Abstract
Adults with type 2 diabetes (T2DM) and low socioeconomic status (SES) have high rates of medication nonadherence, and, in turn, suboptimal glycemic control (hemoglobin A1c [HbA1c]). We tested the initial efficacy of a short message service (SMS) text messaging and interactive voice response (IVR) intervention to promote adherence among this high-risk group. Eighty low SES, diverse adults with T2DM used the MEssaging for Diabetes (MED) SMS/IVR intervention for three months. We used a pre-post single group design to explore adherence changes over three months, and a quasi-experimental design to test the impact of MED on HbA1c among the intervention group relative to a matched, archival control group. Compared to baseline, adherence improved at one (AOR: 3.88, 95% CI: 1.79, 10.86) and at two months (AOR: 3.76, 95% CI: 1.75, 17.44), but not at three months. HbA1c remained stable, with no differences at three months between the intervention group and the control group. MED had a positive, short-term impact on adherence, which did not translate to improvements in HbA1c. Future research should explore the longer-term impact of SMS/IVR interventions on the medication adherence of high risk adults with T2DM.
Keywords: diabetes, medication adherence, vulnerable populations, behavioral interventions, mobile health, health promotion
Among adults with type 2 diabetes (T2DM), medication nonadherence is common (Kirkman et al., 2015), and associated with suboptimal glycemic control (Aikens & Piette, 2013). In the U.S., racial/ethnic minorities and/or persons with low socioeconomic status (SES) have particularly high rates of medication nonadherence and suboptimal glycemic control compared to persons who are non-Hispanic White or have higher SES (Adams et al., 2015). However, few interventions have targeted adherence to improve the glycemic control of low SES, non-Whites with T2DM (Adams et al., 2015).
Leveraging the functionality shared by all cell phones can improve the health of low SES populations (McInnes et al., 2013). Approximately 90% of U.S. adults use cell phones (Duggan, 2013); however, adults with diabetes are less likely to use smartphones, especially those with low SES (Blondon et al., 2014). Short message service (SMS) text messaging and voice communications are not phone-type or operating system dependent and are used equally across racial/ethnic and SES groups (Duggan, 2013), making them accessible to the widest audience.
We designed (Osborn & Mulvaney, 2013) and evaluated the MEssaging for Diabetes (MED) SMS and interactive voice response (IVR) intervention to promote medication adherence among low SES, diverse adults with T2DM. We used a pre-post single group design to explore medication adherence changes over three months, and used a quasi-experimental design to test the impact of MED on glycemic control among the intervention group relative to a matched, archival control group from the same clinic.
Methods
MED Intervention
The MED intervention uses the SuperEgo mobile communications platform (Mulvaney et al., 2012) to deliver and tailor text messages and voice communications to promote medication adherence (Nelson et al., 2015; Osborn & Mulvaney, 2013). MED addresses users’ unique barriers to adherence. We used the research literature to compile a comprehensive list of 34 adherence barriers, and had diabetes experts iteratively review and reduce this list to 17 barriers. These same experts developed text messages addressing each barrier. During enrollment, we assessed each user’s barriers and then sent tailored text messages addressing his/her three highest ranked barriers (see Measures).The majority of barriers assessed in MED map onto psychological constructs. For example, an attitudinal/belief barrier is “believing medication(s) are not important”; a text message addressing this barrier is: “It’s important that you take your diabetes medications as prescribed. Doing so might help you live longer.” An example of a self-efficacy-related barrier is “forgetfulness” and a corresponding text message is: “If you have a hard time remembering to take your diabetes medications, try linking when you take your medications to other activities you do every day.” A perceived social support barrier is “a lack of social support” and a text message addressing this is: “Everyone needs someone to talk to about their diabetes. How can your family or friends help you with your diabetes? Let them know.” For the full list of 17 barriers assessed in MED and corresponding example text messages, see Osborn and Mulvaney (2013).
