Abstract
Borrelia burgdorferi, the etiological agent of Lyme disease, does not produce lipopolysaccharide but expresses a large number of lipoproteins on its cell surface. These outer membrane lipoproteins are highly immunogenic and have been used for serodiagnosis of Lyme disease. Recent studies have shown that highly conserved cytosolic proteins such as enolase and elongation factor Tu (EF-Tu) unexpectedly localized on the surface of bacteria including B. burgdorferi, and surface-localized enolase has shown to contribute to the enzootic cycle of B. burgdorferi. In this study, we studied the immunogenicity, surface localization, and function of B. burgdorferi EF-Tu. We found that EF-Tu is highly immunogenic in mice, and EF-Tu antibodies were readily detected in Lyme disease patients. On the other hand, active immunization studies showed that EF-Tu antibodies did not protect mice from infection when challenged with B. burgdorferi via either needle inoculation or tick bites. Borrelial mouse-tick cycle studies showed that EF-Tu antibodies also did not block B. burgdorferi migration and survival in ticks. Consistent with these findings, we found that EF-Tu primarily localizes in the protoplasmic cylinder of spirochetes and is not on the surface of B. burgdorferi. Taken together, our studies suggest that B. burgdorferi EF-Tu is not surfaced exposed, but it is highly immunogenic and is a potential serodiagnostic marker for Lyme borreliosis.
Keywords: B. burgdorferi, EF-Tu, elongation factor Tu, immunogenic, Lyme disease, moonlighting protein
Introduction
Borrelia burgdorferi, the pathogenic spirochete that causes Lyme disease, is a tick-borne parasitic bacteria whose natural reservoir is a variety of small mammals and avian species.1 B. burgdorferi expresses a large number of outer surface lipoproteins that are the major interface in interacting with host environments. Accumulated evidence has demonstrated that these surface lipoproteins play a central role for the successful maintenance of B. burgdorferi within the enzootic life cycle involving Ixodes ticks and mammals.1,2,3 Spirochetes differentially regulate these surface lipoproteins during the transitions between ticks and mammals.4,5,6,7 B. burgdorferi lipoproteins are also highly immunogenic, and some of the surface lipoproteins have been used as serodiagnostic markers.8,9,10
While much work has been focusing on surface lipoproteins, proteome analysis has revealed that some classical cytosolic proteins of B. burgdorferi are associated with membrane fractions.11,12 These proteins are so-called moonlighting protein in which a single protein has dual or moonlighting functions.13,14,15,16 One such protein is enolase, a known cytosolic enzyme that catalyzes the conversion of 2-phosphoglycerate into phosphoenolpyruvate for glycolysis. However, a fraction of enolase has been found on the cell surface of several microorganisms, where it interacts with extracellular matrix component proteins and plays a role in pathogens' colonization and dissemination.17,18,19 Recently, several reports showed that B. burgdorferi enolase is associated with cell surface and outer membrane vesicles of spirochetes.12,20,21 Borrelia enolase is capable of binding to plasminogen/plasmin in vitro, which may assist spirochetes to disseminate during mammalian infection. In addition, B. burgdorferi enolase also triggered an antibody response, and such response reduced acquisition of spirochetes by ticks from mice.21
Another moonlighting protein found in pathogenic bacteria is elongation factor Tu (EF-Tu). EF-Tu is a cytosolic GTP binding protein and an essential component of protein synthesis apparatus.22 EF-Tu also serves as a novel moonlighting protein that exhibits multiple biological functions involved in bacterial pathogenesis. It is found on the surface of several pathogenic bacteria and is involved in adhesion, invasion, and modulation of the host immune system.23,24,25,26,27,28,29,30,31 For example, surface EF-Tu of Mycoplasma pneumoniae serves as a fibronectin binding protein and facilitates the interaction with extracellular matrix.27 Surface EF-Tu from Leptospira interrogans, Streptococcus pneumoniae, and Pseudomonas aeruginosa binds to the complement regulators factor H and plasminogen and has been proposed to contribute to tissue invasion and host immune evasion.23,24,25 Surface EF-Tu in Franciscella tularensis interacts with nucleolin and plays a role in adhesion and invasion of monocyte-like cells.26 Furthermore, proteome analysis has demonstrated that EF-Tu is an immunogen that elicits antibody response during infection by several bacteria.30,32,33
The B. burgdorferi genome has one copy of tuf gene (bb0476) which encodes for EF-Tu.34 Since EF-Tu has been found on the surface in other organisms and plays a role in pathogenesis, we investigated the possible moonlighting nature of B. burgdorferi EF-Tu. We demonstrated that EF-Tu is highly immunogenic in mice infected with B. burgdorferi and in Lyme disease patients. Immunization of mice with rEF-Tu did not elicit a protective response against challenge with B. burgdorferi. However, EF-Tu did not appear to be localized on the surface of B. burgdorferi. Our results suggest that protoplasmic EF-Tu is an immunoreactive protein that may be a potential serodiagnostic marker during early stages of B. burgdorferi infection.
Material and methods
Bacteria
Low-passage, virulent B. burgdorferi strain 5A4NP1 (kindly provided by Hiroki Kawabata and Steven Norris, University of Texas Health Science Center, Houston, USA) was derived from wild-type strain B31 by inserting a kanamycin resistance marker in the restriction modification gene bbe02 on plasmid lp25.35 Strain B31-A3 (kindly provided by Patricia Rosa, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), USA) is a clonal low-passage infectious strain which contains all of the plasmids described for parental strain B31 MI except cp9 B31-MI.36 Spirochetes were grown using standard Barbour–Stoenner–Kelly II (BSK-II) medium containing the relevant antibiotic. Cultures were maintained at 37 °C, pH 7.5 in a 5% CO2 incubator and were passaged no more than two times from the original stocks.
