Abstract
Setting: For 30 years, Malawi has experienced a dual epidemic of human immunodeficiency virus (HIV) infection and tuberculosis (TB) that has recently begun to be attenuated by the scale-up of antiretroviral therapy (ART).
Objective: To report on the correlation between ART scale-up and annual national TB case notification rates (CNR) in Malawi, stratified by HIV-positive and HIV-negative status, from 2005 to 2015.
Design: A retrospective descriptive ecological study using aggregate data from national reports.
Results: From 2005 to 2015, ART was scaled up in Malawi from 28 470 to 618 488 total patients, with population coverage increasing from 2.4% to 52.2%. During this time, annual TB notifications declined by 35%, from 26 344 to 17 104, and the TB CNR per 100 000 population declined by 49%, from 206 to 105. HIV testing uptake increased from 51% to 92%. In known HIV-positive TB patients, the CNR decreased from a high of 1247/100 000 to 710/100 000, a 43% decrease. In known HIV-negative TB patients, the CNR also decreased, from a high of 66/100 000 to 49/100 000, a 26% decrease.
Conclusion: TB case notifications have continued to decline in association with ART scale-up, with the decline seen more in HIV-positive than HIV-negative TB. These findings have programmatic implications for national TB control efforts.
Keywords: antiretroviral therapy, TB, Malawi, human immunodeficiency virus, HIV/AIDS
Abstract
Contexte : Pendant 30 ans, le Malawi a connu une double épidémie du virus de l'immunodéficience humaine (VIH) et de la tuberculose (TB) qui s'est atténuée récemment avec l'expansion du traitement antirétroviral (TAR).
Objectif : Etablir la corrélation entre l'expansion du TAR et les notifications annuelles de cas de TB, stratifiés en fonction de leur statut VIH positif ou négatif, au Malawi, de 2005 à 2015.
Schéma : Une étude rétrospective descriptive écologique reposant sur les données agrégées des rapports nationaux.
Résultats : De 2005 à 2015, le Malawi a étendu le TAR de 28 470 à 618 488 patients, avec une couverture de la population passant de 2,4% à 52,2%. Pendant ce temps, les notifications annuelles de TB ont décliné de 35%, de 26 344 à 17 104, et le taux de notification des cas de TB par 100 000 population a décliné de 49%, de 206 à 105. L'utilisation du test VIH a augmenté de 51% à 92%. Chez les patients TB-VIH positifs, les taux de notification des cas ont diminué d'un niveau élevé de 1247/100 000 à 710/100 000 (diminution de 43%). Chez les patients TB-VIH négatifs connus, les taux de notification des cas ont également décru d'un taux élevé de 66/100 000 à 49/100 000 (diminution de 26%).
Conclusion : Les notifications de cas de TB ont continué à décliner en association avec l'expansion du TAR, avec un déclin davantage constaté chez les patients TB-VIH positifs que chez les patients TB-VIH négatifs. Ces constatations ont des implications pour les programmes nationaux de lutte contre la TB.
Abstract
Marco de referencia: Durante 30 años se ha presentado en Malawi una epidemia doble de infección por el virus de la inmunodeficiencia humana (VIH) y tuberculosis (TB), que se ha moderado en tiempos recientes gracias a la ampliación de escala de administración del tratamiento antirretrovírico (TAR).
Objetivo: Evaluar la relación entre la ampliación de escala del TAR y la tasa anual nacional de notificación de casos de TB, estratificada por la situación frente al VIH, en Malawi del 2005 al 2015.
Método: Fue este un estudio retrospectivo descriptivo ecológico a partir de los datos agregados de notificación a escala nacional.
Resultados: Del 2005 al 2015 se amplió la escala de administración del TAR en Malawi de 28 470 a 618 488 casos, con un aumento de la cobertura del 2,4% al 52,2% de la población. Durante este período disminuyó un 35% la notificación anual de TB, de 26 344 a 17 104 casos, y un 49% la tasa de notificación, que pasó de 206 por 100 000 habitantes a 105/100 000. La aceptación de la prueba diagnóstica del VIH aumentó del 51% al 92%. En los pacientes positivos frente al VIH, la tasa de notificación de TB disminuyó de 1 247/100 000 a 710/100 000 (disminución del 43%). En los pacientes negativos frente al VIH, las tasas de notificación de TB también disminuyeron de 66/100 000 a 49/100 000 (disminución del 26%).