In summary, MED delivers three elements: (1) a daily, one-way text message addressing user-specific adherence barriers (described above); (2) a daily, two-way text message asking the user if he/she took all of his/her diabetes medication that day (requests a “yes” or “no” response); and (3) a weekly IVR call. The IVR call provides aggregated adherence feedback based on responses to the daily, two-way text message, a motivational message, and problem solving questions to facilitate future adherence success. Daily one-way texts are sent at a varied time each day, daily two-way texts are sent at the user’s bedtime, and weekly IVR calls are sent at a time specified by the user.
Participants
We used a combination of advertisements and assistance from clinic staff to recruit patients who were at least 18 years of age, English-speaking, diagnosed with T2DM, prescribed diabetes medication(s), and had a cell phone with SMS capabilities. Exclusion criteria included a pre-existing diagnosis of dementia, auditory limitations precluding IVR participation, and the inability to see and respond to text messages. The Vanderbilt University Institutional Review Board approved all study procedures prior to enrollment.
Procedures
At baseline, trained research assistants (RAs) verbally administered self-report items and response options to collect participants’ age, race/ethnicity, insulin status, years since a diabetes diagnosis, responses to items assessing 17 barriers to medication adherence, and responses to the Summary of Diabetes Self-Care Activities medications subscale (SDSCA-MS), (Toobert et al., 2000). RAs entered barrier responses into SuperEgo’s web-based form to identify a user’s top three barriers for delivering daily, one-way text messages to overcome these barriers. A clinic phlebotomist performed a blood-drawn hemoglobin A1c (HbA1c) test to assess participants’ glycemic control.
Participants received MED for three months. RAs called participants at one and two months to re-administer the SDSCA-MS and items assessing adherence barriers to account for previous barriers being reduced, and new barriers being present, prompting the delivery of new tailored text messages to address newly-identified barriers.
At three months, participants returned to the clinic to complete self-report measures and a second blood-drawn HbA1c test. Participants received up to US$130 compensation: $10 for completing the baseline asssesment, $15 for each of the telephone assessments (one month and two months), $30 for the three-month assessment, and $60 for responding to daily, two-way text messages ($1/response for up to 5 responses/week). Finally, we used the clinic’s electronic medical record to match each MED participant with two archival controls at baseline (2:1 ratio, based on race, gender, and baseline glycemic control), gathering these patients’ recorded HbA1c data at three months to compare changes in glycemic control between groups.
Measures
Demographic and clinical characteristics
were assessed at baseline. We collected self-reported age, gender, race, ethnicity, income, education (i.e., years in school), insurance status, insulin status, and diabetes duration (i.e., years since a diabetes diagnosis).
Barriers to medication adherence
were assessed at baseline and at one, two, and three month(s) using items from the Diabetes Medication Knowledge Questionnaire (McPherson et al., 2008), the Medicines for Diabetes Questionnaire (Farmer et al., 2006), the Barriers to Diabetes Adherence measure (Mulvaney et al., 2011), and the Medication Adherence Self-Efficacy Scale (Ogedegbe et al., 2003). Barriers were tagged to one or more items on each scale and responses to items were standardized to create scoring consistency. Barrier scores were then ranked and users received tailored one-way text messages addressing their three highest ranked barriers.
Engagement
was assessed with users’ responses to daily, two-way text messages. We calculated engagement by dividing each user’s number of responses to two-way text messages by the total number of text messages sent.
Medication adherence
was assessed at baseline and at one, two, and three month(s) using the SDSCA-MS (Toobert et al., 2000). The SDSCA-MS consists of 2 items: 1) “On how many of the last seven days did you take your medication?” and 2) “On how many of the last seven days did you take the correct number of (pills/injections) for your medication?” Response options range from 0–7, with higher scores indicating better adherence. We averaged responses to both items to create a total score for each of the four assessment points. We defined people scoring <7 as suboptimally adherent. The SDSCA-MS is a valid and reliable measure of medication adherence and is associated with glycemic control (Mayberry et al., 2013; Toobert et al., 2000).
