Highlights
-
•
Development of new drugs against trypanosomes is a crucial but unmet need.
-
•
Membrane transport, endocytosis and related processes have been proposed as drug targets.
-
•
Recent insights uncovered the mode of action of two drugs that are already in the clinic.
-
•
Both of these drugs, suramin and pentamidine, bind surface proteins.
-
•
It is possible that endocytosis is a common component of sensitivity to suramin and pentamidine.
Abstract
Pathogenic protozoa are evolutionarily highly divergent from their metazoan hosts, reflected in many aspects of their biology. One particularly important parasite taxon is the trypanosomatids. Multiple transmission modes, distinct life cycles and exploitation of many host species attests to great prowess as parasites, and adaptability for efficient, chronic infection. Genome sequencing has begun uncovering how trypanosomatids are well suited to parasitism, and recent genetic screening and cell biology are revealing new aspects of how to control these organisms and prevent disease. Importantly, several lines of evidence suggest that membrane transport processes are central for the sensitivity towards several frontline drugs.
Current Opinion in Microbiology 2016, 34:97–103
This review comes from a themed issue on Parasitic and fungal diseases
Edited by Gero Steinberg
For a complete overview see the Issue and the Editorial
Available online 9th September 2016
http://dx.doi.org/10.1016/j.mib.2016.08.005
1369-5274/© 2016 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
African trypanosomes: novelty and conservation
Trypanosomatids cause a very broad range of diseases afflicting humans, animals, livestock, fish, plants and wild animals. Evidence has emerged for preadaptation to parasitism during evolution of the group and ongoing genetic modifications to suit distinct modes of infection and immune evasion [1••, 2••]. Trypanosomes are classified as Excavata, and branched early from the eukaryotic lineage [3]. This fuelled considerable optimism that when the genomes of these organisms were characterized, a wealth of drug and potential therapeutic targets would emerge. Approximately forty percent of trypanosome protein-coding genes appear either lineage-specific or of such great divergence that they present a viable target, despite having an ortholog in higher eukaryotes [4, 5]. This promise has, however, failed to emerge for several reasons, not least of which is translating initial hit compound activity against specific protein targets to leads with promising activity against whole cells, that is, trypanocidal or trypanostatic activity. Consequently, many efforts identifying new drugs remain focused on classical approaches such as phenotypic screens and do not, at least a priori, engage with either genetic divergence or those cell biological aspects unique to the kinetoplastids [6•]. Even compounds emerging from various screening efforts, with promising in vitro activity have experienced low rates of translation into viable (pre)-clinical candidates. However, serendipitously, it has emerged that many drugs presently used against these parasites, and specifically the African trypanosomes, do target rather well known unique aspects of trypanosome cell biology, and/or require these features, for their specificity and high potency.
The trypanosome cell is elongated, with a morphology supported by a sophisticated and elaborate subpellicular microtubule array [7, 8]. This feature essentially precludes budding of conventional transport vesicles from the vast majority of the plasma membrane, and all membrane flow to and from the surface is restricted to the flagellar pocket. Thus, drugs that do not effectively diffuse across membranes, must reach their intracellular targets via the flagellar pocket or cross the membrane via an alternate mechanism, that is, a transporter or channel. The flagellar pocket crucially is devoid of the microtubule array, and while the membrane is continuous with the bulk plasma membrane, it has a distinct protein and lipid composition and is physically delineated by a complex collar surrounding the pocket neck, which likely also restricts fluid phase diffusion [8, 9].
Trypanosomes possess a conventional endomembrane system, including a Golgi complex, early and recycling endosomes, late endosomes incorporating the ESCRT/multi-vesicular body system and a terminal endosome or lysosome, albeit somewhat streamlined, with several of these organelles probably present in interphase cells as single copy [10, 11]. Several features are highly unique, for example, the mammalian-infective form of T. brucei has an extreme rate of endocytosis, capable of turning over the plasma membrane many times per hour, contributing towards removal of surface-bound immune effectors, aiding immune evasion [12•]. The surface is dominated by the variant surface glycoprotein (VSG), a GPI-anchored homodimer comprising 90% of surface protein. Other surface proteins possess trans-membrane domains, but, importantly, are often highly divergent and trypanosome-specific [13•, 14].
Trypanosome endocytosis is exclusively clathrin-dependent, setting it apart from higher eukaryotes where multiple modes of endocytosis operate [15]. Furthermore, the widely conserved heterotetrameric (AP)-2 adaptin complex is absent and is inversely correlated with the presence of VSG and thus antigenic variation, the principal mechanism of immune evasion [16, 12•]. Additional clathrin adaptor proteins are present and include both ENTH and ANTH-domain phosphoinositide-binding proteins [17] and a cohort of trypanosomatid-specific proteins [18]. Sorting surface trans-membrane proteins requires ubiquitylation, likely performed by divergent ubiquitin ligases, although these remain unidentified [19••]. Whilst these details indicate a distinct endocytic system, the level of conservation with, for example, Saccharomyces cerevisiae, is considerable and the divergence certainly appears less extreme than in Apicomplexan parasites, where entire compartments and pathways have been repurposed [20, 21•].