Recombinant protein
The B. burgdorferi tuf gene bb0476 encoding EF-Tu from strain B31 was amplified by polymerase chain reaction (PCR) using the primer pair Sa-bb0476-5 (forward) and Sa-bb0476-3 (reverse; Table 1). The resulting amplicon was cloned into pET100/D-TOPO (Invitrogen, Carlsbard, CA, USA) to generate plasmid pSCEF-TuCT2, which was then entirely sequenced on both strands to rule out the possible introduction of undesired mutations during PCR and cloning procedures. EF-Tu-6×His fusion protein was expressed in Escherichia coli Rosetta (DE3) (Novagen, Madison, WI, USA). Briefly, exponentially grown cells (A600, 0.5) were induced for 5 h at 37 °C with 0.1 mM (final concentration) of isopropyl-β-d-thiogalactopyranoside. Cells were pelleted and resuspended in a buffer containing 400 mM NaCl, 40 mM NaH2PO4 (pH 7.2), 500 mM DTT, and 100 mM proteinase inhibitor phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, St. Louis, MO, USA). Cells were then lysed by brief sonication and debris was cleared by centrifugation. The resulting supernatant was loaded onto nickel-charged resin (Ni NTA, Qiagen, Valencia, CA, USA) for affinity purification. After washing, proteins were eluted with 500 mM imidazole and then concentrated with 40% ammonium sulfate. Proteins were collected by centrifugation, dissolved in buffer solution (50 mM potassium phosphate, 10% glycerol, 0.1 mM EDTA buffer), and dialyzed against the same buffer overnight at 4 °C. The dialysate was centrifuged at 5000×g for 15 min, and the supernatant was filtered in 10 kDa molecular mass cutoff membranes (Fisher Scientific, Pittsburgh, PA, USA) prior to use. Protein purity was assessed by Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was measured using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA).
Table 1. Primers used in the study.
| Primer | Sequence (5' → 3') |
|---|---|
| Sa-bb0476-5 | CACCATGAAATTTAGGAGGTTAGTCATGGC |
| Sa-bb0476-3 | CTATTCCAATATCTCAAGAATTCTTCCTG |
| PRYY7 | GCAGCCATATGGCAAAAGAAGTTTTTCAA |
| PRYY8 | CGTCTGCAGTTAAGCGTAATCTGGAACATCGTATGGGTATTCCAATATCTCAAGAATTCTTCCT |
| qTactin-F | CGGGACCTGACCGACTACCTGATG |
| qTactin-R | CTCCTTGATGTCGCGGACAATTTC |
| flaB-XF F | GCTCCTTCCTGTTGAACACCC |
| flaB-XF R | CTTTTCTCTGGTGAGGGAGCTC |
| nidogen F | CCAGCCACAGAATCACATCC |
| nidogen R | GGACATACTCTGCTGCCATC |
Construction of a shuttle vector for constitutively expression of EF-Tu
To constitutively express the wild-type tuf, the DNA fragment of tuf was amplified by PCR from B. burgdorferi B31-A3 using primers PRYY7 and PRYY8 with restriction enzyme sites NdeI and PstI and a HA epitope tag sequence, respectively. The PCR products were digested with NdeI and PstI and inserted into the pBSV2-derived shuttle vector pJD55 under the control of a constitutive borrelial promoter, PflaB. The resulting shuttle vector, pYY003, was verified by sequencing and then transformed into B31-A3 as described previously.2 The transformants (B31-A3/PflaB-tuf-HA) were selected in a 96-well tissue culture plates (200 μL/well) containing liquid BSK-II medium and relevant antibiotics (200 μg/mL kanamycin). The level of expression of EF-Tu in B. burgdorferi transformants was also verified by immunoblotting.
SDS-PAGE and immunoblotting
SDS-PAGE and immunoblotting were performed as previously described.37 Monoclonal antibodies directed against OspA (14D2-27) and the loading control FlaB (8H3-33)38 were used at a dilution of 1:1000 and 1:500, respectively. A polyclonal mouse antibody against EF-Tu (1:15 000 dilution) was used to detect recombinant EF-Tu and EF-Tu in B31 whole-cell lysates using nitrocellulose membranes (Bio-Rad). HA.11 monoclonal antibody (1:1000; clone 16B12; Covance, Princeton NJ, USA) was used to detect HA-tagged EF-Tu in whole-cell lysates. OspA, FlaB, and/or EF-Tu antibodies were detected with either horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or HRP-conjugated goat anti-human IgM, IgG, and IgA secondary antibody (1:1000; Kirkegaard & Perry Laboratories Gaithersburg, MD, USA). Detection of peroxidase activity was determined in membranes using both 4-chloro-1-napthol and H2O2 (Fisher Scientific) solution or an enhanced chemiluminenscent method (Pierce ECL Western Blotting Substrate, Thermo Scientific, Rockford, IL, USA).
Sera from four C3H/HeN mice infected with 5A4NP1 (106 spirochetes/mouse) were pooled and used to assess the appearance of antibody response against recombinant EF-Tu protein. Sera were diluted 1:600 in 1× PBS −0.05% Tween-20 (PBS-Tween) and incubated at room temperature (RT) for 2 h with nitrocellulose membrane strips containing approximately 500 ng of recombinant EF-Tu protein per strip.
Lyme disease patient sera were from the Center for Disease Control and Prevention (CDC) Lyme disease evaluation panel, which consists of 42 serum samples (including both positive and negative). We used sera from 10 Lyme disease patients that tested positive for Lyme disease by enzyme-linked immuno-sorbent assay (ELISA) and Western blot. Control sera were collected from health individuals in areas of the USA where Lyme disease is not endemic. All human sera were diluted 1:100 in PBS-Tween for immunoblotting,39 as described above. Each strip contains 500 ng of recombinant EF-Tu.
Quantitative PCR (qPCR)
For quantification of B. burgdorferi DNA in mice and ticks, total DNA was isolated from joint tissue and fed larvae (3–6 data points for each group, three tick per data point) using DNeasy blood and tissue kit as described in the manufacturer's protocols (Qiagen). qPCR of genomic DNA was performed using the Platinum SYBR green qPCR SuperMix (Invitrogen). The oligonucleotide primer pairs used to detect flaB, mouse nidogen, and tick actin were flaB-XF F/R, nidogen F/R, and qTactin-F/R, respectively (Table 1).40,41,42 Reactions were carried out on an ABI Prism 7000 real-time PCR machine (Applied Biosystems, Pleasanton, CA, USA). Calculations of DNA copy number of flaB were normalized with the copy numbers of either the mouse nidogen gene or the tick actin gene, as indicated in Results.