Conclusión: La notificación de casos de TB ha continuado su disminución en paralelo con la ampliación de escala de administración del TAR; la disminución es mayor en los pacientes positivos frente al VIH que en los pacientes negativos. Estos resultados tienen consecuencias programáticas sobre las iniciativas nacionales de control de la TB.
Human immunodeficiency virus (HIV) infection is the most important risk factor for the development of tuberculosis (TB) in persons infected with Mycobacterium tuberculosis, and has been responsible for the huge upsurge in TB cases in affected southern African countries over the last 25 years. Antiretroviral therapy (ART) reverses the immune dysfunction associated with HIV, and with initiation of treatment there is rapid functional recovery of mycobacteria-specific immune responses, which results in enhanced capacity to restrict mycobacterial growth.1,2 As a result, ART has a potent TB preventive effect.
A systematic review and meta-analysis of 11 studies from 2002 to 2011 showed that ART was associated with a 65% reduction in TB incidence across all baseline CD4 counts in people living with HIV (PLHIV).3 More recent studies have confirmed these findings and have also highlighted the TB preventive benefits of early initiation of ART.4–7
At the programme level, although PLHIV are often initiated late on ART and with low CD4 counts, studies from Malawi and Swaziland have shown that when high ART coverage rates are reached among HIV-infected populations, the TB case notification rate (CNR) decreases.8–12 In a study from Malawi reporting data up to 2012, there was some preliminary evidence to suggest that while the declines in TB were most apparent in those with HIV infection, there might also be a decline in patients who were HIV-negative.10 This led to suggestions that the overall decrease in HIV-associated TB in the community might have led to reduced community transmission of M. tuberculosis and thus fewer cases of TB in the non-HIV-infected population.
We have now collected national aggregate data on the number of PLHIV and the number of ART and TB case notifications stratified by HIV status up to 2015, and we use these data to report on the correlation between ART scale-up and annual national TB case notifications in Malawi from 2005 to 2015, with particular interest in whether there have been changes in HIV-positive as well as HIV-negative TB.
METHODS
Study design
This was a retrospective descriptive ecological study using aggregate data from national reports.
Setting
Malawi is one of the poorest countries in the world, with a rapidly growing population currently estimated at 17 million and a gross national income per capita of US$730.13 National scale-up of ART commenced in 2004, with the number of patients alive and retained on treatment reported quarterly by the HIV Department of the Ministry of Health (Lilongwe). In line with national and international guidelines,14,15 PLHIV are currently eligible for ART if they are pregnant or breast feeding, have World Health Organization (WHO) clinical Stage 3 or 4 disease, or have a CD4 cell count below the nationally agreed threshold (⩽250 cells/μl before 2010, ⩽350 cells/μl between 2010 and 2014 and ⩽500 cells/μl thereafter). At the start of national scale-up, first-line treatment mainly consisted of a fixed-dose combination of stavudine-lamivudine-nevirapine; from 2011 there was a gradual countrywide change to tenofovir-lamivudine-efavirenz.
Malawi has had an established national TB control programme (NTP) since 1985, with case finding, diagnosis, registration, treatment and treatment outcomes consistently following agreed international guidelines.16 Following published operational research showing the benefit of HIV testing and cotrimoxazole preventive therapy for patients found to be HIV-positive,17,18 HIV testing of TB patients was routinely scaled up, with uptake and results of testing formally and routinely captured at the national level from 2005 onwards.
Study population
The study population included the estimated annual number of PLHIV in Malawi, the number (adults and children) recorded alive and retained on ART at the end of each year, and all adults and children registered nationally each year with TB, stratified by HIV status, from 2005 to 2015.
Sources of data, variables and analysis
Sources of aggregate data were the national annual reports from the NTP and the HIV Department, Ministry of Health, Malawi. With the changes in ART eligibility criteria during the study period, ART coverage was calculated using the total HIV-infected population as the denominator. This was derived from national epidemiological projections using the Estimation and Projection package and Spectrum software from the Joint United Nations Programme on AIDS (UNAIDS, Geneva, Switzerland)19 and based on national population estimates obtained from the Malawi National Statistics Office (http://www.nsomalawi.mw/publications.html). Annual TB case notifications were documented and TB case rates per 100 000 population were derived from national population estimates. TB patients included registered cases stratified by HIV status: known HIV-positive, known HIV-negative and unknown HIV status. The numbers of patients with known HIV-positive TB and with known HIV-negative TB/100 000 HIV-positive and HIV-negative Malawi populations, respectively, were calculated based on the annual data from the UNAIDS Spectrum software and the Malawi National Statistics Office, as described above.19 Data were analysed descriptively.