Membrane transport activity is rather different between life stages and species of parasite, which may in part explain the differential sensitivity to some front-line drugs. Specifically, the bloodstream forms of T. brucei have much greater endocytic transport rates compared with the insect forms, and this correlates with sensitivity to suramin and pentamidine for example, with procyclic cells being much less sensitive to either drug, although other changes to the surface composition are considerable and may also contribute to the differential sensitivity. Although pentamidine is almost exclusively used against T. brucei gambiense, the West African form of the disease, and suramin usually for the East African T. brucei rhodesiense, both subspecies are fully sensitive to either drug. Both drugs are used exclusively to treat early stage (haemolymphatic) trypanosomiasis, because neither drug penetrates sufficiently into the central nervous system; it is not known how trypanosomes adapt after crossing the blood–brain barrier, and whether this might impact drug sensitivity.
In addition to endocytic mechanisms for entry into the cell, trypanosomes also possess a considerable array of surface nucleoside and nucleobase transporters, together with hexose and amino acid permeases, plus a small family of aquaporins [22]. These systems obviously also present a potential mechanism for accumulation of drugs as well as natural metabolites, whilst themselves also being proteins that are subject to turnover by the endocytic system. By contrast to many surface proteins, the transporters appear to be more broadly conserved with higher eukaryotes. Significantly, traffic focused at the flagellar pocket, high rates of endocytosis, novel surface protein composition and the presence of conserved transporters all directly contribute towards sensitivity of African trypanosomes to drugs that have been in clinical use since the 1920s.
A grandfather therapeutic: suramin
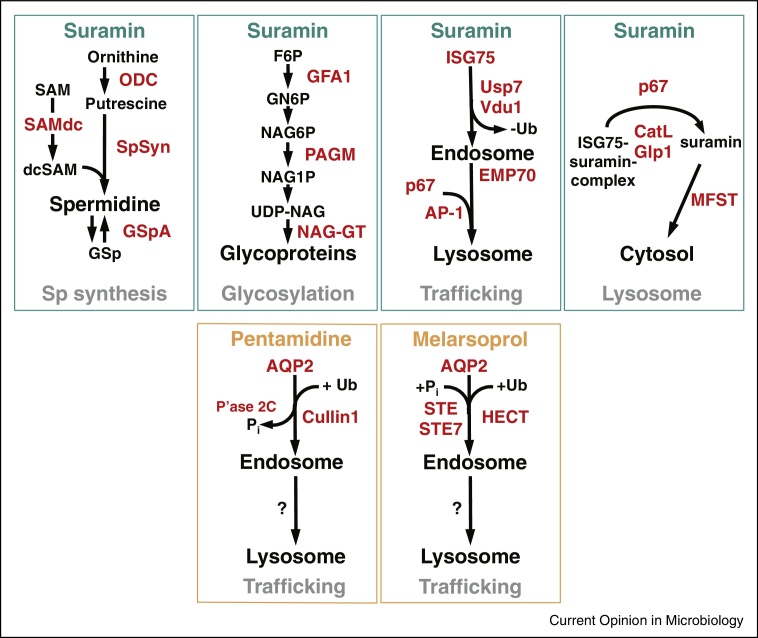
Suramin emerged from early synthetic chemistry and development of aniline dyes [23]. The trypanocidal diazo dyes, trypan red and trypan blue, were developed by Bayer in 1916 and led to suramin, still a frontline treatment against some forms of trypanosomiasis [24]. High molecular weight and negative charge prevent passive membrane diffusion, suggesting specific uptake. A thousand-fold reduced potency against insect stage parasites suggested involvement of endocytosis, as endocytic trafficking is much decreased in this life stage [25]. However, extensive surface proteome remodelling between life stages also suggests the possibility of bloodstream stage-specific expression of a ‘suramin-receptor’ [14]. Genome-wide loss-of-function screens identified multiple genes that sensitize trypanosomes to suramin [26••] (Figure 2), many of which have roles and/or locations at the endocytic pathway, for example, invariant surface glycoprotein 75 (ISG75), two deubiquitylating enzymes (DUBs) Usp7 and Vdu1, the AP-1 adaptin complex and the lysosomal protein p67 [27]. An ISG75-dependent pathway is required for lysosomal delivery of suramin while an AP-1-dependent path is likely connected to lysosomal composition and a requirement for transport of p67 and other factors (Figure 1) [19••]. ISG75 stability is regulated by ubiquitylation [28] and evidence that trypanosome Usp7 and Vdu1 modulate ISG75 turnover is consistent with this model [19••]. Significantly, the suramin-uptake pathway is highly specific and does not involve the closely related invariant surface glycoprotein 65 (ISG65) family.
