclusions
This study is the first to demonstrate the phytochemical and bacteriological effects of an entire commercial supply chain on rocket leaves. It is clear from our results that total GSL concentration increases post processing. Importantly, glucoerucin and glucoraphanin are not significantly reduced by processing, suggesting that GSLs are not lost due to leaching or myrosinase action in wash water as we originally hypothesised. The reason for this is likely a form of abiotic stress response to harvesting and processing, but the underlying genetic mechanisms responsible have yet to be elucidated in rocket.
ITCs (particularly sulforaphane) increase significantly during shelf life in E. sativa, and this could have positive health benefits for the end consumer. We have elucidated significant changes in AA composition of leaves, and that free sugars remain stable throughout processing and shelf life. The fluctuations in abundance of GSLs, ITCs and AAs may have important implications for consumer acceptance and sensory properties of leaves.
We have demonstrated a possible link between GSL and AA concentrations with the bacterial load of leaves. At present it is unknown by what mechanism this is achieved, but further study and identification of bacterial strains on rocket leaves may provide insight. We hypothesise that bacterial populations have evolved to survive on GSL-producing plants, perhaps utilising GSLs as a carbon source and free AAs as a nitrogen source. We speculate that such native bacterial loads are non-pathogenic, however their presence and metabolism of sulphur-containing compounds (such as GSLs) within sealed bags may produce off-odors that consumers might reject (Spadafora et al., 2016).
We have demonstrated that GSL/ITC profiles observed in controlled studies are not fully representative of commercially processed material. The data presented here illustrate how dynamic GSL profiles are over time. Future studies may wish to consider the impact of the whole supply chain when attempting to analyse crops for phytochemicals, and not just the point of harvest. Similarly, the two major species of rocket display several differences in phytochemistry that require further study and verification through a combination of sensory and chemical analyses.
Acknowledgements
Thanks to Dr. Lorraine Shaw of Bakkavor for help arranging the field and processing trial. Special thanks to the staff of the Bakkavor rocket supplier, and to Chris Jeffes and Emma George (Bakkavor). Thanks to Sue Kennedy and Marie-Laure Bayard for organising seed productions. Additional thanks: Melisa Kupaza for assistance collecting samples; Dr. Stella Lignou (University of Reading) for advice on CE; Dr. Nicholas Michael (University of Reading) for assistance and advice with LC-MS/MS; and Dr. Stephen Elmore (University of Reading) for assistance with GC-MS.
Dr. Luke Bell was supported by a BBSRC Case Award (Reference BB/J012629/1) in partnership with Elsoms Seeds Ltd. (Spalding, UK) and Bakkavor Group Ltd. (Spalding, UK).
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foodchem.2016.11.154.
Appendix A. Supplementary data
References
- Alan J., Renwick A. The chemical world of crucivores: Lures, treats and traps. Entomologia Experimentalis et Applicata. 2002;104:35–42. [Google Scholar]
- Albaser A., Kazana E., Bennett M., Cebeci F., Luang-In V., Spanu P.D., Rossiter J.T. Discovery of a bacterial glycoside hydrolase family 3 (GH3) β-glucosidase with myrosinase activity from a Citrobacter strain isolated from soil. Journal of Agricultural and Food Chemistry. 2016;64(7):1520–1527. doi: 10.1021/acs.jafc.5b05381. [DOI] [PubMed] [Google Scholar]
- Arora R., Sharma D., Kumar R., Singh B., Vig A.P., Arora S. Evaluating extraction conditions of glucosinolate hydrolytic products from seeds of Eruca sativa (Mill.) Thell. using GC-MS. Journal of Food Science. 2014;79(10) doi: 10.1111/1750-3841.12579. C1964–9. [DOI] [PubMed] [Google Scholar]