Ethics approval
Ethics approval for the study was obtained from the Malawi National Health Science Research Committee (Lilongwe). The Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France) waived the need for further international ethics approval. As records and reports of aggregate data were used, informed patient consent was not necessary.
RESULTS
The number of people alive and receiving ART from 2005 to 2015 increased progressively from 28 470 to 618 488, with ART coverage among PLHIV rising from 2.4% to 52.2% (Figure 1).
FIGURE 1.
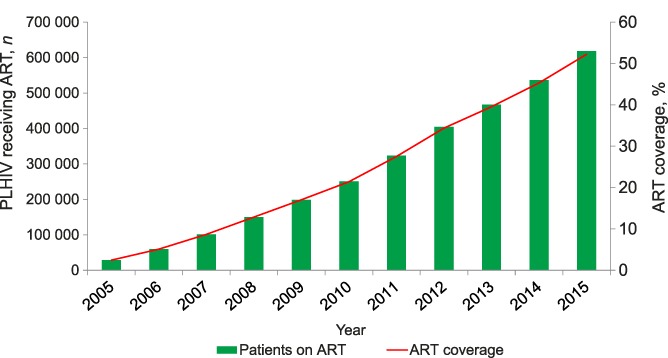
Numbers and coverage of PLHIV receiving ART, Malawi, 2005–2015. PLHIV = people living with the human immunodeficiency virus; ART = antiretroviral treatment.
The trends in TB case notifications and TB CNR (/100 000) are shown in Figure 2. Absolute numbers of TB cases peaked in 2006 at 26 344 and had declined progressively to 17 104 by 2015, a 35% decrease. This trend was mirrored by a decline in the TB CNR, from a high of 206/100 000 in 2006 to 105/100 000 in 2015, a 49% decrease.
FIGURE 2.
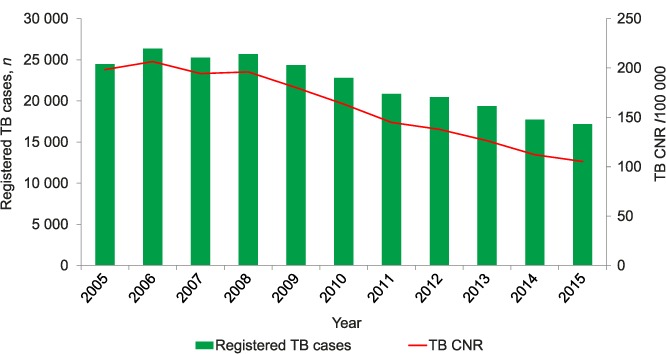
Numbers of registered TB cases and CNRs, Malawi, 2005–2015. TB = tuberculosis; CNR = case notification rate.
Trends in absolute numbers of notified TB patients who were HIV-tested, along with HIV status and, for those known to be HIV-positive, the uptake of ART, are shown in Figure 3. The proportion of TB patients who were HIV-tested in the country increased from 51% in 2005 to 92% in 2015, and was stable between 2012 and 2015 (92–93%). The proportion of TB patients with positive HIV status declined from 69% in 2005 to 53% in 2015, with the period 2010–2015 seeing a continual year-on-year decline from 64% to 53%. The proportion of HIV-positive TB patients started on ART remained at <50% between 2005 and 2009, but increased progressively from 2010 onwards, from 54% to 94% in 2015. For the 7 years when HIV testing rates were >85% (2009–2015), the absolute number of HIV-positive TB patients decreased from 13 558 to 8408 (a 38% decrease), while the overall change in absolute numbers of HIV-negative TB patients was small, decreasing from 7483 to 7420 (a 0.7% decrease).
FIGURE 3.
