Abstract
The WH2 (Wiscott–Aldridge syndrome protein homology domain 2) repeat is an actin interacting motif found in monomer sequestering and filament assembly proteins. We have stabilized the prototypical WH2 family member, thymosin-β4 (Tβ4), with respect to actin, by creating a hybrid between gelsolin domain 1 and the C-terminal half of Tβ4 (G1-Tβ4). This hybrid protein sequesters actin monomers, severs actin filaments and acts as a leaky barbed end cap. Here, we present the structure of the G1-Tβ4:actin complex at 2 Å resolution. The structure reveals that Tβ4 sequesters by capping both ends of the actin monomer, and that exchange of actin between Tβ4 and profilin is mediated by a minor overlap in binding sites. The structure implies that multiple WH2 motif-containing proteins will associate longitudinally with actin filaments. Finally, we discuss the role of the WH2 motif in arp2/3 activation.
Keywords: protein crystallography, SCAR, VCA, WASp, WH2
Introduction
Thymosin-β4 (Tβ4) is the ubiquitous actin monomer sequestering protein in mammalian cells (dos Remedios et al, 2003). It provides a buffer of ATP–actin monomers that participates in actin polymerization-reliant processes such as cell movement and cytokinesis. Tβ4 has a moderate affinity for ATP–actin (Kd=1 μM) and, in cells, is often present at concentrations in excess of the actin monomers (Hartwig, 1992; Weber et al, 1992). The reservoir of actin monomers, stored as the Tβ4:actin complex, can be dissociated by profilin (Pantaloni and Carlier, 1993). The profilin:actin pool is then free to join the barbed end of a filament and allow elongation (Pantaloni and Carlier, 1993; Kang et al, 1999). In the presence of barbed end capping proteins, both Tβ4 and profilin function as sequestering proteins, maintaining a high concentration of nonpolymerized actin.
Tβ4 displays an enigmatic binding behavior towards actin. Profilin, vitamin D-binding protein (DBP), gelsolin domain 1 and DNase I all have been shown to compete directly with Tβ4 in binding to actin (Ballweber et al, 1997, 1998; Safer et al, 1997). However, DNase I can form a ternary complex with Tβ4:actin in the presence of crosslinking agents (Ballweber et al, 1997), and affinity chromatography, ultracentrifugation and fluorescence anisotropy data all point towards the competition between profilin and Tβ4 being mediated through a ternary complex with actin (Yarmola et al, 2001). Furthermore, at concentrations higher than 20 μM, Tβ4 is no longer simply a sequestering protein, but shows weak cooperative binding to actin filaments (Kd=5–10 mM; Carlier et al, 1996). The filament-bound Tβ4 appears to weaken the actin–actin contacts across the filament, changing the filament twist, often separating the strands and aggregating filaments (Carlier et al, 1996; Ballweber et al, 2002). Hence, Tβ4 has been proposed to inhibit polymerization by interfering with lateral actin–actin bonds rather than longitudinal contacts (Carlier et al, 1996).
Tβ4 is one of the several small (5 kDa, 41–44 residues) closely related β-thymosins (Huff et al, 2001). Tβ9, Tβ10 and Tβ15 bind approximately twice as strongly to actin when compared to Tβ4 (Jean et al, 1994; Sun et al, 1996; Eadie et al, 2000). Increasing the affinity of the protein for actin, by exchanging Tβ4 for Tβ10, or increasing the concentration of Tβ4, causes loss of F-actin. In vivo, the resultant, larger available pool of sequestered actin leads to increased cell motility (Eadie et al, 2000). In some non-mammalian eukaryotes, the Tβ4 actin-binding motif has been multiplied. The amoeba protein, actobindin, consists of two Tβ4-like motifs, whereas Drosophila ciboulot has three such repeats. The multiplication of the actin-binding motif results in proteins that are not only able to cap actin filament pointed ends but also are able to participate directly in barbed end elongation (Boquet et al, 2000; Hertzog et al, 2002).
The Tβ4 protein family is a distinct subfamily of a broader group of related proteins, the WH2 motif-containing proteins (Wiscott–Aldridge syndrome protein (WASp) homology domain 2; Paunola et al, 2002). This family includes WASp, WAVE/Scar (WASp family verprolin homologous protein/suppressor of cAMP receptor), WIP (WASp interacting protein) and CAP (adenylyl cyclase-associated protein). The WH2 motif represents a small fraction of these large, multidomain proteins and it resides adjacent to polyproline-rich motifs, which in some cases have been shown to bind profilin. WASp and WAVE are activators of the arp2/3 complex that yield their bound actin for barbed end elongation (Welch and Mullins, 2002). CAP has been identified as an actin monomer sequestering protein; however, its colocalization with other actin-binding proteins, cofilin and aip1 (actin interacting protein 1), suggests a more complex role (Bertling et al, 2004).
