Abstract
Rationale
Chronic kidney disease (CKD) patients develop hyperhomocysteinemia (HHcy) and have a higher cardiovascular mortality than those without HHcy by 10-fold.
Objective
We investigated monocyte (MC) differentiation in human CKD and cardiovascular disease (CVD).
Methods and Results
We identified CD40 as a CKD-related MC activation gene using CKD-MC-mRNA array analysis and classified CD40 MC (CD40+CD14+) as a stronger inflammatory subset than the intermediate MC (CD14++CD16+) subset. We recruited 27 CVD/CKD patients and 14 healthy subjects and found that CD40/CD40 classical/CD40 intermediate MC (CD40+CD14+/CD40+CD14++CD16−/CD40+CD14++CD16+), plasma homocysteine (Hcy), S-adenosylhomocysteine (SAH) and S-adenosylmethionine (SAM) levels were higher in CVD and further elevated in CVD+CKD. CD40 and CD40 intermediate subsets were positively correlated with plasma/cellular Hcy levels and SAH and SAM but negatively correlated with estimated glomerular filtration rate (eGFR). HHcy was established as a likely mediator for CKD-induced CD40 intermediate MC, and reduced SAH/SAM was established for CKD-induced CD40/CD40 intermediate MC. Soluble CD40 ligand (sCD40L), TNFα/IL-6/IFNγ levels were elevated in CVD/CKD. CKD serum/Hcy/CD40L/TNFα/IL-6/IFNγ-induced CD40/CD40 intermediate MC in PBMC. Hcy and CKD serum-induced CD40 MC were prevented by neutralizing antibodies against CD40L/TNFα/IL-6. DNA hypomethylation was found on NFκB consensus element in CD40 promoter in WBC from CKD patients with lower SAM/SAH ratios. Finally, Hcy inhibited DNA methyltransferase-1 activity and promoted CD40 intermediate MC differentiation which was reversed by folic acid in PBMC.
Conclusion
CD40 MC is a novel inflammatory MC subset that appears to be a biomarker for CKD severity. HHcy mediates CD40 MC differentiation via sCD40L induction and CD40 DNA hypomethylation in CKD.
Keywords: CD40 monocyte, homocysteine, DNA methylation, chronic kidney disease, CD40/CD 40L
INTRODUCTION
Homocysteine (Hcy) and its metabolite, S-adenosylhomocysteine (SAH), are uremic toxins that accumulate in the plasma of patients with chronic kidney disease (CKD) because of impaired extrarenal metabolism.1 Cardiovascular disease (CVD) is prevalent in CKD patients and account for 50% of deaths in the end-stage renal disease (ESRD).2 CKD patients have a 10–30 times higher cardiovascular mortality.2 Hyperhomocysteinemia (HHcy) has been established as an independent risk factor for CVD and a cause of cardiovascular events in CKD3 and can be used as a biomarker to predict the prognosis of CVD outcome in CKD.4 Hcy-lowering therapy was initially reported not beneficial to general vascular outcomes in secondary prevention trials,5, 6 VISP and HOPE2, but were reported later to have significantly reduced stroke recurrence and death in the post-hoc analysis of the same trails7, 8. The causative effect of HHcy on CVD is supported by several important trails including a large primary prevention trial, CSPPT,9 and a large population-based cohort study.10 These two studies showed that folic acid therapy reduced overall CVD and stroke incidence in hypertensive patients and that folic acid fortification reduced stroke mortality in the US general population, respectively; however, the mechanism underlying the induction of CVD by HHcy in CKD patient population is unknown.
Monocytes (MCs) are highly plastic and heterogeneous. Their functional phenotype could change in response to environmental stimuli such as uremic toxins. It is recently recognized that MC can be divided into three subsets, classical (CD14++CD16−), intermediate (CD14++CD16+), and non-classical (CD14+CD16++), based on cell surface marker expression.11 The intermediate MC subset is known to be a cellular hallmark of chronic inflammation that is associated with the burden of CVD12,CVD in CKD13, and death in the ESRD.14
We and others have reported that HHcy induces systemic and vascular inflammation15 and cytokine production,16 and that it also potentially accelerates atherosclerosis via inflammatory MC differentiation.17 We demonstrated that HHcy induces both inflammatory MC (Ly6Chigh+middle), the counterpart of human inflammatory intermediate MC subset in mice, and MC-derived inflammatory M1 macrophages (Mϕ) in cystathionine β-synthase (CBS) gene deficient mice compound with streptozotosin-induced hyperglycemia. We provided evidence showing that HHcy-induced DNA hypomethylation may be responsible for inflammatory MC differentiation.18
Hcy is a metabolite of methionine, and it can be converted to SAH, a potent inhibitor for methyltransferase.19 Uremia-associated HHcy is associated with altered epigenetic regulation20 and global DNA methylation change.21 Promoter DNA hypomethylation of p66Shc (SHC1), a stress responsible protein, was identified in blood cells from CKD subjects.22 Differential DNA methylation patterns were found in genes related to inflammation and oxidation in diseased kidneys.23 The mechanism of HHcy-related DNA methylation and its contribution to inflammatory MC differentiation in CKD-related CVD have not been studied.
In this study, we identified CD40 MC as a biomarker of CKD, found the metabolic connection between Hcy and methylation metabolites, and discovered their relation to inflammatory MC differentiation in CVD patients with or without CKD.
METHODS
Human subjects
We analyzed blood samples from 27 patients’ with CVD (13 CVD and 14 CVD+CKD) from the Temple University Vascular Surgery and Nephrology practice and 14 healthy donors with no history of CKD and CVD from the Thrombosis Center at Temple University. Case subjects had defined clinical and objective investigational evidence of all type of vascular disease in which MC counts and HHcy were identified to be independent risk factors.24,25,26 Conditions thought to influence Hcy concentrations and MC counts (e.g. recent systemic infection or thyroid disease) served as exclusion criteria for both cases and control subjects. CKD stage estimation and demographics/clinical information are described in Online Table I and Online Table II. For subjects with stage-5 CKD, blood samples were collected before using the hemodialysis. (more details in Supplement Methods).
Microarray analysis
Microarray data (GSE43484) were analyzed in the R statistical environment using “Biobase”, “GEOquery”, and “limma” Bioconductor projects and interpreted by DAVID tools. Genome-wide gene expression profiles were established from freshly isolated peripheral blood monocytes from CKD stage 4–5 patients and healthy subjects with a median age of 59 years.27 (more details in Supplement Methods).
Metabolite (Hcy, SAM, SAH) analyses
WBCs were isolated after RBC lysis. Plasma and cellular metabolites were measured using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS).18(more details in Supplement Methods).
Plasma sCD40L measurement
Platelets are the predominant source of sCD40L in plasma. Freeze-thawing of the prepared plasma causes lysis of residual platelets. On the day of blood collection, platelets in plasma were removed. Plasma levels of CD40L were measured by ELISA.
PBMC cultivation and differentiation
PBMCs were isolated from 50ml of whole blood and treated with human recombinant-CD40L, TNFα or IL-6, mouse IgG, mouse anti-human IL-6, mouse anti-human TNFα, or folic acid. (more details in Supplement Methods).
Blood MC isolation, cultivation
CD14+ MC was isolated as described,28 with modification. Cells were washed and stained with anti-CD14 antibody. Approximately 3~5x106 cells were isolated with a 95% purity of CD14+ MC from 30x106 PBMCs.
Serum cytokine array
serum cytokine levels were determined by using a commercially available array kit according to manufacturer instructions (Human cytokine array Q1, RayBiotech).
CD40 promoter DNA methylation mapping in WBC
1x106 WBC were used for genomic DNA preparation and CD40 core promoter methylation mapping. (more details in Supplement Methods).
DNMT protein and activity analysis
PBMC were cultured as above. Nuclear protein was extracted and assayed for DNMT protein levels and DNMTs activity as described29. (more details in Supplement Methods).
CpG island and core promoter mapping
A promoter CpG island was searched using a CpG Island Search engine (http://cpgislands.usc.edu). Transcription factor binding sites were mapped as identified previously30, 31 and predicted by database TESS. (more details in Supplement Methods).
Mediation analysis
We investigated the mediation effects of plasma/cellular Hcy and SAM/SAH ratio in the three CKD-induced inflammatory MC subsets, i.e., intermediate MC, CD40 MC/CD40 intermediate MC. The direct and total residual effects of eGFR on MC subsets were estimated using the standard mediation method by testing the cellular/plasma Hcy or SAM/SAH as a mediator32–34. (more details in Supplement Methods).