Proteinase K accessibility assay
Proteinase K (PK) accessibility assays were performed as described.43,44 Briefly, B. burgdorferi B31-A3 or B31-A3/PflaB-tuf-HA (1 × 108/mL) were gently washed three times in PBS (pH 7.4) and collected by centrifugation at 4000×g for 30 min. Washed spirochetes were then gently resuspended in 2.5 mL of PBS and split into five equal 500 μL volume samples. Four samples were treated with proteinase K (25, 50, 100, or 200 μg of PK; Sigma, St Louis, MO) for 1 h at RT while one sample was incubated with 1× phosphate-buffered saline (PBS) as a control. After incubation, samples were treated with 10 μL of PMSF (Sigma) to inactivate PK activity. Samples were subsequently pelleted by centrifugation at 10 000×g for 10 min and resuspended in PBS for SDS-PAGE and immunoblotting.
TX-114 phase partitioning
To determine whether EF-Tu has the amphiphilic properties expected of a membrane protein, B. burgdorferi B31-A3 cells (109 organisms) were harvested and phase-partitioned as described previously.45 The resulting protein pellets were resuspended in PBS and subjected to SDS-PAGE and immunoblot analysis using the polyclonal antibody directed against EF-Tu or the monoclonal antibody against OspA, which is a well-characterized amphiphilic lipoprotein.
Isolation of outer membrane vesicles
Isolation of the outer membrane vesicles (OMVs) of B. burgdorferi was performed as described.46,47 Briefly, 5 × 1010−1011 B. burgdorferi cells were washed twice in 1× PBS (pH 7.4) containing 0.1% bovine serum albumin (BSA), resuspended, and incubated on a rocker at RT for 2 h in ice-cold 25 mM citrate buffer (pH 3.2) containing 0.1% BSA. The resulting outer membrane vesicle and protoplasmic cylinder (PC) fractions were separated and purified on a sucrose density gradient as detailed.46 The OMVs vesicles were monitored for purity by immunoblotting using antibodies against OspA and FlaB.
Immunofluorescence assay (IFA)
To determine whether EF-Tu is surface-exposed, B31-A3 and B31-A3/PflaB-tuf-HA spirochetes from mid-logarithmic phase cultures were probed in solution as previously described.48,49 Briefly, unfixed live spirochetes were incubated at RT for 1 h in blocking solution (2% BSA in 1× PBS with 5 mM MgCL2) containing the primary antibodies of interest. A polyclonal antibody against EF-Tu and a monoclonal antibody directed against HA.11 were used at a final dilution of 1:40 and 1:1000, respectively. Monoclonal antibodies directed against OspA and FlaB were used at a final dilution of 1:40. After the primary incubation, spirochetes were washed twice and gently resuspended in blocking solution, and then 20 µL of cell mixtures were added on silylated microscope slides and air dried (CEL Associates, Pearland, TX, USA). Slides were then incubated with a 1:1000 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Inc, West Grove, PA, USA) for 1 h at 37 °C in a dark, humid chamber. Slides were gently washed with blocking solution and then mounted with antifade light mounting medium (Molecular Probes, Eugene, OR). As an additional control for this unfixed IFA experiments, spirochetes were incubated for 1 h with BacTrace FITC-conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) at a 1:100 dilution in blocking solution. The presence of fluorescent spirochetes was confirmed with an Axio Imager.A2 fluorescence microscope (Carl Zeiss, Jena, DE).
To detect the presence of EF-Tu, FlaB, and OspA in spirochetes, we also used an alternative IFA method48,50 in which spirochetes were placed onto silylated slides, air dried, and fixed and permeabilized by immersion in acetone for 10 min. This fixed IFA method was also used to detect the presence of fixed spirochetes in smears of fed tick larvae.50 Slides were then incubated at 37 °C for 1 h with blocking solution (PBS-Tween 20 with 5% goat serum) in a humid chamber. Fixed spirochetes were incubated at RT for 1 h in blocking solution containing the primary antibodies of interest and then followed by washes and incubation with a secondary antibody, as described above.
Active immunization and infection studies
Groups of C3H/HeN mice were immunized with 50 μg recombinant EF-Tu in PBS (1:1) with adjuvant (complete Freund's adjuvant; Sigma). Mice received two boosts of 50 μg recombinant EF-Tu in PBS (1:1) with incomplete Freund's adjuvant (Sigma) at 14-day intervals. One week after the final boost, mice were inoculated with B. burgdorferi strain 5A4NP1 with a dose of 104 spirochetes per mouse. Mice were euthanized at ether two weeks or five weeks post-inoculation. Infectivity was assessed by culturing ear pinna, joints, and heart tissues in BSK-II medium supplemented with the relevant antibiotic (200 μg/mL kanamycin) as well as the Borrelia antibiotic cocktail (50 μg/mL rifampicin, 20 μg/mL phosphomycin and 2.5 μg/mL amphotericin). A single growth-positive culture occurred within one week post-harvest and was used as the criterion to determine positive mouse infection. All experiments were performed with the approval of the Indiana University Institutional Animal Care and Use Committee.
For tick studies, pathogen-free Ixodes scapularis larvae were obtained from the Tick-Rearing Center at Oklahoma State University (Stillwater, OK, USA). The tick-mouse experiments were conducted in the Vector-borne Diseases Laboratory at Indiana University School of Medicine (IUSM). At three weeks post-challenge with B. burgdorferi strain 5A4NP1, unfed larvae were placed on immunized mice (∼50 larvae per mouse). At 72–96 h, these larvae had fully engorged and naturally dropped off the mice. A subset of fed larvae was subjected to IFA and qPCR analysis to determine B. burgdorferi acquisition and loads. The remaining fed larvae were maintained in a humidified chamber and allowed to molt to the nymphal stage (about five weeks). Two months after molting, unfed nymphs were then allowed to feed on immunized mice (∼three nymphs per mouse) and collected within 48 h after repletion. Two weeks after tick feeding, mouse tissues were collected and tested for infection by culture as described above.
