Abstract
Excessive placental inflammation is associated with several pathological conditions, including stillbirth and fetal growth restriction (FGR). While infection is a known cause of inflammation, a significant proportion of pregnancies have evidence of inflammation without any detectable infection. Inflammation can also be triggered by endogenous mediators, called damage associated molecular pattern (DAMPs) or alarmins. One of these DAMPs, uric acid is increased in the maternal circulation in pathological pregnancies and is a known agonist of the Nlrp3 inflammasome and inducer of inflammation. However its effects within the placenta and on pregnancy outcome remain largely unknown. We found that uric acid crystals (monosodium urate, MSU, crystals) induces a pro-inflammatory profile in isolated human term cytotrophoblast cells, with a predominant secretion of IL-1β and IL-6, a result confirmed in human term placental explants. Pro-inflammatory effects of MSU crystals were shown to be IL-1-dependent using a caspase-1 inhibitor (inhibits IL-1 maturation) and IL-1Ra (inhibits IL-1 signaling). The pro-inflammatory effect of MSU crystals was accompanied by trophoblast apoptosis and decreased syncytialisation. Correspondingly, administration of MSU crystals to rats during late gestation induced placental inflammation and was associated with fetal growth restriction. These results make a strong case for an active pro-inflammatory role of MSU crystals at the maternal-fetal interface in pathological pregnancies, and highlight a key mediating role of IL-1. Furthermore, our study describes a novel in vivo animal model of non-infectious inflammation during pregnancy, which is triggered by MSU crystals and leads to reduced fetal growth.
Keywords: Alarmin, inflammation, pregnancy, placenta, fetal growth restriction, interleukin-1
Introduction
Prenatal inflammation is an important clinical problem associated with devastating short and long-term consequences for the developing fetus1–3. In human pregnancy exposure to inflammation increases the incidence of stillbirth, and surviving fetuses are at increased risk of fetal growth restriction (FGR) and preterm birth, with increased incidence of neonatal death4–8. These serious complications of pregnancy affect approximately 10-12% of all pregnancies in industrialized countries4,5,7. Furthermore, prenatal exposure to inflammation is associated with fetal programming of cardiovascular diseases and with a higher incidence of neurodevelopmental disorders, including cerebral palsy and autism1,2. Currently available treatments only aim to alleviate the symptoms of these disorders, as the causes are still speculative.
These effects of prenatal inflammation are most likely mediated through placental dysfunction. The causal link between inflammation and altered placental function has been shown primarily using animal models of infection-induced inflammation9,10. Infection is a well-known cause of inflammation but, in many cases of pathological pregnancies, there is no detectable infection whilst evidence of inflammation, such as increased levels of cytokines, is present2,11. Other than infection, a plausible cause of inflammation is alarmins or damage associated molecular pattern (DAMPs)12,13, such as uric acid crystals (monosodium urate, MSU, crystals), high-mobility group box 1 (HMGB1) and cell-free fetal DNA (cffDNA), amongst others, which are increasingly associated with pathological pregnancies14–18. DAMPs are endogenous mediators that can induce inflammation through the same receptors as pathogens, namely the Toll-like receptors (TLRs) and NOD-like receptors (NLRs)12,13,19,20. However, little is currently known about the causal link between alarmins and pregnancy pathologies and the mechanisms involved remain mostly unresolved.
The DAMP uric acid has been shown to be elevated in the maternal circulation of many pregnancy pathologies, especially preeclampsia (PE)14,15,21–23. Furthermore, hyperuricemia has been associated with poor maternal and fetal outcomes21. MSU crystals can exert pro-inflammatory effects in humans and animals24,25; however, the effects of MSU crystals at the maternal-fetal interface are still mostly unknown. Studies using human first trimester trophoblast cell lines showed that interleukin (IL)-1β was produced in response to MSU crystals via the activation of the Nlrp3 inflammasome26,27,28. Furthermore, it has been reported that uric acid decreased system A amino acid transporter activity in third trimester placental explants29; a decrease in the activity of this transporter in the placenta has previously been associated with FGR30. Together, mechanistic and clinical evidence points toward a direct effect of uric acid on the placenta, which could lead to inflammation and contribute to the pathogenesis of pregnancy disorders.
Our objective was to investigate the effect of MSU crystals on human placenta late in gestation, with the hypothesis that MSU crystals causes placental inflammation and dysfunction that leads to FGR. Herein, we show that MSU crystals induces an IL-1-dependent pro-inflammatory response in human term cytotrophoblasts and placental explants associated with abrogated syncytialisation and increased trophoblast apoptosis. Alongside, administration of MSU crystals in vivo to pregnant rats led to FGR, which was characterized by a pro-inflammatory profile and immune cell infiltration of the placenta. Hence, our work strongly supports a causal role of uric acid in placental inflammation and dysfunction observed in pathological pregnancies.
Methods
Ethical approval
Approval was obtained from North West Research Ethics Committee in Manchester, UK (Ref: 08/H1010/55), Yale University’s Human Research Protection Program, and the Sainte-Justine Hospital Ethic Board (Ref: 3988) for placentas from uncomplicated term pregnancies. All participants provided written informed consent, except at Yale University as this was not required due to de-identified samples of discarded tissue. Animal work was approved by Institutional Animal Care Committee at the Sainte-Justine Research Center and in line with the guidelines of the Canadian Council of Animal Care.