RESULTS
CKD induces CD40 MC that expresses stronger inflammatory markers
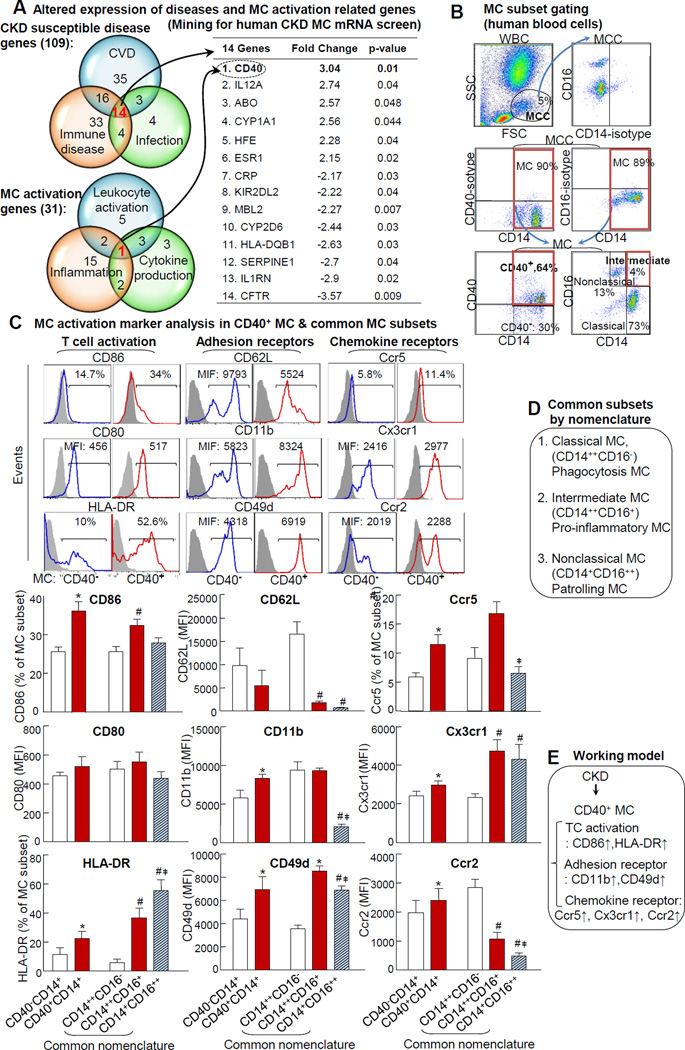
Using a database mining strategy, we analyzed human CKD-MC-mRNA screening data and compared gene expression profiles between stage 4~5 CKD patients and healthy subjects.27 We discovered 109 genes with altered mRNA levels in at least one of the three CKD susceptible disease categories, and 14 of them were in all three disease categories. Thirty-one genes were altered in at least one of three MC activation gene categories. Interestingly, CD40 is the only gene that belongs to all three MC activation related categories (Fig. 1A). This implies that CD40 is a potential marker for MC activation in the CKD patient population. We established gating methods for CD40 MC (CD40+CD14+) in Fig. 1B. CD40 expression was observed in 65% MC compared to the paired isotype control. To validate whether CD40 expression is related to inflammatory feature of MC, we examined the expression of 9 inflammatory markers in CD40 MC by co-staining using antibodies against T cell activation surface markers CD86/CD80/HLA-DR, adhesion receptors CD62L/CD49d/CD11b35, and chemokine receptors Ccr2/Ccr5/Cx3cr112(Fig. 1C). CD40+CD14+ MC expressed higher levels of inflammatory markers CD86/HLA-DR/CD11b/CD49d/Ccr2/Ccr5/Cx3cr1 compared to CD40−CD14+ MC. In general, CD40 MC is similar to that in intermediate MC (CD14++CD16+), which was denoted as inflammatory MC subset on the common nomenclature (Fig 1D) with respect to inflammatory markers. Interestingly, T cell activation marker CD86 and chemokine receptor Ccr2 were higher in CD40 MC than that in intermediate MC (Fig. 1C).
Figure 1. CKD induces CD40 expressing in MC and CD40+CD14+ MC inflammatory classification.
A. Disease and MC activation related genes are altered in human CKD MC. Data mining analysis was performed by DAVID tool (as described in Material and Methods) using human CKD MC mRNA screening database. 14 genes with altered expression were identified and jointly associated with three CKD susceptible diseases as shown in first Venn diagram and is listed in the table. CD40 is the sole gene that was induced in CKD MC and jointly associated with three MC activation related categories, as shown in the second Venn diagram. B. MC subset gating. C. CD40 MC classification and MC activation marker analysis. WBCs from healthy subjects were isolated and stained with anti-CD14, −CD16, and -CD40 mAbs, and co-stained with surface markers for T cell activation (CD80, CD86, & HLA-DR), adhesion receptors (CD62L, CD11b, & CD49d), and chemokine receptors (Ccr5, Cx3cr1, & Ccr2). MCs are defined as CD14+ cells from MCC. Representative dot plots and histograms depict CD40+ cells. MC activation markers were quantified in CD40− vs CD40+ MC or common MC subsets (CD14++CD16−CD14++CD16+CD14+CD16++ MC) and analyzed by flow cytometry. C. Common subsets by nomenclature. Schematic description summarizes the commonly recognized MC classification and function (common nomenclature). D. Working model. Values represent mean ± SEM (n=4); *p<0.05 vs CD40−CD14+ MC; # p<0.05 vs CD14++CD16− MC; ‡ p<0.05 vs. CD14++CD16+ MC. CKD, chronic kidney disease; CVD, cardiovascular disease; MC, monocyte. MCC, monocyte cloud.
Macrophage (Mϕ), CD40 MC and CD40 intermediate MC are increased in CKD and CVD subjects
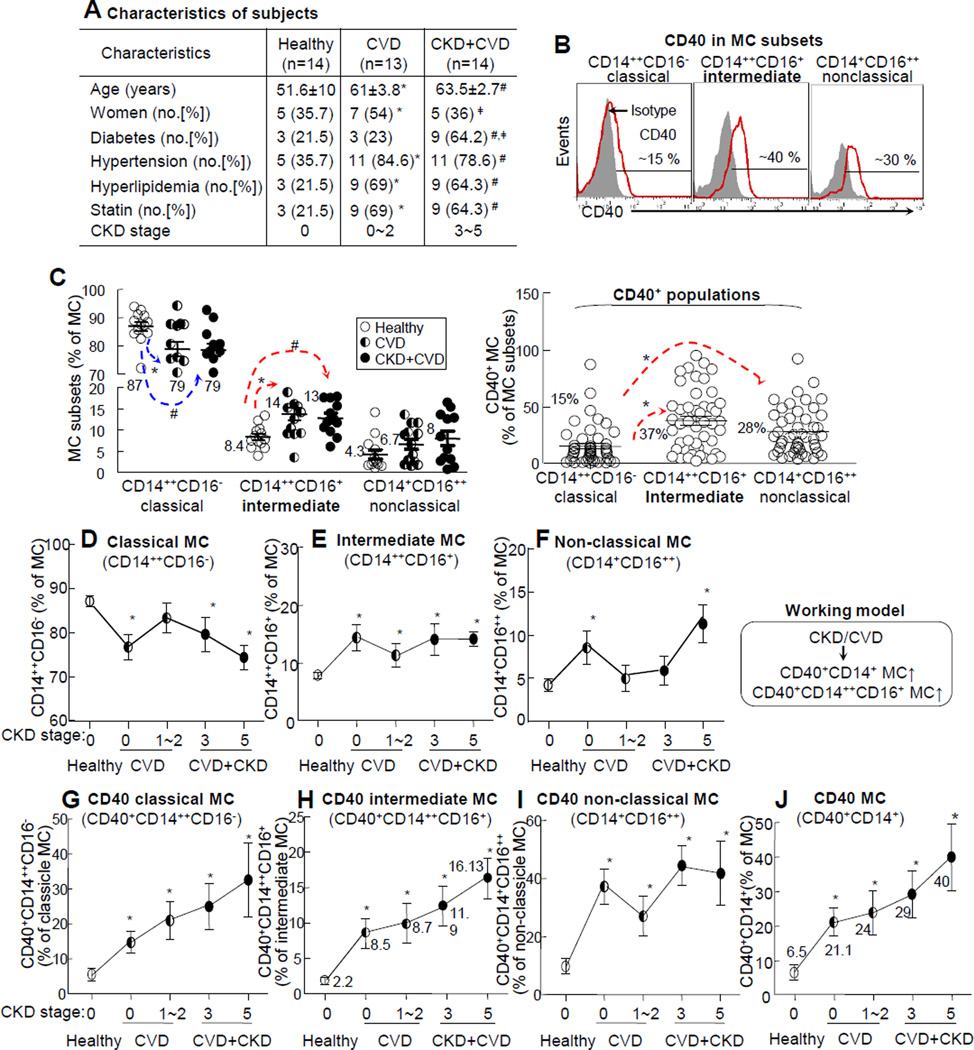
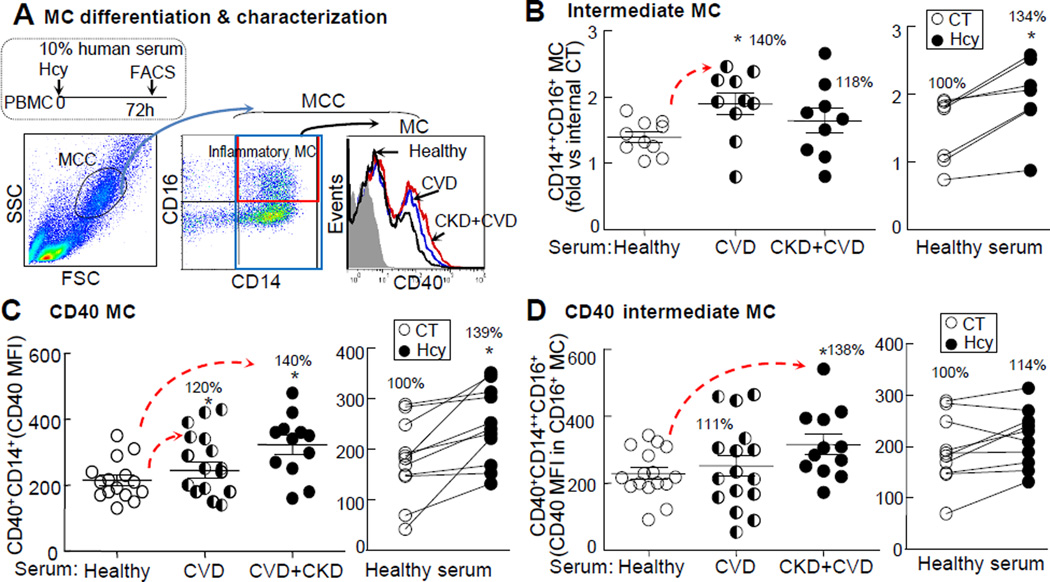
We characterized circulating MC (CD14+), Mϕ (CD14+CD16+), and dendritic cells (DC, HLA-DR+CD16−) in human subjects (Online. Fig. II-A) and found that Mϕ was elevated from 3.5%±0.35% in PBMC in healthy subjects to 5.8%±0.72% in CKD+CVD patients (1.7-fold induction), while MC and DC were not significantly different between the two groups. CD40 Mϕ and CD40 MC were significantly increased in CVD+CKD patients (Online. Fig. II-B). The intermediate MC subset was previously considered to be the inflammatory MC and a predictor of cardiovascular event in CKD subjects.13, 14 The classical MC subset (CD14++CD16−) and non-classical MC subset (CD14+CD16++) were thought to carry out phagocytosis and patrolling functions based on common nomenclature (Fig. 1D). We characterized circulating MC subsets in all subjects (Fig. 2B). MC cloud is 5~10% of WBC and contains approximately 85% MC. Compared to that in healthy subjects, the classical MC subset was decreased from 87%±1.5% of MC to 79%±2.5% and 79%±2.3% for CVD and CKD+CVD patients, respectively. The intermediate MC subset was increased from 8.4%±0.7% of MC in healthy subjects to 14%±1.5% and 13%±1.1% in CVD and CKD+CVD patients, respectively, while non-classical MC was not significantly different between the three groups. CD40+ population was 37% in the intermediate MC subset, which was significantly higher than that in the classical (15%) and non-classical (28%) MC subsets (Fig. 2C). When compared to the healthy subjects, classical MC was lower whereas intermediate and non-classical MC were higher in CVD; however, it was not further increased in CVD+CKD patients (Fig. 2D/E/F). Nonetheless, CD40 MC, CD40 classical and CD40 intermediate subsets were elevated in CVD and CVD+CKD patients and appeared to increase linearly with the elevation of CKD severity (Fig. 2G/H/J).