In aqueous solutions, Tβ4 does not adopt a single folded structure (Domanski et al, 2004). NMR studies in water at 14°C show Tβ4 to consist of a single helix, residues 5–16 (Czisch et al, 1993). In contrast, circular dichroism analysis suggests isolated Tβ4 to have an average of six helical residues and its actin-bound form to contain twice that number (Safer et al, 1997). Mutagenesis analysis reveals the functionality of the first helix of Tβ4 to terminate at residue 16 (Simenel et al, 2000). The NMR structures of Tβ4 and Tβ9 have been solved in alcohol/water mixes. Both consist of two helices, comprising residues 4–16 and 30–40 in Tβ4 and residues 4–27 and 32–41 in Tβ9 (Zarbock et al, 1990; Stoll et al, 1997). More recently, NMR studies have confirmed the existence of the two helices in the actin-bound form of Tβ4 (Domanski et al, 2004), and the interactions of the N-terminal helix with actin have been defined by the crystal structure of ciboulot:actin complex (Hertzog et al, 2004).
The absence of a crystal structure for an actin-bound C-terminal half of a WH2 motif, combined with Tβ4's flexible nature and modest affinity for actin, led us to seek methods to stabilize the motif with respect to actin. Recently, we elucidated the structure of a gelsolin fragment in complex with actin (Irobi et al, 2003), from which we proposed that a section of the gelsolin:actin recognition determinant is homologous with the central region of the WH2 motif. In the present study, we have constructed a hybrid protein based on this homology, consisting of the first domain of gelsolin (G1) and the C-terminal half of Tβ4. The crystal structure of the hybrid protein in complex with actin (G1-Tβ4:actin) reveals for the first time the actin-binding conformation of the C-terminal half of Tβ4 and provides a framework for understanding the role of the WH2 motif in its wide range of protein settings.
Results
G1-Tβ4 severs and caps actin filaments
In a first step to determine the activity of this hybrid protein, we compared the effect of the G1-Tβ4 (consisting of gelsolin residues 27–152 and Tβ4 residues 21–43) with G1+ (gelsolin residues 25–160) on actin assembly (Figure 1A and B). At substoichiometric concentrations, G1+ and G1-Tβ4 inhibit actin polymerization in a concentration-dependent manner, suggesting that both proteins cap the barbed ends of actin filaments, although G1-Tβ4 exhibited a lower efficiency. Hence, the presence of the C-terminal half of Tβ4, within G1-Tβ4, reduces the efficiency of capping at the barbed ends of actin filaments. Subsequently, we characterized the severing activities of G1+ and G1-Tβ4 (Figure 1C). A constant concentration of actin filaments was incubated for 1 min with increasing concentrations of G1+ or G1-Tβ4, these mixtures were then used as seeds for elongation of pyrene-labeled actin monomers. The initial rate of elongation increased with the concentration of G1+ (Figure 1C, black) indicative of G1+ severing actin filaments and elongation from the free pointed ends. The severing activity of G1+ was confirmed by visualizing actin filaments with rhodamine phalloidin by light microscopy (Figure 1D and E; Blanchoin et al, 2000). G1+ generated short filaments (Figure 1E; mean length=1.3 μm) in comparison to actin filaments alone (Figure 1D; mean length=10 μm). Similarly, G1-Tβ4 severs actin filaments (Figure 1F; mean length=1.9 μm). However, in the elongation assay, the initial rate of elongation increases to a maximum before declining (Figure 1C, red), suggesting that G1-Tβ4 is more than a simple severing protein and may also interfere with pointed end assembly. These data suggest that while the presence of G1, within G1-Tβ4, dominates the activity of the hybrid protein, the Tβ4 portion also recognizes its binding site on actin monomers reducing the efficiency of the complex in capping the barbed ends of actin filaments.
Figure 1.