Primary cytotrophoblast isolation
Primary cytotrophoblast cells were isolated from term placentas from uncomplicated pregnancies obtained after caesarean section without labor using a well established method developed by Kliman et al.31 with slight modifications. Briefly, villous tissue was dissected, minced and rinsed in phosphate-buffered saline (PBS) prior to 3 enzymatic digestions in Hanks’ balances salt solution (HBSS) with trypsin and DNase. Cytotrophoblast were isolated from these digestions after separation by centrifugation on a discontinuous Percoll gradient. Cytotrophoblasts were plated at 2x106 cells/ml in DMEM-F12 supplemented with 10% FBS and penicillin/streptomycin and washed with PBS to remove non-adherent cells after 12h to remove residual syncytiotrophoblast. Cell purity was assessed by staining for the trophoblast specific parker cytokeratin 7 (CK7) and 98% of the cells were CK7+ (data not shown). Additional sets of experiments were performed with added negative purification after Percoll separation with anti-HLA-ABC antibody (Affymetrix, CA, USA) and magnetic beads to ensure removal of immune cells. There was no differences observed in cytokines secretion between cells with or without this purification step and therefore the results were combined. Cytotrophoblast were treated with uric acid crystals (MSU crystals, 100μg/ml; Invivogen, CA, USA; same concentration as previously published26 and representing the levels observed in severe PE), recombinant human IL-1β (rhIL-1β, 10ng/ml; Peprotech, Qc, Canada), caspase-1 inhibitor (10μM; Z-WEHD-FMK, R&D Systems, USA) or IL-1 receptor antagonist (IL-1Ra, 1μg/ml; Sobi-Swedish Orphan Biovitrum, ON, Canada) for 24 or 48h in Opti-MEM (Life Technologies, ON, Canada). Supernatants were collected and kept at -80°C until analyzed. For immunohistochemical (IHC) or immunofluorescence (IF) analysis or cytotrophoblasts, cells were fixed with cold MeOH and kept at -20°C until processed.
Placental explant culture and treatment
Villous tissue was obtained from term uncomplicated pregnancies after caesarean section without labor (n=9) within 30 min after delivery and explants were prepared as previously described32 with slight modifications. Briefly, biopsies of the chorionic villi were dissected and washed with PBS to remove any blood. Tissue was cut into small pieces (approx. 2-3mm) and three pieces (i.e. explants) were placed into netwells (74 μm mesh; Corning, Sigma-Aldrich, ON, Canada) in 1.5ml of culture media (RPMI, 5% heat-inactivated FBS, 100μg/ml streptomycin, 100IU/ml penicillin, 1μg/ml insulin, 0.1μg/ml hydrocortisone, 0.1μg/ml retinol acetate, 0.05mg/ml gentamycin; all chemical are from Sigma-Aldrich, ON, Canada). Explants were cultured at 37°C with 5% CO2 with daily media change. On day 4, explants were treated with MSU crystals (100μg/ml; Invivogen, CA, USA), rhIL-1β (10ng/ml; Peprotech, Qc, Canada), caspase-1 inhibitor (10μM; Z-WEHD-FMK, R&D Systems, USA) or IL-1Ra (1μg/ml; Sobi-Swedish Orphan Biovitrum, ON, Canada) for 24 or 48h in Opti-MEM (Life Technologies, ON, Canada). Explants were processed for histology (fixed for 24h in 4% formalin and paraffin embedded) or protein analysis (supernatants and explants frozen and the stored at -80°C until extraction and analysis).
In vivo animal model
Timed mated Sprague-Dawley rats were obtained at gestational day 13 (G13) (Charles River Laboratories, Qc, Canada) and kept in a controlled 20°C environment with a 12h light/dark cycle and access to food and water ad libitum. Pregnant dams were injected intraperitoneally (i.p.) every 12h starting at G18 until G21 with uric acid crystals (monosodium urate (MSU) crystals; 250, 500 or 100μg/kg/12h; Sigma-Aldrich, ON, Canada) combined with potassium oxonate (125mg/kg/day; Sigma-Aldrich, ON, Canada). Potassium oxonate is needed to block the enzyme uricase, which is absent in human but present in rodents and rapidly degrades uric acid33. No differences between vehicle (PBS) or potassium oxonate injected animals were observed in term of uric acid levels or inflammatory markers in the placenta (or fetal weight). Therefore both were combined and are shown as vehicle-injected in the results. Six dams/litters were used in each experimental group.
Protein extraction and analysis
Cells, explants or tissues were homogenized in lysis buffer containing 1% Triton X-100 (Sigma-Aldrich, ON, Canada) and protease inhibitor cocktail (Calbiochem, Millipore, ON, Canada), and centrifuged at 13000 rpm for 10 min at 4°C. Supernatants were collected and stored at -20°C until analysis. Protein concentration was determined using the Bradford assay (BioRad, ON, Canada). Levels of human (h) and rat (r) cytokines (hIL-1β, hIL-6, monocyte chemoattractant protein (hMCP)-1, rIL-1β, tumor necrosis factor (TNF)-α, rIL-6) were determined by DuoSet ELISAs (R&D Systems, MN, USA) following manufacturer’s instructions. Multiplex assay (BioPlex, BioRad, ON, Canada) was performed using a custom panel of 16 human cytokines (IL-1β, IL-6, IL-10, IL-17, IFNγ, TNFα, G-CSF, GM-CSF) and chemokines (IL-8, MCP-1/CCL2, MIP1α/CCL3, MIP1β/CCL4, RANTES/CCL5, VEGF, GROα/CXCL1, IP-10/CXCL10) on cell supernatants according to manufacturer’s instructions. Caspase-1 activity was detected in culture supernatant using a caspase-1 specific kit following manufacturer instructions (Caspase-Glo 1 Inflammasome Assay, Promega, USA). Western blotting was used to detect caspase-1 using the following protocol. Proteins (50μg) were loaded onto an SDS-PAGE gel and, after separation, transferred to a 0.45μm nitrocellulose membrane. Membranes were blocked and incubated overnight at 4°C with the primary antibody (caspase-1, Santa Cruz Biotechnology, CA, USA). After washing they were incubated with goat anti-mouse HRP-conjugated secondary antibody (BioRad, ON, Canada) and signal was detected by chemiluminescence (clarity western ECL substrate, BioRad, ON, Canada).
Histological analysis
5μm-thick paraffin sections were processed for hematoxylin & eosin (H&E) staining and immunohistochemistry (IHC) as previously described17. The following antibodies were used: M30 (caspase-cleaved cytokeratin 8, Roche, USA), desmoplakin (Sigma-Aldrich, UK), CD68 (Serotec, ON, Canada), IL-1β (Santa Cruz Biotechnology, CA, USA), polymorphonuclear antibody (PMN, Cedarlane, ON, Canada). Matched secondary antibody HRP-conjugated (anti-rabbit-HRP, or anti-mouse-HRP, Bio-Rad, ON, Canada) were used, revealed using 3, 3-diaminobenzidine (DAB, VWR, Canada) and sections were counterstained with hematoxylin. For IF, fluo-conjugated secondary antibody was used (anti-mouse Alexa 488, Life Technologies, UK) and mounted with mounting media countaining DAPI (Life Technologies, UK). Images were obtained with a slide scanner (Axioscan, Zeiss, ON, Canada) or an Olympus upright BX51, CoolSnap ES camera and MetaVue software, for immunofluorescence.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical comparison between multiple groups was assessed using one-way ANOVA with Dunnett’s multiple comparison test. Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software, CA, USA) and a p<0.05 was considered significant.