Figure 2. CD40 MC subsets markers CKD stages in CVD subjects.
Peripheral blood was collected from human subjects for MC subset and blood chemistry analyses. WBCs were isolated after red blood cell lysis and stained with antibodies against CD14, CD16, and CD40 for flow cytometric analysis. A. Characteristics of subjects. Diabetes were determined based on if the patient was currently receiving hypoglycemic therapy or it their fasting glucose level was ≥ 126 mg/dL. Hypertension, yyperlipidemia and CKD stage were determined as described in Supplement Material and Methods. Representative dot plots depict three commonly recognized MC subsets; CD14++CD16− classical, CD14++CD16+ intermediate, and CD14+CD16++ nonclassical MC. B. CD40 MC gating in MC subsets. CD 40 MC and common MC subsets are gated as described in Fig 1B. The percentage of CD40+ cells in three MC subsets was quantified. D, E, F. MC subsets. Common MC subsets were quantified and profiled in all subject groups. G, H, I. CD40 MC subsets. J. Total CD40 MC. CD40+ cells in common MC subsets and total CD40 MC were quantified and profiled in all subject groups. Arrows indicate the direction of significant changes. The schematic description summarizes the working model. Values represent mean ± SEM; *p<0.05 vs healthy; #p<0.05 vs CVD. PBMC, peripheral blood mononuclear cells; WBC, white blood cells; MCC, monocyte cloud; MC, monocyte.
Plasma glucose are increased in CKD+CVD patients, but not correlated with inflammatory MC
Plasma glucose level were increased from 116mg/dL in healthy subjects to 151mg/dL in CVD+CKD patients (a 30% induction, Online Figure III-E). It was weakly correlated with CD40 intermediate MC (r<=0.35, p=0.03) and not correlated with intermediate MC and CD40 MC by simple regression analysis (r<=0.35, p=0.03 and r<=0.35, p=0.03) (Online Figure III-G).
Hcy levels are increased in the plasma and WBC in CKD+CVD, and positively correlated with inflammatory MC subsets and hypomethylation status
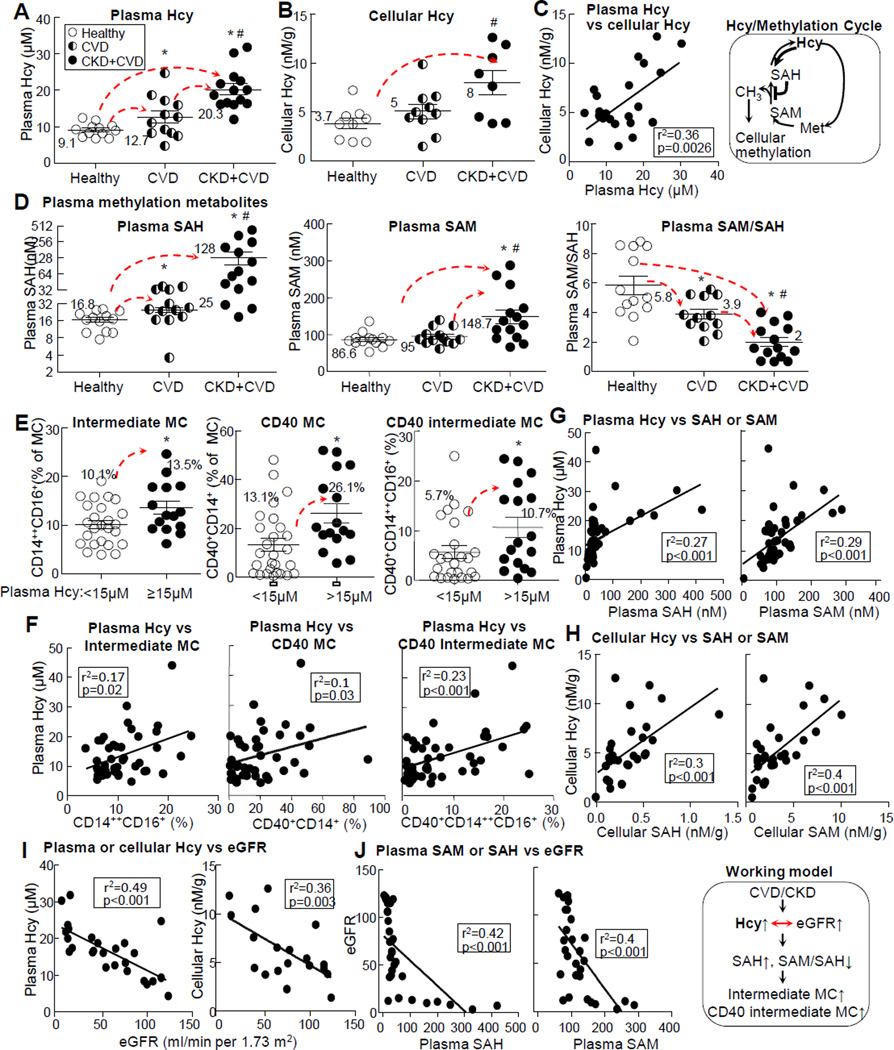
We examined relevant traditional risk factors for CVD/CKD and methylation metabolites and found that plasma Hcy levels were increased from 9.1μM in healthy subjects to 12.7μM and 20.3μM in CVD and CVD+CKD patients, respectively (Fig. 3A, Online Fig. III-A). It was positively correlated with plasma SAH and SAM (Fig. 3G) and all three inflammatory MC subsets (intermediate, CD40, and CD40 intermediate MC) (Fig. 3F), while being negatively correlated with eGFR (Fig. 3I). Cellular Hcy level was increased from 3.7nM/g in healthy subjects to 8nM/g in CVD+CKD patients (Fig. 3B), positively correlated with plasma Hcy (Fig. 3C) and cellular SAH and SAM (Fig. 3H), and negatively correlated with eGFR (Fig. 3I). We considered the Hcy metabolic cycle as the methylation cycle due to the fact that its metabolites (SAH and SAM) determine methylation status (Fig. 3 schematic) and examined SAH and SAM in the plasma and WBC. Compared to the healthy subjects, CVD and CKD+CVD patients’ plasma SAH levels were increased from 16.8nM±1.67nM to 25nM±2.6nM and 128nM±34nM (1.48 and 5.12-fold induction) (Fig. 3D, Online Fig. III-B), and plasma SAM levels were increased from 86.6nM±5.5 to 95nM±6.2 and 148.7nM±18.4 (1.7 and 1.56-fold induction), respectively (Fig. 3D, Online Fig. III-C). Plasma SAM/SAH ratio, an indicator of methylation status, was decreased from 5.8 to 3.9 and 2 in CVD and CKD+CVD patients (1.48 and 2-fold reduction) (Fig. 3D, Online Fig. III-D), respectively. We divided human subjects into normal (plasma Hcy level <15μmol/L) and HHcy (plasma Hcy level ≥15μmol/L) groups36 and found that intermediate, CD40, and CD40 intermediate MC were increased from 10.1%±0.8%, 13.1%±2.6% and 5.7%±1.3% of MC in normal group to 13.5%±1.3%, 26.1%±3.9% and 10.7%±2% in HHcy group, respectively (Fig. 3E); they were positively correlated with plasma Hcy levels (Fig. 3F). Plasma/cellular Hcy levels were positively correlated with plasma/cellular SAH and SAM levels, respectively (Fig. 3G-H). eGFR level was negatively correlated with plasma/cellular Hcy, plasma methylation metabolites SAM and SAH (Fig. 3I-J), and CD40 and CD40 intermediate MC (Online Fig. III-H), respectively. Plasma total cholesterol levels were not significantly different, but plasma glucose levels were increased from 116mg/dL in healthy subjects to 151mg/dL in CKD+CVD patients (Online Fig. IVE-F).
Figure 3. Hcy levels are increased in CKD patients, and positively correlated with intermediate, CD40, and CD40 intermediate MC subsets.