Figure 2.
Mode of action of trypanocidal drugs. Summary of biochemical pathways linked to drug action for suramin, pentamidine and melarsoprol. Proteins sensitizing to the respective drug, as identified in a genome-wide loss-of-function screen [26••] are drawn in red. (Pi: inorganic phosphate, Ub: ubiquitin).
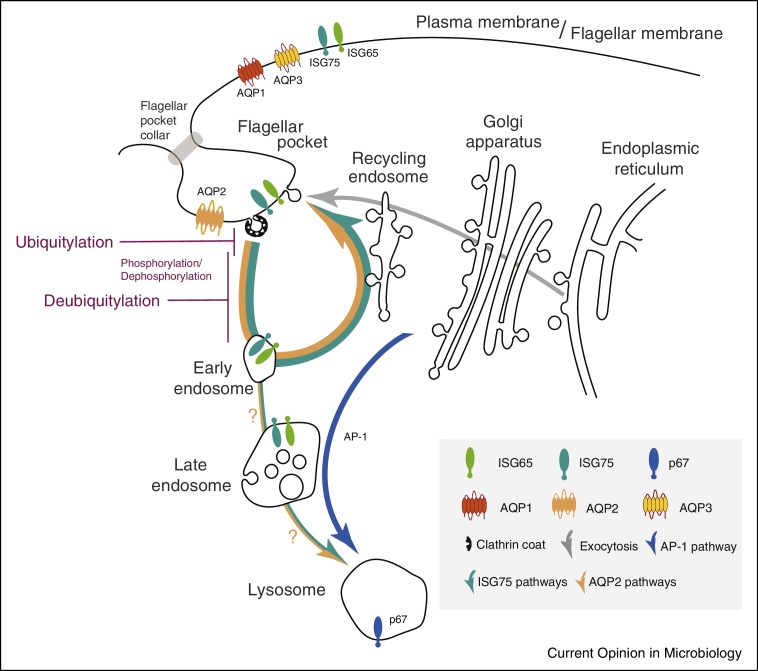
Figure 1.
The trypanosome endosomal system. A simplified schematic of the trypanosome endomembrane system is shown, with the flagellar pocket at top left. Teal and orange arrows indicate degradative and recycling trafficking routes, blue putative AP-1-mediated transport from the Golgi complex to the lysosome and gray exocytic/biosynthetic pathways. The predominant locations of ISG75, ISG65, aquaglyceroporins and p67 (the major lysosomal protein) are indicated by icons. Evidence suggests that ISG75 is ubiquitylated at, or close to the surface (magenta) and deubiquitylation by TbUsp7 and/or TbVdu1 is proposed to take place before the sorting step at the early endosome that selects for the recycling or degradative arm of the endocytic system. TbVdu1 is known to associate with structures in this region, whilst TbUsp7 is likely cytosolic. AQP2 localization is restricted to the flagellar pocket, while AQP1 and AQP3 are predominantly on the flagellar membrane and plasma membrane, respectively. AQP2 has been recently described as high-affinity pentamidine receptor and this raises the possibility of endocytotic uptake.
Together these observations are consistent with a hypothesis that ISG75 is the suramin receptor, but failure to demonstrate binding in vitro to recombinant ISG75 (unpublished data, MZ, MCF) suggests that additional factors may be involved. Suramin binds various serum proteins with high affinity, including Low Density Lipoprotein (LDL), and the influence of LDL on suramin uptake has led to a proposed model of an LDL-dependent receptor-mediated pathway for suramin internalization [29]. However, this was overturned by the mutation of trafficking pathway components [25]. Formal proof of receptor identity and precise mechanisms for suramin uptake remain elusive, but what is clear is the essential role for endocytosis and a protein with an itinerary that includes transport through the endosomal system. Significantly, the lysosomal proteases CatL and CBP1 are also implicated for suramin-sensitivity, and potentially these proteases are required for degradation of ISG75 to release suramin into the lysosome [30]. Notably, recent evidence indicates that CatL also degrades human serum trypanolytic factors and that a lysosomal inhibitor of a cysteine peptidase, modulates this protease activity [31•].