Results
MSU crystals induced an inflammatory profile in primary term cytotrophoblasts and placental explants
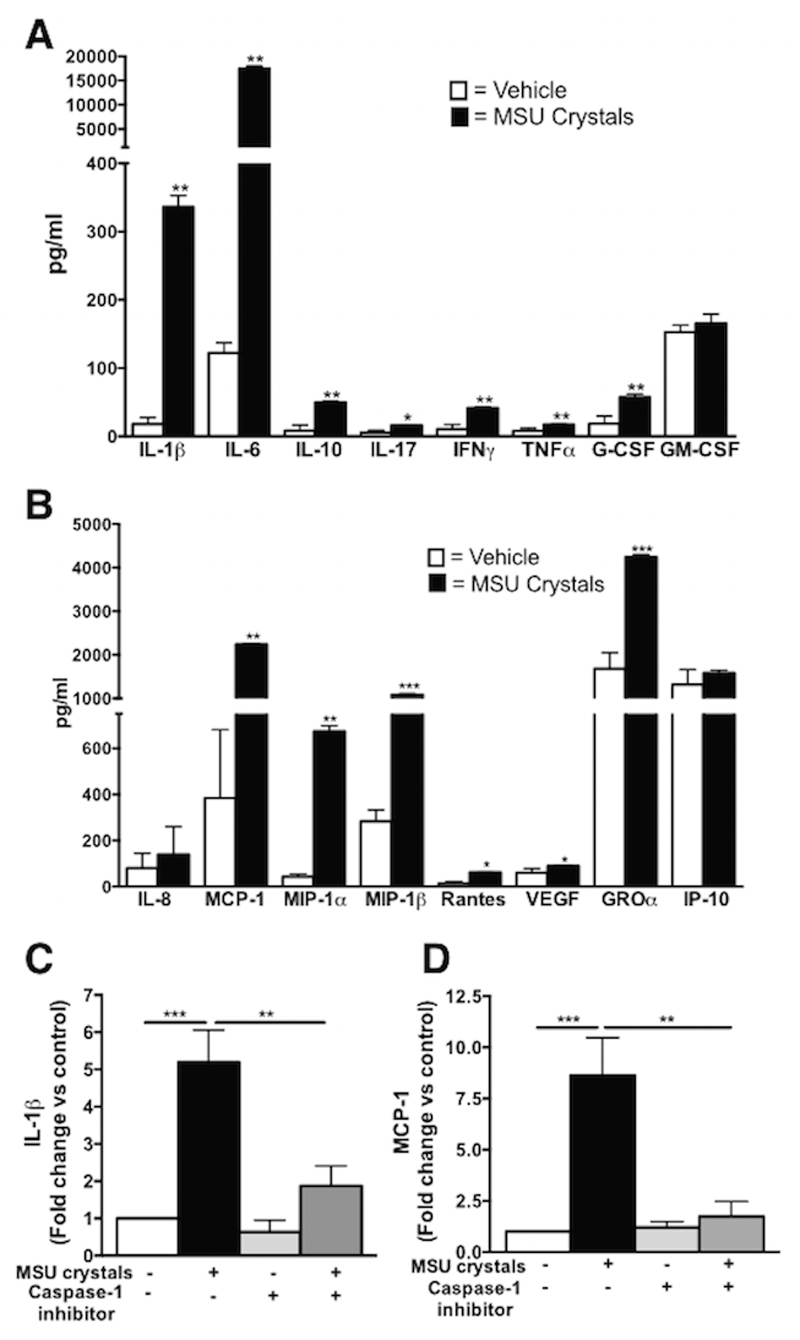
We determined the inflammatory profile induced by MSU crystals in primary cytotrophoblasts (n=6-9) isolated from term human placentas from uncomplicated pregnancies. Exposure of cytotrophoblasts to MSU crystals for 48h induced the secretion of several pro-inflammatory mediators, primarily IL-1β (18.6-fold increase, p<0.01 vs vehicle) and IL-6 (143-fold increase, p<0.01 vs vehicle), but also IFN-γ, TNF-α, IL-17, G-CSF as well as the anti-inflammatory cytokine IL-10 (Fig 1A). No effect was seen on the secretion of GM-CSF (Fig 1A). Elevated secretion of several chemokines was also observed, especially MCP-1 (5.8-fold increase, p<0.01 vs vehicle), and also MIP-1β, MIP-1α and GROα (3.9, 15.3, 2.5-fold increase respectively, p<0.01 vs vehicle, Fig 1B). RANTES and VEGF were also significantly upregulated whilst IP-10 and IL-8 levels were unaffected (Fig 1B).
Figure 1. Inflammatory profile of human term cytotrophoblast after MSU crystals exposure is IL-1 dependent.
MSU crystals induced the secretion of multiple cytokines, primarily IL-1β and IL-6 in cytotrophoblast (A) as well as chemokines, including MCP-1 and MIP-1α and MIP-1β (B). IL-1β secretion by cytotrophoblast exposed to MSU crystals was elevated and this effect was blocked by concomitant treatment with the caspase-1 inhibitor (C). This was also observed for secretion of the chemokine MCP-1 (D). (n=6). Results presented as mean ± SEM. Statistical analysis by t-test with welch correction (A and B) and one-way ANOVA with Dunnett’s post-test (C and D). *p<0.05, **p<0.01, ***p<0.001.