- Bell L., Methven L., Signore A., Oruna-Concha M.J., Wagstaff C. Analysis of seven salad rocket (Eruca sativa) accessions: The relationships between sensory attributes and volatile and non-volatile compounds. Food Chemistry. 2017;218:181–191. doi: 10.1016/j.foodchem.2016.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Oruna-Concha M.J., Wagstaff C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chemistry. 2015;172:852–861. doi: 10.1016/j.foodchem.2014.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Spadafora N.D., Müller C.T., Wagstaff C., Rogers H.J. Use of TD-GC-TOF-MS to assess volatile composition during post-harvest storage in seven accessions of rocket salad (Eruca sativa) Food Chemistry. 2016;194:626–636. doi: 10.1016/j.foodchem.2015.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L., Wagstaff C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia) Journal of Agricultural and Food Chemistry. 2014;62(20):4481–4492. doi: 10.1021/jf501096x. [DOI] [PubMed] [Google Scholar]
- Brown P.D., Tokuhisa J.G., Reichelt M., Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62(3):471–481. doi: 10.1016/s0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Earl S., Harrison E., Mathas E., Navabpour S., Page T., Pink D. The molecular analysis of leaf senescence – A genomics approach. Plant Biotechnology Journal. 2003;1(1):3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Cataldi T.R.I., Rubino A., Lelario F., Bufo S.A. Naturally occuring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21(14):2374–2388. doi: 10.1002/rcm.3101. [DOI] [PubMed] [Google Scholar]
- Cerny M.S., Taube E., Battaglia R. Identification of bis(4-isothiocyanatobutyl) disulfide and its precursor from rocket salad (Eruca sativa) Journal of Agricultural and Food Chemistry. 1996;44(12):3835–3839. [Google Scholar]
- Chiang W.C.K., Pusateri D.J., Leitz R.E.A. Gas chromatography mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. Journal of Agricultural and Food Chemistry. 1998;46(3):1018–1021. [Google Scholar]
- Clarke D.B. Glucosinolates, structures and analysis in food. Analytical Methods. 2010;2(4):310–325. [Google Scholar]
- Dekker M., Verkerk R., Jongen W. Predictive modelling of health aspects in the food production chain: a case study of glucosinolates in cabbage. Trends in Food Science & Technology. 2000;11(4–5):174–181. [Google Scholar]
- Ghawi S.K., Methven L., Niranjan K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba) Food Chemistry. 2013;138(2–3):1734–1741. doi: 10.1016/j.foodchem.2012.10.119. [DOI] [PubMed] [Google Scholar]
- Graser G., Schneider B., Oldham N.J., Gershenzon J. The methionine chain elongation pathway in the biosynthesis of glucosinolates in Eruca sativa (Brassicaceae) Archives of Biochemistry and Biophysics. 2000;378(2):411–419. doi: 10.1006/abbi.2000.1812. [DOI] [PubMed] [Google Scholar]
- Guo L., Yang R., Wang Z., Guo Q., Gu Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. Journal of Functional Foods. 2014;9:70–77. [Google Scholar]
- Hall M.K.D., Jobling J.J., Rogers G.S. Factors affecting growth of perennial wall rocket and annual garden rocket. International Journal of Vegetable Science. 2012;18(4):393–411. [Google Scholar]
- Hall M.K.D., Jobling J.J., Rogers G.S. Influence of storage temperature on the seasonal shelf life of perennial wall rocket and annual garden rocket. International Journal of Vegetable Science. 2013;19(1):83–95. [Google Scholar]
- Jin J., Koroleva O.A., Gibson T., Swanston J., Magan J., Zhang Y., Wagstaff C. Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. Journal of Agricultural and Food Chemistry. 2009;57(12):5227–5234. doi: 10.1021/jf9002973. [DOI] [PubMed] [Google Scholar]
- Lelario F., Bianco G., Bufo S.A., Cataldi T.R.I. Establishing the occurrence of major and minor glucosinolates in Brassicaceae by LC-ESI-hybrid linear ion-trap and Fourier-transform ion cyclotron resonance mass spectrometry. Phytochemistry. 2012;73:74–83. doi: 10.1016/j.phytochem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Lokke M.M., Seefeldt H.F., Edelenbos M. Freshness and sensory quality of packaged wild rocket. Postharvest Biology and Technology. 2012;73:99–106. [Google Scholar]