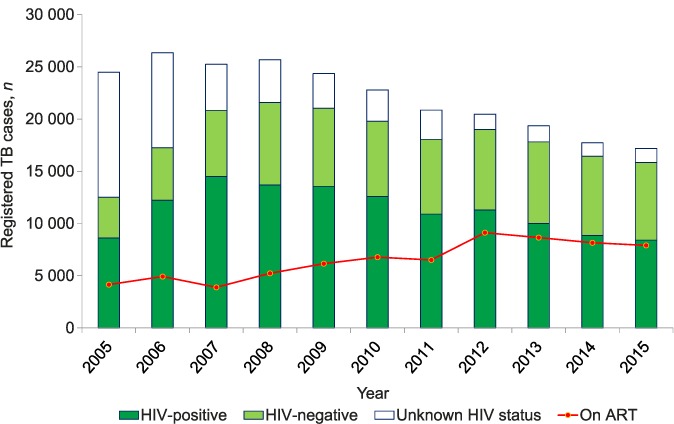
Trends in HIV testing, HIV status and ART uptake among registered TB patients, Malawi, 2005–2015. TB = tuberculosis; HIV = human immunodeficiency virus; ART = antiretroviral treatment.
Trends in HIV-positive TB cases/100 000 PLHIV and HIV-negative TB cases/100 000 people not infected with HIV are shown in Figure 4A and B. The initial increase in CNR/100 000 in the first 3 years reflects the progressive increase in TB patients being HIV tested, from 51% to 65% to 83%. Thereafter, HIV testing rates were always >83%. In known HIV-positive TB patients, there was a progressive decrease in the CNR, from a high of 1247/100 000 in 2007 to 710/100 000 in 2015, a 43% decrease. In known HIV-negative TB patients, there was also a decrease in the CNR from a high of 66/100 000 in 2008 to 49/100 000 in 2015, a 26% decrease.
FIGURE 4.
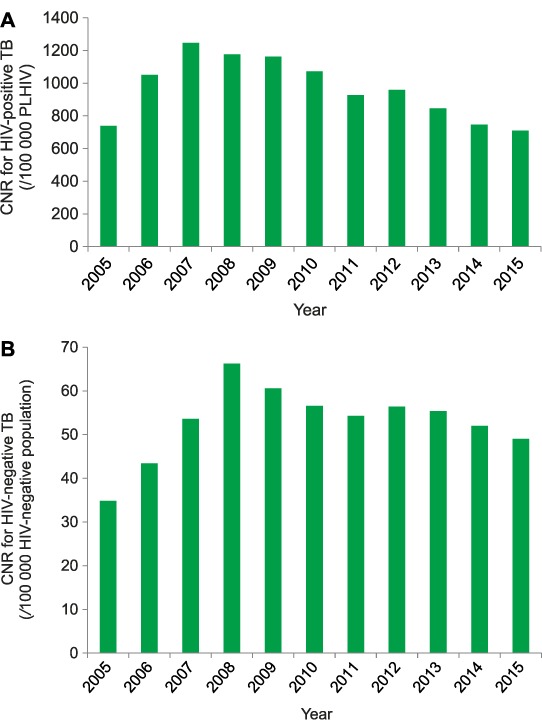
CNRs for HIV-positive and HIV-negative TB, Malawi, 2005–2015. A) For HIV-positive TB: case rates are known HIV-positive TB cases/100 000 PLHIV in Malawi. B) For HIV-negative TB: case rates are known HIV-negative TB cases/100 000 people not infected with HIV in Malawi. CNR = case notification rate; HIV = human immunodeficiency virus; TB = tuberculosis; PLHIV = people living with HIV.
