Abstract
Setting: Greater Banjul area of The Gambia.
Objectives: To evaluate uptake, adherence and completion of treatment among tuberculosis (TB) exposed children in The Gambia when isoniazid preventive treatment (IPT) is delivered at home
Design: Child (age <5 years) contacts of adults with smear-positive TB were prospectively enrolled. Following symptom screening, tuberculin skin testing and clinical evaluation where indicated, those without disease were placed on daily isoniazid, provided monthly at home. Adherence was assessed by pill counts and IsoScreen™ urine test.
Results: Of 404 contacts aged <5 years, 368 (91.1%) were offered IPT. Of the 328 (89.4%) for whom consent was received and who commenced IPT, 18 (5.5%) dropped out and 310 (94.5%) remained on IPT to the end of the 6-month regimen. Altogether, 255/328 children (77.7%, 95%CI 73.2–82.2) completed all 6 months, with good adherence. The IsoScreen test was positive in 85.3% (435/510) of all tests among those defined as having good adherence by pill count and in 16% (8/50) of those defined as having poor adherence (P < 0.001). A cascade of care analysis showed an overall completion rate with good adherence of 61% for all child contacts.
Conclusion: Home-delivered IPT among child contacts of adults with smear-positive TB in The Gambia achieved verifiable high uptake and adherence rates. System rather than patient factors are likely to determine the success of IPT at national level.
Keywords: adherence, IsoScreen, IPT, prophylaxis, cascade of care
Abstract
Contexte : Région du Grand Banjul en Gambie.
Objectifs : Evaluer la couverture, l'adhésion et l'achèvement du traitement parmi des enfants exposés à la tuberculose (TB) en Gambie quand le traitement préventif par isoniazide (TPI) est donné à domicile.
Schéma : Les enfants âgés de <5 ans, contacts d'adultes atteints de TB à frottis positif, ont été enrôlés de manière prospective. Après dépistage sur les symptômes, test cutané à la tuberculine et évaluation clinique quand cela était indiqué, les enfants non malades ont été mis sous isoniazide, fourni une fois par mois à domicile. L'adhésion a été évaluée par un comptage des comprimés et par un test urinaire IsoScreen™.
Résultats : Sur 404 contacts âgés de <5 ans, 368 (91,1%) ont été invités à bénéficier du TPI, et 328 (89,4%) ont consenti et commencé le TPI. Sur ces 328 enfants, 18 (5,5%) ont abandonné et 310 (94,5%) sont restés sous TPI jusqu'à la fin du 6e mois. Au total, 255/328 enfants (77,7% ; IC95% 73,2–82,2) ont achevé les 6 mois de traitement avec une bonne adhésion. Le test IsoScreen a été positif chez 85,3% (435/510) de tous les tests parmi ceux définis comme ayant une bonne adhésion par le comptage des comprimés et chez 16% (8/50) de ceux définis comme ayant une adhésion médiocre (P < 0,001). L'analyse de la « cascade des soins » a montré, pour tous les enfants contacts, un taux de bonne adhésion d'ensemble de 61%.
Conclusion: L'administration à domicile du TPI à des enfants contacts d'adultes atteints de TB à frottis positif en Gambie a abouti à une bonne couverture et à un bon taux d'adhésion, tous deux vérifiables. Ce sont les facteurs de système plutôt que ceux liés au patient qui sont susceptibles de déterminer le succès du TPI au niveau national.
Abstract
Marco de referencia: La zona del Gran Banjul en Gambia.
Objetivos: Evaluar la aceptación del tratamiento preventivo con isoniazida (TPI), su cumplimiento y su compleción por parte de los niños expuestos en Gambia, cuando se suministra el tratamiento en los hogares.
Método: Se incluyeron en el estudio de manera prospectiva los niños menores de 5 años de edad que eran contactos de un adulto con diagnóstico de tuberculosis (TB) y baciloscopia positiva. Luego de la detección sistemática a partir de los síntomas, se practicaron la prueba cutánea de la tuberculina y la evaluación clínica cuando estaban indicadas; en caso de ausencia de enfermedad activa se inició el tratamiento diario con isoniazida, la cual se suministraba en el hogar cada mes. Se evaluó el cumplimiento en función del recuento de los comprimidos y la prueba IsoScreen™ en muestras de orina.