Despite these advances the mechanism of suramin toxicity remains elusive. Suramin exhibits considerable polypharmacology and can bind and inhibit a wide variety of distinct protein families (see e.g. [32, 33, 34]). Although suramin inhibits all seven trypanosome glycolytic enzymes plus cytosolic pyruvate kinase (PYK) in the micromolar range [35], it inhibits bloodstream stage trypanosome proliferation at nanomolar concentrations [26••], making it unlikely that glycolysis represents the primary target. However, endocytosis may accumulate the drug to higher intracellular levels than the external concentration; importantly the highly charged suramin molecule, once internalized, cannot easily escape from the cell. Again, this represents a remarkable impact from unique aspects of trypanosome cell biology in enabling specific toxicity, and is currently beyond the ability of rational drug design to predict or replicate. However, these features do indicate potential cellular aspects that are exploitable.
Old and still in the clinic: pentamidine and melarsoprol
Aromatic diamidine drugs including pentamidine, and organic arsenicals like melarsoprol, are highly cytotoxic to most cells they enter. Therefore toxicity is predominantly determined by uptake across the plasma membrane, and selectivity rests on the expression of efficient cell-surface transporters by the parasite. Conversely, mutations in such transporters diminishing transport rates or loss of substrate recognition can lead to drug resistance [36]. Pentamidine and melarsoprol, despite very different structures, exhibit cross-resistance in Trypanosoma brucei [37]. Since pentamidine and melarsoprol have distinct cellular targets, cross resistance may reside within the uptake mechanism. An unusual aminopurine transporter, TbAT1/P2, a member of the highly conserved Equilibrative Nucleoside Transporter family, facilitates uptake of both [37]. Deletion or mutation of the TbAT1/P2 gene reduces the sensitivity of T. brucei to diamidines, particularly diminazene, and melaminophenyl arsenicals [38, 39, 40, 41, 42].
Significantly, loss of TbAT1/P2 alone does not recreate the high levels of melarsoprol-pentamidine cross-resistance (MPXR) observed [39, 43]. T. brucei expresses additional transport systems for pentamidine and a genome wide RNAi screen identified the TbAQP2/AQP3 locus as the genetic determinant for MPXR [26••, 44•]. Further analyses revealed that aquaglyceroporin 2 (AQP2) determines MPXR [44•, 45•]. Further, re-introduction of a wild-type TbAQP2 allele in even the most resistant strains fully restores drug sensitivity [45•, 46••, 47•, 41]. Indeed, expressing TbAQP2 in Leishmania mexicana promastigotes profoundly increases their sensitivity to both pentamidine and melarsoprol [45•].
However, the translocation mechanism of TbAQP2 is less obvious as aquaglyceroporins are not known to allow passage of molecules of high molecular weight like melarsoprol or pentamidine. TbAQP2 does have a unique selectivity filter; several large, aromatic amino acids that normally restrict the pore are replaced by smaller side chain residues, and a key arginine is replaced with leucine [44•]. These changes are expected to enlarge the pore size and thus allow passage of cations, including the highly flexible pentamidine molecule [36]. However, an alternative model, in which AQP2 binds pentamidine and mediates internalization via receptor-mediated endocytosis, was recently proposed [48••]. This is an attractive alternative, marrying the implausibility of large-molecule transport by TbAQP2, an unusually high affinity for pentamidine, the high endocytosis of bloodstream trypanosomes [12•] and localization of TbAQP2 to the flagellar pocket [44•]. The flagellar pocket localization is consistent with uptake by endocytosis (Figure 1). Thus, uptake of both suramin and pentamidine, and potentially even melarsoprol, may depend upon their affinities for surface receptors, with subsequent membrane trafficking (Figure 2).
The genes identified through genome-wide screens [26••] for pentamidine/melarsoprol and suramin sensitivity do feature a significant overlap but this does not implicate common endocytic components. However, additional evidence from these screens is consistent with involvement of trafficking factors downstream of AQP2 [26••]. Indeed, for both pentamidine and melarsoprol, the screens identified proteins likely involved in phosphorylation (Phosphatase 2C) and ubiquitylation (Cullin1) (Figure 2), post-translational modifications implicated in regulating endocytosis of mammalian AQP [49]. This is reminiscent of the DUBs that sensitize trypanosomes to suramin and regulate ISG75 [19••]. However, if these proteins are involved in the promotion of AQP2 internalization and turnover, knockdown could change pentamidine sensitivity regardless of whether the drug binds the channel or translocates through it. Finally, the endocytosis model does not explain all previous observations on pentamidine uptake in T. brucei, such as a 10-fold higher rate of high-affinity pentamidine uptake in procyclic forms than in bloodstream forms [50] despite TbAQP2 distribution across the entire procyclic cell surface [44•], or the high uptake rate when TbAQP2 is expressed in Leishmania promastigotes [45•]. Both procyclic and promastigotes have much lower endocytosis than the bloodstream T. brucei [25, 51], and further study is essential to settle the mechanism of TbAQP2-mediated drug transport.
