Abstract
In vertebrates, MCM2–7 and Cdc45 are required for DNA replication initiation, but it is unknown whether they are also required for elongation, as in yeast. Moreover, although MCM2–7 is a prime candidate for the eukaryotic replicative DNA helicase, a demonstration that MCM2–7 unwinds DNA during replication is lacking. Here, we use Xenopus egg extracts to investigate the roles of MCM7 and Cdc45 in DNA replication. A fragment of the retinoblastoma protein, Rb1−400, was used to neutralize MCM7, and antibodies were used to neutralize Cdc45. When added immediately after origin unwinding, or after significant DNA synthesis, both inhibitors blocked further DNA replication, indicating that MCM7 and Cdc45 are required throughout replication elongation in vertebrates. We next exploited the fact that inhibition of DNA polymerase by aphidicolin causes extensive chromosome unwinding, likely due to uncoupling of the replicative DNA helicase. Strikingly, Rb1−400 and Cdc45 antibodies both abolished unwinding by the uncoupled helicase. These results provide new support for the model that MCM2–7 is the replicative DNA helicase, and they indicate that Cdc45 functions as a helicase co-factor.
Keywords: Cdc45, DNA helicase, DNA replication, MCM2–7, Xenopus
Introduction
Vertebrate cells contain a vast amount of DNA that is faithfully replicated during every mitotic cell division (for reviews, see Waga and Stillman, 1998; Bell and Dutta, 2002; Mendez and Stillman, 2003). The first step in DNA replication, pre-RC assembly, occurs at thousands of origins during the G1 phase of the cell cycle when a six-subunit origin recognition complex (ORC) bound to origins recruits the initiation factors Cdc6, Cdt1, and MCM2–7. Once MCM2–7 is loaded, ORC becomes dispensable, and the MCM2–7 complex serves as the platform on which further initiation events take place (Hua and Newport, 1998; Rowles et al, 1999; Shimada et al, 2002).
In S phase, pre-RCs are acted upon by two protein kinases and a multitude of replication initiation factors, which together are required for origin unwinding. In metazoans, MCM10 and the protein kinase Cdc7/Dbf4 are the first factors to load onto pre-RCs, and their loading is MCM2–7-dependent (Jares and Blow, 2000; Walter, 2000; Wohlschlegel et al, 2002). MCM10 and Cdc7 enable the loading of several additional factors such as GINS and Cdc45, whose binding is also dependent on Cdk2/Cyclin E. The binding of Cdc45 and GINS to pre-RCs is interdependent and converts this structure into a pre-Initiation Complex (or pre-IC) (Mimura and Takisawa, 1998; Zou and Stillman, 1998; 2000; Jares and Blow, 2000; Walter, 2000; Kubota et al, 2003; Takayama et al, 2003). Formation of the pre-IC is the last known event that occurs before origin unwinding, which is accompanied by chromatin loading of the single-stranded DNA-binding protein RPA (Tanaka and Nasmyth, 1998; Mimura et al, 2000; Walter and Newport, 2000). Once the origin has been sufficiently unwound, DNA polymerase α loads and synthesizes an RNA primer, which it then extends to form a short DNA primer. The presence of a DNA primer allows loading of the processivity factor PCNA by the RFC complex, followed by pol δ.
The final stage of DNA replication, elongation, involves the coordinated synthesis of nascent strands. Studies in yeast clearly show that, in addition to DNA polymerases, elongation also requires MCM2-7, Cdc45, and GINS (Labib et al, 2000; Tercero et al, 2000; Kanemaki et al, 2003), all of which localize to replication forks (Aparicio et al, 1997; Kanemaki et al, 2003; Takayama et al, 2003). It is unknown whether MCM2–7, Cdc45, or GINS are also required for elongation in metazoans. Given the results in yeast, it is surprising that immunofluorescence studies in vertebrate cells failed to detect colocalization of MCM2–7 with sites of ongoing DNA replication (Todorov et al, 1994; Krude et al, 1996; Romanowski et al, 1996).
The requirement for MCM2–7 in elongation in yeast is consistent with it being the eukaryotic replicative DNA helicase (Labib and Diffley, 2001). Like the replicative DNA helicases DnaB and Large T antigen, all six MCM subunits are members of the AAA+ family of ATPases, and the MCM2–7 complex adopts a ring-like structure (Chong et al, 2000; Fletcher et al, 2003). In yeast and mammals, a purified MCM4/6/7 subcomplex exhibits helicase activity in oligo-nucleotide displacement assays (You et al, 1999; Lee and Hurwitz, 2000; Kaplan et al, 2003). However, MCM2–7 is inactive as a helicase, and the purified MCM4/6/7 complex is inhibited by MCM2 and MCM3/5 (Ishimi et al, 1998; Lee and Hurwitz, 2000), which is surprising given the requirement for all six MCM subunits in fork progression in vivo (Labib et al, 2000). These observations have led to models in which MCM4/6/7 is the motor that unwinds DNA, whereas the other subunits serve regulatory functions. A definitive demonstration that MCM2–7 is the eukaryotic replicative DNA helicase will require biochemical reconstitution of DNA replication or a demonstration that MCM2–7 performs helicase activity at the replication fork (Labib and Diffley, 2001). Unlike the MCM2–7 complex, Cdc45 contains no known sequence motifs, and its molecular mechanism is unclear. Interestingly, Cdc45 is found in a complex with MCM2–7 on chromatin in yeast and in Xenopus egg extracts (Zou and Stillman, 1998; Mimura et al, 2000), suggesting that it might regulate the activity of MCM2–7.
To study chromosomal DNA replication, we use a soluble cell-free system derived from Xenopus eggs. Sperm chromatin or plasmid DNA is first incubated in a high speed supernatant of egg cytoplasm (HSS), leading to pre-RC assembly. Subsequently, a highly concentrated nucleoplasmic extract (NPE) prepared from synthetic nuclei is added, which supplies Cdk2/Cyclin E and Cdc7/Dbf4, as well as other activities (Walter, 2000; Wohlschlegel et al, 2002; Prokhorova et al, 2003) and a complete round of DNA replication ensues. Using this system, origin unwinding can be detected on plasmids via negative supercoiling, or on sperm via RPA loading (Walter and Newport, 2000). Interestingly, in the presence of aphidicolin, which inhibits replicative DNA polymerases, the degree of negative supercoiling and RPA loading are dramatically enhanced, indicating a high degree of DNA unwinding, or ‘hyperunwinding' (Walter, 2000; Walter and Newport, 2000). Since hyperunwinding depends on prior initiation of DNA replication, and is rapid and extensive, it likely reflects the action of the replicative DNA helicase after it has become uncoupled from the stalled replication complex. Thus, aphidicolin-induced helicase uncoupling represents a potentially powerful assay to study the eukaryotic replicative DNA helicase in the context of replication-competent chromatin.
We wanted to determine whether Cdc45 and the MCM complex are required after pre-IC formation in Xenopus egg extracts. To inactivate the chromatin-bound MCM complex after pre-ICs had formed, we used the N-terminal 400 amino acids of Rb (Rb1–400), a domain that was previously shown to interact with MCM7 and thereby inhibit DNA replication in Xenopus egg extracts at an unknown step (Sterner et al, 1998). When we added Rb1–400 to DNA replication complexes synchronized immediately after origin unwinding or during elongation, it completely inhibited further DNA replication, indicating a role for MCM7 in elongation. Moreover, at both of these stages, Rb1–400 inhibited the activity of the uncoupled DNA helicase in the presence of aphidicolin. These experiments show a direct role for MCM7 in DNA unwinding in the context of replication-competent chromatin, and thus provide new support for the idea that the MCM complex is the replicative DNA helicase. To determine whether Cdc45 is required for elongation, we inactivated chromatin-bound Cdc45 using antibodies. Cdc45 antibodies inhibited DNA replication and DNA unwinding by the uncoupled DNA helicase when added to replication complexes synchronized immediately after origin unwinding, or during elongation. The data show that Cdc45 is required for chromosome unwinding during elongation, and they are consistent with a model in which Cdc45 stimulates the helicase activity of the MCM2–7 complex.