Resultados: En los 404 contactos menores de 5 años de edad, se ofreció el TPI a 368 niños (91,1%) y 328 lo aceptaron y comenzaron a recibirlo (89,4%). De este grupo, 18 niños abandonaron el tratamiento (5,5%) y 310 recibían aun el medicamento al final del 6 mes (94,5%). De los 328 niños, 255 terminaron los 6 meses de tratamiento, con un cumplimiento satisfactorio (77,7%; IC del 95% de 73,2 hasta 82,2). La prueba IsoScreen fue positiva en el 85,3% (435/510) de los casos definidos con cumplimiento adecuado según el recuento de comprimidos y en el 16% (8/50) de los casos cuyo cumplimiento se consideró deficiente (P < 0,001). El análisis de la trayectoria asistencial reveló que en todos los contactos la tasa global de compleción con cumplimiento satisfactorio fue 61%.
Conclusión: El TPI suministrado en el hogar a los niños que son contactos de un adulto con diagnóstico de TB y baciloscopia positiva alcanza altas tasas de aceptación y de cumplimiento que se pueden verificar. Los factores que determinan el éxito del TPI a escala nacional dependen del sistema de salud y no del paciente.
The current elimination goals for tuberculosis (TB) include a focus on individuals who are latently infected with Mycobacterium tuberculosis to reduce the number of new TB cases and subsequent M. tuberculosis transmission.1 This strategy requires isoniazid preventive therapy (IPT) or an alternative regimen. Initial targets are those at most risk of disease progression, such as young children and human immunodeficiency virus (HIV) infected individuals in contact with an infectious adult with TB.2
The above World Health Organization (WHO) recommendations have not, however, been implemented in most of the high-burden TB settings where IPT is most needed. In many places where IPT has been implemented and evaluated, the impact is suboptimal and the operational challenges are formidable.3–6 Considering the cascade of care as a whole, it is estimated that, even where IPT is part of routine practice, only a minority of eligible children complete a course of treatment.7 Not only is uptake poor, but adherence rates tend to be less than 30% among those who do commence IPT.8,9 The measurement of adherence can also be unreliable, relying heavily on reports from care givers, how often care givers return for more medication, and pill counts.10,11 Furthermore, interventions to improve IPT delivery frequently do not result in improved uptake, adherence and treatment completion.12 A recent meta-analysis of the cascade of care for treatment of latent tuberculous infection (LTBI), which indicated where patients get lost in the system for various reasons, focused primarily on adults.13
As IPT implementation now has a higher priority within the agenda for TB control,14 and as implementation via TB clinics appears to be challenging, we developed a home-based IPT programme among child contacts of recently diagnosed adult sputum smear-positive TB cases and assessed its impact on uptake, completion and adherence within the cascade of care in The Gambia. We also measured the isoniazid (INH) metabolites in urine to assess the reliability of our adherence measures.
METHODS
Study sites
The study was carried out in the Greater Banjul area of The Gambia between November 2013 and May 2015. This is a mixed urban-to-rural area, including the capital city of Banjul, and has a population of approximately 700 000. The setting has been described elsewhere.15
Participants
Child contacts aged <5 years living in the same household as adult sputum smear-positive TB cases were recruited. A household was defined as a group of individuals eating from the same pot and living in the same building.16
Symptom screening, tuberculin skin testing and clinical evaluation
Adults with newly diagnosed sputum smear-positive TB were identified at the National Leprosy/TB Control Programme (NLTP) clinics and were asked for their consent to a household visit. After the project was explained to the parents/care givers, written informed consent was obtained. A standard symptom-screening questionnaire for TB was then administered to ascertain if any child living in the same household had a cough of ⩾2 weeks' duration in association with at least one of the following symptoms: weight loss, failure to gain weight, fever or night sweats. The tuberculin skin test (TST) was performed using the Mantoux method (2 tuberculin units of purified protein derivative RT23, Statens Serum Institute, Copenhagen, Denmark) and read 48–72 h later. A positive TST was defined as an induration of ⩾10 mm, in line with WHO recommendations.17 Child contacts with symptoms suggestive of TB and/or a positive TST were referred to a dedicated childhood TB clinic for further evaluation. All children diagnosed with TB disease were referred to the TB clinics of the NLTP for DOTS-based treatment.