Perspectives
Serendipity will always be an important component of discovery, and drug uptake is a good exemplar. Recognising the specific binding properties of aniline dyes to fabrics in the early years of synthetic chemistry was rightly interpreted as representing specificity, and ultimately evolved into Ehrlich's concept of the ‘magic bullet’ that kills a pathogen but not the host. Ehrlich also reported on the activities of trypan blue and trypan red, two trypanocidal compounds leading to the development of suramin. The unique features of the flagellar pocket and high endocytic rate have long been considered as potential routes to therapy and demonstrations that innate immune mechanisms also rely on endocytic trafficking has strengthened this [52]. Many experimental interventions compromising endocytic trafficking lead to the rapid death of African trypanosomes [53], and it is rather a curious realisation that the properties of this system were being exploited by highly effective therapeutic agents developed almost a century ago. There is much scope for exploiting these drug uptake mechanisms to deliver new drugs, especially given the surprising variety of compounds that they internalize. Notably, drug-loaded nanoparticles, coated with a nanobody specifically targeting the trypanosome surface, can be used to bypass the usual delivery route altogether and deliver drugs via endocytosis [47•]. Indeed, this approach actually increases the efficacy of pentamidine, and a similar approach is likely to be effective for delivering other drugs. Harnessing these features represents attractive and tractable opportunities, as does direct screening for inhibitors of the novel components that control trafficking and surface protein transport. Finally, there are at least two components required for trypanocidal activity, entry and activity against a target; rather unexpectedly it is emerging that it is the former that may be more crucial for specificity, at least for current drugs.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Work in the authors’ laboratories on aspects of drug mode of action and cell biology are supported by the Medical Research Council (MR/K008749/1 to MCF, MR/K000500/1 to DH, 84733 to HPdK, and MR/L018853/1 to MCF and DH) and Wellcome Trust (105021/Z/14/Z to MCF and DH and 100320/Z/12/Z to DH).
References
- 1••.Jackson A.P., Otto T.D., Aslett M., Armstrong S.D., Bringaud F., Schlacht A., Hartley C., Sanders M., Wastling J.M., Dacks J.B. Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr Biol. 2016;26:161–172. doi: 10.1016/j.cub.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sequencing and annotation of a basal trypanosomatid genome. Comparisons between the B. saltans genome and other kinetoplastids uncovers several possible adaptive processes that facilitate the acquisition of parasitism.
- 2••.Manna P.T., Kelly S., Field M.C. Adaptin evolution in kinetoplastids and emergence of the variant surface glycoprotein coat in African trypanosomatids. Mol Phylogenet Evol. 2013;67:123–128. doi: 10.1016/j.ympev.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comparative genomics that reveals specific changes to the endocytic apparatus that are correlated with the evolution of antigenic variation and suggests that features of the trafficking apparatus are potentially involved in the origins of the specific immune evasion mechanisms and hence drug sensitivity for African trypanosomes.
- 3.Adl S.M., Simpson A.G., Lane C.E., Lukeš J., Bass D., Bowser S.S., Brown M.W., Burki F., Dunthorn M., Hampl V. The revised classification of eukaryotes. J Eukaryot Microbiol. 2012;59:429–493. doi: 10.1111/j.1550-7408.2012.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D.C., Lennard N.J., Caler E., Hamlin N.E., Haas B. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 5.Jackson A.P., Goyard S., Xia D., Foth B.J., Sanders M., Wastling J.M., Minoprio P., Berriman M. Global gene expression profiling through the complete life cycle of Trypanosoma vivax. PLoS Negl Trop Dis. 2015;9:e0003975. doi: 10.1371/journal.pntd.0003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Peña I., Pilar Manzano M., Cantizani J., Kessler A., Alonso-Padilla J., Bardera A.I., Alvarez E., Colmenarejo G., Cotillo I., Roquero I. New compound sets identified from high throughput phenotypic screening against three kinetoplastid parasites: an open resource. Sci Rep. 2015;5:8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]; Open resource emerging as the result of screening of hundreds of thousands of compounds against trypanosomatids.