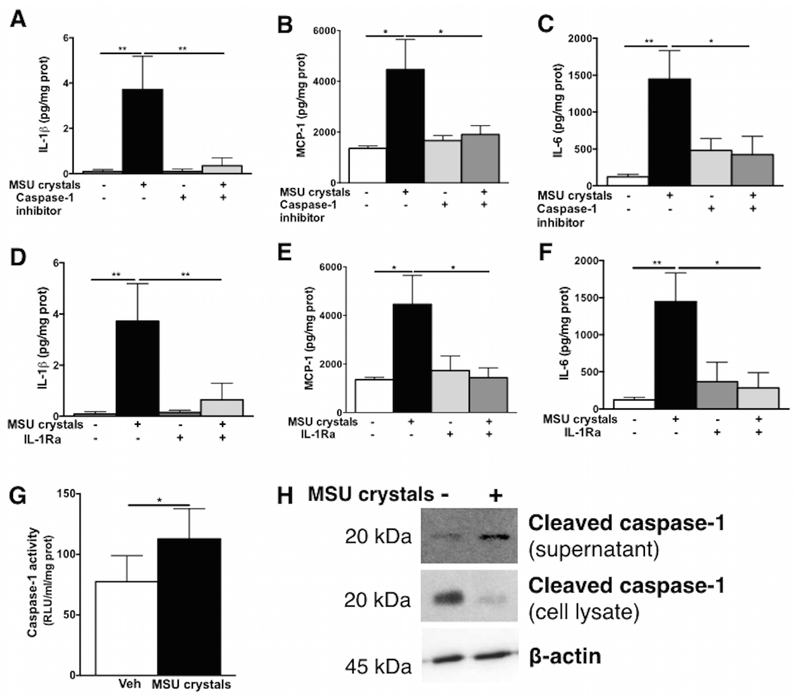
Since IL-1β is a well-known mediator of MSU crystals actions in immune cells, and it was strongly induced in term cytotrophoblasts in response to MSU crystals, we addressed the mechanisms of IL-1β production. The secretion of IL-1β induced by MSU crystals in cytotrophoblasts was dependent on the common processor of pro-IL-1β, caspase-1, which was ascertained using a caspase-1 inhibitor (Fig 1C). The secretion of another inflammatory mediator, MCP-1, was also decreased following caspase-1 inhibition (Fig 1D) suggesting an upstream role of IL-1 in the induction of inflammatory mediators in cytotrophoblasts in response to MSU crystals. A similar inflammatory effect of MSU crystals was observed in term placental explants in which MSU crystals significantly induced IL-1β, MCP-1 and IL-6 secretion via caspase-1 (Fig 2A-C) and IL-1 receptor (Fig 2D-F) as shown with a caspase-1 inhibitor and IL-1Ra, respectively, confirming the central role of IL-1β in placental inflammation induced by MSU crystals. Furthermore, caspase-1 activation was significantly elevated in the supernatant following MSU crystals exposure (Fig 2G) concomitantly to increased cleaved caspase-1 in the supernatant and decreased levels within the cells (Fig 2H). Basal activation of caspase-1 was observed in term placental explants (Fig 2G-H) due to the constant release of alarmins (i.e. fetal DNA, trophoblast debris) in such a culture setting and to the trophoblast own production of uric acid as previously reported27,28.
Figure 2. IL-1 dependent effects of MSU crystals on the inflammatory profile of term placental explants.
In human term placental explants, IL-1β (A), MCP-1 (B) and IL-6 (C) secretion were all induced by MSU crystals and abrogated with the use of the caspase-1 inhibitor. The specificity to the IL-1 pathway was confirmed using the IL-1 receptor antagonist (IL-1Ra) which also decreased the secretion of IL-1β (D), MCP-1 (E) and IL-6 (F). Increased caspase-1 activity was detected in the supernatant (G) alongside increased cleaved caspase-1 in the supernatant and decreased in the lysate detected by western blot (representative example shown in H). (n=4-6) Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test (A-F) or t-test with welch correction (G). *p<0.05; **p<0.01.
MSU crystals induced trophoblast apoptosis and blocked syncytialisation in an IL-1-dependent manner
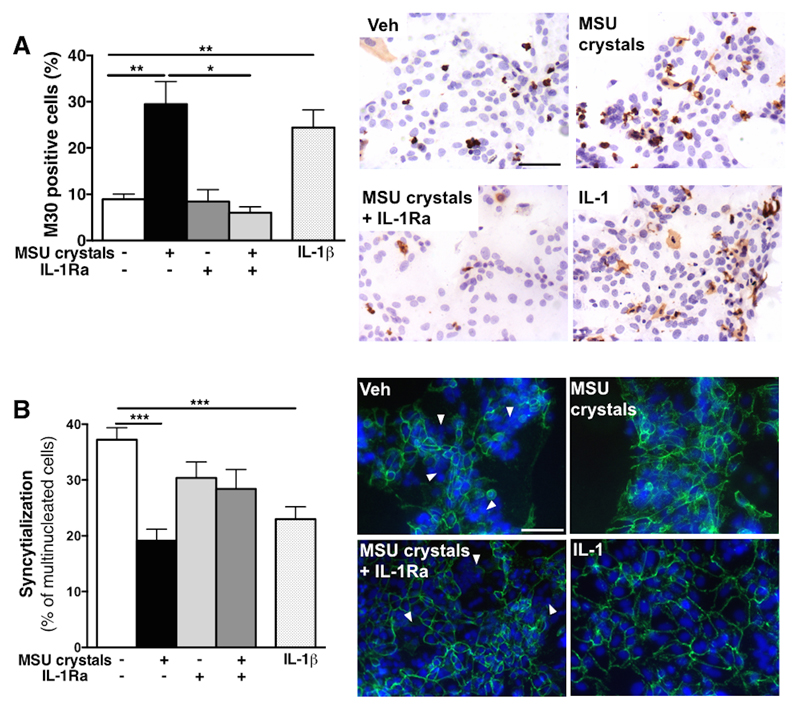
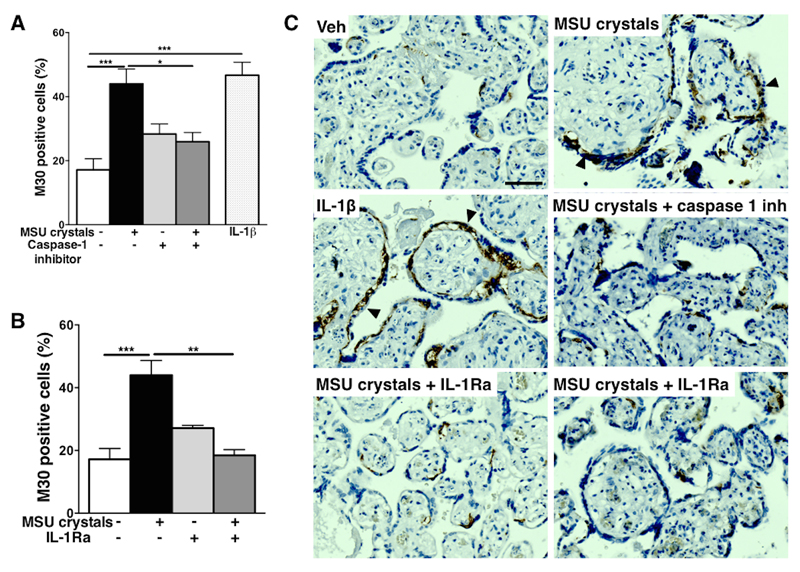
Both MSU crystals and IL-1β significantly induced cell death in cytotrophoblast cultures, as seen by the 3.3-fold and 2.7-fold increase, respectively, in the percentage of cells positive for the apoptotic marker M30 at 48h (Fig 3A). Treatment of MSU crystals-exposed cytotrophoblasts with IL-1Ra was protective, with decreased percentage of M30+ apoptotic cells (Fig 3A). MSU crystals exposure also significantly blocked syncytialisation (i.e. decreased the percentage of multinucleated cells) to a similar extent of that observed following direct IL-1β exposure (Fig 3B). Blockade of IL-1 signaling pathway with IL-1Ra increased the percentage of multinucleated cells (syncytialisation) (Fig 3B), although this did not reach significance (p=0.069). Consistent with these findings in cytotrophoblast, treatment with MSU crystals or IL-1β induced apoptosis in term placental explants, (Fig 4A, B) which was mainly observed in cytotrophoblast cells (arrowheads in Fig 4C). MSU crystals-induced apoptosis was again demonstrated to be IL-1-dependent using caspase-1 inhibitor and IL-1Ra (Fig 4A-C).