DISCUSSION
This study has a number of interesting findings. First, our initial reports from Malawi on the pronounced inverse correlation between the national scale-up of ART and national TB case notifications are confirmed.10,12 As ART coverage increases, the absolute numbers of TB cases and the TB CNR/100 000 have continued to decline. This is in line with other reports and publications from southern Africa.11
Second, the proportion of TB patients being tested for HIV is now consistently greater than 90%, and in the last 2 years over 90% of patients with HIV-associated TB have been initiated on ART. We have no information from this aggregate data set about the time of starting ART, or indeed whether ART was already being taken at the time TB was diagnosed. Nevertheless, having PLHIV on ART during anti-tuberculosis treatment should improve TB treatment outcomes—provided there is no resistance to the antiretroviral drugs—and should also help to prevent recurrent TB developing after successful completion of anti-tuberculosis treatment.8,20
Third, while there has been a large decrease in the absolute number and proportion of HIV-infected TB patients in the last 7 years, the decrease in absolute numbers of TB patients without HIV infection was marginal. However, when TB-HIV status was assessed in relation to the CNR/100 000, there were large decreases both in HIV-positive TB cases/100 000 PLHIV and in the CNR in HIV-negative TB cases/100 000 people without HIV, although these decreases were less marked compared with HIV-positive cases. These results confirm our first and preliminary findings in Malawi for 2012,10 and suggest that a reduction in HIV-positive TB cases has a beneficial effect on transmission of M. tuberculosis in the community and thus fewer cases of TB in the non-HIV-infected population. Similar findings have recently been reported from a 15-year observational study in Kenya, where there was a 28–44% decrease in HIV-positive TB and an 11–26% decrease in HIV-negative TB.21
The strengths of this study are the comprehensive national reports, which for TB have integrated HIV parameters since 2005, and the recognised quality of Malawi's ART data.22 The limitations relate mainly to the aggregate nature of the data, which rely on accurate TB case notifications from Malawi's many TB registration centres, and which prevent detailed examination and explanation of some of the observations, such as time of ART initiation in HIV-infected TB patients. We also cannot exclude the possibility that the decrease in TB notifications might have occurred as a result of other changes in the last 12 years, such as improved socio-economic status, better coverage or quality of TB diagnosis, or implementation of isoniazid preventive therapy (IPT). However, we have no evidence of any marked improvement in socio-economic conditions of the rural population. Changes in coverage or quality of TB diagnosis, such as the introduction of Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), might have been expected to increase rather than reduce TB case notifications, and to date there has been no significant scale-up of IPT.
There are some important implications for moving forward. First, in line with the WHO's rapid advice in 2015 and recently released 2016 guidelines to start all PLHIV on ART regardless of clinical stage or CD4 cell count,23,24 Malawi has made a strategic decision to start what is called ‘HIV test and treat’. Early start of ART has been shown to further reduce the risk of TB in PLHIV,4–7 and this should enhance the gains already made in the past several years. TB is also a major cause of hospitalisation and in-hospital mortality in adults and children globally, and early ART is likely to substantially reduce this burden.25
Second, there are plans to scale up IPT in a phased manner around the country. Two randomised controlled studies in Ivory Coast and South Africa have demonstrated that adding IPT to ART in PLHIV can lead to an additional reduction in TB cases of 30% or higher,7,26 and this intervention may add to the TB preventive effect of ART. This is important because despite the effective intervention of ART, TB CNRs in PLHIV are still much higher than in the non-HIV-infected population.
Third, with HIV-negative TB cases decreasing, but at a relatively slow rate, it is important that the Malawi NTP attend to the basics of TB control, including good infection control, measures to minimise the risk of multidrug-resistant TB and the provision of regular structured supportive supervision for all the registration units in the country.27
Fourth, Malawi needs to start recognising and addressing other factors that increase the risk of TB, such as undernutrition, smoking, alcohol and diabetes mellitus.28 Rapid uncontrolled urbanisation together with major lifestyle changes among populations in sub-Saharan Africa are driving a rapidly increasing epidemic of diabetes mellitus,29 and there is increasing evidence to show that this poses a risk for TB control in terms of rising case numbers and worse treatment outcomes for people undergoing anti-tuberculosis treatment.30
In conclusion, this study provides further evidence of the continuing decline in TB notifications in association with the scale-up of ART in Malawi. There is a large decline in CNRs in HIV-positive TB, although rates are still high compared with the HIV-negative population. CNRs are declining in HIV-negative TB, albeit slowly. While efforts towards scaling up ART must continue to ensure the wide coverage and early start of treatment for PLHIV, Malawi must ensure that it provides high quality core services for TB, and it should also begin to address other determinants of the disease.
Acknowledgments
The authors thank La Fondation Veuve Emile Metz-Tesch (Luxembourg) for their support for the open access publication costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Conflict of interest: none declared.