Peripheral blood was collected from human subjects for MC subset analysis, as described in Fig 2, and for Hcy/methylation metabolite analysis as described in Materials and Methods. Correlation was determined by liner regression analysis. A-C. Plasma Hcy, cellular Hcy, and correlation of plasma Hcy with cellular Hcy. D. Plasma methylation metabolites. E-F. MC subset change in HHcy condition and its correlation with plasma Hcy levels. Subjects were divided into 2 groups, plasma Hcy <15μM (normal Hcy, n=25) and >15μM (HHcy, n=16). MC populations for intermediate MC, CD40, and CD40 intermediate MC subsets were quantified and analyzed for correlation with plasma Hcy. G-H. Correlation of plasma and cellular Hcy with methylation metabolites and MC subsets. I-J. Correlation of eGFR with Hcy and methylation metabolites. Hcy and methylation metabolites are described in the framed methylation cycle. The schematic description in the frame summarizes the working model. Each dot represents one subject. Values represent mean ± SEM; *p<0.05 vs Healthy; # p<0.05 vs CVD. Arrows indicate the direction of significant changes. CVD, cardiovascular disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SAHs-adenosylhomocysteine; SAMs-adenosylmethionine; Hcy, Homocystine; Hcy, Homocysteine; MC, monocyte; HHcy, hyperhomocysteinemia;
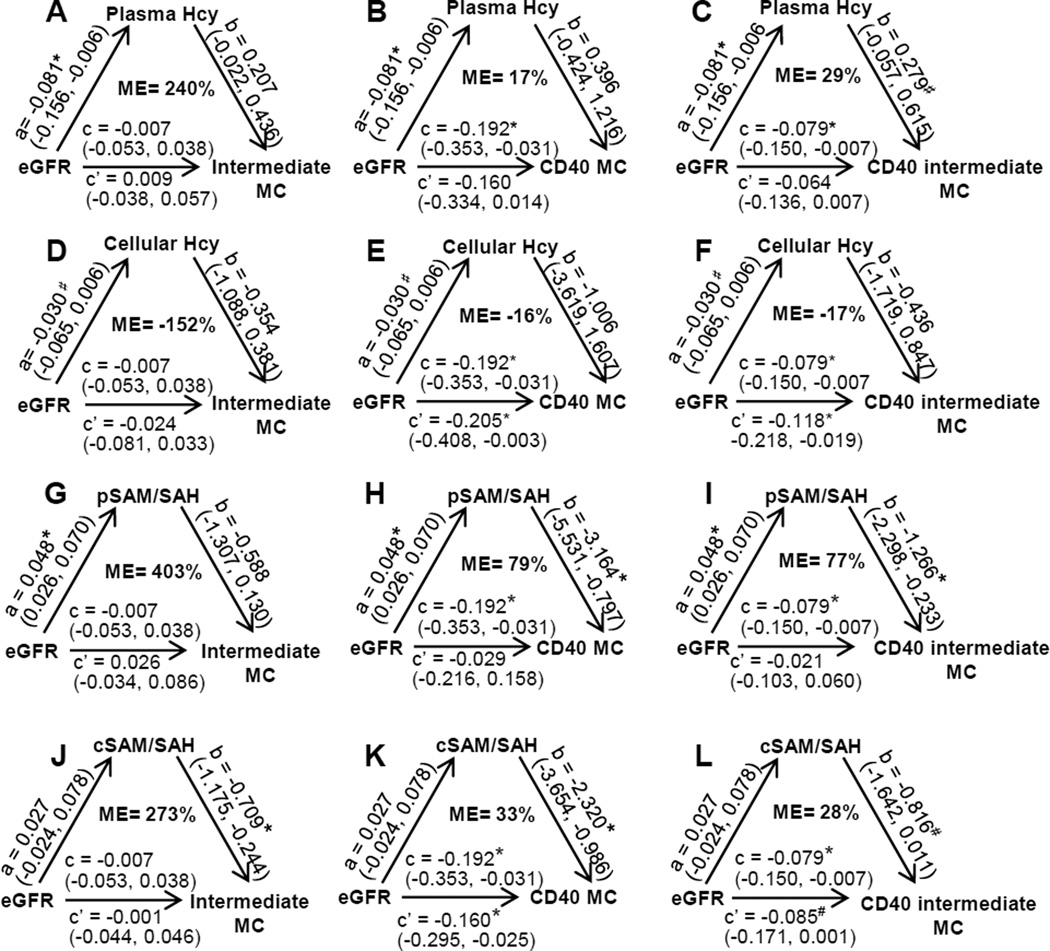
Plasma Hcy level is likely a mediator for CKD-induced CD40 intermediate MC differentiation (mediation analysis with adjustment)
We performed mediation analysis to further determine the role of HHcy in CKD-induced inflammatory MC (Fig. 4). After having adjusted for, age, sex, hypertension (HTN) and hypercholesterolnemia (HC) whenever necessary, we observed a significant direct reduction effect of eGFR on plasma Hcy (a=−0.081*, p<0.05, 95% CI: [0.156, −0.006] (Fig. 4A-C), but not on cellular Hcy (a=−0.030, p>0.10, 95% CI: [−0.065, 0.006]) (Fig. 4D-F). eGFR also demonstrated a significant total negative effect on CD40 MC (c=−0.192*, p<0.05, 95% CI: [−0.353, −0.031]) and CD40 intermediate MC (c=−0.079*, p<0.05, 95% CI: [−0.150, −0.007]), but not on intermediate MC (Fig. 4A-F). Plasma Hcy elevation exhibited a marginally significant mediation effect on CD40 intermediate MC differentiation (b=0.279#, p<0.10, 95% CI: [−0.057, 0.615]), demonstrating a 29% mediation effect on the eGFR deficiency-induced CD40 intermediate MC (ME=29%), the residual effect of eGFR became insignificant (c’=−0.064, p>0.10, 95% CI: [−0.136, 0.007]) in the presence of the mediation effect of plasma Hcy (Fig. 4C). No significant mediation effects were found for plasma Hcy on the other two CD40 MC subsets or for cellular Hcy on the three CD40 MC subsets under consideration (Fig. 4A-B&D-F); however, eGFR showed a consistent residual effect on CD40 MC (c’=−0.205*, p<0.05, 95% CI: [−0.408, −0.003]) and CD40 intermediate MC (c’=−0.118*, p<0.05, 95% CI: [−0.218, −0.118]) even in the presence of the possible mediation effect of cellular Hcy (Fig. 4E-F).
Figure 4. Increased plasma Hcy levels and reduced SAM/SAH are likely key mediators for CKD induced- CD40 MC differentiation (Mediation Analysis, with adjustment).
Peripheral blood was collected from human subjects for MC analysis and for Hcy, SAM, and SAH analysis as described in Fig 2 and in Materials and Methods. Mediation analyses were performed to estimate the direct and mediation/residual effect of eGFR and cellular/plasma Hcy or SAM/SAH ratio on three inflammatory MC subsets (intermediate, CD40 and CD40 intermediate) using the standard mediation method treating cellular/plasma Hcy or cellular/plasma SAM/SAH as a mediator with adjustment as described in Materials and Methods. A-C. Mediation analysis for plasma Hcy. D-F. Mediation analysis for cellular Hcy. G-I. Mediation analysis for plasma SAM/SAH. J-L. Mediation analysis for cellular SAM/SAH. “a” is the direct effect size, i.e., the slope, of eGFR on Hcy or SAM/SAH. “b” is the direct effect of Hcy or SAM/SAH on MC subsets having considered the residual effect, c’, of eGFR on the same variables. “c” is the total effect of eGFR on MC subsets. Numbers in the parentheses indicate the 95% confidence intervals. ME, mediation effect (=a*b/c) in percentage, is the % of mediator (Hcy or SAM/SAH) effect to the total effect of reduced eGFR-induced MC differentiation. *, p-value < 0.05. #p-value < 0.10. eGFR, estimated glomerular filtration rate; Hcy, Homocysteine; MC, monocyte; LC-ESI-MS/MS, liquid chromatography-electrospray ionization-tandem mass spectrometry; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine.
Reduced plasma/cellular SAM/SAH ratio is a stronger mediator for CKD-induced CD40 MC differentiation (mediation analysis with adjustment)
We also performed mediation analysis to determine the role of methylation status (SAM/SAH ratio) on CKD-induced inflammatory MC differentiation (Fig. 4). We observed a significant positive direct effect of eGFR on plasma SAM/SAH (a=0.048*, p<0.05, 95% CI: [0.026, 0.070]) (Fig. 4G-I) but not on cellular SAM/SAH (a=0.027, p>0.10, 95% CI: [0.024, 0.078]) (Fig. 4J-L). Reduced plasma SAM/SAH exhibited a significant mediation effect on CD40 MC and CD40 intermediate MC (b=−3.146*, p<0.05, 95% CI: [−5.531, −0.797] and b=−1.266*, p<0.05, 95% CI: [−2.298, −0.233]), with 79% and 77% mediation effects on the eGFR deficiency-induced CD40 MC and CD40 intermediate MC (ME=79% and 77%). The residual effect of eGFR became insignificant (c’=−0.029, p>0.10, 95% CI: [−0.216, 0.158] and c’=−0.021, p>0.10, 95% CI: [−0.103, 0.060]) in the presence of the mediation effect of plasma SAM/SAH (Fig. 4H-I).
Cellular SAM/SAH ratio reduction exhibited a possible mediation effect on CD40 MC and CD40 intermediate MC differentiation (b=−2.320*, p<0.05, 95% CI: [−3.654, −0.986]; and b=−0.816#, p<0.10, 95% CI: [−1.642, 0.011]) with a likely 33% and 28% mediation effect on the eGFR deficiency-induced CD40 MC and CD40 intermediate MC (ME=33% and 28%), respectively. Moreover, eGFR presented a consistent residual effect on CD40 MC and CD40 intermediate MC (c’=−0.160*, p<0.05, 95% CI: [−0.295, 10.025]; and c’=−0.085*, p<0.05, 95% CI: [−0.171, 0.001]) in the presence of the possible mediation effect of cellular SAM/SAH ratio reduction (Fig. 4J-L); however, such findings were very weak since the direct effect of eGFR on cellular SAM/SAH was not statistically significant (a=0.027, p>0.10, 95% CI: [−0.024, 0.078]) (Fig. 4J-L).
CKD serum and Hcy treatment induce intermediate, CD40 MC, and CD40 intermediate MC differentiation in PBMC
To confirm the mediation effect of HHcy on CKD-induced CD40 MC differentiation, we cultured human PBMC and treated it with patients’ serum and exogenous Hcy (100μΜ). CVD serum and Hcy treatment increased intermediate MC by 1.4 and 1.34-folds (Fig. 5B), respectively. CD40 MC was increased by 1.2 and 1.4-folds in CVD and CKD+CVD serum-treated groups and by 1.4-folds in Hcy-treated group, respectively (Fig. 5C). CD40 intermediate MC was increased by 1.4-folds in CKD+CVD serum-treated group but was not significantly different in CVD serum- and Hcy-treated PBMC (Fig. 5D).