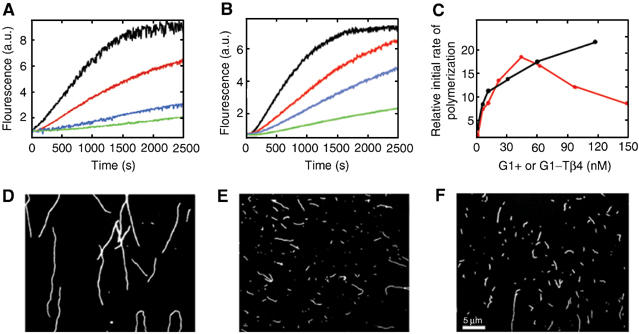
Characterization of the effect of G1+ and G1-Tβ4 on actin filaments. (A, B) Time course of actin polymerization in the presence of G1+ or G1-Tβ4. The fluorescence intensity of 4 μM actin (5% pyrene labeled) was followed during polymerization. (A) Black, actin alone; red, addition of 20 nM G1+; blue, 270 nM G1+; and green, 540 nM G1+. (B) Black, actin alone; red, addition of 50 nM G1-Tβ4; blue, 200 nM G1-Tβ4; and green, 600 nM G1-Tβ4. (C) Elongation assay for the effect of G1+ or G1-Tβ4 on the number of free actin filament ends. Mixtures of G1+ or G1-Tβ4 and 1 μM actin were equilibrated before the addition of 1 μM pyrene–actin monomers and polymerization was followed by fluorescence. The dependence of the relative initial rate of polymerization was calculated from the initial slope of the elongation curves and is plotted against concentration of G1+ (black) and G1-Tβ4 (red). (D–F) Fluorescence micrographs of (D) actin filaments, (E) actin filaments incubated with 80 nM G1+ and (F) actin filaments incubated with 80 nM G1-Tβ4.
Structure of the G1-Tβ4:actin complex
G1-Tβ4 was bound to rabbit muscle actin and purified by gel filtration chromatography. Crystals grown from the complex diffracted to a resolution of 2 Å and resulted in the present structure (Figure 2A and B; Table I). G1 (royal blue) binds to actin (sky blue) in the established manner between subdomains 1 and 3 (McLaughlin et al, 1993). The homologous region between Tβ4 and gelsolin (red, Tβ4 residues 17–23, gelsolin residues 149–155) extends up the face of actin subdomain 1 and includes a short β-sheet-like interaction between gelsolin residue His151 and actin residues 22–25 (Irobi et al, 2003). The Tβ4 sequence (gold) continues in this direction, forming another β-sheet interaction with actin subdomain 1 (Tβ4 residues 24–26 and actin residues 28–30). The chain then travels up the interface between actin subdomains 2 and 4, ending in an α-helix (residues 30–40) that caps the pointed end of the monomer at the junction between subdomains 2 and 4. There is no interpretable electron density for the final four Tβ4 residues. Only one homologous residue is present in both the Tβ4 portion of the G1-Tβ4:actin structure and the previously published ciboulot:actin structure (Glu21 in Tβ4, Ser34 in ciboulot; Hertzog et al, 2004).
Figure 2.
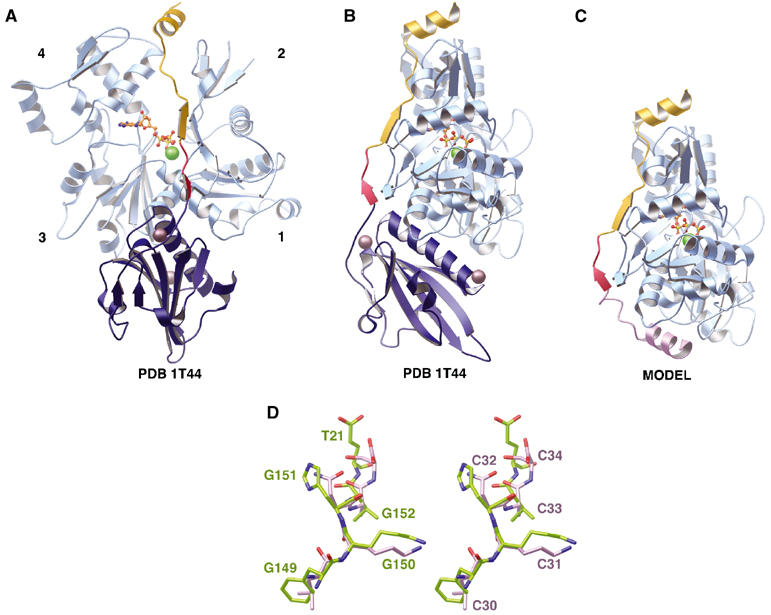
Tβ4 interactions with G-actin. (A) Structure of the G1-Tβ4:actin complex. The actin protomer is shown in sky blue with a bound ATP in orange (ball-and-stick representation) and a calcium ion (green sphere). The actin subdomains are labeled 1–4. The G1 portion of the hybrid (residues 27–149) is shown in royal blue with two associated calcium ions (dark spheres). The Tβ4 portion (residues 21–39) is depicted in gold. The red region of the G1-Tβ4 ribbon represents the G1 sequence that is homologous to the Tβ4 sequence (residues 17–20 in Tβ4). (B) A 90° rotation (clockwise when viewed from above) around the vertical axis compared to (A). (C) Model of Tβ4 bound to actin. Actin and Tβ4 residues 17–39 are taken from the structure in (B). The Tβ4 N-terminus (pink) is modeled by taking the homologous amino acids from the ciboulot:actin structure (PDB code 1SQK; Hertzog et al, 2004) after superimposing the actin structures. (D) Stereo view of the structural overlap within the LKKTET-related motif between the actin-bound forms of G1-Tβ4 and ciboulot. The actin structures from the present structure and the ciboulot:actin structure (PDB code 1SQK; Hertzog et al, 2004) were superimposed. Gelsolin (G) residues Phe149-Lys150-His151-Val152 and Tβ4 (T) residue Glu21 from the hybrid are shown in green and are labeled in the left panel. The homologous ciboulot (C) residues Leu30-Lys31-Asn32-Ala33-Ser34 are shown in pink and are labeled in the right panel.