Results
Rb1–400 inhibits origin unwinding when added after pre-RC formation
To examine what steps of DNA replication after pre-IC formation are dependent on the MCM complex, we sought to inactivate the chromatin-bound complex at progressively later stages of DNA replication. To this end, we took advantage of the previous observation that the retinoblastoma gene product, Rb, binds to MCM7 (Sterner et al, 1998). Using two-hybrid assays and co-immunoprecipitation of mammalian cell extracts, Rb1–400 was found to interact with the C-terminal region of MCM7. Moreover, when Rb1–400 was added to nuclear-assembly egg extracts before sperm chromatin, DNA replication was blocked. The inhibition was reversed when Rb1–400 was pre-incubated with an MCM7 peptide, arguing that inhibition was the result of Rb1–400 binding to the endogenous MCM7 protein (Sterner et al, 1998). It was not determined which step in DNA replication is blocked by Rb1–400.
We examined the effects of Rb1–400 on DNA replication in the nucleus-free system (see Introduction). Initially, Rb1–400 was added to HSS before sperm chromatin. Upon addition of NPE, DNA replication was blocked, and the inhibition was relieved by the MCM7 peptide (data not shown). Further analysis showed that, in the presence of Rb1–400, the MCM complex and Cdc45 failed to load onto chromatin (data not shown). These results show that, when added before sperm chromatin, Rb1–400 can block pre-RC assembly, and they confirm the previous observation that Rb1–400 inhibits DNA replication in Xenopus egg extracts (Sterner et al, 1998).
We next sought to determine what happens when Rb1–400 is added after MCM2–7 complexes have loaded onto chromatin. Sperm chromatin was incubated in HSS to assemble pre-RCs. Subsequently, Rb1–400 was added, followed by NPE. Under these conditions, Rb1–400 inhibited DNA replication five-fold, but when Rb1–400 was pre-incubated with MCM7 peptide, inhibition was relieved (Figure 1A, bar graph). To determine at what stage DNA replication was blocked, chromatin was isolated from NPE containing aphidicolin (NPEaph) to examine which factors loaded in the presence of Rb1–400. As discussed in the Introduction, the hyperloading of RPA in the presence of aphidicolin likely reflects the action of the replicative DNA helicase after it has become uncoupled from the stalled replication complex. In the presence of Rb1–400, MCM7, ORC2, and Cdc45 binding was unaffected, but RPA hyperloading was reduced (Figure 1A, upper panel, compare lanes 1 and 2), and the effect was reversed when Rb1–400 was preincubated with MCM7 peptide (Figure 1A, lane 3). Importantly, Rb1–400 did not affect the binding of RPA to an immobilized single-stranded DNA oligonucleotide in extracts (data not shown), indicating that the inhibition of RPA loading by Rb1−400 was due to an effect on chromosome unwinding.
Figure 1.
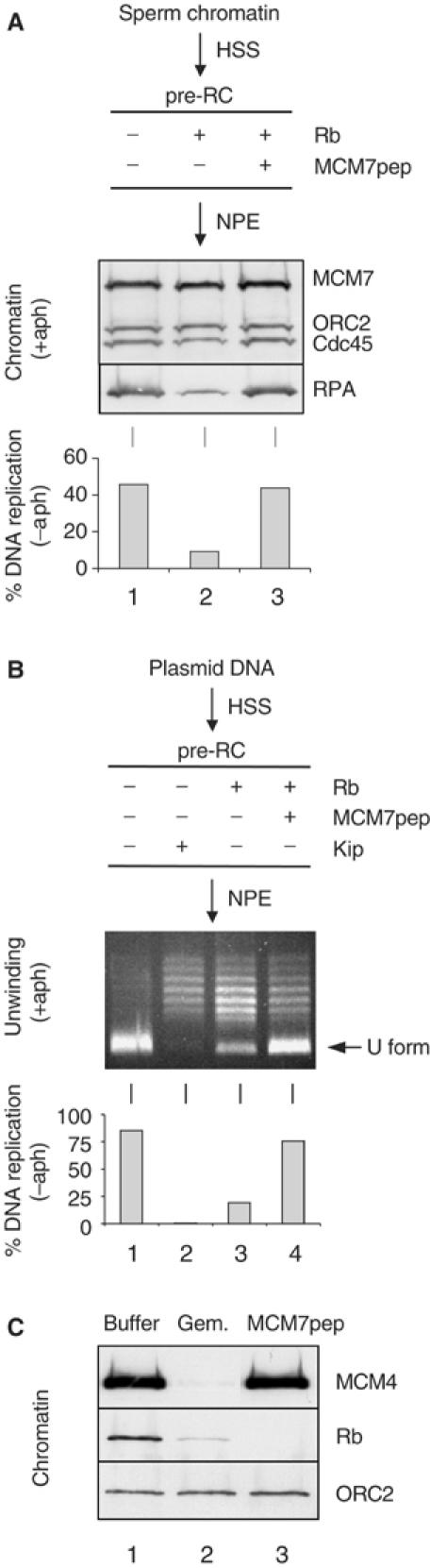
Rb1–400 inhibits origin unwinding after pre-RC formation. (A) Chromatin-loading assay. Sperm chromatin (10 000/μl) was incubated with 2 μl of HSS for 30 min and then supplemented with buffer (lane 1), 800 ng Rb1–400 (lane 2), or 800 ng Rb1–400 preincubated with 1.6 μg MBP-MCM7 peptide fusion (lane 3). After 30 min, 4 μl NPE was added, which contained aphidicolin (50 μg/ml) and buffer (lane 1), 400 ng Rb1–400 (lane 2), or 400 ng Rb1–400 and 800 ng MCM7 peptide (lane 3). After 45 min, the chromatin was purified, and bound proteins were analyzed by Western blot analysis using antibodies against RPA (lower panel), and a mixture of antibodies against MCM7, ORC2, and Cdc45 (upper panel). To measure DNA replication, the same reaction was carried out using NPE lacking aphidicolin but containing [α-32P]dATP (lower panel). (B) DNA topology assay. Same as panel A, except that pBS (40 ng/μl) was used as the DNA template. Lane 2 shows the effect of p27Kip addition. After 30 min incubation with NPE, the DNA was extracted, separated on a chloroquine agarose gel, and stained (top panel). To measure DNA replication, the same reaction was carried out using NPE lacking aphidicolin but containing [α-32P]dATP (lower panel). (C) Sperm chromatin was incubated with HSS supplemented with control buffer (lanes 1 and 3), or 500 nM geminin (lane 2) for 30 min. Subsequently, buffer (lane 1), 800 ng Rb1–400 (lane 2), or 800 ng Rb1–400 preincubated with 1.6 μg MCM7 peptide (lane 3) was added. After further 30 min, chromatin-bound proteins were analyzed with antibodies against MCM4, Rb, or ORC2.