Figure 5. CKD serum and Hcy induce inflammatory MC differentiation in PBMC.
A. PBMC-derived MC and gating method. PBMCs were isolated by using density gradient centrifugation to remove granulocytes. Serum was collected from human subjects. PBMCs were cultured with 10% human serum plus DL-Hcy (100μΜ) for 72 h. MC are defined as CD14+ cells from MCC (low granularity (SSC) and medium size (FSC)). Intermediate MC, CD40 MC and CD40 Intermediate MC were characterized. Representative dot plots depict intermediate (CD14++CD16+) MC and the histograms depict CD40+ MC. B, C & D. Inflammatory MC differentiation. Intermediate MC quantification is expressed as the ratio of that in PBMCs treated with patient serum vs healthy subject serum. CD40 MC is quantified using CD40 MFI in CD14+ MC. CD40 intermediate MC is quantified using CD40 MFI in intermediate MC. Representative results of four experiments with duplicates are shown in this figure. Each dot represents one subject. Values represent mean ± SEM; *p<0.05 vs Healthy or CT. Arrows indicate the direction of significant changes. CVD, cardiovascular disease; CKD, chronic kidney disease; PBMC, peripheral blood mononuclear cell; MFI, median fluorescence intensity; CT, control; Hcy, homocysteine.
Plasma soluble CD40L (sCD40L) is increased in CKD and CVD. CD40L induces intermediate and CD40 intermediate MC differentiation in PBMC
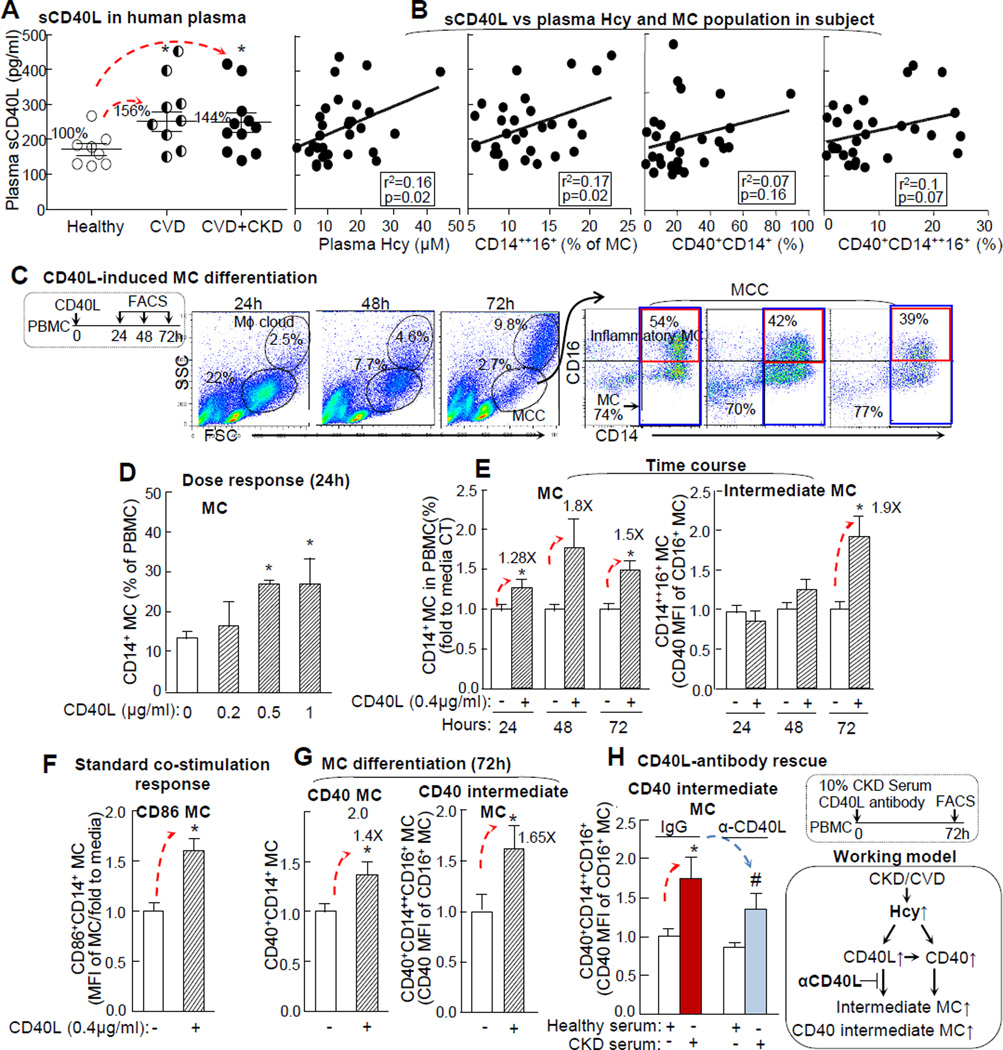
We examined plasma levels of sCD40L in all subjects and found that it was elevated from 171±17 in healthy subjects to 267 ±30 pg/ml in CVD and 247±27 pg/ml in CKD+CVD patients (156% and 144% induction, respectively) (Fig. 6A). In addition, plasma sCD40L levels were positively correlated with plasma Hcy levels and intermediate MC (Fig. 6B). This may imply that elevated CD40L in CVD patients’ serum induces inflammatory MC; hence, we investigated the effect of CD40L on the early stage MC differentiation in IFNγ-primed (72h) PBMC (Fig. 6C). CD40L induced MC differentiation in a dose sensitive manner and elevated MC by 1.3, 1.8, and 1.5-folds at 24, 48, and 72h, respectively (Fig. 6D), and intermediate MC by 1.9-folds at 72h (Fig. 6E). It is known that CD40-CD40L signaling induces CD40 and CD86 expression in MC.37 We confirmed that CD40L increased CD86 and CD40 MC by 1.6- and 1.4-fold, respectively (Fig. 6F/G), and induced CD40 intermediate MC differentiation by 1.7-folds (Fig. 6G). CD40L neutralizing antibody partially reversed CKD serum-induced CD40 intermediate MC from 1.73 to 1.32-folds (Fig. 6H).
Figure 6. CD40L induces CD40 intermediate MC differentiation in PBMC.
A. CD40L in human plasma. B. sCD40L correlation with plasma Hcy, and inflammatory MC subsets. sCD40L levels were measured in human plasma after platelet removal by ELISA. Plasma Hcy levels and MC subsets (intermediate, CD40, and CD40 intermediate MC subsets were examined as described in Fig1/2/3. Each dot represents one subject. C. Gating method for CD40L-induced MC differentiation. D & E. Dose response and time course of CD40L-induced MC differentiation. PBMC was primed with IFNγ (100U/ml) for MC differentiation and treated with human recombinant CD40L (0.4μg/ml) for indicated times. MC (CD14+) and intermediate MC populations are expressed as the fold change to that in CT (n=5). F. Co-stimulation response. G. CD40L-induced CD40 MC subsets differentiation. H. CD40L-antibody rescue. PBMC was treated with CD40L (0.4 μg/ml), in the presence of IFNγ (100U), and anti-CD40L (1μg/ml) , polyclonal mouse IgG antibody (1μg/ml), or 10% pooled CKD serum as a source of CD40L.CD86+CD14+ SMC was determined as the standard CD40L co-stimulation response. MC subsets were quantified as the fold increase to average value of these in CT (n=4). Schematic description summarizes the working model. Values represent mean ± SEM; *p<0.05 vs Healthy and CT. Arrows indicate the direction of significant changes. CVD, cardiovascular disease; CKD, chronic kidney disease; PBMC, peripheral blood mononuclear cell; MFI, median fluorescence intensity; sCD40L, Soluble CD40 ligand.