Table 1.
Data collection and refinement statistics
| Wavelength | 1.0052 |
| Space group | P21 |
| Unit cell | a=56.85 Å, b=69.34 Å, c=80.23 Å, |
| α=γ=90.0°, β=94.93° | |
| Resolution (Å) | 20.0–2.0 (2.1–2.0) |
| Unique reflections | 39 223 |
| Redundancy | 3.3 (3.3) |
| Completeness (%) | 98.2 |
| Average I/σ | 11.4 (3.6) |
| Rmerge a (%) | 11.4 (19.8) |
| Rfactor b (%) | 14.4 (13.0) |
| Rfree c (%) | 19.5 (20.0) |
| Nonhydrogen atoms (G1, Tβ4, actin, ATP, Ca, water) | 1000, 157, 2836, 31, 3, 627 |
| Residues (G1, Tβ4, actin) | 28–152, 21–39, 6–38, 51–375 |
| Mean temperature factors (Å2) | |
| G1, Tβ4, actin, ATP, water | 19.0, 34.7, 21.0, 16.6, 26.5 |
| R.m.s. deviation bonds (Å) | 0.014 |
| R.m.s. deviation angles (deg) |
1.43 |
| Rmerge (∑∣I−〈I〉∣/∑〈I〉). | |
| Rfactor (∑∣∣Fo∣−∣Fc∣∣/∑∣Fo∣). | |
| Based on 5% of the data. | |
Crystallographic statistics indicate that this is a well-refined structure (Table I). Electron density maps show good density in the Tβ4 portion of this construct (Figure 3A), which displays an intimate association with actin, forming many specific interactions (Table II). The average B-factor within the Tβ4 portion is higher than for the rest of the structure, 34.7 Å2 compared to 21.0 and 19.0 Å2 for actin and G1, respectively. These values are in line with those refined for ciboulot:actin (38.6 Å2 for ciboulot and 25.6 Å2 for actin; Hertzog et al, 2004). The structure of WH2 motif lacks a hydrophobic core and thus displays relatively higher mobility, reflected in the average B-factors, even when bound to actin. Of residues 22–39, only four do not form side-chain interactions with actin (Ser30, Glu32, Glu35 and Gln39). However, Ser30 forms the hydrogen bond to Glu32 that initiates the Tβ4 C-terminal helix, leaving only Glu35 and Gln39 without functional roles.
Figure 3.

Conformational changes in the β-thymosin family on binding actin. (A) Stereo view of a representative portion of the 2Fo–Fc electron density covering Tβ4 residues 23–39, contoured at 1.2σ. The orientation is similar to that in Figure 2B. (B) The solution structure of Tβ9 (PDB code 1HJ0; Stoll et al, 1997). (C) Solution structure of Tβ4 (Czisch et al, 1993). (D) Model of actin-bound Tβ4 from Figure 2C. In each representation, the models are aligned based on the C-terminal minus-end capping helix and colored as in Figure 2B.
Table 2.