- 7.Field M.C., Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 8.Lacomble S., Vaughan S., Gadelha C., Morphew M.K., Shaw M.K., McIntosh J.R., Gull K. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J Cell Sci. 2009;122:1081–1090. doi: 10.1242/jcs.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadelha C., Rothery S., Morphew M., McIntosh J.R., Severs N.J., Gull K. Membrane domains and flagellar pocket boundaries are influenced by the cytoskeleton in African trypanosomes. Proc Natl Acad Sci U S A. 2009;106:17425–17430. doi: 10.1073/pnas.0909289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung K.F., Dacks J.B., Field M.C. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic. 2008;9:1698–1716. doi: 10.1111/j.1600-0854.2008.00797.x. [DOI] [PubMed] [Google Scholar]
- 11.Silverman J.S., Muratore K.A., Bangs J.D. Characterization of the late endosomal ESCRT machinery in Trypanosoma brucei. Traffic. 2013;14:1078–1090. doi: 10.1111/tra.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Manna P.T., Gadelha C., Puttick A.E., Field M.C. ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis. J Cell Sci. 2015;128:2130–2142. doi: 10.1242/jcs.167726. [DOI] [PMC free article] [PubMed] [Google Scholar]; Expands the list of likely novel proteins involved in endocytosis in trypanosomes, and indicates the presence of novel molecular mechanisms.
- 13•.Gadelha C., Zhang W., Chamberlain J.W., Chait B.T., Wickstead B., Field M.C. Architecture of a host–parasite interface: complex targeting mechanisms revealed through proteomics. Mol Cell Proteomics. 2015;14:1911–1926. doi: 10.1074/mcp.M114.047647. [DOI] [PMC free article] [PubMed] [Google Scholar]; Described a much expanded set of surface proteins for African trypanosomes, as well as demonstrated the presence of microdomains and the high level of diversity of the surface proteome.
- 14.Shimogawa M.M., Saada E.A., Vashisht A.A., Barshop W.D., Wohlschlegel J.A., Hill K.L. Cell surface proteomics provides insight into stage-specific remodeling of the host–parasite interface in Trypanosoma brucei. Mol Cell Proteomics. 2015;14:1977–1988. doi: 10.1074/mcp.M114.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 16.Manna P.T., Boehm C., Leung K.F., Natesan S.K., Field M.C. Life and times: synthesis, trafficking, and evolution of VSG. Trends Parasitol. 2014;30:251–258. doi: 10.1016/j.pt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manna P.T., Field M.C. Phosphoinositides, kinases and adaptors coordinating endocytosis in Trypanosoma brucei. Commun Integr Biol. 2016;8:e1082691. doi: 10.1080/19420889.2015.1082691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adung’a V.O., Gadelha C., Field M.C. Proteomic analysis of clathrin interactions in trypanosomes reveals dynamic evolution of endocytosis. Traffic. 2013;14:440–457. doi: 10.1111/tra.12040. [DOI] [PubMed] [Google Scholar]
- 19••.Zoltner M., Leung K.F., Alsford S., Horn D., Field M.C. Modulation of the surface proteome through multiple ubiquitylation pathways in African Trypanosomes. PLoS Pathog. 2015;11:e1005236. doi: 10.1371/journal.ppat.1005236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of the presence of two pathways required for sensitivity to suramin in trypanosomes, both of which rely on intracellular transport. The first route involves uptake, mediated by invariant surface glycoproteins and the second is required for delivery of lysosomal proteins to their destination.
- 20.Tomavo S. Evolutionary repurposing of endosomal systems for apical organelle biogenesis in Toxoplasma gondii. Int J Parasitol. 2014;44:133–138. doi: 10.1016/j.ijpara.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 21•.Sangaré L.O., Alayi T.D., Westermann B., Hovasse A., Sindikubwabo F., Callebaut I., Werkmeister E., Lafont F., Slomianny C., Hakimi M.A. Unconventional endosome-like compartment and retromer complex in Toxoplasma gondii govern parasite integrity and host infection. Nat Commun. 2016;7:11191. doi: 10.1038/ncomms11191. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence for repurposing of compartments and trafficking routes in parasites — a superb example of the connections between selective pressures and modulation of intracellular functions.
- 22.Bassarak B., Uzcátegui N.L., Schönfeld C., Duszenko M. Functional characterization of three aquaglyceroporins from Trypanosoma brucei in osmoregulation and glycerol transport. Cell Physiol Biochem. 2011;27:411–420. doi: 10.1159/000327968. [DOI] [PubMed] [Google Scholar]
- 23.Travis A.S. Models for biological research: the theory and practice of Paul Ehrlich. History Philos Life Sci. 2008;30:79–97. [PubMed] [Google Scholar]
- 24.Steverding D. The development of drugs for treatment of sleeping sickness: a historical review. Parasit Vectors. 2010;3:15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal A., Hall B.S., Field M.C. Evidence for a non-LDL-mediated entry route for the trypanocidal drug suramin in Trypanosoma brucei. Mol Biochem Parasitol. 2002;122:217–221. doi: 10.1016/s0166-6851(02)00096-8. [DOI] [PubMed] [Google Scholar]
- 26••.Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F., Turner D.J., Field M.C., Berriman M., Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]; Genetic screens revealed networks of proteins, including endocytic components, involved in drug uptake in trypanosomes.