Isoniazid prophylaxis and follow-up
All child household contacts aged <5 years in whom TB disease was excluded were provided with IPT at 10 mg/kg/day for 6 months, as recommended by the WHO,2 regardless of their TST result. Field workers delivered the IPT to the homes of the children on a monthly basis and administered a brief questionnaire to capture missed doses, the reasons for missed doses and any adverse events from the medication. To measure adherence, pill counts were performed for all children. An IPT card was specifically developed to record the child's weight and the number of unconsumed doses of medication. All children were assessed every 3 months for 1 year; those with new symptoms suggestive of TB were referred to the childhood TB clinic at the Medical Research Council (MRC) unit. A final home visit was made 1 year after INH completion to ascertain post-IPT status.
IsoScreen testing
In the first year of the study, consecutively recruited children on IPT were asked to provide a monthly urine sample for a qualitative assessment of adherence using the IsoScreenTM test (GFC Diagnostics Ltd, Oxford-shire, UK), a point-of-care colorimetric assay that detects INH and its metabolites utilising a disposable plastic test device and the Arkansas method for metabolite detection.18 Two ml of collected urine was injected into a reaction chamber and mixed with the reagents contained in an effervescent tablet for about 10 s. Dark blue/purple colouration appearing within 5 min indicates a positive result, i.e., the individual has taken INH within the previous 24–48 h. If no INH has been taken, the colour of the urine remains unchanged, indicating a negative result. Care givers were informed about the monthly visits, but the actual days of the visit were unannounced. The results of the IsoScreen testing were recorded on the child's INH card.
Ethics approval
Ethics approval for the study was obtained from the joint Medical Research Council/Gambian government ethics committee (Ref. L2012.E01), Banjul, The Gambia.
Data analysis
Data were double-entered into an Access database (Microsoft Corp, Redmond, WA, USA) and verified using consistency checks. All analyses were performed using STATA/IC 13.1 (StataCorp, College Station, TX, USA). IPT implementation was assessed using a cascade of care approach. To provide a denominator for the uptake component of the cascade, the number of contacts of those TB cases who refused a home visit and the number of children with TB disease among those who had not been assessed in the clinic were estimated. This was performed by simple extrapolation of the average number of child contacts per case and the rate of disease in those assessed to a rate of disease in those not assessed, rounded to the nearest whole number.13 Adherence was divided into three categories: good, reasonable and poor, if respectively >80%, 60–80% or <60% of the pills delivered each month had been taken.19 Treatment completion was defined as consuming >80% of all pills prescribed in each of the 6 months of prophylaxis.20 The proportion of children with a positive IsoScreen test result was compared among the adherence categories using the χ2 test. The data are presented in frequencies, proportions and percentages with their 95% confidence interval (CI).