Tβ4:actin interactions
| Tβ4 residues | Actin residues within 3.9 Å |
|---|---|
| Thr22 | Ala26, Pro27, Val30, Tyr337 |
| Gln23 | Arg28, Ala29, Val30 |
| Gln24 | Val30, Tyr337 |
| Lys25 | Val30, Phe31, Pro32, Asp56, Glu93 |
| Asn26 | Pro32, Gln59, Arg210 |
| Pro27 | Gln59 |
| Leu28 | Arg210, Asp211 |
| Pro29 | Ala204, Pro243 |
| Lys31 | Pro243, Asp244 |
| Thr33 | Arg62, Gly63 |
| Ile34 | Thr202, Glu205, Pro243 |
| Gln36 | Gly63, Ile64 |
| Glu37 | Arg62, Thr202, Thr203, Ala204 |
| Lys38 | Thr202 |
Within the homologous region shared by Tβ4 and gelsolin (Tβ4 residues 17–23; Figure 4), mutagenesis studies have identified Lys18 to be an important binding residue and have suggested that the LKKTET motif (residues 17–22) should not form a helical structure in order to bind to actin (Simenel et al, 2000; Irobi et al, 2003). This motif adopts an extended conformation in the actin-bound structure with gelsolin residue Lys150 (equivalent of Lys18 in Tβ4) interacting with actin residue Asp24. In this structure, Thr6 is the first ordered actin residue, which lies at a distance of 9 Å from gelsolin residue Lys150. This is consistent with the observation that Tβ4 residue Lys18 can be crosslinked to actin residues 1–4 (Safer et al, 1997). Comparison of this structure with the ciboulot:actin structure (Hertzog et al, 2004), a structure that terminates in the LKKTET motif, by superimposition of only the actin structures reveals that gelsolin residues 149–152 and ciboulot residues 30–33 also directly superimpose and confirms the validity of the hybrid design (Figure 2D). In the C-terminus of Tβ4, residue Lys38 has been crosslinked to actin residue Gln41 (Safer et al, 1997). While actin residues 39–50 are disordered in this structure, the distance between actin residue Pro38 and Tβ4 Lys38 is 14.2 Å, verifying that the C-terminus of Tβ4 also binds to an appropriate position on the surface of actin. Tβ4 residues 23–39 are not involved in crystal contacts, and are therefore unlikely to be perturbed by the crystal environment. Taken together, the crosslinking data, the structural similarity of the actin interaction of the LKKTET motif with ciboulot, the crystallographic statistics and the extensive binding interface allow us to assert that the structure of the Tβ4 portion of the G1-Tβ4 construct provides an accurate representation of the binding geometry of the Tβ4:actin complex.
Figure 4.
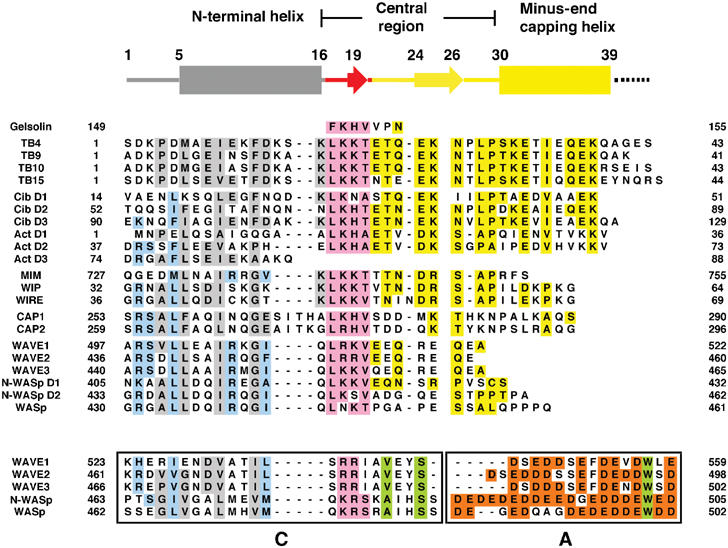
Sequence alignment of the WH2 family of proteins based on structural considerations. Yellow, conserved residues in the Tβ4 structure; pink, conserved residues in the G1 structure; gray, Tβ4 homologous residues in the model; pale blue, conserved residues in the model throughout the WH2 family with the exception of the Tβ4 subfamily; orange, acidic residues within the A region; green, nonacidic conserved residues in the CA regions that are not related to the WH2 family. The CA regions are shown boxed. The C regions appear to have homology with the WH2 motif. The A regions are included for size comparison and show no homology with the WH2 family. All sequences are human, except Tβ9 (bovine), actobindin (amoeba) and ciboulot (Drosophila).
The LKKTET motif also has been proposed to exist in several non-WH2-containing proteins, including the villin head piece, tropomyosin, myosin, fimbrin and α-actinin (Vaduva et al, 1997). Barring a structural rearrangement in this region, away from a helical conformation, these proteins will adopt a different actin-binding mode.
Model of Tβ4:actin complex
A variety of evidence points towards the N-terminal 16 residues of Tβ4, which were not included in the G1-Tβ4 construct, adopting a mainly α-helical conformation. The NMR structures of Tβ4 in water or an alcohol:water mix and the NMR structure of Tβ9 in alcohol:water mix (Figure 3B and C) display a helical nature in this region (Zarbock et al, 1990; Czisch et al, 1993; Stoll et al, 1997). A peptide consisting of Tβ4 residues 5–20 is induced to form a helix in trifluoroethanol solutions, as monitored by circular dichroism, and its affinity for actin is enhanced by an order of magnitude in these solutions (Feinberg et al, 1996). Mutagenesis analysis has demonstrated that this N-terminal helix of Tβ4 functionally terminates at residue 16 and that it interacts with actin through a hydrophobic patch (Met6, Ile9 and Phe12, residues that are particularly well conserved within the WH2 family of proteins; Figure 4), and through Lys14 forming a charged interaction with actin (Van Troys et al, 1996; Simenel et al, 2000). Furthermore, Tβ4 residue Lys3 has been crosslinked directly to actin residue Glu167 (Safer et al, 1997). All these data are consistent with the recently published structure of the N-terminus of ciboulot bound to actin (Hertzog et al, 2004).