MC and T-cell origin inflammatory cytokines were induced in CKD patients and synergistically induced CD40 MC with Hcy
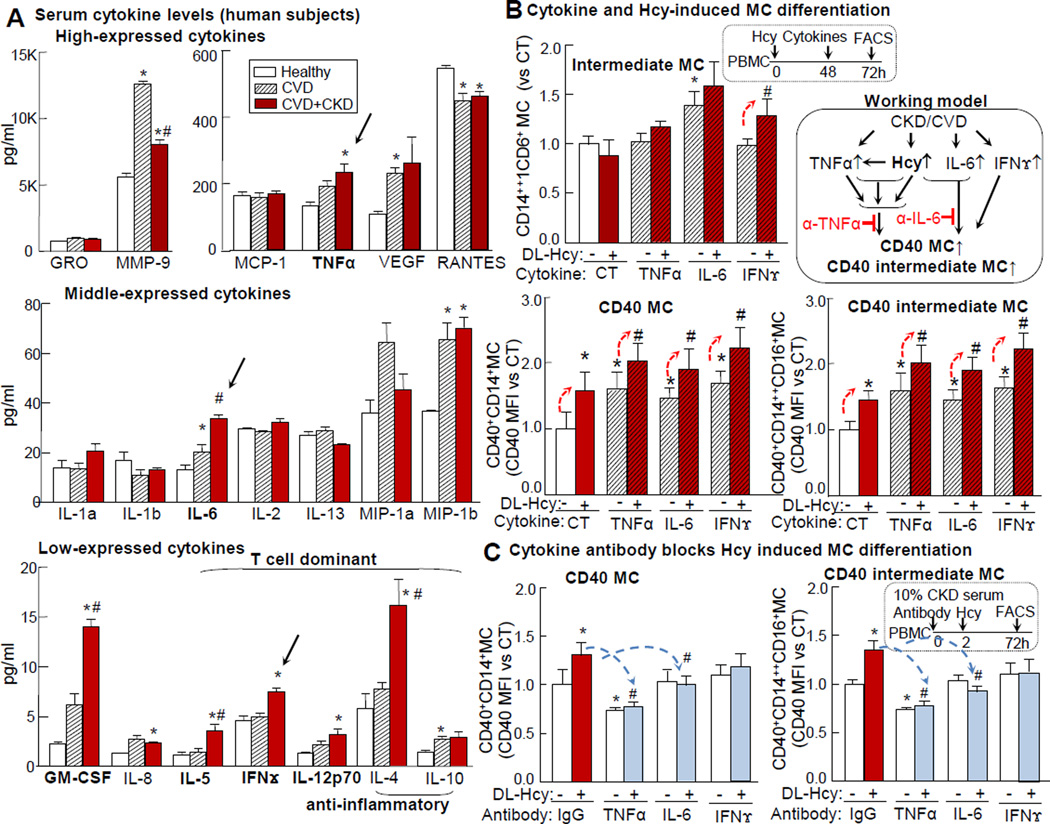
We screened 20 cytokines in the serum of human subjects using ELISA and found that 4 cytokines of MC origin (IL-12p70/GM-CSF/IL-6/TNFα) were elevated in CKD and CVD patients when compared to those of healthy subjects. Similarly, TNFα level was increased from 132±12pg/ml to 190.6±17pg/ml and 232.6±26pg/ml, and IL-6 levels were increased from 13.2±1.9pg/ml to 20.2±3pg/ml and 33.58±1.7pg/ml in CVD and CKD+CVD patients, respectively. The absolute levels of CD4+ T cell-dominant cytokines (IL-4/IFN/IL-5/IL-12p70) were relatively low in the healthy subjects but were elevated in CVD+CKD patients. The IFNγ level was elevated from 4.6±0.4 pg/ml to 4.9±0.4pg/ml and 7.5±0.4pg/ml in CVD and CKD+CVD patients, respectively (Fig. 7A). We further examined the synergistic effect of Hcy and inflammatory cytokines on MC differentiation in PBMC. Intermediate inflammatory MC was induced by IL-6 by 1.4-fold. CD40 MC was induced by Hcy, TNFα, IL-6 and IFNγ by 1.6, 1.6, 1.4, and 1.7-folds, respectively. The combination of Hcy with TNFα/IL-6/IFNγ resulted in further elevation of CD40 MC by 2, 1.9, and 2.3-folds, respectively. CD40 intermediate MC was induced by 1.5-folds in Hcy group, by 1.7, 1.4, and 1.6-folds with TNFα, IL-6, and IFNγ treatment. The combination of Hcy with TNFα/IL-6/IFNγ resulted in further elevation of CD40 intermediate MC by 2/1.9/2.3-folds (Fig. 7B), respectively. Neutralizing antibodies against TNFα and IL-6 completely reversed Hcy-induced CD40 MC and CD40 intermediate MC (Fig. 7C). The IFNγ antibody had no significant effect on Hcy-induced CD40 MC, which may be due to relatively low levels of IFNγ in CKD serum.
Figure 7. Inflammatory cytokines TNFα, IL-6, and IFNγ are induced in CKD+CVD subjects and synergized in CD40 intermediate MC differentiation with Hcy.
Peripheral blood was collected from human subjects and screened for serum cytokine levels. PBMCs were isolated from healthy donors and cultured for MC differentiation study as described in Fig 5. A. Serum cytokine levels. Serum from 5 human subjects was pooled as one assay sample and analyzed for cytokines in quadruplicates using Quantibody multiplex ELISA cytokine array (n=2–3). B. Cytokine potentiated Hcy-induced CD40 MC differentiation. PBMC was treated with DL-Hcy (100μM) and inflammatory cytokines: IFNγ (100U/ml), TNFα (10ng/ml), IL-6 (100ng/ml) (n=6). C. TNFα and IL-6 antibodies blocked Hcy-induced CD40 MC in CKD. PBMC was treated with anti-IL-6, −TNFα, polyclonal mIgG antibody, and DL-Hcy (100μM) in the presence of pooled 10% CKD serum as a source of inflammatory cytokines (C) (n=4). Quantification of MC subsets are expressed as the fold change to average value of these in CT. Schematic description summarizes the working hypothesis. Values represent mean ± SEM; *p<0.05 vs Healthy, #p<0.05 vs CVD; *p<0.05 vs CT, #p<0.05 vs Hcy. Arrows indicate the direction of significant changes. MC, monocyte; PBMC, peripheral blood mononuclear cell; Hcy, homocysteine; MFI, median fluorescence intensity.
DNA hypomethylation in CD40 promoter and DNMT1 suppression contribute to Hcy-induced inflammatory MC differentiation
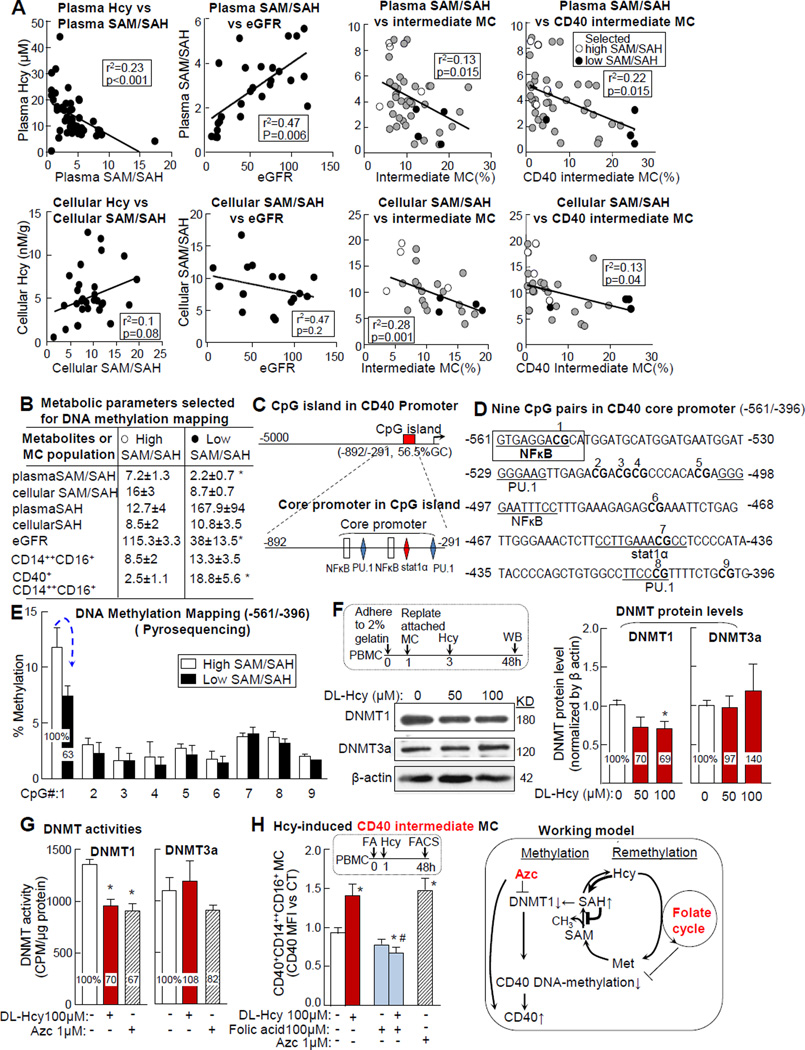
Plasma Hcy levels were negatively correlated with plasma SAM/SAH ratio, which was also negatively correlated with eGFR. Cellular Hcy levels were associated, but not significantly correlated with, cellular SAM/SAH. Plasma/cellular SAM/SAH ratios were negatively correlated with intermediate and CD40 intermediate MC in CKD and CVD patients (Fig. 8A). Because decreased SAM/SAH ratios indicate hypomethylation status, we mapped DNA methylation in CD40 promoter in selected subjects with high or low plasma SAM/SAH ratios (7.2±1.3 vs 2.2±0.7) (Fig. 8 A&B). We identified a CpG island (-892/-291) on the CD40 promoter (Fig. 8C), which has an IFNγ/TNFα-responsible core promoter (-561/-396)30 containing 5 cis-transactive consensus elements (2xNFκB, 2xPU.1, and a Stat1) and 9 potential methylation sites (CpG) (Fig. 8D). We mapped DNA methylation in the CD40 core promoter region using bisulfite converting and pyrosequencing. DNA methylation at site 1 CpG dinucleotide, a NFκB binding site, was reduced from 11.8% in high SAM/SAH subjects to 7.4% in low SAM/SAH subjects (Fig. 8E). Furthermore, protein levels and activity of DNA methyltransferase (DNMT) 1, but not 3a, were reduced to 70% in MC treated with Hcy (Fig. 8F-G). AZC was used as a specific DNMT inhibitor control. Finally, Hcy-induced CD40 intermediate MC was significantly reduced by folic acid, which provides methylation power to re-methylate Hcy and converts it back to methionine (Fig. 8H).
Figure 8. CD40 promoter DNA hypomethylation and DNMT1 suppression contribute to Hcy-induced inflammatory MC differentiation.
Peripheral blood was collected from human subjects for metabolite analysis for MC population characterization, and for DNA methylation mapping as described in Fig 1/2/3. PBMCs were isolated from healthy donors and cultured as described in Fig 5. A. Plasma SAM/SAH correlation with Hcy, eGFR and MC subsets. Plasma SAM/SAH was used as an indicator of methylation status. Correlation was determined by liner regression analysis. Subjects with high or low SAM/SAH were selected as representatives for DNA methylation mapping (shown in E) and indicated by empty and black circles to distinguish from other subjects (gray circle). Each dot represents one subject. B. Metabolite & MC features of subjects selected for DNA methylation mapping. Metabolites and CD40 intermediate MC data from high or low SAM/SAH subjects (4 individuals in each group) are listed in the table. C. CD40 promoter analysis. One CpG island was identified in CD40 promoter by computational analysis. The previously identified core promoter is located in the CpG island and contains 5 transcription factor binding sites. D. Nine CpG pairs in core promoter. Cis-transactive consensus elements (underline) for corresponding transcription factors and 9 CpG pairs are located in the CD40 core promoter region (-561/-396). E. DNA methylation mapping. WBCs from high and low SAM/SAH subjects (empty and black dots in A&B) were used for genomic DNA extraction and DNA methylation mapping by bisulfite converting and pyrosequencing. The percentage of methylated cytosine of #1–9 CpG pairs was quantified. F-G. DNMT protein levels and activity. MC was enriched by replating from PBMC and treated with DL-Hcy. DNMT1 and 3a protein levels and activities were examined (n=4). H. Hcy-induced CD40 intermediate MC. PBMCs were isolated, primed as described in Fig 5, and treated with folic acid prior to DL-Hcy (n=6). Azc, DNMT1 inhibitor, was used as negative control. Schematic description summarizes the working model. Values represent mean ± SEM; *p<0.05 vs CT and high SAM/SAH, #p<0.05 vs. Hcy. Arrows indicate the direction of significant changes. PBMC, peripheral blood mononuclear cell; MC, monocyte; Hcy, homocysteine; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; DNMT, DNA methyltransferase; Azc, 5-azacytidine; Met, Methionine.