Figure 3. IL-1-dependent effects of MSU crystals of cytotrophoblast viability and syncytialization.
Term human cytotrophoblast exposed to MSU crystals (100μg/ml) showed increased number of M30+ apoptotic cells (brown staining in A) to a similar extent has exposure with IL-1β and this effect was dependent on IL-1 since blocking the IL-1 pathway using IL-1Ra protected cytotrophoblast against MSU crystals-induced cell death (A). Syncytialisation (addressed by staining for desmoplakin and quantifying the percentage of multinucleated cells counted (white arrows)) was decreased by MSU crystals or IL-1β treatment (B). (n=4-6). Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test. Scale bar: 50μm. *p<0.05, **p<0.01, ***p<0.001.
Figure 4. Effects of MSU crystals on human term placental explants.
Term human placental explants exposed to MSU crystals or IL-1β displayed elevated apoptosis which was blocked by caspase-1 inhibition (A) and IL-1Ra treatment (B). Representative example of M30+ cells in explants are shown (C) with positive cells being mostly trophoblast (black arrowheads). (n=6) Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test. Scale bar: 50μm. *p<0.05, **p<0.01, ***p<0.001.
Administration of MSU crystals during late gestation induced placental inflammation and FGR in rats
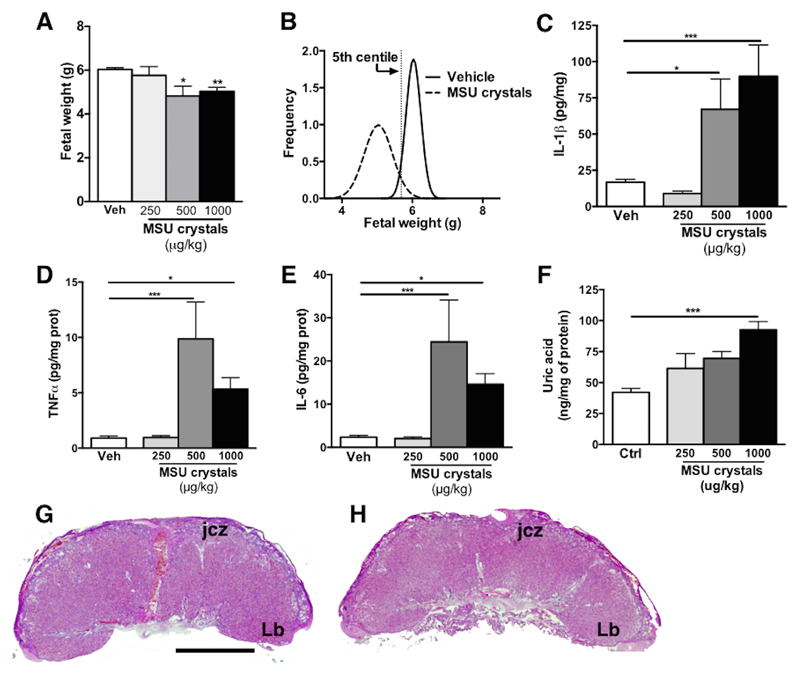
Pregnant dams exposed to MSU crystals during late gestation presented evidence of decreased fetal weight; there was an overall 20% decrease in fetal weight at G22 (term gestation corresponding to G22-23 in rats) in pups from dams exposed to 500 or 1000μg/kg/12h of MSU crystals (4.82 ± 0.45 and 5.03g ± 0.18 respectively as compared to 6.03 ± 0.09 in vehicle-exposed; Fig 5A). Over 80% of the pups from MSU crystals exposed dams (1000μg/kg, Fig 5B) were below the 5th centile of the weight of pups from vehicle-exposed dams, whereas in those exposed to the lower dose of MSU crystals (250μg/kg) only 20% of the pups were growth restricted (data not shown). MSU crystals exposure was associated with placental inflammation, with significantly increased levels of IL-1β (5.4-fold increase vs vehicle, p<0.001), TNF-α (5.9-fold increase vs vehicle, p<0.05) and IL-6 (6.2-fold increase vs vehicle, p<0.05) proteins in placentas from dams exposed to the highest dose of MSU crystals (1000μg/kg; Fig 5C-E). Uric acid levels were significantly elevated within the placentas of dams exposed to the highest dose of MSU crystals (1000μg/kg, p<0.001) (Fig 5F). However, uric acid levels in the maternal circulation was not significantly elevated by i.p. injection of MSU crystals combined with potassium oxonate (1.6 ± 0.27 mg/dL in dams exposed to MSU crystals 1000μg/kg vs 1.0 ± 0.39 mg/dL in vehicle exposed, p = 0.27). Preterm birth was not observed in any of the experimental conditions.