To confirm by another method that Rb1–400 inhibits origin unwinding when added to assembled pre-RCs, we used a DNA topology assay (Walter and Newport, 2000). DNA replication is carried out using a circular plasmid, such as pBluescript (pBS), as the DNA template. Upon initiation of DNA replication, the plasmid becomes transiently underwound, generating a negatively supercoiled species that is readily detected by its rapid mobility during electrophoresis (U-form DNA). Addition of aphidicolin to the NPE traps all the DNA in the U form, and each plasmid undergoes much more extensive unwinding (‘hyperunwinding') than in the absence of aphidicolin. Hyperunwinding in the presence of aphidicolin is sensitive to the Cdk2 inhibitor p27Kip (Walter and Newport, 2000; Figure 1B, upper panel, compare lanes 1 and 2), indicating that it reflects uncoupling of the replicative DNA helicase after initiation. To determine whether Rb1–400 affected origin unwinding when added after pre-RC formation, pBS was incubated in HSS to form pre-RCs. Subsequently, Rb1–400 was added, followed by NPE containing aphidicolin. Rb1–400 significantly reduced the generation of U-form DNA (Figure 1B, lanes 1 and 3), and inhibition was largely alleviated when Rb1–400 was pre-incubated with MCM7 peptide (Figure 1B, lane 4). Consistent with the inhibition of origin unwinding, Rb1–400 also blocked DNA replication of pBS when it was added after pre-RC formation (Figure 1B, bar graph).
We wanted to verify that Rb1–400 targeted MCM7 on chromatin. Sperm chromatin was incubated with HSS to load the MCM complex, followed by addition of Rb1–400. Subsequently, the chromatin was isolated and blotted for Rb. Figure 1C (lane 1) shows that Rb1–400 cosedimented with the chromatin. Importantly, in the presence of geminin, which blocks MCM2–7 chromatin loading by targeting Cdt1 (Wohlschlegel et al, 2000; Tada et al, 2001), Rb1–400 loading was reduced (Figure 1C, lane 2). When Rb1–400 was pre-incubated with MCM7 peptide, Rb1–400 binding was also severely reduced (Figure 1C, lane 3). These results argue that Rb1–400 exerts its inhibitory effects in DNA replication and origin unwinding by binding to MCM7 on chromatin. Together, the data in this section indicate that MCM7 is required for origin unwinding independently of its role in pre-IC formation.
Rb1–400 inhibits chromosome unwinding by a previously activated helicase
The origin-unwinding defect seen in Figure 1 could be explained in two ways. First, Rb1–400 might block an unknown step after pre-IC formation that is required to activate the replicative DNA helicase, or Rb1–400 might block the fully activated helicase itself. To distinguish between these possibilities, we added Rb1–400 to chromatin containing a previously activated helicase. To this end, sperm chromatin was incubated with HSS, followed by NPE containing actinomycin D (NPEactD). ActD abolishes DNA replication in Xenopus egg extracts (Michael et al, 2000; our data not shown), likely due to its inhibition of the RNA priming activity of DNA pol α (Grosse and Krauss, 1985). Consistent with such a mechanism, actD does not affect chromatin loading of Cdc45 (Edwards et al, 2002) or DNA polymerase α (data not shown). Importantly, in the presence of actD, RPA was also loaded onto chromatin, and the loading was Cdk2-dependent, demonstrating that it required initiation (Figure 2A, compare lanes 5 and 6). The amount of RPA loaded in the presence of actD was similar to the peak level seen on chromatin during an unperturbed S phase (Figure 2A, compare lanes 2 and 5), indicating that unwinding in NPEactD was significant, but it was far less than what is observed during hyperunwinding in aphidicolin extract (Figure 2A, compare lanes 5 and 7). The reason why actD does not support hyperunwinding is unclear. One possibility is that its intercalating activity (Kamitori and Takusagawa, 1992) prevents the ability of the helicase to travel more than a short distance along DNA. Whatever the precise mechanism of actD, the data show that it arrests DNA replication after a Cdk2-dependent helicase has been allowed to unwind a limited amount of DNA (Figure 2B, top).
Figure 2.
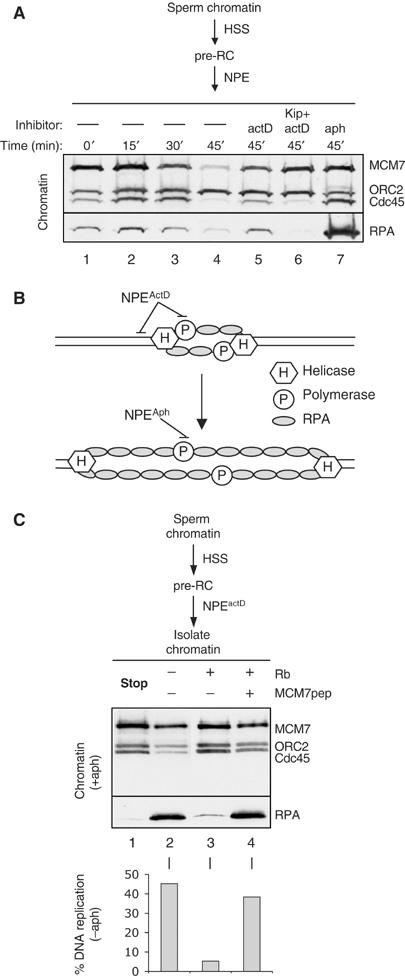
Rb1–400 protein inhibits DNA replication and chromosome unwinding after initiation. (A) RPA loading in the presence of actinomycin D is Cdk2-dependent. Sperm chromatin was incubated with HSS, followed by unsupplemented NPE (lanes 1–4), or NPE containing 10 μM actinomycin D (lanes 5), actinomycin D and p27Kip (lane 6), or aphidicolin (lane 7). At the indicated times, chromatin was isolated and blotted for MCM7, ORC2, Cdc45, and RPA. (B) Model for the actinomycin D and aphidicolin arrest points. (C) Sperm chromatin was incubated with HSS, followed by NPEactD, and isolated (lane 1), or isolated and then incubated with buffer (lane 2), 800 ng Rb1−400 (lane 3), or 800 ng Rb1−400 preincubated with 1.6 μg MCM7 peptide. Isolation leads to permanent immobilization of the sperm on the tube. After 30 min, the supernatant was replaced with 5 μl fresh NPEaph containing buffer (lane 2), 400 ng Rb1−400 (lane 3), or 400 ng Rb1−400 preincubated with 800 ng MCM7 peptide. After 45 min, chromatin was washed and blotted for MCM7, ORC2, Cdc45, and RPA34 (upper panel). Identical reactions were carried out in which the second incubation with NPE lacked aphidicolin but contained [α-32P]dATP to measure DNA replication (bar graph).
When chromatin containing the activated helicase was transferred from NPEactD to fresh NPE, DNA replication was efficient, indicating that a replication complex stalled in actD can resume DNA synthesis (Figure 2C, bar graph, column 2). When the chromatin incubated in NPEactD was first exposed to Rb1–400 before transfer to fresh NPE, DNA replication was severely inhibited, but this inhibition was not observed when Rb1–400 was first pre-incubated with MCM7 peptide (Figure 2C, bar graph, compare columns 3 and 4). Therefore, MCM7 is still required for DNA replication after helicase activation. The experiment was repeated, but chromatin was transferred from NPEactD into NPEaph, and chromatin association of RPA was measured. There was a large increase in RPA binding when chromatin was transferred from NPEactD into NPEaph (Figure 2C, upper panel, compare lanes 1 and 2). This increase was dependent on the presence of aphidicolin because it did not occur when chromatin was transferred into fresh NPEactD (Supplementary Figure S1). Moreover, it did not involve new initiation events because it still occurred when chromatin was transferred into NPEaph that also contained the Cdk2 inhibitor, p27Kip (Supplementary Figure S1). Therefore, a helicase arrested in the presence of actD can subsequently become uncoupled from the replication fork and carry out hyperunwinding in the presence of aphidicolin (illustrated in Figure 2B). When chromatin incubated in NPEactD was exposed to Rb1–400 before transfer into NPEaph, RPA hyperloading was strongly reduced, and the effect was reversed by MCM7 peptide (Figure 2C, upper panel, lanes 3 and 4). The data indicate that, even after the replicative DNA helicase has been activated, DNA replication still requires MCM7 due to a direct involvement of this protein in chromosome unwinding.