RESULTS
IPT uptake and characteristics of those commenced on IPT
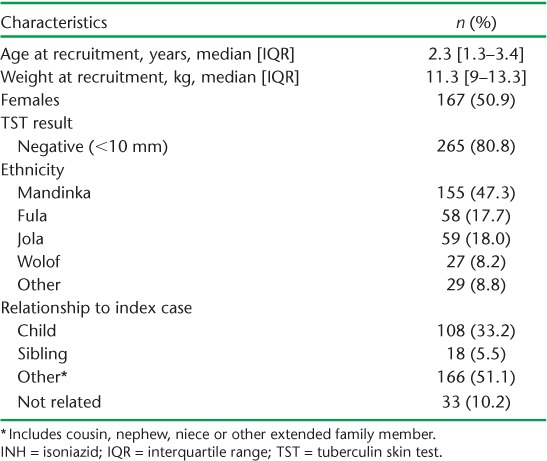
Over the study period, 301/330 adults with sputum smear-positive TB consented to contact tracing in their households. Altogether 404 children aged <5 years and living in the same household as an adult index case were screened at community level. Of these, 163 symptomatic and/or TST-positive children were referred for further evaluation in the clinic, and 153/163 (94%) attended the appointment. Of this group, 26 (16.9%) were diagnosed with active TB. The remaining 368 children were eligible for IPT and consent was sought from their parent or legal guardian, of whom 25 refused, 14 moved out of the study area after consenting and one child died of a brief febrile illness thought to be malaria in the first month of prophylaxis (Figure 1). Of 328 children initiated on IPT, 50.6% were females; the median age (interquartile range [IQR]) at recruitment was 2.3 years (IQR 1.3–3.4) (Table 1).
FIGURE 1.

Flow chart of screening and recruitment of children on INH prophylaxis. INH = isoniazid; TB = tuberculosis.
TABLE 1.
Characteristics of children placed on INH prophylaxis
Completion of IPT
Of the 328 children commenced on IPT, 318 (96.9%) started within 1 month of the diagnosis of their respective index cases. IPT was administered by the mothers in 92.3% (303/328) of the cases, by the fathers in 2.4% (8/325), and by grandmothers and siblings in 0.9% of cases each.
Eighteen of the 328 children dropped out of IPT, leaving 310 children remaining on prophylaxis at the end of 6 months. There was no difference in the characteristics of those who dropped out and those who completed prophylaxis. A final 255 of the 328 children (77.7%, 95%CI 73.2–82.2) completed the 6 months of IPT with good adherence.
Adherence to treatment
During the study period, 59 040 doses of IPT were dispensed, of which 53 573 (90.7%) were consumed. Based on pill counts, 78.9% (95%CI 74.5–83.2) of all medications were consumed by children with good adherence, 15.4% (95%CI 11.5–19.3) by children with reasonable adherence, and 5.6% (95%CI 3.1–8.1) by children with poor adherence. Table 2 shows the monthly adherence of children on IPT as determined by pill count. Less than 10% of children on prophylaxis in any month had poor adherence. Overall, the proportion of children with positive IsoScreen test results was similar to the proportion with good adherence: good adherence by pill count was 77.4% (95%CI 74.2–80.6) compared to 76.6% (95%CI 73.4–79.8) with positive urine test results.
TABLE 2.
Adherence to INH prophylaxis determined by monthly pill count
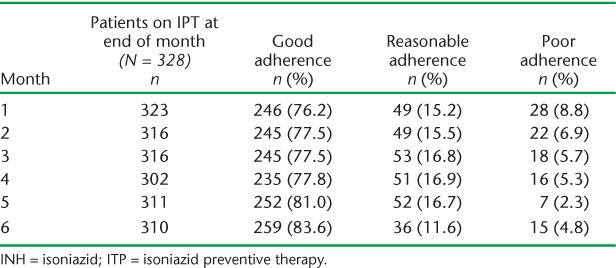
The proportion of children with good adherence remained high, at 76.2% in the first month, and increased gradually over time to 83% in month 6. Spot urine samples were collected from the first 141 children recruited into the study, and 658 episodes of pill counts and urine tests were carried out simultaneously. Among these children, 85.3% (435/510) of all tests among those defined as having good adherence by pill count were positive, compared to 16% (8/50) of tests among those defined as having poor adherence (P < 0.001). Over the 6 months of IPT, 80.6–93.3% of those with good adherence by pill count were also positive by IsoScreen, compared to 0–25.0% of those with poor adherence (Table 3). Across the three adherence categories there was a significant trend in IsoScreen positivity (P < 0.002) in each month of IPT.
TABLE 3.