Glycemic control
(HbA1c) was assessed at baseline and at three months using a blood-drawn lab test. An HbA1c test provides information about a person’s average levels of blood glucose over the past three months. For a person diagnosed with diabetes, a level <7% is considered optimal, whereas ≥7% is considered suboptimal.
Statistical Analysis
First, we calculated descriptive statistics with median and interquartile ranges (IQR) or mean and standard deviations for continuous variables, and frequency and proportions for categorical variables. We used locally weighted scatterplot smoothing (LOWESS) to graphically visualize our SDSCA-MS data over time. To assess changes in adherence among MED participants, we used a proportional odds logistic regression model for the ordinal SDSCA-MS with correction for clustering (repeated measures) via bootstrapped confidence intervals. Regression models included all available observations at each time point (i.e., n=80 at baseline, n=47 at one month, n=24 at two months, and n=52 at three months). We also performed a pooled logistic regression model to explore whether baseline covariates (i.e., age, gender, race, education, income, insurance status, diabetes duration, and insulin status) and time point were associated with missingness (or attrition) of SDSCA-MS data (yes vs no) across the intervention. Diabetes duration was not included in the final model because of collinearity with age.
In an exploratory analysis, a Spearman rank correlation coefficient examined the relationship between text message engagement and changes in medication adherence (i.e., the slope of the SDSCA-MS calculated for each participant with data from two or more time points).
Next, we used a two-arm intervention vs. matched control design to explore the initial efficacy of MED on HbA1c. We used complete case analysis restricted to MED participants with three-month HbA1c data (n=52) and their archival matched pairs. In unadjusted analysis, we used Wilcoxon rank sum tests to compare HbA1c change from baseline to three months between groups. Among matched pairs, we used analysis of covariance (ANCOVA) to compare three-month HbA1c adjusted for baseline HbA1c. Using all available longitudinal data we performed a multivariable generalized least squares model accounting for correlated data to assess whether HbA1c slopes differed by condition. We included condition and time (baseline and three months) as a cross-product and adjusted for a priori age, gender, race and insulin use. HbA1c was log-transformed to meet linear regression assumptions.
Results
Sample Characteristics
We previously reported sample characteristics for the 80 participants who received MED (Nelson et al., 2015). Participants were, on average, 50.1 ± 10.5 years old and 69% non-White. Nearly two-thirds (70.1%) had annual household incomes less than $20K and 20% had less than a high school degree. At baseline, the average SDSCA-MS was 6.1 ± 1.2, and the average HbA1c was 67 mmol/mol (8.3 ± 2.0%). Table 1 reports characteristics by condition.
Table 1.
Patient characteristics by conditiona
| Variable | Matched Control (n = 160)b |
Intervention (n = 80) |
|---|---|---|
| Age, years | 55.3 ± 12.3 | 50.1 ± 10.5 |
| Gender | ||
| Male | 71 (44.4) | 26 (32.5) |
| Female | 89 (55.6) | 54 (67.5) |
| Race | ||
| Caucasian/White | 49 (31.6) | 25 (31.3) |
| Non-Caucasian/Non-White | 106 (68.4) | 55 (68.8) |
| Education, years | 12.94 ± 2.29 | |
| < High school graduate | N/A | 16 (20) |
| High school graduate | N/A | 29 (36.3) |
| > High school graduate | N/A | 35 (43.8) |
| Annual Household Income (in US$) | ||
| <10,000 | N/A | 29 (36.3) |
| 10,000–20,000 | N/A | 27 (33.8) |
| >20,000 | N/A | 24 (30) |
| Insurance status | ||
| Private | N/A | 14 (17.5) |
| Public | N/A | 38 (47.5) |
| None | N/A | 28 (35) |
| Diabetes duration, years | N/A | 7.5 [5 , 13] |
| Insulin status, taking insulin | 64 (40) | 29 (36.3) |
| SDSCA-MS | ||
| Baseline | N/A | 6.1 ± 1.2 |
| 1-month | N/A | 6.5 ± 1.4 |
| 2-month | N/A | 6.8 ± 0.4 |
| 3-month | N/A | 6.2 ± 1.3 |
| Glycemic control (HbA1c, %) | ||
| Baseline | 67 mmol/mol (8.3±2.0%) | 67 mmol/mol (8.3±2.0%) |
| 3-month | 63 mmol/mol (8.0±1.9%) | 64 mmol/mol (8.0±2.0%)c |
| Δ HbA1c | −0.291 ± 1.418 | 0.044 ± 1.252 |
Note. SDSCA-MS, Summary of Diabetes Self-Care Activities - Medications Subscale; HbA1c, Hemoglobin A1c
Mean and ± Standard deviations, Median and interquartiles ranges (IQR), or frequency number and percent are reported
Data on matched control participants is limited to data available in the medical record.