Rb1−400 inhibits DNA replication and chromosome unwinding during replication elongation
Although there is origin unwinding of DNA templates incubated in NPEactD(Figure 2A), there is no DNA synthesis (Michael et al, 2000; our data not shown). Therefore, replication complexes assembled in these extracts have not entered the elongation phase of DNA replication. To address whether Rb1–400 inhibits DNA replication of chromatin engaged in elongation, we synchronized replication complexes in elongation mode by lowering the reaction temperature from 22 to 19°C, which caused S phase to be extended by at least 30 min (Figure 3A). To determine the synchrony of origin firing at 19°C, we added p27Kip 20 min after the addition of NPE and found that it had no effect on the kinetics or efficiency of DNA replication (Figure 3B, compare circles and triangles), whereas when p27Kip was added at the same time as NPE DNA replication was completely blocked (Figure 3B, squares). We know that Cdk2 remained inactive throughout the experiment because a fresh, licensed template added after p27Kip did not undergo DNA replication (data not shown). The data indicate that, at 19°C, all origins fire within 20 min of NPE addition, and the time following Cdk2 addition comprises the elongation phase of DNA replication.
Figure 3.
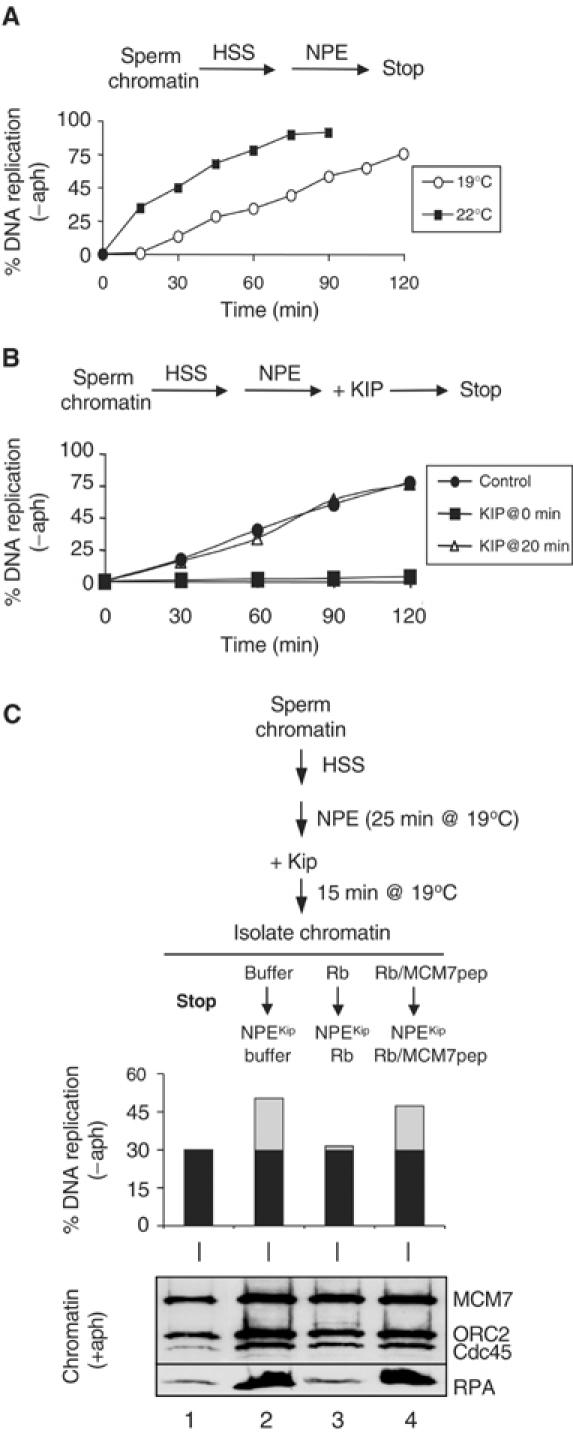
Rb1–400 inhibits DNA replication and chromosome unwinding during elongation. (A) DNA replication kinetics at 22 and 19°C. Sperm chromatin was pre-incubated with HSS, NPE was added, reactions were transferred to 22°C (squares) or 19°C (circles), and DNA replication was measured. (B) All origins fire within 20 min of NPE addition at 19°C. Sperm chromatin was incubated with HSS, followed by NPE addition and transfer to 19°C. Buffer (circles) or p27Kip was added 0 min (squares) and 20 min (triangles) after NPE. (C) Rb1–400 inhibits the elongation complex. Sperm chromatin was incubated with HSS, followed by addition of NPE and transfer to 19°C. After 25 min, p27Kip was added, and after a further 15 min chromatin was isolated (lane 1). Chromatin was exposed to buffer (lane 2), Rb1–400 (lane 3), or Rb1–400/MCM7 (lane 4). Finally, fresh NPE containing aphidicolin, p27Kip, and buffer (lane 2), Rb1–400 (lane 3), or Rb1–400/MCM7 peptide (lane 4) was added to measure chromatin binding (lower panel). To measure DNA replication (bar graph), aphidicolin was omitted and both NPEs contained [α-32P]dATP.
We used the low-temperature synchronization to address whether Rb1–400 inhibits DNA replication of elongating complexes. Pre-RCs were assembled in HSS and allowed to initiate DNA replication in NPE at 19°C. After 25 min, p27Kip was added to inhibit additional initiation events, and after a further 15 min, the chromatin was isolated. At this stage, 30% of the input DNA had replicated (Figure 3C, bar graph, column 1). Individual aliquots of chromatin were then mixed with buffer, Rb1–400, or Rb1–400 and MCM7 peptide, followed by fresh NPE, which was supplemented with p27Kip to prevent new initiation events. In the buffer control, addition of NPEKip allowed a further 20% DNA replication (Figure 3C, column 2). It should be noted that the relatively low efficiency of DNA replication observed after chromatin isolation is due to nonspecific inactivation of chromatin (Walter et al, 1998), but it does not affect our conclusions. In contrast, only ∼1% further replication was observed in the presence of Rb1–400, and 16% additional DNA replication was seen in the presence of Rb1–400 and MCM7 peptide (Figure 3C, columns 3 and 4). These results indicate that MCM7 is required for replication elongation.
To determine the effects of Rb1–400 on chromosome unwinding by elongating complexes, chromatin was treated as described above, but it was incubated with NPEKip+aph in the final step to measure RPA hyperloading. As seen in Figure 3C (lower panel), transfer of elongating complexes into NPEKip+aph caused hyperloading of RPA, indicating that the helicase can become uncoupled from elongating complexes (compare lanes 1 and 2). As with DNA replication, Rb1−400 blocked RPA hyperloading, and the effect was reversed by MCM7 peptide (Figure 3C, lanes 3 and 4). The data show that Rb1–400 inhibits DNA replication and chromosome unwinding of elongating replication complexes, consistent with the idea that MCM7 is directly required for chromosome unwinding in these complexes.
In Figure 3C, it was conceivable that Rb inhibited DNA replication of the isolated chromatin due to a requirement for MCM7 in restarting the replication fork upon transfer to fresh NPE. However, this scenario is unlikely, since Rb (but not Rb pre-incubated with MCM7 peptide) inhibited DNA replication when added to elongating complexes that had not been isolated from the extract (Supplementary Figure S2).