Figure 5. Maternal treatment with MSU crystals during late gestation in rats lead to placental inflammation and FGR.
Intraperitoneal administration of MSU crystals (500μg/kg/12h or 1000μg/kg/12h) at the end of gestation in rats significantly decreased fetal weight (A) with over 80% of the pups being below the 5th centile of pups from vehicle-exposed dams (B; R square value for vehicle curve: 0.98 and MSU crystals curve: 0.87). The highest doses of MSU crystals led to increased placental inflammation with increased levels of IL-1β (C), TNFα (D) and IL-6 (E) proteins. Levels of uric acid were significantly elevated in the placenta from dams exposed to the highest dose of MSU crystals (1000μg/kg/12h) (F). There was no difference between vehicle (G) and MSU crystals (H) exposed dams in morphological analysis of their placentas. At least six dams were used in each experimental group (48 to 84 fetuses in each experimental group). Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test. Scale bar: 1000µm. Jcz = Junctional zone; Lb = labyrinth. *p<0.05, **p<0.01, ***p<0.001.
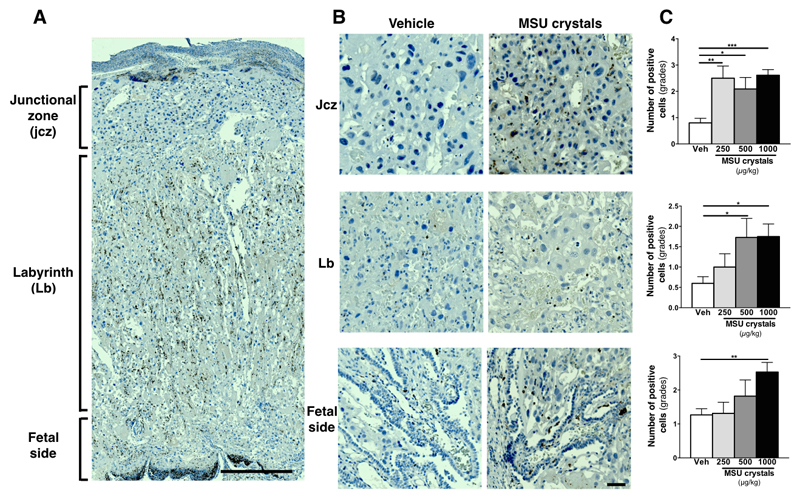
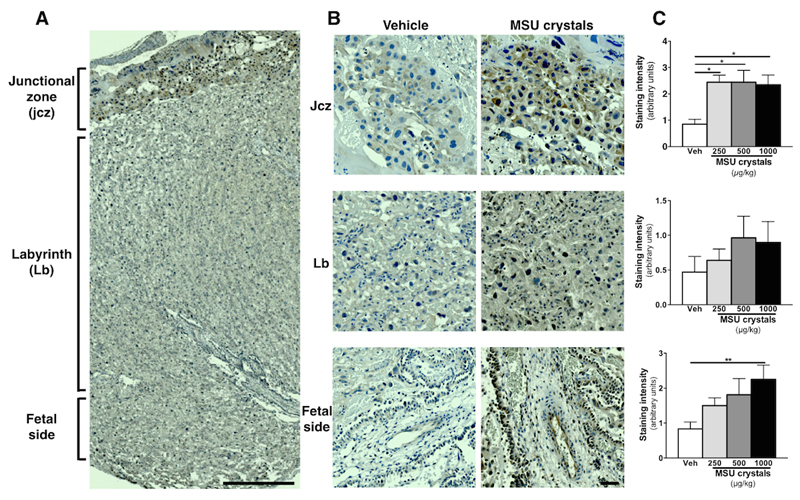
There were no significant differences in placental weight or gross morphological analysis between control and MSU crystals treated animals (Fig 5G, H); however, immunohistochemical analysis revealed increased numbers of immune cells within placenta (Fig 6). This was predominantly observed within the junctional zone, where all doses of MSU crystals led to a significant elevation of monocytes/macrophages (CD68+ cells) and within the labyrinth (fetal side), where only the highest dose of MSU crystals induced a significant increased in CD68+ monocytes/macrophages (Fig 6C). There was also an elevated number of macrophages within the labyrinth zone in placenta from dams exposed to the two highest dose of MSU crystals (Fig 6C). There were only a small number of neutrophils (polymorphonuclear, PMN) detected and these cell numbers were similar in all the experimental groups (data not shown). No immunoreactivity for CD3 (lymphocytes) was detected (data not shown). Similarly to the ELISA data (see Fig 5C), IL-1β immunoreactivity was elevated within the placentas exposed to MSU crystals, mainly in the junctional zone and in the labyrinth (fetal side) (Fig 7). The majority of positive cells were CD68+ immune cells, with some immunostaining in trophoblast (data not shown).
Figure 6. Histological analysis of CD68+ macrophage in the placenta following MSU crystals treatment.
Injection of MSU crystals during gestation in rats increased the number of CD68+ macrophages in the junctional zone, the labyrinth and on the fetal side (A, with higher magnification in B; 1000μg/kg/12h of MSU crystals shown in the pictures). Semi-quantitative analysis of the numbers of CD68+ cells is shown in C. Gradation scale: 0 = less than 5; 1 = 6-10; 2 = 11-15; 3 = 16-20 and 4 = more than 20 (n = 6 dams/conditions; 3 placentas/dams). Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test. *p<0.05, **p<0.01. Scale bar: 500µm (A); 50µm (B).
Figure 7. Histological analysis of IL-1β staining in the placenta following MSU crystals treatment.