52 participants completed 3-month follow-up, and their baseline was 8.0 ± 1.9%
Outcomes
Medication adherence
Fig. 1 displays SDSCA-MS data by month using LOWESS. Compared to baseline, medication adherence improved at one (adjusted odds ratio [AOR]: 3.88, 95%CI: 1.79, 10.86) and two months (AOR: 3.76, 95%CI: 1.75, 17.44), but not at three months (AOR: 1.49, 95%CI: 0.66, 3.10); see Figure 1). In logistic regression analysis examining missingness of SDSCA-MS data, gender, race, education, income, insurance status, and insulin use were not significantly associated with missing data. Participant age (p=0.04 overall and p=−0.01 for non-linearity) and time point (p<0.001) post-baseline were statistically significant. Younger and older participants were less likely to provide SDSCA-MS data across time points, and participants in general were less likely to provide data at two months compared to both one month (AOR: 3.91, 95%CI: 2.15, 7.10) and three months (AOR: 5.29, 95%CI: 2.81, 9.97). In exploratory analyses, responding to text messages was marginally correlated with improved adherence (rho=0.23, p=0.07).
Fig. 1.
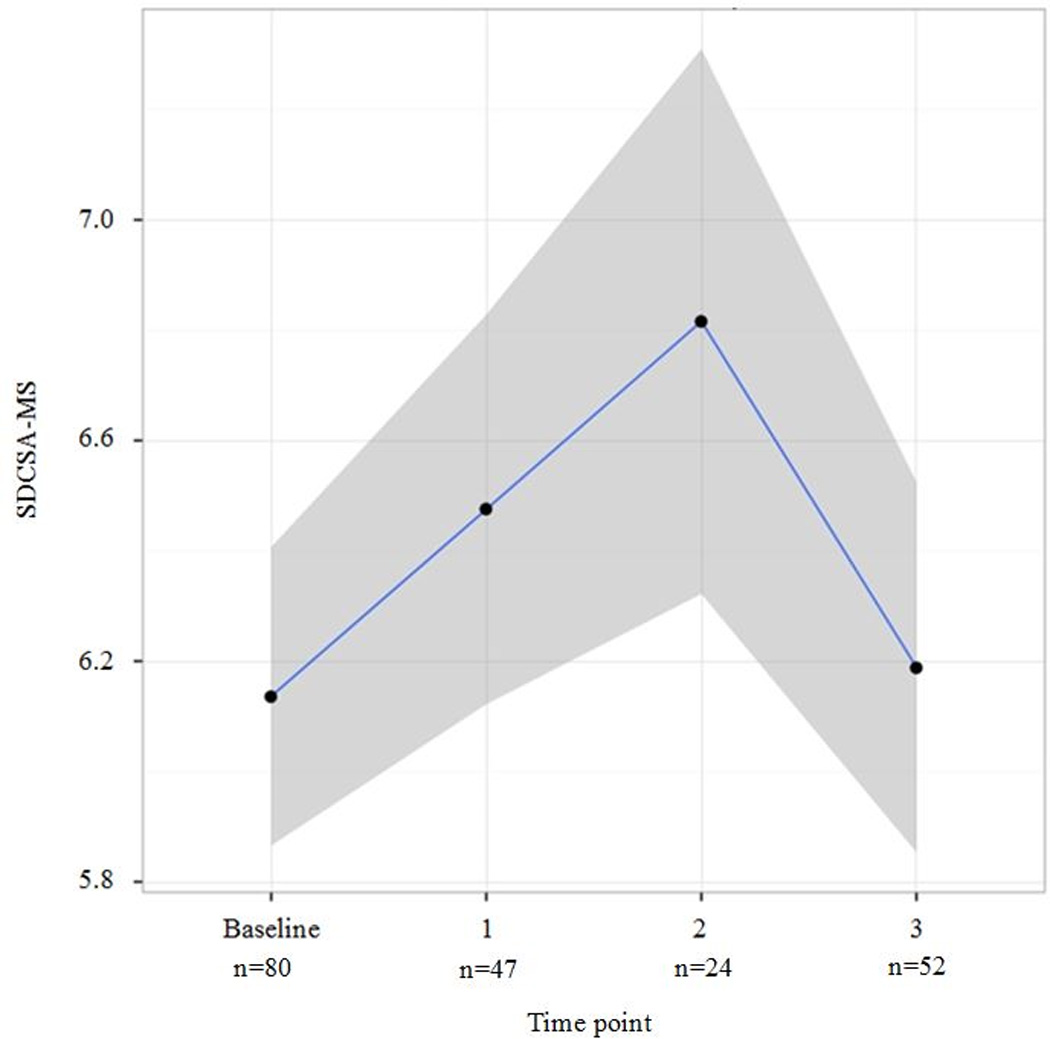
LOWESS Smoothed estimatingof SDSCA-MS over time point measured. To depict the data, we used non-parametric LOWESS (locally weighted scatterplot smoothing) smoothed fit curve with 95% confidence interval region (grey bands). SDSCA-MS, Summary of Diabetes Self-Care Activities- medications subscale
Glycemic control