Previously, it was shown that multiple MCM2–7 complexes bind to chromatin in a distributed pattern in Xenopus egg extracts (Edwards et al, 2002; Harvey and Newport, 2003). Therefore, it was conceivable that Rb1–400 might block DNA replication and chromosome unwinding by associating with multiple MCM2–7 complexes, thereby creating a chromatin structure that cannot be traversed by the replication complex. If this model were correct, chromatin with a lower density of MCM2–7 complexes should be impervious to inhibition by Rb1–400. To generate such chromatin, we incubated sperm in HSS that was diluted 10-fold with buffer. Under these conditions, the amount of chromatin-bound MCM2–7 complexes was severely reduced compared to undiluted HSS (Figure 4A, compare lanes 1 and 3), but the residual MCM2–7 loading was still geminin-sensitive (Figure 4A, lane 4). To test whether Rb1–400 still inhibits DNA replication of chromatin containing low levels of MCM2–7, we performed a low-temperature synchronization in which we used either undiluted or 10-fold diluted HSS. Figure 4B shows that DNA replication of chromatin assembled in dilute HSS was ∼50% of control (compare columns 1 and 2), consistent with previous findings that MCM complexes are present on chromatin in a large functional excess (Mahbubani et al, 1997; Edwards et al, 2002), and replication was still completely geminin-sensitive (compare columns 2 and 4). Importantly, despite the low level of MCM2–7 bound, Rb1–400 inhibited DNA replication of chromatin assembled in dilute HSS to the same extent as control chromatin (compare columns 1 and 5 with 2 and 6), and in each case MCM7 peptide rescued DNA replication (columns 7 and 8). This result argues against the idea that inhibition by Rb1–400 results from its association with multiple MCM complexes to generate a repressive chromatin structure.
Figure 4.
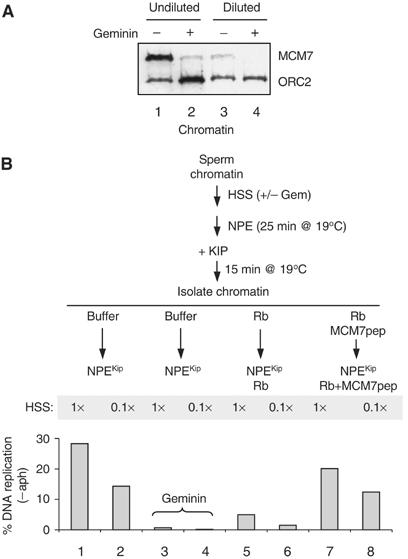
Rb1–400 inhibits DNA replication of chromatin containing low levels of MCM2–7. (A) Effect of HSS dilution on MCM2–7 loading. Sperm chromatin was incubated with HSS (lanes 1 and 2), or HSS that was diluted 10-fold with ELB (lanes 3 and 4), in the presence (lanes 2 and 4) and absence (lanes 1 and 3) of geminin. After 30 min, the chromatin was isolated and blotted for MCM7 and ORC2. (B) Effect of Rb1−400 on chromatin containing low levels of MCM2–7. Sperm chromatin was incubated with HSS (1 ×) or 10 times diluted HSS (0.1 ×), some of which contained 500 nM geminin (lanes 3 and 4). Subsequently, NPE was added at 19°C, and after 25 min p27Kip was added. After a further 15 min, the chromatin was isolated and exposed to buffer, Rb1−400, or Rb1−400/MCM7 peptide. Fresh NPE containing buffer, Rb1−400 or Rb1−400/MCM7 peptide was added, and DNA replication was measured after 45 min.
Cdc45 is required for DNA replication and chromosome unwinding after initiation
In Cdc45-depleted egg extracts, pre-IC formation is defective, and no origin unwinding is detected (Mimura and Takisawa, 1998; Mimura et al, 2000; Walter and Newport, 2000). To determine whether Cdc45 is required after pre-IC formation, we used a high-titer polyclonal antibody raised against the Cdc45 protein (Walter and Newport, 2000). When added to egg extracts before sperm chromatin, this antibody blocked Cdc45 loading and inhibited DNA replication (data not shown). To determine whether Cdc45 is still required after helicase activation, we incubated sperm chromatin in NPEactD, which allows Cdc45 loading and limited chromosome unwinding (Figure 2B). After isolation, the chromatin was incubated with buffer or purified anti-Cdc45 IgG. The chromatin was washed and supplemented with fresh NPE lacking antibody but containing [α]32P-dATP. As shown in the bar graph in Figure 5A, Cdc45 antibody eliminated DNA replication (compare columns 2 and 3), and the inhibitory effect was reversed by pre-incubating the antibody with recombinant Cdc45 protein (column 4). In a control experiment, we found that anti-geminin antibody did not inhibit DNA replication (data not shown). We repeated the same experiment but used NPEaph for the final incubation, and found that Cdc45 antibody blocked RPA hyperloading (Figure 5A, upper panel). The apparent excess of ORC2 present in lane 4 represents the purified his-tagged Cdc45 protein, which sticks nonspecifically to chromatin, and is detected because we blot for ORC2 and Cdc45 simultaneously. The results in Figure 5A argue that Cdc45 plays a crucial role in chromosome unwinding by origin-proximal replication complexes.
Figure 5.
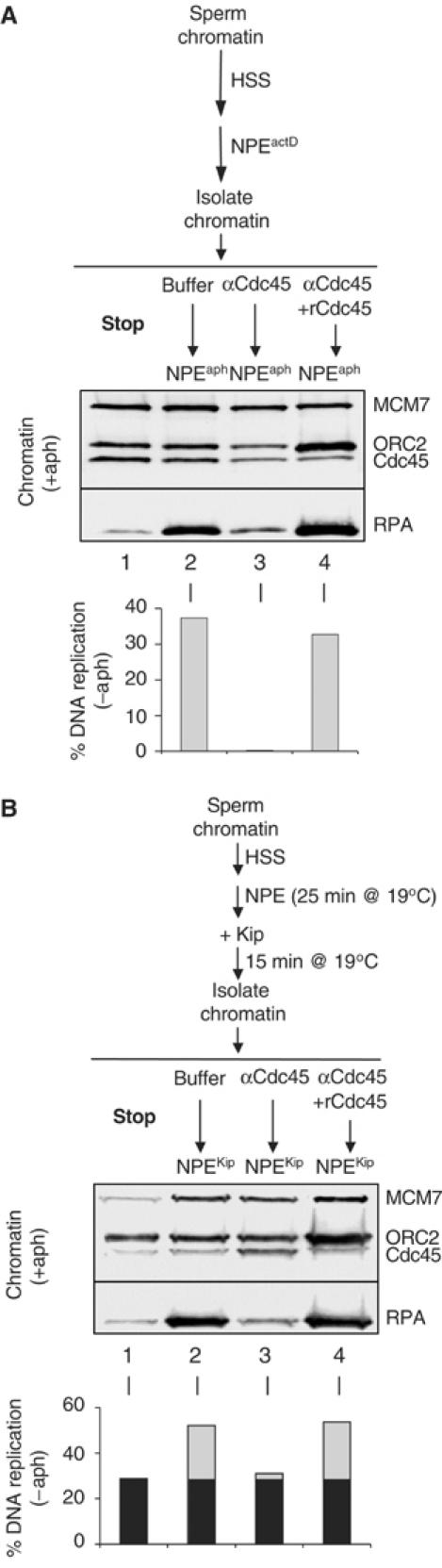
Cdc45 is required for chromosome unwinding during elongation. (A) Cdc45 is required for chromosome unwinding near origins. After incubation in HSS followed by NPEactD, chromatin was isolated and stopped (lane 1), or isolated and exposed to buffer (lane 2), 0.4 μg/μl Cdc45 IgG (lane 3), or Cdc45 IgG that had been pre-incubated with 90 ng/μl purified His-tagged Cdc45 protein (lane 4). After 30 min, the chromatin was washed, and fresh NPE was added, which contained aphidicolin to measure chromatin loading of RPA or [α-32P]dATP to measure DNA replication. (B) The same low-temperature synchronization approach used in Figure 3C was used, except that inhibition was carried out using Cdc45 antibodies as in (A), and DNA replication (bar graph), or chromatin loading of MCM7, ORC2, Cdc45, and RPA in the presence of aphidicolin (upper panel) was measured.