Injection of MSU crystals during gestation increased the staining intensity of IL-1β primarily in the junctional zone but also on the fetal side and in the labyrinth. Representative image shown in A, with higher magnification of each region in B and quantification in C (n = 6 dams/conditions; 3 placentas/dams). (n = 6 dams/conditions; 3 placentas/dams). Results presented as mean ± SEM. Statistical analysis by one-way ANOVA with Dunnett’s multiple comparison post-test. *p<0.05, **p<0.01, ***p<0.001. Scale bar: 500µm (A); 50µm (B).
Discussion
Elevated uric acid has been strongly associated with several pathologies specific to pregnancy, such as PE, but also more recently, to FGR14,15,17,21–23. Even though uric acid crystals - MSU crystals are a known inducer of inflammation in inflammatory cells, its role at the maternal fetal interface and potential involvement in prenatal inflammation is mostly unknown. We now provide evidence that this DAMP has a causative role in placental inflammation and dysfunction, as seen by the inflammatory profile, increased trophoblast death and impaired syncytialization in primary cytotrophoblasts and in placental explants. Furthermore, we confirmed the effects of MSU crystals in vivo in a newly developed animal model of non-infectious inflammation during pregnancy that led to reduced fetal growth with placental inflammation.
Inflammation occurring during pregnancy has been strongly associated with pregnancy pathologies. Infection-induced inflammation leading to FGR, preterm birth or PE-like symptoms has been shown in animal models and studied in depth9,10,34. However, in an important proportion of women with pregnancy pathologies, infection is not detected even though inflammation (such as elevated cytokines) is still present. Alarmins or DAMPs can also induce inflammation, through TLRs and NLRs, the same receptors activated by pathogens12,13,19,20, and are increasingly associated with pathological pregnancies14–18,21–23,29. Uric acid in particular has been strongly associated to PE with elevated circulating levels detected in the maternal circulation14,15,21,29. Furthermore, our group also showed that uric acid was elevated in other pathologies of pregnancy, such as pregnancies with reduced fetal movements17, without any hypertension or abnormal renal function. The inflammatory actions of MSU crystals have been described in immune cells, as an activator of the NALP3 inflammasome, upstream of caspase-1, leading to the production of interleukin IL-1β25,35,36. Information on the action of MSU crystals at the maternal-fetal interface is scarce but it has been published that it does induce IL-1β secretion in a first trimester trophoblast cell line through activation of caspase-1 and of the NLRP3 inflammasome26–28. NLRP3 activation by MSU crystals was also observed in monocytes from PE patients37. This is in line with our current work, wherein we showed the role of caspase-1 in MSU crystals-induced IL-1β secretion in term primary cytotrophoblast cells and placental explants. The fact that caspase-1 inhibition completely blocked cytokine secretion in the presence of MSU crystals suggest that IL-1β, and not IL-1α, is involved since caspase-1 is not needed for IL-1α maturation. These findings also suggest that the secretion, and extracellular actions of IL-1β, are needed for the negative actions of MSU crystals on placental cells. In term of placental function, uric acid has been shown to decrease placental system A nutrient transport in explants29, which is in line with the effects on placental function we observed such as trophoblast apoptosis as well as decreased syncytialisation. This strongly supports altered placental function with abrogated syncytiotrophoblast renewal mediated by MSU crystals action on placental cells.
Commonly used animal models of inflammation during pregnancy involve infectious/pathogenic induced inflammation, with bacterial lipopolysaccharide (LPS) or the viral mimic polyinosinic:polycytidylic acid (poly I:C) being the most widely used10,38. These models have been very useful to show the causal link between prenatal inflammation and the negative effects on fetal development, and especially to highlight the deleterious actions of inflammatory mediators on the placenta, central to the observed negative fetal effects10,38,39. However, these models do not reproduce the most common cases observed in clinical situation in which infection is not detectable but markers of inflammation are still present. Recently, animal models were developed first using fetal DNA as a stimuli leading to inflammation and fetal demise40 whereas another group used high-mobility group box 1 (HMGB1) administration in the amniotic fluid which induced preterm labor41. Both HMGB1 and cffDNA were shown by ourselves and others to be elevated in pregnancy pathologies14–18 in association with placental dysfunction. These evidence points towards an important role of endogenous mediators of inflammation in pregnancy pathologies. In the current study we showed that MSU crystals administration to the rat reduces fetal growth in a dose dependent fashion. This is the first demonstration of such an effect and strongly suggests that uric acid is a potent inducer of placental inflammation/dysfunction leading to FGR, rather than just being elevated as a consequence of the pathology. This is supported by human studies that reported uric acid to be associated with the levels of inflammatory mediators in PE patients42 and the levels of uric acid in the first and second trimester were shown to correlate with pregnancy complications and birthweight42,43.
In conclusion, this study support the hypothesis that MSU crystals causes placental inflammation and dysfunction that leads to FGR. The work demonstrates the negative effects of MSU crystals within the human placenta, inducing inflammation and apoptosis whilst blocking syncytialisation. Furthermore we showed a causal role of MSU crystals in reducing fetal growth in an animal model of non-infectious infection during pregnancy. Importantly, we showed that the deleterious effects of MSU crystals were mediated via IL-1, which has many implications for the development of an effective therapy to tackle obstetrical disorders with placental dysfunction. Together these results strongly suggest an important role of MSU crystals and IL-1 in triggering placental inflammation with potential involvement in placental dysfunction leading to FGR.
Acknowledgments
We would like to thank Dr Mark Dilworth and Dr Susan Greenwood, University of Manchester, UK, for helpful discussion. We would like to thank Sophie Perrault and Lise-Angela Ouellet at the CHU Sainte-Justine, Montreal, Canada and research midwives at St-Mary’s Hospital, Manchester, UK for their help with patient recruitment, as well as all the patients who participated in this study. We are grateful to Adriana Carbonaro (CHU Sainte-Justine Research Center, Universite de Montreal) for technical help.
Funding: This work was supported by funding from the SickKids Foundation/Canadian Institute for Health Research – Institute of Human Development Child and Youth Health (CIHR – IHDCYH) (SG), Reseau Quebecois en Reproduction (RQR) (SG), Fondation du CHU Sainte-Justine (SG), Fonds de Recherche Sante Quebec (FRQS, SG), Vanier Canada Graduate Scholarship (MNV), Tommy’s – the baby charity (SG, RLJ, CPS), NICHD-NIH (Grant # R01HD049446, VMA) and MRC project grant (MR/N010892/1; SG, RLJ, CPS).