The MED (n=52) and matched control groups (n=104) had similar, stable HbA1c change at three months (deltaintervention Median: 0.0 [IQR: −0.40, 0.60] vs. deltacontrol Median: −0.10, [IQR: −0.50, 0.40], p=.40). The intervention median HbA1c was 57 mmol/mol (7.4%) [IQR: 50 mmol/mol (6.7%), 73 mmol/mol (8.8%)] vs. the control median HbA1c which was 55 mmol/mol (7.2%), [IQR: 46 mmol/mol (6.4%), 75 mmol/mol (9.0%)], p=.44. Using ANCOVA adjusted for baseline HbA1c and covariates, three-month HbA1c did not differ between groups (p=0.416). Using all available data for longitudinal analysis, there was no significant slope difference in HbA1c between groups (p value for interaction = 0.218).
Discussion
We tested the initial efficacy of the MED tailored, SMS/IVR-delivered intervention among low SES, diverse adults with T2DM. Medication adherence improved at one and two months post-baseline, but did not persist to three months, and engagement with MED was marginally associated with improved adherence. However, glycemic control remained stable at three months and did not differ from matched, archival controls.
Few medication adherence interventions improve HbA1c (Sapkota et al., 2015), which might be due to their short duration (Sapkota et al., 2015). Short-term adherence improvements are unlikely to be fully incorporated in a glycemic marker assessing three-month average blood glucose levels. Therefore, SMS intervention studies examining longer-term effects are needed.
Although we observed improved adherence at one and two months, effects did not persist to three months. This may be because adherence was high at baseline (6.1 ± 1.2 days per week), leaving little room for ongoing improvement. Future studies should include patients whose adherence is below the recommended 80% threshold (Karve et al., 2009; Lau & Nau, 2004), and are more likely to benefit from the intervention.