- 27.Peck R.F., Shiflett A.M., Schwartz K.J., McCann A., Hajduk S.L., Bangs J.D. The LAMP-like protein p67 plays an essential role in the lysosome of African trypanosomes. Mol Microbiol. 2008;68:933–946. doi: 10.1111/j.1365-2958.2008.06195.x. [DOI] [PubMed] [Google Scholar]
- 28.Leung K.F., Riley F.S., Carrington M., Field M.C. Ubiquitylation and developmental regulation of invariant surface protein expression in trypanosomes. Eukaryot Cell. 2011;10:916–931. doi: 10.1128/EC.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vansterkenburg E.L., Coppens I., Wilting J., Bos O.J., Fischer M.J., Janssen L.H., Opperdoes F.R. The uptake of the trypanocidal drug suramin in combination with low-density lipoproteins by Trypanosoma brucei and its possible mode of action. Acta Trop. 1993;54:237–250. doi: 10.1016/0001-706x(93)90096-t. [DOI] [PubMed] [Google Scholar]
- 30.Alsford S., Field M.C., Horn D. Receptor-mediated endocytosis for drug delivery in African trypanosomes: fulfilling Paul Ehrlich's vision of chemotherapy. Trends Parasitol. 2013;29:207–212. doi: 10.1016/j.pt.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 31•.Alsford S., Currier R.B., Guerra-Assunção J.A., Clark T.G., Horn D. Cathepsin-L can resist lysis by human serum in Trypanosoma brucei brucei. PLoS Pathog. 2014;15:e1004130. doi: 10.1371/journal.ppat.1004130. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reveals lysosomal protein–protein interactions determining sensitivity to human serum trypanolytic factors and highlights the importance of lysosomal proteases for toxin delivery and stability in African trypanosomes.
- 32.Wu K., Chong R.A., Yu Q., Bai J., Spratt D.E., Ching K., Lee C., Miao H., Tappin I., Hurwitz J. Suramin inhibits cullin-RING E3 ubiquitin ligases. Proc Natl Acad Sci U S A. 2016;113:2011–2018. doi: 10.1073/pnas.1601089113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvador G.H., Dreyer T.R., Cavalcante W.L., Matioli F.F., Dos Santos J.I., Velazquez-Campoy A., Gallacci M., Fontes M.R. Structural and functional evidence for membrane docking and disruption sites on phospholipase A2-like proteins revealed by complexation with the inhibitor suramin. Acta Crystallogr D Biol Crystallogr. 2015;71:2066–2078. doi: 10.1107/S1399004715014443. [DOI] [PubMed] [Google Scholar]
- 34.Opoku-Temeng C., Sintim H.O. Potent inhibition of cyclic diadenylate monophosphate cyclase by the antiparasitic drug, suramin. Chem Commun (Camb) 2016;52:3754–3757. doi: 10.1039/c5cc10446g. [DOI] [PubMed] [Google Scholar]
- 35.Morgan H.P., McNae I.W., Nowicki M.W., Zhong W., Michels P.A., Auld D.S., Fothergill-Gilmore L.A., Walkinshaw M.D. The trypanocidal drug suramin and other trypan blue mimetics are inhibitors of pyruvate kinases and bind to the adenosine site. J Biol Chem. 2011;286:31232–31240. doi: 10.1074/jbc.M110.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munday J.C., Settimo L., De Koning H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Frontiers Pharmacol. 2015;6:32. doi: 10.3389/fphar.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker N., De Koning H.P., Mäser P., Horn D. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 2013;29:110–118. doi: 10.1016/j.pt.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mäser P., Sütterlin C., Kralli A., Kaminsky R. A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science. 1999;285:242–244. doi: 10.1126/science.285.5425.242. [DOI] [PubMed] [Google Scholar]
- 39.Matovu E., Stewart M., Geiser F., Brun R., Mäser P., Wallace L.J.M., Burchmore R.J., Enyaru J.C.K., Barrett M.P., Kaminsky R. The mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matovu E., Geiser F., Schneider V., Mäser P., Enyaru J.C., Kaminsky R., Gallati S., Seebeck T. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol Biochem Parasitol. 2001;117:73–81. doi: 10.1016/s0166-6851(01)00332-2. [DOI] [PubMed] [Google Scholar]
- 41.Graf F.E., Ludin P., Arquint C., Schmidt R.S., Schaub N., Kunz-Renggli C., Munday J.C., Krezdorn J., Baker N., Horn D. Comparative genomics of drug resistance of the sleeping sickness parasite Trypanosoma brucei rhodesiense. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munday J.C., Tagoe D.N.A., Eze A.A., Krezdorn J.A., Rojas López K.E., Alkhaldi A.A.M., McDonald F., Still J., Alzahrani K.J., Settimo L. Functional analysis of drug resistance-1 associated mutations in the Trypanosoma brucei Adenosine Transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol Microbiol. 2015;96:887–900. doi: 10.1111/mmi.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bridges D., Gould M.K., Nerima B., Mäser P., Burchmore R.J.S., De Koning H.P. Loss of the high affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol. 2007;71:1098–1108. doi: 10.1124/mol.106.031351. [DOI] [PubMed] [Google Scholar]
- 44•.Baker N., Glover L., Munday J.C., Aguinaga Andrés D., Barrett M.P., De Koning H.P., Horn D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc Natl Acad Sci USA. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated a role for AQP2, and not AQP3, in melarsoprol and pentamidine uptake. Also that AQP2 is concentrated in the trypanosome flagellar pocket.