Statistics
Data are presented as the mean ± SEM and were analyzed with the Prism 5.0 statistical program (GraphPad Software, Inc., San Diego, CA, USA). Statistical comparisons were performed by using a Student t test. A P value ≤ 0.05 was considered significant.
Results
B. burgdorferi EF-Tu is recognized by mouse and human sera during infection
To determine whether B. burgdorferi EF-Tu is immunogenic during mammalian infection, we first purified recombinant EF-Tu (rEF-Tu) from E. coli (Figure 1A). We then infected mice sera from infected mice with B. burgdorferi were tested for reactivity to recombinant EF-Tu (rEF-Tu). We found that antibodies against rEF-Tu were detectable in the serum of infected mice as early as two weeks after needle inoculation. Seroreactivity to rEF-Tu was detectable even after 6 weeks or 11 weeks (data not shown) post-infection (Figure 1B), suggesting that B. burgdorferi anti-EF-Tu antibodies are maintained after two months of infection. This result indicates that B. burgdorferi EF-Tu is an antigenic protein that elicits antibody response during murine infection.
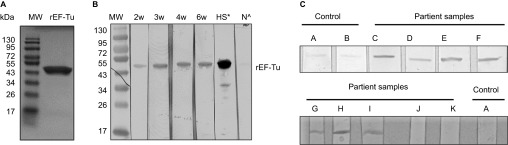
Figure 1.
Serologic reactivity of recombinant EF-Tu (rEF-Tu) in infected mice and Lyme disease patients. (A) SDS-PAGE showing purified recombinant EF-Tu protein. Predicted size and observed size for B. burgdorferi EF-Tu is 43 kDa and 47 kDa, respectively. (B) Immunoblotting of rEF-Tu using sera collected from C3H/HeN mice infected with B. burgdorferi strain 5A4NP1. Mouse sera used were collected at 2, 3, 4, 6 weeks of post-infection (1:600 dilution). *, HS stands for hyperimmune sera from mice immunized with rEF-Tu (αEF-Tu, 1:10000 dilution). ^, N stands for sera from naïve mice (1:600 dilution). (C) Representative immunoblotting results showing serologic reactivity of rEF-Tu in Lyme disease patients. Strips C-K are rEF-Tu probed with sera from ten different Lyme disease patients randomly selected from the CDC Lyme disease patient sera panel (1:100 dilution).
To assess whether Lyme disease patients elicit antibody response against EF-Tu of B. burgdorferi, rEF-Tu was immunoblotted with Lyme disease patient sera from CDC Lyme disease evaluation panel. We found that 70% of samples tested (seven out ten patient sera) were reactive to rEF-Tu (Figure 1C and Table 2). Seroreactivity to rEF-Tu was detected in samples from seropositive patients with different clinical manifestations associated with Lyme disease, including erythema migrans, arthritis, and/or neurologic symptoms. Four samples exhibited strong reactivity to rEF-TU and were also seropositive by ELISA and Western blot with presence of IgM and IgG antibodies against B. burgdorferi antigens (Table 2). Two samples exhibited strong reactivity to rEF-Tu were also seropositive by ELISA and Western blot with presence of IgM antibodies against B. burgdorferi antigens (Table 2). One sample was also reactive to rEF-TU and tested positive by ELISA and Western blot with evidence of IgG when blotted against B. burgdorferi antigens (Table 2). These results indicate that B. burgdorferi EF-Tu is an attractive serological target for Lyme disease diagnosis.
Table 2. Seroreactivity of Lyme disease patient serum against recombinant EF-Tu protein.
| Reactivitya | ||||
|---|---|---|---|---|
| Western blot | ||||
| Serum IDb | EF-Tua | ELISA | IgM | IgG |
| 902668 (C) | + | + | + | + |
| 920057 (D) | + | + | + | + |
| 911222 (E) | + | + | + | − |
| 902111 (F) | + | + | − | + |
| 911351 (G) | + | + | + | − |
| 910544 (H) | + | + | + | + |
| 911348 (I) | + | + | + | + |
| 910865 (J) | − | + | + | + |
| 931414 (K) | − | + | + | + |
| 910533 | − | + | + | + |
+: Seropositive and −: seronegative results. All Lyme disease patient samples tested positive to ELISA and/or Western blot. Sera from three healthy donors were used as negative controls for immunoblotting as indicated in material and methods.
Serum identification number used by the CDC Lyme disease evaluation panel, and letters in parentheses indicated strip identification from immunoblot results in Figure 1C.
Active immunization with rEF-Tu does not protect mice from B. burgdorferi infection
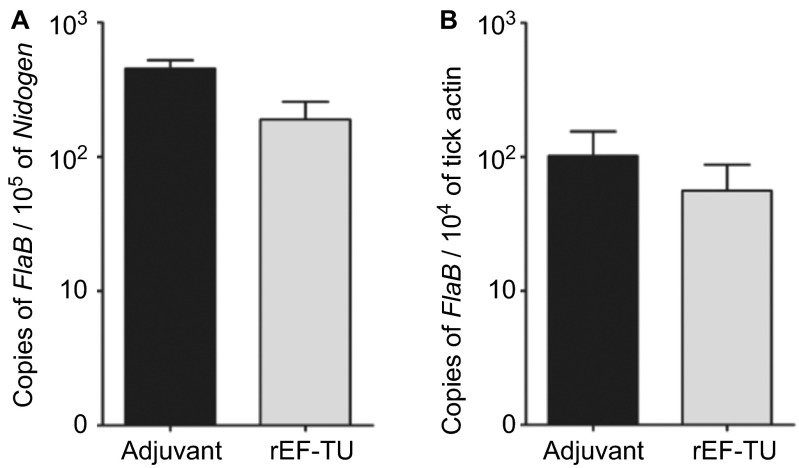
The finding of EF-Tu as an antigenic protein prompted us to assess whether immunization of mice with rEF-Tu could elicit protective immunity against B. burgdorferi infection. C3H/HeN mice were actively immunized with rEF-Tu or adjuvant alone (control), and antibody titers were verified by immunoblot assays. Mice developed an antibody titer to rEF-Tu of 1:15,000 before intradermal challenged with infectious B. burgdorferi strain 5A4NP1 (104 spirochetes/mouse). Two and five weeks post-challenge, mouse ear punch biopsies, tibiotarsal joints, and hearts were cultured for detection of B. burgdorferi. Culture results showed that all mice were infected with B. burgdorferi (Table 3). To further quantify the spirochete load in mice, joint tissues were subjected to DNA extraction and real-time qPCR. No significant difference in spirochete loads were detected in joints from immunized mice when compared to control mice (Figure 2A). Thus, active immunization with rEF-Tu does not protect mice upon needle inoculated with B. burgdorferi.