Adherence by pill count and urine test result in the IsoScreen™ cohort

Of 314 responses to the adherence questionnaire administered among care givers, 247 (78.7%) stated that the main reason for failure to administer the pills was forgetfulness. The second major reason, given by 30 (9.6%) of the care givers, was travel. Other reasons included the child being sick (11 [3.5%]), refusing medication (7 [2.2%]) and misplacement of the medications (5 [1.6%]).
Side effects
No side effects were reported throughout the study period.
One-year follow-up
Of the 310 children who completed their course of IPT, 12 (3.9%) were lost to follow-up at 1 year after IPT. One child aged 18 months died of a sudden febrile illness not related to INH or TB. One child who dropped out of IPT in the first month developed clinically diagnosed TB by the ninth month of follow-up. All the other children remained well.
Cascade of care summary analysis
To identify where improvements may be needed in the continuum of care of child contacts eligible for IPT, we used a cascade of care approach, as summarised in Figure 2. We estimated uptake and completion of IPT among all eligible children in the households of the 330 adults with TB approached during the study. After excluding 27 children with TB disease (26 diagnosed after clinical evaluation and one estimated from among those who failed to attend the clinic), 418 children remained eligible for IPT, of whom 328 (78.5%) accepted IPT and 255 (61%) completed 6 months of IPT with good adherence.
FIGURE 2.

Cascade of care of child contacts eligible for IPT. IPT = isoniazid preventive therapy.
DISCUSSION
In this study, we implemented and evaluated a home-based IPT programme in The Gambia. Uptake of IPT was high (78%), and 77.7% of all child contacts who initiated IPT completed the 6-month course with good adherence. A further 15.4% had reasonable adherence, and 5.6% had poor adherence. We also found that pill count reliably reflected adherence in this population. The most frequent reason for non-adherence was forgetfulness. The cascade of care analysis showed that approximately 61% of the estimated original number of contacts eligible for IPT completed 6 months of IPT with good adherence, and provides insight into the potential public health impact of the programme.
Adherence is a major determinant of efficacy of IPT.21 High IPT uptake in The Gambia is encouraging as the country moves to expand these services nationwide. In high TB burden countries, the proportion of those commencing and completing at least 4 months of IPT is approximately 15%,6,7 and even in research conditions adherence to unsupervised IPT in high-burden countries has been relatively low. Good adherence to IPT was achieved by approximately 24% in South Africa,4,22 25.6% in Indonesia8 and 32.5% in Southern Ethiopia.9 In contrast, in another research study conducted in Guinea Bissau, West Africa, where IPT was also delivered to the homes of child contacts for 9 months, the proportion achieving good adherence according to pill counts was 76%,20 similar to our study. Interestingly, when INH was delivered at home to children in Ethiopia, adherence was very poor, with only 33% of children taking their medications for up to 4 months.9 More site-specific research is required to identify the best locally applicable approach to optimise adherence.23
The understanding and willingness shown by carers, especially mothers, to administer IPT provides a potential solid base for programmatic home-delivered IPT. As the project is now being transferred to the government programme, the priority is to enable sustained delivery of INH to homes of child contacts. In the current organisation of the health system in The Gambia, community health workers and assistants living within the communities play a major role in public health. It is intended to provide training for this group to deliver IPT—and monitor IPT delivery—to homes of contacts at minimal or no additional cost to the NTP, as they are already involved as TB treatment supporters to the index cases.
The use of IsoScreen enabled us to objectively assess whether pill counts are a reliable way to assess adherence in The Gambia, and we found excellent agreement. Our results should be interpreted with caution, however, as some of the negative test results came from individuals with good adherence by pill count but who had missed doses only in the most recent days. The sensitivity of the IsoScreen test decreases with the passage of time from ingestion of the medication.24 Similarly, some poorly adherent individuals may have taken IPT prior to their IsoScreen test. As the day for IsoScreen testing was not announced in advance, however, it is unlikely that differential bias was introduced. A negative IsoScreen result has been reported in fast acetylators;25 we did not assess acetylator status.