We constructed a model of whole Tβ4 bound to actin, based on the present structure, in three steps (Figure 2C). First, gelsolin residues 27–148 were removed. Second, Tβ4 residues LKKT replaced the homologous gelsolin region FKHV. Finally, ciboulot residues equivalent to Tβ4 residues 1–16, from the superimposed ciboulot:actin structure, were included (Hertzog et al, 2004). The model predicts the Tβ4 N-terminal helix to approximate the path of the long helix from G1, albeit with opposite polarity (Figure 2B and C).
Comparison of the NMR structures of Tβ4 and Tβ9 determined in alcohol/water mixes with the actin-bound model of Tβ4 (Figure 3B–D) illustrates a number of similarities (Zarbock et al, 1990; Stoll et al, 1997). Each structure contains a C-terminal helix preceded by an unstructured region, and an N-terminal helix. Despite these similarities, the relationships among these structural elements are very different. Tβ4 is known to be flexible in aqueous solution, adopting the stable actin-bound conformation only in the presence of actin (Safer and Chowrashi, 1997; Domanski et al, 2004). Hence, Figure 3B and C represents a sample of possible Tβ4 solution conformations that are able to fold on the surface of actin to adopt the single actin-bound structure, modeled in Figure 3D. The large percentage of the surface of Tβ4 in contact with actin reflects an enthalpic compensation for the unfavorable entropy change associated with formation of the complex.
Discussion
Competition with other actin-binding proteins
Tβ4 and gelsolin directly compete in binding to actin (Figure 2) through sharing an extensive, overlapping binding site (Ballweber et al, 1997). Similarly, the structures of gelsolin domain 4 (G4) and DBP in complex with actin (not shown) confirm that gelsolin and DBP sterically compete with Tβ4 for the surface of actin (Robinson et al, 1999; Otterbein et al, 2002). Superposition of actin-bound structures demonstrates smaller overlap of the binding sites for DNase I and profilin with Tβ4 than observed for gelsolin and DBP (Figure 5A and B), in line with crosslinking data (Kabsch et al, 1990; Schutt et al, 1993; Ballweber et al, 1997). The actin-binding sites of profilin and the Tβ4 model show minor steric overlap. Biochemically, both profilin (residue Arg88; Korenbaum et al, 1998) and Tβ4 (residue Lys3; Safer et al, 1997) have been shown to bind to actin residue Glu167. Hence, competition between profilin and N-terminus of Tβ4 for actin (Yarmola et al, 2001), probably mediated through residue Glu167, is a likely mechanism for harvesting the Tβ4 store of ATP–actin during cell movement.
Figure 5.
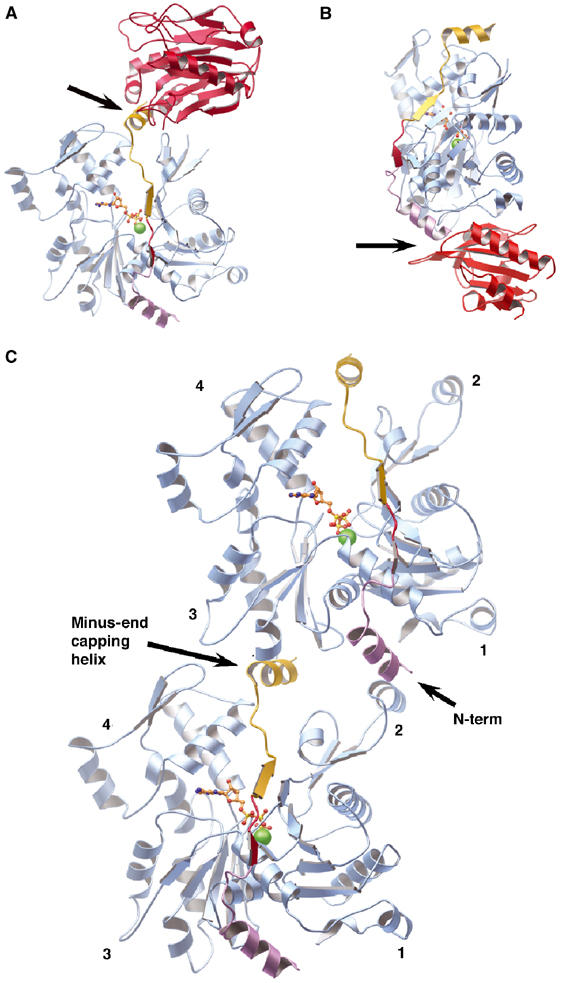
Competition for the Tβ4 actin-binding site. (A) Model of the competition between Tβ4 and DNase I for binding actin. The structure of actin:DNase I (PDB code 1ATN; Kabsch et al, 1990) and the Tβ4:actin model are superimposed, with only one actin shown. DNase I is drawn in red. The arrow indicates a structural clash. (B) Model of the competition between Tβ4 and profilin for binding actin. The structure of profilin:actin (PDB code 2BTF; Schutt et al, 1993) has been superimposed on the Tβ4:actin model, and profilin is depicted in red. The arrow indicates a structural clash. (C) Model of the interactions of the WH2 domain family with F-actin. Tβ4 docked onto the side of an actin filament based on superimposing the actins from two copies of the Tβ4:actin model (Figure 2C) on two actins from a modified version of the Holmes model of the filament (Holmes et al, 1990). The actins are colored sky blue and Tβ4s are painted as in Figure 2A. This representation shows that capping by the N-terminal helix and the minus-end capping helix prevent the Tβ4:actin complex from joining either end of a filament. The model also demonstrates that multiple WH2 repeat proteins will bind actin protomers in a longitudinal manner, along the axis of the filament.