Footnotes
The authors have no conflict of interest to disclose
References
- 1.Mwaniki MK, Atieno M, Lawn JE, Newton CRJC. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet EOG, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and long-term neurodevelopment of very preterm infants. Am J Obstet Gynecol. 2012;206:489.e1–7. doi: 10.1016/j.ajog.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Derricott H, Jones RL, Greenwood SL, Batra G, Evans MJ, Heazell AEP. Characterizing Villitis of Unknown Etiology and Inflammation in Stillbirth. Am J Pathol. 2016;186:952–961. doi: 10.1016/j.ajpath.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 6.Hulthén Varli I, Petersson K, Kublickas M, Papadogiannakis N. Both acute and chronic placental inflammation are overrepresented in term stillbirths: a case-control study. Infect Dis Obstet Gynecol. 2012;2012:293867. doi: 10.1155/2012/293867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenwald EC, Stark AR. Management and Outcomes of Very Low Birth Weight. N Engl J Med. 2008;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 10.Girard S, Tremblay L, Lepage M, Sébire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 11.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000;47:185–196. doi: 10.1016/s0165-0378(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 13.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powers RW, Bodnar LM, Ness RB, Cooper KM, Gallaher MJ, Frank MP, Daftary AR, Roberts JM. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194:160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(Suppl A):S67–72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard S, Heazell AEP, Derricott H, Allan SM, Sibley CP, Abrahams VM, Jones RL. Circulating Cytokines and Alarmins Associated with Placental Inflammation in High-Risk Pregnancies. Am J Reprod Immunol. 2014;72:422–434. doi: 10.1111/aji.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naruse K, Sado T, Noguchi T, Tsunemi T, Yoshida S, Akasaka J, Koike N, Oi H, Kobayashi H. Peripheral RAGE (receptor for advanced glycation endproducts)-ligands in normal pregnancy and preeclampsia: novel markers of inflammatory response. J Reprod Immunol. 2012;93:69–74. doi: 10.1016/j.jri.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Shaw MH, Reimer T, Kim Y-G, Nuñez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins TL-A, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119:484–492. doi: 10.1111/j.1471-0528.2011.03232.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao T, Zablith NR, Burns DH, Skinner CD, Koski KG. Second trimester amniotic fluid transferrin and uric acid predict infant birth outcomes. Prenat Diagn. 2008;28:810–814. doi: 10.1002/pd.1981. [DOI] [PubMed] [Google Scholar]
- 23.Laughon SK, Catov J, Roberts JM. Uric acid concentrations are associated with insulin resistance and birthweight in normotensive pregnant women. Am J Obstet Gynecol. 2009;201:582.e1–6. doi: 10.1016/j.ajog.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577–582. [PubMed] [Google Scholar]
- 25.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9:13–23. doi: 10.1038/nrrheum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Uric acid induces trophoblast IL-1β production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. 2011;65:542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. A Role for Uric Acid and the Nalp3 Inflammasome in Antiphospholipid Antibody-Induced IL-1β Production by Human First Trimester Trophoblast. PLoS ONE. 2013;8:e65237. doi: 10.1371/journal.pone.0065237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han CS, Herrin MA, Pitruzzello MC, Mulla MJ, Werner EF, Pettker CM, Flannery CA, Abrahams VM. Glucose and Metformin Modulate Human First Trimester Trophoblast Function: a Model and Potential Therapy for Diabetes-Associated Uteroplacental Insufficiency. Am J Reprod Immunol. 2015;73:362–371. doi: 10.1111/aji.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bainbridge SA, von Versen-Höynck F, Roberts JM. Uric acid inhibits placental system A amino acid uptake. Placenta. 2009;30:195–200. doi: 10.1016/j.placenta.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Malhendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of the fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 32.Simán CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1116–22. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 33.Stavric B, Nera EA. Use of the uricase-inhibited rat as an animal model in toxicology. Clin Toxicol. 1978;13:47–74. doi: 10.3109/15563657808988228. [DOI] [PubMed] [Google Scholar]
- 34.Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211:165–179. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 36.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matias ML, Romao M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araújo JP, Peraçoli JC, de Oliveira L, Peraçoli MT. Endogenous and Uric Acid-Induced Activation of NLRP3 Inflammasome in Pregnant Women with Preeclampsia. PLoS ONE. 2015;10:e0129095. doi: 10.1371/journal.pone.0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Girard S, Tremblay L, Lepage M, Sébire G. Early detection of placental inflammation by MRI enabling protection by clinically relevant IL-1Ra administration. Am J Obstet Gynecol. 2012;206:358.e1–9. doi: 10.1016/j.ajog.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Scharfe-Nugent A, Corr SC, Carpenter SB, Keogh L, Doyle B, Martin C, Fitzgerald KA, Daly S, O'Leary JJ, O'Neill LAJ. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188:5706–5712. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016;75:3–7. doi: 10.1111/aji.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Zheng D-Y, Yang J-M, Wang M, Zhang X-T, Sun L, Yun XG. Maternal serum uric acid concentration is associated with the expression of tumour necrosis factor-α and intercellular adhesion molecule-1 in patients with preeclampsia. J Hum Hypertens. 2015:1–7. doi: 10.1038/jhh.2015.110. [DOI] [PubMed] [Google Scholar]
- 43.Laughon SK, Catov J, Powers RW, Roberts JM, Gandley RE. First trimester uric acid and adverse pregnancy outcomes. Am J Hypertens. 2011;24:489–495. doi: 10.1038/ajh.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fotiou M, Michaelidou AM, Athanasiadis AP, Menexes G, Symeonidou M, Koulourida V, Ganidou M, Theodoridis TD, Tarlatzis BC. Second trimester amniotic fluid glucose, uric acid, phosphate, potassium, and sodium concentrations in relation to maternal pre-pregnancy BMI and birth weight centiles. J Matern Fetal Neonatal Med. 2014:1–6. doi: 10.3109/14767058.2014.937692. [DOI] [PubMed] [Google Scholar]