DISCUSSION
In this comprehensive clinically driven mechanistic study, we investigated inflammatory MC differentiation in human CVD patients, with or without CKD, and in cultured PBMC. We identified CD40 MC as an inflammatory MC subset that is elevated in CVD+CKD patients and induced by CKD patient serum. CD40 is termed as TNF receptor family member 5 and expressed in antigen presenting cells, including MC, Mϕ, and dendritic cells. CD40-CD40L signaling activates T-cells by enhancing co-stimulatory CD80/CD86 and facilitates myeloid cell maturation.38 We found that CD40+ MC expressed similar or higher levels of inflammatory markers (Fig. 1) compared to the currently recognized inflammatory intermediate MC. Similar to the patrolling non-classical MC, the CD40− MC exhibited anti-inflammatory features with reduced inflammatory markers. We conclude that CD40+ MC is a novel and stronger inflammatory subset compared to the currently recognized inflammatory intermediate MC.
We found that unlike the intermediate MC, CD40 classical/intermediate MC and CD40 MC are linearly increased with the elevation of CKD severity (Fig. 2G/H/J). We propose that CD40 MC can be used as a diagnosis and prognosis marker for CKD and inflammatory disease, more accurately than the intermediate MC, to predict and represent CKD severity.
In the efforts to identify the metabolic mediator responsible for inflammatory MC differentiation in CKD, we found that plasma Hcy and its metabolite, SAH, were increased in CKD and positively correlated with three inflammatory MC subsets (Fig. 3). The negative correlation between eGFR and Hcy metabolites supported a strong metabolic link between kidney function and Hcy metabolites. This is not influenced by the clearance of metabolites since blood samples were collected from ESRD patients before dialysis.
Our data suggest that CKD is a stronger inducer for CD40 MC differentiation than is an increased glucose level. eGFR has a significant direct effect on CD40 MC and CD40 intermediate MC differentiation (Fig 4B/C/E/F). Plasma glucose levels are significantly increased in CVD+CKD patients (Online Fig III-E), but its correlation with CD40 MC and CD40 intermediate MC is very weak (Online Fig III-G).
The mediation effect of plasma Hcy elevation was further supported by mediation analysis (Fig. 4). HHcy has a direct effect on CD40 intermediate MC differentiation and a 29% mediation effect on eGFR deficiency-induced CD40 intermediate MC differentiation. The direct and mediating effect of Hcy on CD40 MC differentiation was further validated in PBMC by using exogenous Hcy treatment. We found that both DL-Hcy (100 μM) and CKD serum induced CD40 MC by 140% and 139% (Fig. 5C), respectively. This is consistent with our previous findings in mouse models of HHcy.15,17,18 This study suggests that HHcy mediates CKD-induced CD40 MC differentiation and can be a potential therapeutic target for CKD. The role of CD40 in inflammatory MC differentiation and vascular disease is supported by a previous report showing that CD40 deficiency reduced blood inflammatory MC and atherosclerosis in apoE-/- mice.39
We discovered that reduced plasma/cellular SAM/SAH ratio has a stronger direct effect on CD40 MC differentiation than that of increased plasma Hcy levels. Reduced plasma SAM/SAH has a 77% and 79% mediation effect on eGFR deficiency-induced CD40 intermediate MC and CD40 MC differentiation. These data suggest that SAM/SAH ratios may be influenced by other metabolic pathway, independent from Hcy metabolism, and may be a more potent mechanism for CD40 MC differentiation.
We demonstrated that plasma CD40L levels are positively correlated with plasma HHcy and circulating inflammatory MC in CVD and CKD subjects and that CD40L induces CD40 MC differentiation in PBMC (Fig. 6). Plasma sCD40L is considered to be an essential inflammatory biomarker for CVD. It is mostly generated by activated platelets and elevated in stage-5 CKD patients with CVD.40 Consistent with our findings, sCD40L levels were found to be elevated in HHcy patients and positively correlated with Hcy.36 Our study is the first to connect CD40L elevation with MC differentiation and to validate this connection in experimental models. We discovered that CD40 system blocking, such as neutralizing antibody against CD40L, reverses CKD patient serum induced CD40 MC differentiation and could be a potential therapeutic strategy to reduce inflammatory response in patients with CKD and CVD.
In addition to sCD40L, previous reports suggested that IFNγ induces CD40 transcription in microglia/macrophages.30 We found that circulating inflammatory cytokines TNFα, IL-6, and IFNγ are increased in CKD and CVD patients. TNFα antibody reversed CKD serum- and CKD serum+Hcy-induced CD40 MC, while IL-6 antibody only blocks such effect induced by CKD serum+Hcy (Fig. 7). We propose a model that HHcy and TNFα directly induce CD40 MC differentiation and that IL-6 promotes HHcy-induced MC in CKD (Fig 7 working model). Taken together, our data indicate that soluble CD40L and inflammatory cytokines in combination with Hcy synergize inflammatory MC differentiation in CKD.
The hypomethylation status in CKD was likely due to SAH elevation as it dictated SAM/SAH ratio reduction regardless the increased plasma SAM levels observed (Fig. 3D). In some Hcy-lowering trials, unchanged plasma SAH levels and SAM/SAH ratios have been suggested to be the explanation for the absence of benefits in CVD prevention.41 Our findings provided a metabolic basis for future clinical trial design. We found that SAH levels are exponentially increased in stage-5 CKD subjects (Fig. 3G & new Supplement Fig. III-B), indicating that SAH is a pathogenic factor. We propose that future clinical trials should consider SAH and methylation targeted therapy, especially for stage-5 CKD, to improve the benefit of Hcy-lowering therapy. The pathogenic effect of SAH is also supported by its biochemical feature, as the Ki for SAH, the inhibitor constant for its concentration inhibiting 50% of maximal reaction rate, is smaller than the Km for SAM, the Michaelis constant for substrate concentration for 50% of maximal reaction rate, for most of the methyltransferase (MT).42 Therefore, SAH has a higher affinity for MT. Lower SAH concentration is needed to compete with SAM for binding to MT. In this study, we demonstrated that HHcy and SAM/SAH correlate with inflammatory MC subsets in patients with CVD and CKD (Fig. 3, 4 & 8). We propose that HHcy and SAH are metabolic sensors that determine hypomethylation status and are responsible for inflammatory MC differentiation and the progression of CVD and CKD. These data are consistent with our previous findings, showing that hypomethylation induces inflammatory MC and Mϕ in mice,18 and lead us to hypothesize that HHcy induces both sCD40L and CD40 expression via hypomethylation related mechanism in CKD.
We tested the DNA hypomethylation hypothesis in HHcy- and CKC-induced CD40 MC differentiation. We discovered that CpG methylation in CD40 core promoter at NFκB binding site is reduced in patients with low SAM/SAH ratios and that Hcy-induced CD40 intermediate MC differentiation can be rescued using a re-methylation reagent folic acid in PBMC (Fig. 8). These findings are consistent with the notions that NFκB is essential for MC differentiation and that altered DNA methylation at the NFκB binding site influences intestinal metaplasia, epithelial dedifferentiation, and carcinogenesis.43
In summary, we have found that CD40 MC, CD40 classical MC, and CD40 intermediate MC are induced in CVD and further elevated with the progress of CKD, which is likely mediated by elevated plasma Hcy levels and reduced SAM/SAH ratios. HHcy induces CD40 MC via soluble CD40L induction and CD40 promoter DNA hypomethylation due to SAH induction and DNMT1 suppression (Online Fig. I, working model). It is known that CD40-CD40L interaction leads to enhanced T-cell co-stimulation37, myeloid cell maturation, and B-cell proliferation.44 Clinical trials targeting CD40 using monoclonal antibodies, Dacetuzumab,45 are ongoing for B-cell lymphoma to inhibit B-cell proliferation and 4D1146 for immunosuppressant after organ transplantation to inhibit antigen presenting MC maturation. Our study presents a novel model for CD40-CD40L interaction in MC differentiation.
Our study is a typical clinically driven mechanistic research that employed multilayers of sophisticated discovery tools and approaches. It incorporated comprehensive genetic, immunological, molecular and cellular, biological, metabolical, and statistical strategies. We provided substantial evidence ranging from gene and novel MC subset identification to metabolic profiling, mediator discovery, and DNA methylation characterization in CKD patients. Importantly, we used multiple rescue strategies that reversed CKD serum and Hcy-induced inflammatory MC differentiation and presented novel therapeutic strategies for treating CKD and CVD, including α-CD40L, α-TNFα, α-IL6 and folic acid therapies. Our work established a solid foundation for further research into inflammatory response and cardiovascular disease in CKD.
Future studies will validate the key mechanistic findings in a larger sample size and/or in different disease populations; such studies should identify the regulatory mechanism underlying CD40 MC differentiation and the crosstalk between metabolic, cellular, and molecular changes in CKD. Continued research on CD40 MC differentiation could lead to the discovery of novel therapeutic targets for inflammatory disease, especially for CKD-related CVD.
Supplementary Material
Novelty and Significance.
What Is Known?
CD14++CD16+, an inflammatory monocyte subset, is increased in human chronic kidney disease (CKD) and cardiovascular disease (CVD).
CD40 is expressed in antigen presenting cells and CD40-CD40L signaling activates T-cells.
Plasma homocysteine levels are increased in human CKD and CVD.
What New Information Does This Article Contribute?