In summary, the uptake of and adherence to IPT in our study were high by international standards, suggesting that system rather than patient factors will be the main determinants of success when IPT management is transferred to be fully operational under the NLTP. IPT was highly acceptable to care givers and children in The Gambia, and home delivery will present an opportunity for health education on TB and other topics of public health importance, such as malnutrition, sanitation and oral rehydration. Training of health workers in childhood TB and IPT will be required to ensure success.26,27 The cascade of care summary showed that there are opportunities for improvement for the IPT programme to have maximal public health impact. Further studies could explore the reasons why some households do not uptake IPT and whether an intervention to reduce care giver forgetfulness, such as cell phone text reminders,28 could be beneficial.
Acknowledgments
The authors are grateful to the National Leprosy and Tuberculosis Control Programme of The Gambia (Banjul), the childhood TB field team of the Medical Research Council (MRC) Unit (Banjul) and all the children and their families who participated in the study. This work was funded by an MRC Programme grant to BK (MR/K011944/1). Research at the MRC–The Gambia is jointly funded by the UK MRC (London, UK) and the UK Department for International Development (DFID, London, UK) under the MRC/DFID concordant agreement, and is also part of the European & Developing Countries Clinical Trials Partnership programme (The Hague, The Netherlands) supported by the European Union.
Footnotes
Conflicts of interest: none declared.
References
- 1.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–286. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.9. [PubMed] [Google Scholar]
- 3.Pothukuchi M, Nagaraja S B, Kelamane S et al. Tuberculosis contact screening and isoniazid preventive therapy in a South Indian district: operational issues for programmatic consideration. PLOS ONE. 2011;6:e22500. doi: 10.1371/journal.pone.0022500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marais B J, van Zyl S, Schaaf H S, van Aardt M, Gie R P, Beyers N. Adherence to isoniazid preventive chemotherapy: a prospective community based study. Arch Dis Child. 2006;91:762–765. doi: 10.1136/adc.2006.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall C, Sukijthamapan P, dos Santos R et al. Challenges to delivery of isoniazid preventive therapy in a cohort of children exposed to tuberculosis in Timor-Leste. Trop Med Int Health. 2015;20:730–736. doi: 10.1111/tmi.12479. [DOI] [PubMed] [Google Scholar]
- 6.van Wyk S S, Reid A J, Mandalakas A M et al. Operational challenges in managing isoniazid preventive therapy in child contacts: a high-burden setting perspective. BMC Public Health. 2011;11:544. doi: 10.1186/1471-2458-11-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Wyk S S, Hamade H, Hesseling A C, Beyers N, Enarson D A, Mandalakas A M. Recording isoniazid preventive therapy delivery to children: operational challenges. Int J Tuberc Lung Dis. 2010;14:650–653. [PubMed] [Google Scholar]
- 8.Rutherford M E, Ruslami R, Maharani W et al. Adherence to isoniazid preventive therapy in Indonesian children: a quantitative and qualitative investigation. BMC Research Notes. 2012;5:7. doi: 10.1186/1756-0500-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garie K T, Yassin M A, Cuevas L E. Lack of adherence to isoniazid chemoprophylaxis in children in contact with adults with tuberculosis in Southern Ethiopia. PLOS ONE. 2011;6:e26452. doi: 10.1371/journal.pone.0026452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman M, Hesseling A, Beyers N et al. Routine programmatic delivery of isoniazid preventive therapy to children in Cape Town, South Africa. Public Health Action. 2013;3:199–203. doi: 10.5588/pha.13.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford M E, Ruslami R, Anselmo M. Management of children exposed to Mycobacterium tuberculosis: a public health evaluation in West Java, Indonesia. Bull World Health Organ. 