To determine whether Cdc45 is required for the elongation phase of DNA replication, we used low-temperature synchronization. DNA replication was allowed to initiate in NPE for 25 min at 19°C, p27Kip was added, and after a further 15 min, chromatin was isolated, or incubated with buffer, Cdc45 antibody, or Cdc45 antibody pre-incubated with Cdc45 protein. After incubation, excess supernatant was removed and replaced with fresh NPEKip to measure DNA replication (Figure 5B, bar graph), or NPEKip+aph to measure helicase-uncoupled hyperunwinding (Figure 5B, upper panel). Whereas the buffer control exhibited significant additional DNA replication and hyperunwinding upon transfer of chromatin to the second NPE (Figure 5B, compare conditions 1 and 2), the reaction containing Cdc45 antibody did not (condition 3), and preincubation of Cdc45 antibody with Cdc45 protein reversed the inhibition (condition 4). These results argue that Cdc45 is required for chromosome unwinding during elongation.
It is noteworthy that, in Figure 5, the Cdc45 antibody completely inhibited DNA replication and unwinding, even though no Cdc45 antibody was added with the second NPE, which contains high concentrations of Cdc45 protein. This result suggests that the Cdc45 initially loaded onto chromatin (either in actD or at 19°C) has a very slow off-rate. However, it was possible that the antibody locked the replication complex into a conformation in which Cdc45 was not able to dissociate. To further explore the Cdc45 off-rate, we loaded Cdc45 onto chromatin in NPEactD, and then transferred the chromatin to fresh NPE that was Cdc45 depleted. DNA replication in the Cdc45-depleted NPE was reduced only about 50% compared to the mock-depleted extract (Figure 6, bar graph, columns 1 and 2). A similarly modest effect was observed on RPA hyperloading and Cdc45 binding itself (Figure 6, lower panel). Importantly, the depleted NPE was unable to support DNA replication, Cdc45 loading, or RPA hyperloading of naive chromatin (Figure 6, compare conditions 3 and 4), demonstrating that it lacked functional levels of Cdc45. We conclude that chromatin-bound Cdc45 has a slow off-rate and that extensive DNA replication can occur without Cdc45 dissociation. Interestingly, the fact that replication is reduced by 50% when no free Cdc45 is present implies that free Cdc45 can normally reload onto replication complexes that have lost the protein, as seen in yeast (Tercero et al, 2000).
Figure 6.
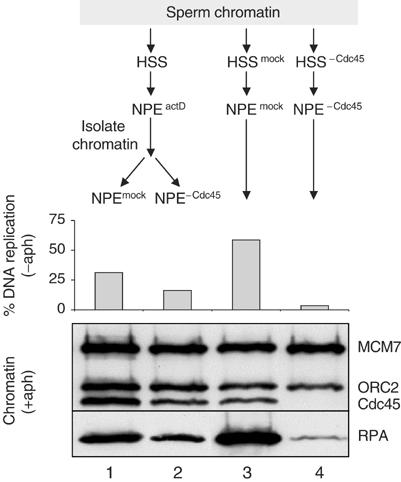
Cdc45 has a slow off-rate from chromatin. Sperm chromatin containing the activated helicase was generated and purified as in Figure 2C. Subsequently, it was incubated with mock-depleted (lane 1) or Cdc45-depleted NPE (lane 2). In the same experiment, sperm chromatin was incubated with mock-depleted HSS and NPE (lane 3) or Cdc45-depleted HSS and NPE (lane 4). At 45 min after the final NPE addition, DNA replication and chromatin loading in the presence of aphidicolin were measured.
Discussion
Previous results using Xenopus egg extracts showed that the MCM complex is required for pre-RC and pre-IC assembly (Cdc45 loading) in metazoans, but it was unclear whether MCM participates in subsequent steps of DNA replication including origin unwinding and elongation. In this report, we used Rb1–400 to target the MCM7 protein after pre-RC assembly. Rb1–400 inhibited DNA replication when it was added after pre-RC formation, immediately after origin unwinding, and after 30% of the input DNA had replicated. These data indicate that MCM7 is required for elongation in metazoans. We also examined the effects of Rb1–400 on DNA unwinding by uncoupled DNA helicases. First, we showed that Rb1–400 added immediately after pre-RC formation allowed Cdc45 loading but blocked aphidicolin-induced hyperunwinding, suggesting that MCM7 is required for the activity of the helicase that unwinds the origin. To rule out that Rb1–400 inhibits an unknown helicase activation step, Rb1–400 was added after the helicase had been activated in the presence of actD. The hyperunwinding observed when replication complexes are transferred from NPEactD to NPEaph likely reflects uncoupling of the helicase that unwound the origin. Based on the inhibition of this helicase by Rb1–400, we infer that MCM7 is part of the molecular machine that unwinds origins. Finally, replication complexes were allowed to synthesize 30% of the DNA before transfer of the chromatin to the extract containing aphidicolin to induce hyperunwinding. Inhibition of hyperunwinding in this setting by Rb1−400 argues that MCM7 is required for unwinding of DNA along the entire replicon.
Although the simplest interpretation of the inhibitory effects of Rb1–400 is that it targets MCM7 and thereby prevents chromosome unwinding, alternative explanations exist. First, it is possible that Rb1–400 binds to MCM7 and then disrupts another protein at the replication fork, which is the actual helicase. Given the great stability with which the eukaryotic replicative DNA helicase is expected to bind to DNA, this scenario appears unlikely. Second, the binding of Rb1–400 to the multitude of chromatin-bound MCM2–7 complexes might create a repressive chromatin structure that cannot be traversed by the replicative DNA helicase. However, we showed that chromatin containing much lower levels of MCM2–7 was still susceptible to inhibition by Rb1–400. Third, Rb might cause inhibition by targeting a protein other than MCM7. However, such a factor would have to closely resemble MCM7, since the inhibitory effect of Rb1–400 is completely reversed by an MCM7 peptide. In addition, we showed that loading of Rb1–400 onto chromatin (the most likely site of the target) was dependent on chromatin-bound MCM2-7 and sensitive to MCM7 peptide. Finally, the inhibition of DNA replication and chromosome unwinding by Rb1−400 was reversed by co-addition of Cdk4/Cyclin D (M Pacek, Andrew Gladden, Alan Diehl, and JC Walter, unpublished results). Since the interaction between MCM7 and Rb is also sensitive to Cdk4/Cyclin D (Gladden and Diehl, 2003), this finding provides further evidence that Rb1–400 specifically targets MCM7 in extracts.
Our results provide a new class of support for the idea that the MCM complex is the eukaryotic replicative DNA helicase. Previous work showed that: (1) MCM2–7 travels with and is required for progression of the replication fork in yeast (Aparicio et al, 1997; Labib et al, 2000); (2) MCM complexes are structurally similar to replicative DNA helicases (Chong et al, 2000; Fletcher et al, 2003); (3) MCM4/6/7 subassemblies exhibit helicase activity in vitro (You et al, 1999; Lee and Hurwitz, 2000; Kaplan et al, 2003). We now show that MCM7 is directly required for DNA unwinding in the context of replication-competent chromatin. It is important to note that our data do not address whether an MCM2–7 holocomplex, an MCM4/6/7 subcomplex, or some other MCM7-containing complex unwinds chromosomes. However, given that at least five MCM subunits are required for elongation in Saccharomyces cerevisiae (Labib et al, 2000), the simplest interpretation of our results is that the MCM2–7 holocomplex is the replicative DNA helicase and that MCM7 is an essential component of this enzyme.