Classified CD40+ monocytes as a stronger inflammatory monocyte subset, which can predict and represent CKD severity.
Hyperhomocyteinemia and reduced S-adenosylmethionine/S-adenosylhomocysteine (SAM/SAH) ratio mediate CKD-induced CD40+ monocyte differentiation.
CD40 ligand is elevated in CVD/CKD and can induce inflammatory monocyte differentiation. Homocysteine mediated CKD-induced CD40+ monocyte differentiation via SAH elevation determined hypomethylation status (reduced SAM/SAH ratio) and CD40 DNA hypomethylation on NF-κB consensus element.
Homocyteine suppressed DNMT1 activity and induced CD40 intermediate monocyte differentiation in PBMC.
Folic Acid prevented homocysteine-induced CD40 intermediate MC differentiation in PBMC.
In this study, we investigated monocyte differentiation in human CKD and CVD. We identified CD40 as a CKD-related monocyte activation gene and classified CD40+CD14+ monocytes as a stronger inflammatory subset than the currently recognized inflammatory intermediate CD14++CD16+ monocytes. We found that CD40 and CD40 intermediate monocytes, homocysteine, SAH and SAM levels were increased in CVD and further elevated in CVD+CKD patients. We established hyperhomocysteinemia as a mediator for CKD-induced CD40 intermediate monocyte differentiation. We propose CD40L as a mediator for CKD/homocysteine-induced CD40 monocyte differentiation because it was increased in CVD/CKD and the antibody against CD40 prevented CKD patient serum/homocysteine-induced CD40 monocyte differentiation. We characterized DNA methylation and identified hypomethylation on the NF-κB site in CD40 promoter in white blood cells from CKD subjects with lower SAM/SAH ratios. Finally, homocysteine inhibited DNA methyltransferase-1 activity and promoted CD40 intermediate MC differentiation, which was reversed by folic acid in peripheral blood mononuclear cells.
Acknowledgments
The authors thank A. C. Kolli and M. N. Wright for providing healthy donor and patient material and clinical data.
SOURCES OF FUNDING
This work was supported in part by National Institutes of Health (NIH) Grants number: HL67033, HL77288, HL82774, HL110764, HL117654, DK104116 and HL131460 (HW); HL9445, HL108910 and HL116917 (XFY), HL131460 to HW/EC/XFY, and by the National Science Foundation of China 81330004 (YJ).
Footnotes
Nonstandard Abbreviations and Acronyms: None.
DISCLOSURE
None.
REFERENCES
- 1.van Guldener C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrol Dial Transplant. 2006;21:1161–1166. doi: 10.1093/ndt/gfl044. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: a meta-analysis. Am J Kidney Dis. 2009;54:478–489. doi: 10.1053/j.ajkd.2009.01.266. [DOI] [PubMed] [Google Scholar]
- 4.Mallamaci F, Zoccali C, Tripepi G, Fermo I, Benedetto FA, Cataliotti A, Bellanuova I, Malatino LS, Soldarini A. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002;61:609–614. doi: 10.1046/j.1523-1755.2002.00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J, Jr Heart Outcomes Prevention Evaluation I. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 6.Loland KH, Bleie O, Blix AJ, Strand E, Ueland PM, Refsum H, Ebbing M, Nordrehaug JE, Nygard O. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: a Western Norway B Vitamin Intervention Trial (WENBIT) substudy. Am J Cardiol. 2010;105:1577–1584. doi: 10.1016/j.amjcard.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E Heart Outcomes Prevention Evaluation I. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365–1372. doi: 10.1161/STROKEAHA.108.529503. [DOI] [PubMed] [Google Scholar]
- 8.Hankey GJ, Eikelboom JW, Yi Q, Lees KR, Chen C, Xavier D, Navarro JC, Ranawaka UK, Uddin W, Ricci S, Gommans J, Schmidt R group Vts. Antiplatelet therapy and the effects of B vitamins in patients with previous stroke or transient ischaemic attack: a post-hoc subanalysis of VITATOPS, a randomised, placebo-controlled trial. Lancet Neurol. 2012;11:512–520. doi: 10.1016/S1474-4422(12)70091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF Investigators C. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, Johansen H, Friedman JM. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113:1335–1343. doi: 10.1161/CIRCULATIONAHA.105.570846. [DOI] [PubMed] [Google Scholar]
- 11.Zawada AM, Rogacev KS, Schirmer SH, Sester M, Bohm M, Fliser D, Heine GH. Monocyte heterogeneity in human cardiovascular disease. Immunobiology. 2012;217:1273–1284. doi: 10.1016/j.imbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 14.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Kohler H, Girndt M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 15.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice. Circ Res. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ Res. 2003;93:311–320. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET, Han Y, Yang XF, Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamaluddin MS, Yang X, Wang H. Hyperhomocysteinemia, DNA methylation and vascular disease. Clin Chem Lab Med. 2007;45:1660–1666. doi: 10.1515/CCLM.2007.350. [DOI] [PubMed] [Google Scholar]
- 20.Zawada AM, Rogacev KS, Heine GH. Clinical relevance of epigenetic dysregulation in chronic kidney disease-associated cardiovascular disease. Nephrol Dial Transplant. 2013;28:1663–1671. doi: 10.1093/ndt/gft042. [DOI] [PubMed] [Google Scholar]
- 21.Ingrosso D, Cimmino A, Perna AF, Masella L, De Santo NG, De Bonis ML, Vacca M, D'Esposito M, D'Urso M, Galletti P, Zappia V. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet. 2003;361:1693–1699. doi: 10.1016/S0140-6736(03)13372-7. [DOI] [PubMed] [Google Scholar]
- 22.Geisel J, Schorr H, Heine GH, Bodis M, Hubner U, Knapp JP, Herrmann W. Decreased p66Shc promoter methylation in patients with end-stage renal disease. Clin Chem Lab Med. 2007;45:1764–1770. doi: 10.1515/CCLM.2007.357. [DOI] [PubMed] [Google Scholar]
- 23.Smyth LJ, McKay GJ, Maxwell AP, McKnight AJ. DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics. 2014;9:366–376. doi: 10.4161/epi.27161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Wei Zuo S, Li Y, Jia X, Jia SH, Zhang T, Xiang Song Y, Qi Wei Y, Xiong J, Hua Hu Y, Guo W. Hyperhomocysteinaemia is an independent risk factor of abdominal aortic aneurysm in a Chinese Han population. Sci Rep. 2016;6:17966. doi: 10.1038/srep17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andras A, Stansby G, Hansrani M. Homocysteine lowering interventions for peripheral arterial disease and bypass grafts. Cochrane Database Syst Rev. 2013;7:CD003285. doi: 10.1002/14651858.CD003285.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999-2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 27.Al-Chaqmaqchi HA, Moshfegh A, Dadfar E, Paulsson J, Hassan M, Jacobson SH, Lundahl J. Activation of Wnt/beta-catenin pathway in monocytes derived from chronic kidney disease patients. PLoS One. 2013;8:e68937. doi: 10.1371/journal.pone.0068937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freundlich B, Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 29.Jamaluddin MD, Chen I, Yang F, Jiang X, Jan M, Liu X, Schafer AI, Durante W, Yang X, Wang H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood. 2007;110:3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen VT, Benveniste EN. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J Biol Chem. 2000;275:23674–23684. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen VT, Benveniste EN. Critical role of tumor necrosis factor-alpha and NF-kappa B in interferon-gamma -induced CD40 expression in microglia/macrophages. J Biol Chem. 2002;277:13796–13803. doi: 10.1074/jbc.M111906200. [DOI] [PubMed] [Google Scholar]
- 32.CM J. Process Analysis: Estimating mediation in treatment evaluations. Evaluation Review. 1981;(5):602–619. [Google Scholar]
- 33.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 34.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Manivannan A, Crane I, Dawson R, Liversidge J. Critical but divergent roles for CD62L and CD44 in directing blood monocyte trafficking in vivo during inflammation. Blood. 2008;112:1166–1174. doi: 10.1182/blood-2007-06-098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prontera C, Martelli N, Evangelista V, D'Urbano E, Manarini S, Recchiuti A, Dragani A, Passeri C, Davi G, Romano M. Homocysteine modulates the CD40/CD40L system. J Am Coll Cardiol. 2007;49:2182–2190. doi: 10.1016/j.jacc.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103:1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 39.Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM, Naik SH, Boon L, Oufella HA, Mallat Z, Ahonen CL, Noelle RJ, de Winther MP, Daemen MJ, Biessen EA, Weber C. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim PS, Wu MY, Chien SW, Wu TK, Liu CS, Hu CY, Chang HC, Pai MA. Elevated circulating levels of soluble CD-40 ligand in haemodialysis patients with symptomatic coronary heart disease. Nephrology (Carlton) 2008;13:677–683. doi: 10.1111/j.1440-1797.2008.00999.x. [DOI] [PubMed] [Google Scholar]
- 41.Becker A, Smulders YM, Teerlink T, Struys EA, de Meer K, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. S-adenosylhomocysteine and the ratio of S-adenosylmethionine to S-adenosylhomocysteine are not related to folate, cobalamin and vitamin B6 concentrations. Eur J Clin Invest. 2003;33:17–25. doi: 10.1046/j.1365-2362.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- 42.Steve Clarke KB. Homocystine in healthy and disease; s-adenosylmethionine-dependent methyltransferase. Cambridge university press. 2001 [Google Scholar]
- 43.Rau TT, Rogler A, Frischauf M, Jung A, Konturek PC, Dimmler A, Faller G, Sehnert B, El-Rifai W, Hartmann A, Voll RE, Schneider-Stock R. Methylation-dependent activation of CDX1 through NF-kappaB: a link from inflammation to intestinal metaplasia in the human stomach. Am J Pathol. 2012;181:487–498. doi: 10.1016/j.ajpath.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, Mixan BJ, Lee WP, Lin Z, Valdez P, Wahl AF, Grewal IS. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 46.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, Shimamura T, Takahashi N, Miura T, Okimura K, Itoh T, Shimizu A, Furukawa H, Todo S. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.