Mechanism of actin sequestration
The mechanism of Tβ4 sequestration of actin monomers is illustrated by superimposing copies of the Tβ4:actin model (Figure 2C) onto two actin protomers in a model of the actin filament. We have constructed a model of an ADP–actin filament (ADP model) by overlaying the structure of rhodamine-modified ADP–actin onto each actin protomer in a modified version of the Holmes model for F-actin (Figure 5C; Holmes et al, 1990; Otterbein et al, 2001). The N-terminus and the C-terminal helix of Tβ4 are observed to prevent sterically the Tβ4-bound actin from joining either the pointed end or barbed end of an actin filament, respectively, in line with our functional data. Hence, Tβ4 precludes actin from establishing longitudinal contacts with a filament (Hertzog et al, 2004) and not by disrupting lateral contacts (Carlier et al, 1996). This mechanism agrees with earlier models that arose from crosslinking data, in that Tβ4 caps both ends of an actin monomer (Safer et al, 1997; Hertzog et al, 2004). However, the C-terminal cap lies between actin subdomains 2 and 4 rather than being bound exclusively by subdomain 2 as had been predicted.
Tβ4 interacts at high concentrations with actin filaments (Carlier et al, 1996). This is not predicted by the Tβ4:actin model (Figure 5C). A structural change, involving either the loss of binding of the extremities of Tβ4 or a change in actin filament conformation, is required to overcome the capping nature of the termini of Tβ4 in order to allow filament binding. The change in twist of Tβ4 decorated filaments, from crossover spacing of 36.0–40.5 nm, revealed by electron microscopy, may at least partially accommodate Tβ4 (Ballweber et al, 2002).
Actin nucleotide exchange is diminished on binding Tβ4, leading to the notion that Tβ4 closes the nucleotide-binding cleft (De La Cruz et al, 2000). The width of the nucleotide cleft in this structure falls into the normal range refined for other actin structures, with the exception of the profilin-bound open state of ATP–actin (Chik et al, 1996). It should be noted that most other actin structures also have been elucidated in the presence of agents that lower nucleotide exchange, for example, gelsolin and DNase I (Kabsch et al, 1990; Robinson et al, 1999; Irobi et al, 2003). This structure shows that the C-terminal capping helix of Tβ4 occupies a position between actin subdomains 2 and 4 that locks actin into the standard conformation. Hence, prevention of the dynamic opening and closing of the two halves of actin inhibits nucleotide exchange.
The Tβ4 binding interface also suggests how Tβ4 preferentially binds to MgATP–actin (50-fold higher affinity than for MgADP–actin; Carlier et al, 1993), by sensing conformational changes in actin subdomain 2 relative to subdomain 4. Indeed, the ADP–actin:ciboulot structure lacks density for the capping helix indicative of a compromised binding site (Hertzog et al, 2004). However, missing in metastasis (MIM), a WH2-containing protein that lacks a capping helix also, displays nucleotide specificity (Mattila et al, 2003). Hence, nucleotide-sensing elements may reside in both termini of Tβ4 (Hertzog et al, 2004).
Members of the Tβ4 family of proteins share strong sequence homology and sequester actin in a similar manner. The functional differences between Tβ4 and Tβ15 have been determined to lie in the last 10 amino acids (Eadie et al, 2000). In the present structure, only one residue varies between the two proteins (Q39E), a residue that is not in contact with actin, and the final four residues are disordered (Figure 4). Within this disordered region, Gly41 may serve to terminate the Tβ4 C-terminal helix. Thus, the Tβ15 helix may be longer and provide additional interaction with actin.