The conclusion that MCM2–7 is required for elongation in vertebrates is noteworthy because previous immunofluorescence (IF) studies failed to place the MCM2–7 complex at sites of ongoing DNA replication (Todorov et al, 1994; Krude et al, 1996; Romanowski et al, 1996). Previously, we and others showed that, in Xenopus egg extracts, a large number of MCM2–7 complexes are widely distributed on chromatin, and that only some of these are activated for DNA replication (Edwards et al, 2002; Harvey and Newport, 2003). Evidence for a similar phenomenon exists in mammalian cells (see Discussion in Edwards et al, 2002). We therefore postulated that the MCM2–7 complexes present at the replication fork are invisible by IF due to the large number of latent complexes present elsewhere. Our current demonstration that MCM7 is required for replication elongation in metazoans supports this interpretation since it implies that there must be MCM complexes present at the replication fork.
Like MCM2–7, Cdc45 has been shown to travel with and be required for replication fork progression in yeast (Aparicio et al, 1997; Tercero et al, 2000), and Cdc45 and MCM2–7 are found in the same complex on chromatin (Zou and Stillman, 1998; Mimura et al, 2000). When MCM2–7 is recovered from chromatin that was assembled in the absence of Cdc45 in Xenopus egg extracts, MCM2–7-associated helicase activity is reduced (Masuda et al, 2003). These data are consistent with Cdc45 playing a role in pre-IC formation, but do not demonstrate an ongoing requirement for Cdc45 in helicase activity. We added Cdc45 antibody to replication complexes arrested immediately after origin unwinding or during elongation. In both cases, Cdc45 antibody blocked further DNA replication, demonstrating a requirement for Cdc45 in elongation. Importantly, Cdc45 antibodies also blocked hyperunwinding in the presence of aphidicolin, indicating that this protein is required for the activity of the replicative DNA helicase. Since Cdc45 itself has no recognizable ATPase motifs, the simplest interpretation of these results is that it functions as a helicase co-factor. Interestingly, once Cdc45 has been loaded, transfer of chromatin into Cdc45-deficient extract caused only an ∼50% reduction in DNA replication, suggesting that Cdc45 does not rapidly cycle on and off the chromatin.
Although we used Rb1–400 primarily as a tool to target the MCM2-7 complex, it is interesting to consider the in vivo role of this interaction. The canonical function of Rb is to bind to E2F and prevent transcription of S-phase-specific genes (Sherr, 1996). Activation of Cdk4/Cyclin D by growth factors leads to phosphorylation and inactivation of Rb such that cells can proceed into the S phase, and Rb remains phosphorylated for the remainder of the cell cycle. Similar to the Rb–E2F interaction, the Rb–MCM7 interaction is negatively regulated by Cdk4/Cyclin D (Gladden and Diehl, 2003), and this protein kinase abolishes the inhibitory effects of Rb on DNA replication and chromosome unwinding (M Pacek, Andrew Gladden, Alan Diehl, and JC Walter, unpublished observations). Together, these observations indicate that Rb could restrain S phase by an additional mechanism that involves inhibition of the replicative DNA helicase. Interestingly, Cyclin D is destroyed and Rb is dephosphorylated when cells are exposed to DNA-damaging agents (Lan et al, 2002), and recent data indicate that, under these conditions, Rb binds to origins of DNA replication (Avni et al, 2003). Thus, in the G1 phase, and in response to cell cycle checkpoints, the hypophosphorylated form of Rb might inhibit the helicase activity of the MCM2–7 complex and thereby prevent unscheduled DNA replication.
Materials and methods
DNA replication, chromatin binding, and DNA topology assays
For DNA replication assays, HSS was incubated with sperm chromatin (10 000 sperm/μl) for 30 min at 22°C, followed by addition of 2 volumes NPE containing trace amounts of [α-32P]dATP (Walter et al, 1998). Incorporation of [α-32P]dATP was measured using agarose gel electrophoresis (Dasso and Newport, 1990), and the percentage of input DNA replicated was calculated by determining the fraction of free label consumed, assuming a 50 μM endogenous pool of dATP (Blow and Laskey, 1986).
For chromatin-binding assays, the extract containing sperm chromatin (up to 10 μl) was diluted with 60 μl of cold ELB/TX-100 buffer (0.25 M sucrose, 2.5 mM MgCl2, 50 mM KCl, 10 mM HEPES pH 7.7 and 0.2% Triton X-100), layered onto a 180 μl sucrose cushion (ELB containing 0.5 M sucrose) in Beckman 5 × 44 mm microcentrifuge tubes, and isolated by centrifugation for 25 s at 12 000g (Walter et al, 1998). After washing twice with ELB, SDS sample buffer was added.
For the chromatin transfer experiments in Figures 2C, 5A and 6, sperm chromatin was incubated in 2.5 μl of HSS, followed by 5 μl of NPE containing 10 μM actinomycin D (NPEactD), and then isolated and washed as described above. The chromatin was incubated with the appropriate inhibitor for 30 min at 22°C. Subsequently, excess liquid was aspirated, and the chromatin was washed three times in ELB. Next, 5 μl of fresh NPE that contained either 50 μM aphidicolin to measure chromosome unwinding, or trace amounts of [α-32P]dATP to measure DNA replication was added. After 45 min, chromatin loading or DNA replication was measured. The low-temperature synchronization experiment was performed in the same manner, except that, after incubation of sperm chromatin in HSS, NPE lacking actD was added on ice and the reaction was incubated at 19°C for 25 min, followed by the addition of 2 μM p27Kip for another 15 min. The chromatin was then isolated and further processed as described above. The DNA topology assay was carried out as described (Walter and Newport, 2000).
Protein purification and immunological techniques
The 400 amino-acid GST-tagged N-terminal fragment of Rb and the 137 amino-acid MBP-tagged C-terminal MCM7 peptide were purified as described (Sterner et al, 1998). his-Cdc45 was purified as described (Walter and Newport, 2000). Western blotting was carried out using an RPA polyclonal rabbit antibody (Walter and Newport, 2000) or a mixture of ORC2 (Walter and Newport, 1997), MCM7 (Walter and Newport, 2000), and Cdc45 (Walter and Newport, 2000) polyclonal rabbit antibodies. The 34 kDa subunit of RPA is shown. Immunodepletion of Cdc45 protein was carried out as described (Walter and Newport, 2000). The Cdc45 IgG used to inhibit Cdc45 function was generated by acidic elution from rSepharose A FastFlow resin. The concentration of the eluted total IgG preparation was ∼2 mg/ml.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Acknowledgments
We thank Jonathan Horowitz for Rb1–400 and MCM7 peptide expression plasmids, and for anti-Rb antibody. We thank Hisao Masai, Karlene Cimprich, Alan Diehl, Andrew Gladden, and the members of our laboratory for helpful discussions. This work was supported by NIH Grant GM62267 to JCW.