- Martínez-Sánchez A., Allende A., Cortes-Galera Y., Gil M.I. Respiration rate response of four baby leaf Brassica species to cutting at harvest and fresh-cut washing. Postharvest Biology and Technology. 2008;47(3):382–388. [Google Scholar]
- Martinez-Sanchez A., Marin A., Llorach R., Ferreres F., Gil M.I. Controlled atmosphere preserves quality and phytonutrients in wild rocket (Diplotaxis tenuifolia) Postharvest Biology and Technology. 2006;40(1):26–33. [Google Scholar]
- Matusheski N.V., Jeffery E.H. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. Journal of Agricultural and Food Chemistry. 2001;49(12):5743–5749. doi: 10.1021/jf010809a. [DOI] [PubMed] [Google Scholar]
- Nelson G., Chandrashekar J., Hoon M.A., Feng L., Zhao G., Ryba N.J.P., Zuker C.S. An amino-acid taste receptor. Nature. 2002;416(6877):199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Okumoto S., Funck D., Trovato M., Forlani G. Editorial: Amino acids of the glutamate family: Functions beyond primary metabolism. Frontiers in Plant Science. 2016;7:318. doi: 10.3389/fpls.2016.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Arasu M.V., Lee M.-K., Chun J.-H., Seo J.M., Lee S.-W.…Kim S.-J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.) Food Chemistry. 2014;145:77–85. doi: 10.1016/j.foodchem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Rangkadilok N., Tomkins B., Nicolas M.E., Premier R.R., Bennett R.N., Eagling D.R., Taylor P.W.J. The effect of post-harvest and packaging treatments on glucoraphanin concentration in broccoli (Brassica oleracea var. italica) Journal of Agricultural and Food Chemistry. 2002;50(25):7386–7391. doi: 10.1021/jf0203592. [DOI] [PubMed] [Google Scholar]
- Rico D., Martín-Diana A.B., Barat J.M., Barry-Ryan C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends in Food Science and Technology. 2007;18:373–386. [Google Scholar]
- Rochfort S.J., Trenerry V.C., Imsic M., Panozzo J., Jones R. Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry. 2008;69(8):1671–1679. doi: 10.1016/j.phytochem.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Schreiner M., Krumbein A., Ruppel S. Interaction between plants and bacteria: glucosinolates and phyllospheric colonization of cruciferous vegetables by Enterobacter radicincitans DSM 16656. Journal of Molecular Microbiology and Biotechnology. 2009;17:124–135. doi: 10.1159/000226589. [DOI] [PubMed] [Google Scholar]
- Solms J. Taste of amino acids, peptides, and proteins. Journal of Agricultural and Food Chemistry. 1969;17(4):686–688. [Google Scholar]
- Spadafora N.D., Amaro A.L., Pereira M.J., Müller C.T., Pintado M., Rogers H.J. Multi-trait analysis of post-harvest storage in rocket salad (Diplotaxis tenuifolia) links sensorial, volatile and nutritional data. Food Chemistry. 2016;211:114–123. doi: 10.1016/j.foodchem.2016.04.107. [DOI] [PubMed] [Google Scholar]
- Traka M.H., Saha S., Huseby S., Kopriva S., Walley P.G., Barker G.C., Boddupalli, Mithen R.F. Genetic regulation of glucoraphanin accumulation in Beneforté broccoli. The New Phytologist. 2013;198:1085–1095. doi: 10.1111/nph.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn S.F., Berhow M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Industrial Crops and Products. 2005;21(2):193–202. [Google Scholar]
- Verkerk R., Schreiner M., Krumbein A., Ciska E., Holst B., Rowland I.…Dekker M. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Molecular Nutrition & Food Research. 2009;53:S219–S265. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- Vig A.P., Rampal G., Thind T.S., Arora S. Bio-protective effects of glucosinolates – A review. Lwt-Food Science and Technology. 2009;42(10):1561–1572. [Google Scholar]
- Watada A.E., Ko N.P., Minott D.A. Factors affecting quality of fresh-cut horticultural products. Postharvest Biology and Technology. 1996;9(2):115–125. [Google Scholar]
- Winkler S., Faragher J., Franz P., Imsic M., Jones R. Glucoraphanin and flavonoid levels remain stable during simulated transport and marketing of broccoli (Brassica oleracea var. italica) heads. Postharvest Biology and Technology. 2007;43:89–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.