Table 3. Culture results of mice immunized with rEF-TU. Culture results expressed as number of mice infected/total number of mice tested.
| Method of inoculation | Group | Culture resulta |
|---|---|---|
| Needle | Adjuvant | 4/4 |
| Adjuvant + rEF-TU | 6/6 | |
| Nymphal ticks | Adjuvant | 2/2 |
| Adjuvant + rEF-TU | 2/2 |
Mice were euthanized at either two or five weeks post-challenge. Isolation of spirochetes were attempted from ear pinna, tibiotarsal joint, and heart base.
Figure 2.
Active immunization with rEF-Tu does not protect mice from B. burgdorferi, and anti-EF-Tu antibody does not affect spirochetal survival and migration in in the tick vector. (A) Quantitation of spirochete load in mice. Tibio-tarsal joints from mice immunized with rEF-Tu or non-immunized mice (labeled as ‘Adjuvant') were subjected to qPCR analyses (n = 4 mice/group). The B. burgdorferi flaB gene was used as target, and the levels were normalized with the mouse Nidogen gene. Data denote the mean ± the standard error (SEM) from two separate experiments. Differences between mice immunized with rEF-Tu and controls were analyzed using a Student t test (P value ≤ 0.05). (B) qPCR analyses of spirochete load in larvae fed on B. burgdorferi-infected mice that were either immunized with EF-Tu (which contains high levels of anti-EF-Tu antibodies) or non-immunized. Bars represent the mean ± SEM from 18 fed larval ticks collected from immunized mice and 9 fed larval ticks collected from controls (n = 2 mice/group). Data are representative of two separate experiments. Copies of the flaB genes were normalized with tick actin gene.
Needle inoculation and tick challenge of mice may have profoundly different infection outcomes,51 as the in vitro cultivation conditions are different form the environments in which spirochetes encounter during tick feeding. Therefore, we next challenged a second group of EF-Tu-immunized mice with nymphal ticks carrying B. burgdorferi. Similar to what was observed for needle inoculation, all mouse tissues were culture positive for spirochetes two weeks after tick feeding (Table 3). These results indicate that although B. burgdorferi infection elicits antibody response against EF-Tu, immunization with rEF-Tu does not protect mice from B. burgdorferi infection by either needle inoculation or natural tick infection.
Antibody against EF-Tu does not affect spirochetal survival or migration in ticks
It is well known that antibodies against surface antigens of B. burgdorferi are capable of killing spirochetes in ticks during tick feeding or block spirochetal migration between mice and ticks.21,52,53 To determine whether EF-Tu antibody could influence acquisition of spirochetal survival or migration in ticks, naïve larval ticks were fed on mice that were immunized with rEF-Tu and infected with B. burgdorferi as described above. Ticks were allowed to fully engorge, and then collected and subjected to DNA extraction and qPCR analyses. No significant differences in spirochete loads were detected in larvae fed on immunized and control mice (Figure 2B). IFA using anti-B. burgdorferi antibody also did not observe noticeable differences in the number of spirochetes in tick-smears collected from the two groups (data not shown). These results indicate that EF-Tu antibodies did not interfere with B. burgdorferi acquisition by ticks from mice nor spirochetal replication in ticks.
B. burgdorferi EF-Tu is not surface-exposed
To investigate whether EF-Tu can be found on the surface of B. burgdorferi as observed in several other bacteria,15,16,24 we first performed IFA assays (Figure 3A). Fixed (top panel) or unfixed (lower panel) spirochetes were probed with antibody directed against EF-Tu, or with antibody against the known surface lipoprotein OspA or with antibody against the periplasmic protein FlaB. As shown in the top panel of Figure 3A, all three proteins were detected in fixed spirochetes, suggesting that they are readily expressed. In unfixed samples, OspA was readily detected. Both FlaB and EF-Tu were not detected with the corresponding antibodies (Figure 3A, lower panel), suggesting that B. burgdorferi EF-Tu was not surface-localized.
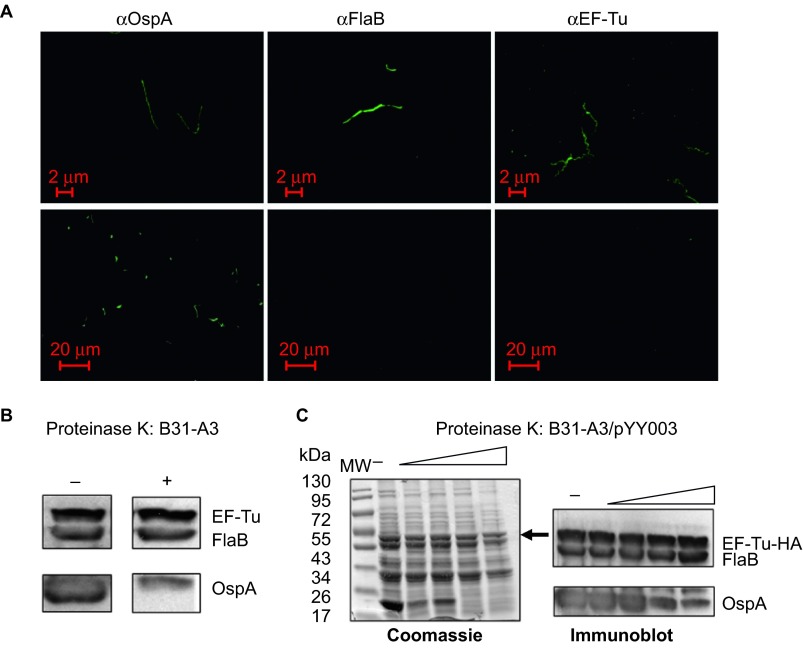
Figure 3.