References
- 1.Lawn S D, Bekker L G, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS. 2005;19:1113–1124. doi: 10.1097/01.aids.0000176211.08581.5a. [DOI] [PubMed] [Google Scholar]
- 2.Kampmann B, Tena-Coki G N, Nicol M P, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS. 2006;20:1011–1018. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 3.Suthar A B, Lawn S D, del Amo J et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLOS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinsztejn B, Hosseinipour M C, Ribaudo H J et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins S E, Jean Juste M A, Koenig S P et al. CD4 deficit and tuberculosis risk persist with delayed antiretroviral therapy: 5-year data from CIPRA HT-001. Int J Tuberc Lung Dis. 2015;19:50–57. doi: 10.5588/ijtld.14.0217. [DOI] [PubMed] [Google Scholar]
- 6.INSIGHT START Study Group. Lundgren J D, Babiker A G et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TEMPRANO ANRS 12136 Study Group. Danel C, Moh R et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 8.Zachariah R, Bemelmans M, Akesson A et al. Reduced tuberculosis case notification associated with scaling up antiretroviral treatment in rural Malawi. Int J Tuberc Lung Dis. 2011;15:933–937. doi: 10.5588/ijtld.10.0666. [DOI] [PubMed] [Google Scholar]
- 9.Middelkoop K, Bekker L-G, Myer L et al. Antiretroviral therapy and TB notification rates in a high HIV prevalence South African community. J Acquir Immune Defic Syndr. 2011;56:263–269. doi: 10.1097/QAI.0b013e31820413b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanyerere H, Mganga A, Harries A D et al. Decline in national tuberculosis notifications with national scale-up of antiretroviral therapy in Malawi. Public Health Action. 2014;4:113–115. doi: 10.5588/pha.14.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haumba S, Dlamini T, Calnan M et al. Declining tuberculosis notification trend associated with strengthened and expanded HIV care in Swaziland. Public Health Action. 2015;5:103–105. doi: 10.5588/pha.15.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanyerere H, Harries A D, Tayler-Smith K et al. The rise and fall of tuberculosis in Malawi: associations with HIV infection and antiretroviral therapy. Trop Med Int Health. 2016;21:101–107. doi: 10.1111/tmi.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. World Health Statistics 2014. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 14.Malawi Ministry of Health. 2014 Clinical management of HIV in children and adults. 2nd ed. Lilongwe, Malawi: Ministry of Health; 2014. http://www.emtct-iatt.org/wp-content/uploads/2015/09/Malawi-HIV-Guidelines-2014.pdf Accessed September 2016. [Google Scholar]
- 15.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 16.World Health Organization. Treatment of tuberculosis guidelines. 4th ed. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 17.Zachariah R, Spielmann M P, Chinji C et al. Voluntary counselling, HIV testing and adjunctive treatment with cotrimoxazole reduces mortality in tuberculosis patients in Thyolo, Malawi. AIDS. 2013;17:1053–1061. doi: 10.1097/00002030-200305020-00015. [DOI] [PubMed] [Google Scholar]
- 18.Mwaungulu F B D, Floyd S, Crampin A C et al. Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organ. 2014;82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 19.Ghys P D, Brown T, Grassly N C et al. The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sex Transm Infect. 2004;80(Suppl 1):i5–i9. doi: 10.1136/sti.2004.010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan F A, Minion J, Al-Motairi A, Benedetti A, Harries A D, Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis. 2012;55:1154–1163. doi: 10.1093/cid/cis630. [DOI] [PubMed] [Google Scholar]
- 21.Yuen C M, Weyenga H O, Kim A A et al. Comparison of trends in tuberculosis incidence among adults living with HIV and adults without HIV—Kenya, 1998–2012. PLOS ONE. 2014;9:e99880. doi: 10.1371/journal.pone.0099880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global Fund for AIDS, TB and Malaria. Report for the Data Quality Audit for HIV/AIDS in Malawi (MLW-1-2-G01-H, MLW-506-G-3-H, MLW-708-G07-H) Geneva, Switzerland: Global Fund; 2010. Final Audit Report, October 26, 2010 (Amended April 5, 2011) [Google Scholar]
- 23.World Health Organization. Guidelines on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO; 2015. [PubMed] [Google Scholar]
- 24.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2nd ed. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
- 25.Ford N, Matteelli A, Shubber Z et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. 2016;19:20 714. doi: 10.7448/IAS.19.1.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangaka M X, Wilkinson R J, Boulle A et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frieden T R, Brudney K F, Harries A D. Global tuberculosis: perspectives, prospects, and priorities. JAMA. 2014;312:1393–1394. doi: 10.1001/jama.2014.11450. [DOI] [PubMed] [Google Scholar]
- 28.Lönnroth K, Castro K G, Chakaya J M et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 29.Mbanya J C N, Motala A A, Sobngwi E, Assah F K, Enoru S T. Diabetes in sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 30.Lönnroth K, Roglic G, Harries A D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2:730–739. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]