2013;91:932–941. doi: 10.2471/BLT.13.118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams L V, Talbot E A, Odato K, Blunt H, Steingart K R. Interventions to improve delivery of isoniazid preventive therapy: an overview of systematic reviews. BMC Infect Dis. 2014;14:281. doi: 10.1186/1471-2334-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsdurf H, Hill P C, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 14.Donald P R, Maher D, Qazi S. A research agenda to promote the management of childhood tuberculosis within national tuberculosis programmes. Int J Tuberc Lung Dis. 2007;11:370–380. [PubMed] [Google Scholar]
- 15.Togun T O, Egere U, Sillah A K et al. Contribution of Xpert® MTB/RIF to the diagnosis of pulmonary tuberculosis among TB-exposed children in The Gambia. Int J Tuberc Lung Dis. 2015;19:1091–1097. doi: 10.5588/ijtld.15.0228. [DOI] [PubMed] [Google Scholar]
- 16.Adetifa I M, Ota M O, Jeffries D J et al. Commercial interferon gamma release assays compared to the tuberculin skin test for diagnosis of latent Mycobacterium tuberculosis infection in childhood contacts in the Gambia. Pediatr Infect Dis J. 2010;29:439–443. doi: 10.1097/INF.0b013e3181cb45da. [DOI] [PubMed] [Google Scholar]
- 17.Stop TB Partnership Childhood TB Subgroup, World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: Introduction and diagnosis of tuberculosis in children. Int J Tuberc Lung Dis. 2006;10:1091–1097. [PubMed] [Google Scholar]
- 18.Whitfield R, Cope G F. Point-of-care test to monitor adherence to anti-tuberculous treatment. Ann Clin Biochem. 2004;41:411–413. doi: 10.1258/0004563041731637. [DOI] [PubMed] [Google Scholar]
- 19.Le Roux S M, Cotton M F, Golub J E, Le Roux D M, Workman L, Zar H J. Adherence to isoniazid prophylaxis among HIV-infected children: a randomized controlled trial comparing two dosing schedules. BMC Medicine. 2009;7:67. doi: 10.1186/1741-7015-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes V F, Wejse C, Oliveira I et al. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective study from Guinea-Bissau. Int J Tuberc Lung Dis. 2011;15:1637–1643. doi: 10.5588/ijtld.10.0558. [DOI] [PubMed] [Google Scholar]
- 21.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 22.van Zyl S, Marais B J, Hesseling A C, Gie R P, Beyers N, Schaaf H S. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10:13–18. [PubMed] [Google Scholar]
- 23.Rutherford M E, Hill P C, Triasih R, Sinfield R, van Crevel R, Graham S M. Preventive therapy in children exposed to Mycobacterium tuberculosis: problems and solutions. Trop Med Int Health. 2012;17:1264–1273. doi: 10.1111/j.1365-3156.2012.03053.x. [DOI] [PubMed] [Google Scholar]
- 24.Amlabu V, Mulligan C, Jele N et al. Isoniazid/acetylisoniazid urine concentrations: markers of adherence to isoniazid preventive therapy in children. Int J Tuberc Lung Dis. 2014;18:528–530. doi: 10.5588/ijtld.13.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerra R L, Conde M B, Efron A et al. Point-of-care Arkansas method for measuring adherence to treatment with isoniazid. Respir Med. 2010;104:754–757. doi: 10.1016/j.rmed.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rekha B, Jagarajamma K, Chandrasekaran V, Wares F, Sivanandham R, Swaminathan S. Improving screening and chemoprophylaxis among child contacts in India's RNTCP: a pilot study. Int J Tuberc Lung Dis. 2013;17:163–168. doi: 10.5588/ijtld.12.0415. [DOI] [PubMed] [Google Scholar]
- 27.Skinner D, Hesseling A C, Francis C, Mandalakas A M. It's hard work, but it's worth it: the task of keeping children adherent to isoniazid preventive therapy. Public Health Action. 2013;3:191–198. doi: 10.5588/pha.13.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbuagbaw L, Thabane L, Ongolo-Zogo P et al. Trends and determining factors associated with adherence to antiretroviral therapy (ART) in Cameroon: a systematic review and analysis of the CAMPS trial. AIDS Res Ther. 2012;9:37. doi: 10.1186/1742-6405-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]