B. burgdorferi EF-Tu is not surface-localized. (A) IFAs of fixed (top panels) or unfixed (bottom panels) wild-type B. burgdorferi strain B31-A3 probed with monoclonal antibodies directed against outer surface protein OspA, periplasmic protein FlaB, or EF-Tu as primary antibodies, and FITC-conjugated goat anti-mouse antibody as secondary antibodies. (B) Proteinase K protection assay of wild-type B. burgdorferi. Intact cells were incubated with proteinase K (200 μg/mL) for 1 h. Washed cells were then subjected immunoblotting using a mixture of antibodies against OspA, FlaB, and EF-Tu. (C) Proteinase K protection assay of B. burgdorferi overexpressing EF-Tu. Intact cells were incubated with proteinase K (25, 50, 100, or 200 μg/mL) for 1 h and then subjected to SDS-PAGE (left panel and black arrow indicates the observed size of B. burgdorferi EF-Tu) or immunoblotting using a mixture of antibodies against OspA, FlaB, and EF-Tu (right panel). Data are representative of four separate experiments.
We also performed proteinase K treatment experiment to assess the surface localization of EF-Tu. Proteinase K can degrade surface-exposed proteins (e.g., OspA) in B. burgdorferi, whereas subsurface proteins are protected from proteolysis (e.g., FlaB). The results showed that the bulk of OspA was degraded by proteinase K (100–200 μg/mL, Figure 3B), while the levels of FlaB and EF-Tu were not affected by proteinase K treatment. This result is consistent with the above IFA finding that EF-Tu is not localized on the surface of B. burgdorferi.
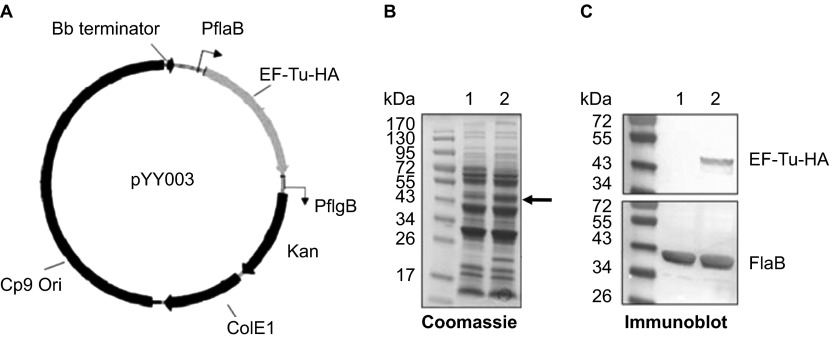
To further evaluate whether B. burgdorferi EF-Tu is surface-exposed, we constructed a B. burgdorferi strain that overexpresses EF-Tu. We reasoned that the fraction of surface EF-Tu may be too low to detect in wild-type strain and if so, overexpressing EF-Tu would amplify the signal and allow detection of such population of EF-Tu. Accordingly, a shuttle vector carrying a tuf gene under the control of a flaB promoter (Figure 4A) was transformed into the wild-type B. burgdorferi strain B31, resulting in a B. burgdorferi strain (B31-A3/PflaB-tuf-HA) which overexpressed EF-Tu (fused with HA tag at the C terminus; Figure 4B and 4C, the band corresponding to EF-Tu-HA was indicated by arrow). This EF-Tu overexpressing strain was then subjected to IFA and proteinase K protection experiments as described above. The IFA result showed that no surface EF-Tu was detected in B. burgdorferi even with overexpression (data not shown). Consistent with this observation, the proteinase K protection result also showed no obvious change of the level of EF-Tu-HA upon proteinase K treatment, i.e., EF-Tu-HA was not accessible by proteinase K (Figure 3C). Taken together, these results suggest that EF-Tu is not surface-localized in B. burgdorferi.
Figure 4.
Construction of the B. burgdorferi strain overexpressing EF-Tu. (A) Diagram of the shuttle vector pYY003 carrying a tuf gene under the control of a strong and constitutive flaB promoter. (B) Coomassie-stained gel showing overexpression of EF-Tu. Lane 1, wild-type strain B31-A3; lane 2, B31-A3/PflaB-tuf-HA. The band corresponding to EF-Tu was indicated by arrow. (C) Confirmation of over-production of HA-tagged EF-Tu by immunoblotting using either HA monoclonal antibody or antibody against FlaB (loading control). Lane 1, wild-type strain B31-A3; lane 2, B31-A3/PflaB-tuf-HA.
EF-Tu is a PC-associated protein in B. burgdorferi
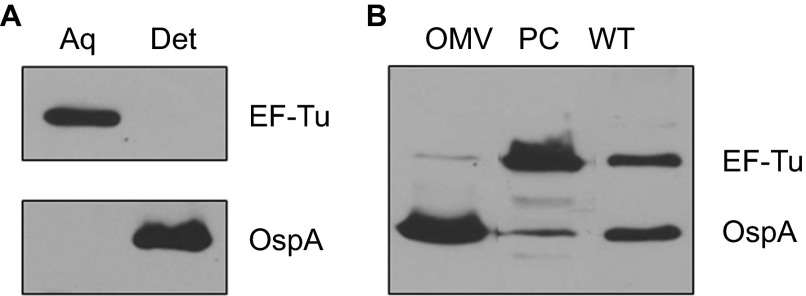
To further examine the cellular localization of EF-Tu, we next performed Triton X-114 (TX-114) phase partitioning studies with B. burgdorferi cells to separate fractions based on their amphiphilic properties. As expected, OspA, a known outer surface lipoprotein, was found in the detergent (membrane-enriched) phase (Figure 5A). In contrast, EF-Tu was not detected in the detergent phase of B. burgdorferi and was partitioned exclusively into the soluble aqueous (non-membrane-enriched) fractions. These data suggest that EF-Tu is a cytoplasmic protein and not associated with membrane of B. burgdorferi.
Figure 5.