Greater engagement with a technology-based intervention is associated with greater benefit (Glasgow et al., 2011; Katz et al., 2012). Engagement with MED via responding to text messages was marginally associated with improved adherence. While we examined the relationship between each participant’s overall rate of responding to text messages with his/her medication adherence slope from at least two to four timepoints (i.e., depending on available data), future work should examine how changes in intervention engagement impacts changes in outcomes over the course of a trial. Moreover, identifying what factors impact engagement will allow for designing interventions that have the broadest appeal and value (Holtz & Lauckner, 2012). For example, we recently reviewed studies reporting on engagement with technology-delivered interventions for adults with T2DM (Nelson et al., 2016). Interventions that either tailored content to users’ needs or used strategies to promote engagement (i.e., prompts and reminders to use the intervention) had better user engagement, whereas usability problems and the inability to afford a technology were barriers to engagement (Nelson et al., 2016).
There are study limitations to acknowledge. Attrition at follow-up was problematic and may have impacted our findings. Retention in intervention trials is challenging, and even more so with low SES populations (Katz et al., 2012). We did not employ a true control condition or random assignment, precluding causal inference about the effect of MED on short-term adherence. Additionally, although younger and older participants were less likely to provide SDSCA-MS data across time points, and attrition was highest at the two-month assessment, we cannot infer whether missingness affects our intervention effect without a control group. Future studies with a control group should consider using analytical methods dealing with missing follow-up data (Harrell, 2015) and implement strategies to avoid missing data. In MED, doubling compensation at the final assessment relative to the one- and two-month assessments may explain participants’ increased return at three months. Using incremental increases in compensation at each follow-up assessment may help minimize attrition over time. Finally, we recruited low SES, diverse adults with T2DM from a single clinic, limiting the generalizability of our findings to other populations.
Leveraging the technology available to the widest audience has far-reaching potential. Our findings provide preliminary support for using SMS and IVR to improve medication adherence among low SES, diverse adults with T2DM.
Acknowledgments
This research was supported by a McKesson Foundation mHealth Award to Drs. Osborn and Mulvaney. Dr. Osborn was also supported by a career development award NIH/NIDDK K01-DK087894 and the Vanderbilt Center for Diabetes Translational Research P30-DK092986. Drs. Osborn and Nelson were both supported by NIH/NIDDK R01-DK100694. Dr. Nelson oversaw analyses and wrote the manuscript. Dr. Mulvaney co-designed the study, oversaw data collection, administered the intervention platform, and edited the manuscript. Mrs. Gebretsadik conducted analyses and edited the manuscript. Dr. Johnson edited the manuscript. Dr. Osborn co-designed the study, oversaw data collection and analyses, and co-wrote and edited the manuscript. The authors thank Cecilia C. Quintero and Dr. Lindsay S. Mayberry for their role in data collection, and the Vine Hill Community Clinic personnel and the participants for their contributions to this research.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the funding entities.
Footnotes
The authors have no conflict of interests or financial disclosures to report.
Contributor Information
Lyndsay A. Nelson, Department of Medicine, Center for Health Behavior and Health Education, Vanderbilt University Medical Center
Shelagh A. Mulvaney, School of Nursing, Center for Diabetes Translational Research, Department of Biomedical Informatics, Department of Pediatrics, Vanderbilt University Medical Center
Tebeb Gebretsadik, Department of Biostatistics, Vanderbilt University Medical Center.