References
- Aparicio OM, Weinstein DM, Bell SP (1997) Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91: 59–69 [DOI] [PubMed] [Google Scholar]
- Avni D, Yang H, Martelli F, Hofmann F, ElShamy WM, Ganesan S, Scully R, Livingston DM (2003) Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol Cell 12: 735–746 [DOI] [PubMed] [Google Scholar]
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA (1986) Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47: 577–587 [DOI] [PubMed] [Google Scholar]
- Chong J, Hayashi M, Simon M, Xu R, Stillman B (2000) A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA 97: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M, Newport JW (1990) Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell 61: 811–823 [DOI] [PubMed] [Google Scholar]
- Edwards MC, Tutter AV, Cvetic C, Gilbert CH, Prokhorova TA, Walter JC (2002) MCM2–7 complexes bind chromatin in a distributed pattern surrounding ORC in Xenopus egg extracts. J Biol Chem 277: 33049–33057 [DOI] [PubMed] [Google Scholar]
- Fletcher RJ, Bishop BE, Leon RP, Sclafani RA, Ogata CM, Chen XS (2003) The structure and function of MCM from archaeal M. thermoautotrophicum. Nat Struct Biol 10: 160–167 [DOI] [PubMed] [Google Scholar]
- Gladden AB, Diehl JA (2003) The cyclin D1-dependent kinase associates with the pre-replication complex and modulates RB.MCM7 binding. J Biol Chem 278: 9754–9760 [DOI] [PubMed] [Google Scholar]
- Grosse F, Krauss G (1985) The primase activity of DNA polymerase alpha from calf thymus. J Biol Chem 260: 1881–1888 [PubMed] [Google Scholar]
- Harvey KJ, Newport J (2003) Metazoan origin selection: origin recognition complex chromatin binding is regulated by CDC6 recruitment and ATP hydrolysis. J Biol Chem 278: 48524–48528 [DOI] [PubMed] [Google Scholar]
- Hua XH, Newport J (1998) Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J Cell Biol 140: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y, Komamura Y, You Z, Kimura H (1998) Biochemical function of mouse minichromosome maintenance 2 protein. J Biol Chem 273: 8369–8375 [DOI] [PubMed] [Google Scholar]
- Jares P, Blow JJ (2000) Xenopus cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev 14: 1528–1540 [PMC free article] [PubMed] [Google Scholar]
- Kamitori S, Takusagawa F (1992) Crystal structure of the 2:1 complex between d(GAAGCTTC) and the anticancer drug actinomycin D. J Mol Biol 225: 445–456 [DOI] [PubMed] [Google Scholar]
- Kanemaki M, Sanchez-Diaz A, Gambus A, Labib K (2003) Functional proteomic identification of DNA replication proteins by induced proteolysis in vivo. Nature 423: 720–724 [DOI] [PubMed] [Google Scholar]
- Kaplan DL, Davey MJ, O'Donnell M (2003) Mcm4,6,7 uses a ‘pump in ring' mechanism to unwind DNA by steric exclusion and actively translocate along a duplex. J Biol Chem 278: 49171–49182 [DOI] [PubMed] [Google Scholar]
- Krude T, Musahl C, Laskey RA, Knippers R (1996) Human replication proteins hCdc21, hCdc46 and P1Mcm3 bind chromatin uniformly before S-phase and are displaced locally during DNA replication. J Cell Sci 109: 309–318 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takase Y, Komori Y, Hashimoto Y, Arata T, Kamimura Y, Araki H, Takisawa H (2003) A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev 17: 1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K, Diffley JF (2001) Is the MCM2–7 complex the eukaryotic DNA replication fork helicase? Curr Opin Genet Dev 11: 64–70 [DOI] [PubMed] [Google Scholar]
- Labib K, Tercero JA, Diffley JF (2000) Uninterrupted MCM2–7 function required for DNA replication fork progression. Science 288: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Lan Z, Sever-Chroneos Z, Strobeck MW, Park CH, Baskaran R, Edelmann W, Leone G, Knudsen ES (2002) DNA damage invokes mismatch repair-dependent cyclin D1 attenuation and retinoblastoma signaling pathways to inhibit CDK2. J Biol Chem 277: 8372–8381 [DOI] [PubMed] [Google Scholar]
- Lee JK, Hurwitz J (2000) Isolation and characterization of various complexes of the minichromosome maintenance proteins of Schizosaccharomyces pombe. J Biol Chem 275: 18871–18878 [DOI] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JP, Chevalier S, Thommes P, Blow JJ (1997) Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol 136: 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Mimura S, Takisawa H (2003) CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45. Genes Cells 8: 145–161 [DOI] [PubMed] [Google Scholar]
- Mendez J, Stillman B (2003) Perpetuating the double helix: molecular machines at eukaryotic DNA replication origins. BioEssays 25: 1158–1167 [DOI] [PubMed] [Google Scholar]
- Michael WM, Ott R, Fanning E, Newport J (2000) Activation of the DNA replication checkpoint through RNA synthesis by primase. Science 289: 2133–2137 [DOI] [PubMed] [Google Scholar]
- Mimura S, Takisawa H (1998) Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk. EMBO J 17: 5699–5707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura S, Masuda T, Matsui T, Takisawa H (2000) Central role for cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts. Genes Cells 5: 439–452 [DOI] [PubMed] [Google Scholar]
- Prokhorova TA, Mowrer K, Gilbert CH, Walter JC (2003) DNA replication of mitotic chromatin in Xenopus egg extracts. Proc Natl Acad Sci USA 100: 13241–13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski P, Madine MA, Laskey RA (1996) XMCM7, a novel member of the Xenopus MCM family, interacts with XMCM3 and colocalizes with it throughout replication (see comments). Proc Natl Acad Sci USA 93: 10189–10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles A, Tada S, Blow JJ (1999) Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J Cell Sci 112: 2011–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cycles. Science 274: 1672–1677 [DOI] [PubMed] [Google Scholar]
- Shimada K, Pasero P, Gasser SM (2002) ORC and the intra-S-phase checkpoint: a threshold regulates Rad53p activation in S phase. Genes Dev 16: 3236–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner JM, Dew Knight S, Musahl C, Kornbluth S, Horowitz JM (1998) Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7. Mol Cell Biol 18: 2748–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ (2001) Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H (2003) GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev 17: 1153–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Nasmyth K (1998) Association of RPA with chromosomal replication origins requires an Mcm protein, and is regulated by Rad53, and cyclin- and Dbf4-dependent kinases. EMBO J 17: 5182–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero JA, Labib K, Diffley JF (2000) DNA synthesis at individual replication forks requires the essential initiation factor Cdc45p. EMBO J 19: 2082–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov IT, Pepperkok R, Philipova RN, Kearsey SE, Ansorge W, Werner D (1994) A human nuclear protein with sequence homology to a family of early S phase proteins is required for entry into S phase and for cell division. J Cell Sci 107: 253–265 [DOI] [PubMed] [Google Scholar]
- Waga S, Stillman B (1998) The DNA replication fork in eukaryotic cells. Annu Rev Biochem 67: 721–751 [DOI] [PubMed] [Google Scholar]
- Walter J, Newport J (2000) Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha. Mol Cell 5: 617–627 [DOI] [PubMed] [Google Scholar]
- Walter J, Newport JW (1997) Regulation of replicon size in Xenopus egg extracts. Science 275: 993–995 [DOI] [PubMed] [Google Scholar]
- Walter J, Sun L, Newport J (1998) Regulated chromosomal DNA replication in the absence of a nucleus. Mol Cell 1: 519–529 [DOI] [PubMed] [Google Scholar]
- Walter JC (2000) Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J Biol Chem 275: 39773–39778 [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dhar SK, Prokhorova TA, Dutta A, Walter JC (2002) Xenopus mcm10 binds to origins of DNA replication after mcm2–7 and stimulates origin binding of cdc45. Mol Cell 9: 233–240 [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A (2000) Inhibition of eukaryotic DNA replication by geminin binding to cdt1. Science 290: 2309–2312 [DOI] [PubMed] [Google Scholar]
- You Z, Komamura Y, Ishimi Y (1999) Biochemical analysis of the intrinsic Mcm4–Mcm6–mcm7 DNA helicase activity. Mol Cell Biol 19: 8003–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Stillman B (1998) Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science 280: 593–596 [DOI] [PubMed] [Google Scholar]
- Zou L, Stillman B (2000) Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase. Mol Cell Biol 20: 3086–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2