B. burgdorferi EF-Tu is a soluble protein associated with the PC fractions. (A) Immunoblot analyses of detergent-enriched (Det) and aqueous-enriched (Aq) phases of B. burgdorferi with antibodies against EF-Tu or OspA. (B) Immunoblot analyses of PC fraction and outer membrane vesicles (OMVs) of B. burgdorferi separated by sucrose density gradient centrifugation. Whole cell lysates of wild-type B. burgdorferi (WT) were used for comparison. Equal amounts of proteins were loaded and immunoblotted with antibodies directed against EF-Tu or OspA.
Because B. burgdorferi is capable to release immunogenic surface proteins as well as cytoplasmic proteins via OMVs,12,46,54 we next assessed whether EF-Tu could be part of the protein cargo that is released in OMVs and contributes to its immunogenicity. Outer membrane vesicles and PC inner membrane associated proteins were obtained by sucrose density gradient centrifugation and subjected to immunoblot analyses. As expected, OspA was predominantly detected in the OMV fraction (Figure 5B). On the other hand, EF-Tu protein was primarily associated with the PC inner membrane-enriched fraction; only a trace amount was detected in the OMV sample (Figure 5B). Collectively, our data show that EF-Tu localizes primarily in the cytoplasm associated with the PC structures.
Discussion
In recent years, a growing number of highly conserved bacterial proteins that are commonly involved in metabolic regulation or cell stress responses have shown additional biological functions involved in bacterial adaptation, virulence, and/or immune modulation.15,16 One such moonlighting protein is enolase that localizes on the surface of B. burgdorferi and has properties associated with spirochetal adhesion and immunogenicity. In this study, we showed that EF-Tu of B. burgdorferi does not appear to be exposed on the cell surface. However, it elicits antibody response in mice as well as in Lyme disease patients, suggesting to be a potential serodiagnostic marker for Lyme disease.
According to the US Centers for Disease Control recommendation, serodiagnosis of Lyme disease should be performed using a two-tiered approach, an ELISA with B. burgdorferi whole-cell lysates, followed by immunoblot confirmation with purified antigens of B. burgdorferi.55 This method was established in 1995 and there are several shortcomings including insensitive detection of early infection and high false positive rates. Early diagnosis is important, as the treatment is most effective during early infection of B. burgdorferi, which prevents development of complicated post-treatment Lyme disease syndrome. Thus, new antigens, especially antigens that may be used for early diagnosis of Lyme disease, are urgently needed. Our immunoblotting results showed that EF-Tu was recognized by antibodies from mouse sera collected at the early and disseminated stage of infection. In addition, 7 out of 10 patients with a history of prior tick exposure or infection and early and late clinical manifestations were seroreactive to rEF-Tu. Thus, our findings suggest that rEF-Tu is recognized by IgG antibodies as this protein reacted with mouse and human sera collected at different stages of Lyme borreliosis. rEF-Tu triggered an antibody response within the first four weeks post-challenge, suggesting that EF-Tu is a potential early marker of Lyme borreliosis. Interestingly, two Lyme disease patients who exclusively had IgM antibodies to B. burgdorferi antigens showed seroreactivity to rEF-Tu. Although a small number IgM seropositive samples were tested, our data suggest the possibility that B. burgdorferi EF-Tu could elicit an antibody response shortly after exposure to spirochetes. This observation is consistent with a previous study showing that Borrelia hermsii EF-Tu was strongly reactive with IgM antibodies during early spirochetal infection in mice.56 Given EF-Tu is a conserved protein, we also analyzed potential cross reactivity of B. burgdorferi EF-Tu with antibodies from other pathogens: B. burgdorferi EF-Tu shares 50–65% identity with EF-Tu from other spirochetal pathogens and non-spirochetal pathogens. Thus, future studies are needed to evaluate whether the immunogenic epitopes in the variable region from B. burgdorferi EF-Tu could be used as potential serodiagnostic markers of early Lyme borreliosis.
Although B. burgdorferi EF-Tu appeared to elicit an antibody response in vivo, several lines of evidences suggest that EF-Tu is not surface-localized. First, the active immunization experiments showed that these anti-EF-Tu antibodies were not bactericidal during Lyme borreliosis as they were unable to reduce spirochetal burden in joints or in engorged tick larvae from immunized mice. Second, the proteinase K and immunofluorescent microscopy findings showed that EF-Tu was not on cell surface. Third, our findings showed that B. burgdorferi EF-Tu predominantly localizes in PC fractions and may present in OMV fractions in small quantities. B. burgdorferi periplasmic and cytoplasmic proteins have shown to be immunogenic in Lyme disease patients.12,57,58,59 Of these proteins, FlaB (41 KDa) and FlaA are highly reactive to IgM antibodies during the first four weeks of illness, and the protoplasmic protein p83/100 (83–100 kDa) is highly reactive to IgG antibodies in patients with late Lyme borreliosis.57,60
In conclusion, our findings demonstrate that B. burgdorferi EF-Tu is not surface-localized but is highly immunogenic that triggers an antibody response during Lyme borreliosis. Thus, B. burgdorferi EF-Tu is a potential candidate for serodiagnosis of Lyme borreliosis.
Acknowledgments
This work was supported by NIH grants AI083640 and AI085242 (to X Frank Yang) and Indiana INGEN and METACyt grants of Indiana University, funded by the Lilly Endowment, Inc (to X Frank Yang). Trainee Sebastian E Carrasco was supported by the NIH, National Research Service Award (NRSA) T32 AI 060519, Immunology and Infectious Disease Program at IUSM. This investigation was partially conducted in a facility with support from research facilities improvement program grant number C06 RR015481-01 from the National Center for Research Resources, NIH.