- 45•.Munday J.C., Eze A.A., Baker N., Glover L., Clucas C., Aguinaga Andrés D., Natto M.J., Teka I.A., McDonald J., Lee R.S. Trypanosoma brucei Aquaglyceroporin 2 is a high affinity transporter for pentamidine and melaminophenyl arsenic drugs and is the main genetic determinant of resistance to these drugs. J Antimicrob Chemother. 2014;69:651–663. doi: 10.1093/jac/dkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]; Establishes AQP2 as a crucial drug transporter in trypanosomes, mediating high-affinity uptake of pentamidine and melaminophenyl arsenicals.
- 46••.Graf F.E., Baker N., Munday J.C., De Koning H.P., Horn D., Mäser P. Chimerization at the AQP2-AQP3 locus is the genetic basis of melarsoprol-pentamidine cross-resistance in clinical Trypanosoma brucei gambiense isolates. Int J Parasitol Drugs Drug Res. 2015;5:65–68. doi: 10.1016/j.ijpddr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Expression of an intact AQP2 gene in a drug-resistant field-isolate fully restores drug-susceptibility.
- 47•.Unciti-Broceta J.D., Arias J.L., Maceira J., Soriano M., Ortiz-González M., Hernández-Quero J., Muñóz-Torres M., de Koning H.P., Magez S., Garcia-Salcedo J.A. Specific cell targeting therapy bypasses drug resistance mechanisms in African trypanosomiasis. PloS Pathog. 2015;11:e1004942. doi: 10.1371/journal.ppat.1004942. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drug-loaded nanoparticles, coated with single domain antibody targeting surface proteins are used to demonstrate the feasibility of drug delivery via endocytosis circumventing the usual delivery routes.
- 48••.Song J., Baker N., Rothert M., Henke B., Jeacock L., Horn D., Beitz E. Pentamidine is not a permeant but a nanomolar inhibitor of the Trypanosoma brucei Aquaglyceroporin-2. PLoS Pathog. 2016;12:e1005436. doi: 10.1371/journal.ppat.1005436. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pentamidine tightly binds and inhibits AQP2. This suggests an alternative to uptake by passing through the AQP channel that may involve endocytosis.
- 49.Moeller H.B., Aroankins T.S., Slengerik-Hansen J., Pisitkun T., Fenton R.A. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J Cell Sci. 2014;127:3174–3183. doi: 10.1242/jcs.150680. [DOI] [PubMed] [Google Scholar]
- 50.Teka I.A., Kazibwe A., El-Sabbagh N., Al-Salabi M.I., Ward C.P., Eze A.A., Munday J.C., Mäser P., Matovu E., Barrett M.P. The diamidine diminazene aceturate is a substrate for the High Affinity Pentamidine Transporter: implications for the development of high resistance levels. Mol Pharmacol. 2011;80:110–116. doi: 10.1124/mol.111.071555. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali H.Z., Harding C.R., Denny P.W. Endocytosis and sphingolipid scavenging in Leishmania mexicana Amastigotes. Biochem Res Int. 2012;2012:691363. doi: 10.1155/2012/691363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecordier L., Uzureau P., Tebabi P., Pérez-Morga D., Nolan D., Schumann Burkard G., Roditi I., Pays E. Identification of Trypanosoma brucei components involved in trypanolysis by normal human serum. Mol Microbiol. 2014;94:625–636. doi: 10.1111/mmi.12783. [DOI] [PubMed] [Google Scholar]
- 53.Stijlemans B., Caljon G., Natesan S.K., Saerens D., Conrath K., Pérez-Morga D., Skepper J.N., Nikolaou A., Brys L., Pays E. High affinity nanobodies against the Trypanosome brucei VSG are potent trypanolytic agents that block endocytosis. PLoS Pathog. 2011;7:e1002072. doi: 10.1371/journal.ppat.1002072. [DOI] [PMC free article] [PubMed] [Google Scholar]