Kevin B. Johnson, Department of Biomedical Informatics, Vanderbilt University Medical Center
Chandra Y. Osborn, Department of Medicine, Center for Health Behavior and Health Education, Center for Diabetes Translational Research, Department of Biomedical Informatics, Vanderbilt University Medical Center
References
- Adams AS, Banerjee S, Ku CJ. Medication adherence and racial differences in diabetes in the USA: An update. Diabetes Manag (Lond) 2015;5(2):79–87. [Google Scholar]
- Aikens JE, Piette JD. Longitudinal association between medication adherence and glycaemic control in Type 2 diabetes. Diabetic Medicine. 2013;30(3):338–344. doi: 10.1111/dme.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondon KS, Hebert PL, Ralston JD. An Exploration of the Potential Reach of Smartphones in Diabetes; Paper presented at the AMIA Annual Symposium Proceedings; 2014. [PMC free article] [PubMed] [Google Scholar]
- Duggan M. Pew Research Center’s Internet & American Life Project. Washington, D.C.: Pew Research Center; 2013. Cell phone activities 2013. [Google Scholar]
- Farmer A, Kinmonth AL, Sutton S. Measuring beliefs about taking hypoglycaemic medication among people with Type 2 diabetes. Diabetic Medicine. 2006;23(3):265–270. doi: 10.1111/j.1464-5491.2005.01778.x. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Christiansen SM, Kurz D, King DK, Woolley T, Faber AJ, Dickman J. Engagement in a diabetes self-management website: usage patterns and generalizability of program use. Journal of Medical Internet Research. 2011;13(1):e9. doi: 10.2196/jmir.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd. Springer; 2015. [Google Scholar]
- Holtz B, Lauckner C. Diabetes management via mobile phones: a systematic review. Telemedicine and e-health. 2012;18(3):175–184. doi: 10.1089/tmj.2011.0119. [DOI] [PubMed] [Google Scholar]
- Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi: 10.1185/03007990903126833. [DOI] [PubMed] [Google Scholar]
- Katz R, Mesfin T, Barr K. Lessons from a community-based mHealth diabetes self-management program: "it's not just about the cell phone". Journal of Health Communication. 2012;(17 Suppl 1):67–72. doi: 10.1080/10810730.2012.650613. [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Rowan-Martin MT, Levin R, Fonseca VA, Schmittdiel JA, Herman WH, Aubert RE. Determinants of adherence to diabetes medications: Findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604–609. doi: 10.2337/dc14-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27(9):2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102(2):96–104. doi: 10.1016/j.diabres.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes DK, Li AE, Hogan TP. Opportunities for engaging low-income, vulnerable populations in health care: a systematic review of homeless persons’ access to and use of information technologies. American Journal of Public Health. 2013;103(S2):e11–e24. doi: 10.2105/AJPH.2013.301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson ML, Smith SW, Powers A, Zuckerman IH. Association between diabetes patients' knowledge about medications and their blood glucose control. Research in Social and Administrative Pharmacy. 2008;4(1):37–45. doi: 10.1016/j.sapharm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. Journal of Telemedicine and Telecare. 2012;18(2):115–118. doi: 10.1258/jtt.2011.111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvaney SA, Hood KK, Schlundt DG, Osborn CY, Johnson KB, Rothman RL, Wallston KA. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract. 2011;94(1):77–83. doi: 10.1016/j.diabres.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LA, Coston TD, Cherrington AL, Osborn CY. Patterns of user engagement with mobile- and web-delivered self-care interventions for adults with T2DM: A review of the literature. Current Diabetes Reports. 2016;16(7):66. doi: 10.1007/s11892-016-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type 2 diabetes. Journal of the American Medical Informatics Association. 2015 doi: 10.1093/jamia/ocv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogedegbe G, Mancuso CA, Allegrante JP, Carlson ME. Development and evaluation of a medication adherence self-efficacy scale in hyptertensive African-American patients. Journal of Clinical Epidemiology. 2003;56(6):520–529. doi: 10.1016/s0895-4356(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Osborn CY, Mulvaney SA. Development and feasibility of a text messaging and interactive voice response intervention for low-income, diverse adults with type 2 diabetes mellitus. Journal of Diabetes Science and Technology. 2013;7(3):612–622. doi: 10.1177/193229681300700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota S, Brien JA, Greenfield J, Aslani P. A systematic review of interventions addressing adherence to anti-diabetic medications in patients with type 2 diabetes--impact on adherence. PLoS One. 2015;10(2):e0118296. doi: 10.1371/journal.pone.0118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]