References
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Micro 2012; 10: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol 1995; 47: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun 2004; 72: 5419–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun 2003; 71: 3371–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol 2011; 65: 479–499. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 2000; 38: 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 2003; 31: 108–112. [DOI] [PubMed] [Google Scholar]
- Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 1993; 167: 392–400. [DOI] [PubMed] [Google Scholar]
- Goettner G, Schulte-Spechtel U, Hillermann R, Liegl G, Wilske B, Fingerle V. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J Clin Microbiol 2005; 43: 3602–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Lenhart TR, Akins DR. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol 2012; 66: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowalk AJ, Nolder C, Clifton DR, Carroll JA. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 2006; 6: 2121–2134. [DOI] [PubMed] [Google Scholar]
- Toledo A, Coleman JL, Kuhlow CJ, Crowley JT, Benach JL. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect Immun 2012; 80: 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Wu H et al. Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 2002; 186: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinost G, Dykhuizen DE, Dattwyler RJ et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 1999; 67: 3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun 2011; 79: 3476–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Xia Y, Cui J et al. The roles of moonlighting proteins in bacteria. Curr Issues Mol Biol 2013. 22; 16: 15–22. [PubMed] [Google Scholar]
- Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. J Immunol 2012; 188: 1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Catt DM, Gregory RL. Streptococcus mutans surface -enolase binds salivary mucin MG2 and human plasminogen. Infect Immun 2004; 72: 6748–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS, Radolph JD. Borrelia: molecular biology, host interaction and pathogenesis. Norfolk: Caister Academic Press, 2010. [Google Scholar]
- Floden AM, Watt JA, Brissette CA. Borrelia burgdorferi enolase is a surface-exposed plasminogen binding protein. PLoS One 2011; 6: e27502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira SV, Smith AA, Qin JH, Pal U. A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks. Infect Immun 2012; 80: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeing TM, Voorhees RM, Kelley AC et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 2009; 326: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert A, Losse J, Gruszin C et al. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J Immunol 2007; 179: 2979–2988. [DOI] [PubMed] [Google Scholar]
- Wolff DG, Castiblanco-Valencia MM, Abe CM et al. Interaction of Leptospira elongation factor Tu with plasminogen and complement factor H: a metabolic leptospiral protein with moonlighting activities. PLoS One 2013; 8: e81818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Hertweck C, Dudda A et al. Tuf of Streptococcus pneumoniae is a surface displayed human complement regulator binding protein. Mol Immunol 2014; 62: 249–264. [DOI] [PubMed] [Google Scholar]
- Barel M, Hovanessian AG, Meibom K, Briand JP, Dupuis M, Charbit A. A novel receptor – ligand pathway for entry of Francisella tularensis in monocyte-like THP-1 cells: interaction between surface nucleolin and bacterial elongation factor Tu. BMC Microbiol 2008; 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Kannan TR, Baseman JB. The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infect Immun 2008; 76: 3116–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo FR, Costa CM, Ramos CA et al. IgG and IgG2 antibodies from cattle naturally infected with Anaplasma marginale recognize the recombinant vaccine candidate antigens VirB9, VirB10, and elongation factor-Tu. Mem Inst Oswaldo Cruz 2008; 103: 186–190. [DOI] [PubMed] [Google Scholar]
- Bunk S, Susnea I, Rupp J et al. Immunoproteomic identification and serological responses to novel Chlamydia pneumoniae antigens that are associated with persistent C. pneumoniae infections. J Immunol 2008; 180: 5490–5498. [DOI] [PubMed] [Google Scholar]
- Nieves W, Heang J, Asakrah S, Honer zu Bentrup K, Roy CJ, Morici LA. Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization. PLoS One 2010; 5: e14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale MN, Echeverria-Valencia G, Romasanta P et al. Description of a novel adhesin of Mycobacterium avium subsp. paratuberculosis. BioMed Res Int 2014; 2014: 729618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MA, Chitale S, Brennan PJ, Pessolani MC. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect Immun 1998; 66: 2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipond C, Suker J, Jones C, Tang C, Feavers IM, Wheeler JX. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics 2006; 6: 3400–3413. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 1997; 390: 580–586. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Norris SJ, Watanabe H. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun 2004; 72: 7147–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AF, Stewart PE, Grimm D et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun 2002; 70: 2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Zhang JJ, Fang X et al. DhhP, a cyclic di-AMP phosphodiesterase of Borrelia burgdorferi, is essential for cell growth and virulence. Infect Immun 2014; 82: 1840–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Goldberg MS, Popova TG et al. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol 2000; 37: 1470–1479. [DOI] [PubMed] [Google Scholar]
- Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, 3rd, Flavell RA. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 1997; 6: 531–539. [DOI] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 2004; 199: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TB, Ma Y, Weis JH, Weis JJ. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol 1999; 37: 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, He M, Pang X, Xu ZC, Piesman J, Yang XF. Characterization of the highly regulated antigen BBA05 in the enzootic cycle of Borrelia burgdorferi. Infect Immun 2010; 78: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Akins DR. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun 2006; 74: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, He M, He JJ, Yang XF. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect Immun 2010; 78: 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, Pal U. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun 2010; 78: 4477–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Shang ES, Foley DM et al. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest 1995; 96: 2380–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Promnares K, Qin J et al. Characterization of multiprotein complexes of the Borrelia burgdorferi outer membrane vesicles. J Proteome Res 2011; 10: 4556–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Akins DR, Bourell KW, Lahdenne P, Norgard MV, Radolf JD. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc Natl Acad Sci USA 1996; 93: 7973–7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Thomas DD, Barbour AG. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun 1995; 63: 1573–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun 2008; 76: 3844–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman KE, Yang X, Wikel SK et al. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun 2000; 68: 4759–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun 1997; 65: 3146–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Telford SR, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med 1996; 183: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire WM, Garon CF. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect Immun 1993; 61: 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ. Laboratory Diagnostic Testing for Borrelia burgdorferi Infection. In: Halperin J (ed). Lyme disease: an evidence-based approach. Croydon: CAB International, 2011: 73–88. [Google Scholar]
- Lopez JE, Porcella SF, Schrumpf ME et al. Identification of conserved antigens for early serodiagnosis of relapsing fever Borrelia. Microbiology 2009; 155: 2641–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev 2005; 18: 484–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwang B, Dewing P, Fikrig E, Flavell RA. The hook protein of Borrelia burgdorferi, encoded by the flgE gene, is serologically recognized in Lyme disease. Clin Diagn Lab Immunol 1995; 2: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Benach JL. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis 1992; 165: 658–666. [DOI] [PubMed] [Google Scholar]
- Rossler D, Eiffert H, Jauris-Heipke S et al. Molecular and immunological characterization of the p83/100 protein of various Borrelia burgdorferi sensu lato strains. Med Microbiol Immunol 1995; 184: 23–32. [DOI] [PubMed] [Google Scholar]