Implications for WH2 family proteins
β-Thymosins are members of a wider family of WH2 domain-containing proteins (Paunola et al, 2002). In general, the sequence similarity of the WH2 motifs in comparison to Tβ4 is clear in the N-terminal half, which includes the N-terminal amphipathic helix and gelsolin-related central region (Figure 4). Sequence similarity in the C-terminal half is less reliable and the minus-end capping helix is absent in many cases. One particular member of note is MIM, a protein containing a C-terminal, truncated WH2 domain that lacks the minus-end capping helix. This protein sequesters actin monomers, but allows barbed end assembly (Mattila et al, 2003). The absence of the minus-end capping helix is consistent with the ability of the MIM:actin complex to join only the barbed end of a filament. Once bound, we propose that a conformational change in the ATP–actin monomer to an ATP F-actin conformation releases MIM in a mechanism paralleling the release of profilin from the profilin:actin complex (dos Remedios et al, 2003). Given the structural relationship of Tβ4 and profilin, with respect to actin (Figure 5B), we suggest that the WH2 N-terminal helix functions as an actin conformation sensor in this process. This mechanism may be a common feature for WH2-containing proteins and an important factor in general actin dynamics. The presence of the WH2 N-terminal actin conformational sensor allows polymerization-promoting proteins to be released after contributing an actin protomer to the filament, while ensuring that actin-sequestering proteins are prevented from capping the barbed end of the filament.
More exotic members of the WH2 family are the multiple repeat proteins, actobindin (two repeats) and ciboulot (three repeats), which show a high degree of amino-acid sequence conservation throughout the Tβ4 motif (Figure 4). Curiously, actobindin appears to also contain a third partial repeat. The sequence homology in each of the Tβ4-like motifs, domains that have no structural core and hence have been conserved purely due to functional pressures, suggests that these proteins will be able to bind multiple actin protomers, as has been described for actobindin (Bubb et al, 1991). Figure 5C, therefore, also represents a model of a double domain Tβ4-like protein, such as actobindin. The C-terminus of the first Tβ4 repeat (gold) is seen to be in close proximity to the N-terminus of the second (pink), and as such, multiple WH2 repeat proteins will associate with longitudinally related actin protomers, with respect to a filament.
Ciboulot and actobindin facilitate the addition of actin monomers to the barbed end but cap the pointed end of F-actin (Boquet et al, 2000; Hertzog et al, 2002). Hertzog et al (2004) demonstrated a second mechanism by which a WH2 domain may participate in barbed end elongation. The C-terminal cap from domain 1 of ciboulot displays lower affinity for actin than that of Tβ4. Thus, the C-terminal cap dynamically moves on and off the bound actin allowing the complex to join the barbed end of a filament.
A third mechanism for these activities can be proposed by considering the proximity of the N-terminal sensor helix and the C-terminal minus-end capping helix within two connected Tβ4-like repeats. The actin conformational sensor from one repeat may be able to release the C-terminal cap from and actin monomer bound to the adjacent C-terminal repeat during barbed end elongation (Figure 5C). At the pointed end of the filament, the final C-terminal minus-end capping helix is not sensitive to filament formation and provides a stable, pointed-end cap.
WH2 motif in Arp2/3 activation
A particularly prominent subset of WH2-containing proteins is the WASp/WAVE family, proteins that contribute an actin monomer to, and activate, the arp2/3 complex during filament branching (Welch and Mullins, 2002). The C-termini of the WASp/WAVE proteins comprise the VCA motif (V, another notation for the WH2 motif; C, a connecting region; and A, an acidic region). During VCA-induced arp2/3 activation, the V region binds to G-actin and the CA regions contact arp2/3 (Panchal et al, 2003). N-WASp, which contains two tandem V regions, will associate with two longitudinally related actin monomers in a similar manner to actobindin (Figures 4 and 5C). Sequence comparison of the C and V motifs shows many similarities, particularly in the N-terminal amphipathic helices (Figure 4). An interesting possibility is that the C region functionally and structurally mimics the WH2 motif, but with specificity for arp2 rather than for actin (Hertzog et al, 2004). This notion is consistent with VCA binding increasing the affinity of arp2/3 for ATP (Le Clainche et al, 2001), as the C region would stabilize a closed form of arp2 in a similar manner to the stabilization of the closed form of actin induced through Tβ4 binding.
Superimposing actin and arp2, from the Tβ4:actin model (Figure 2C) and the arp2/3 structure (Robinson et al, 2001), respectively, provides a model as to how the C region may bind to arp2/3 and affords new insight into the activation mechanism (Figure 6). A major implication of this model is that the C region will dock the V region-bound actin onto arp2, in the daughter filament, in an actin filament-like orientation during arp2/3 activation. Within the model (Figure 6), the N-terminus of the C region lies at the interface between arp2 (yellow) and ARC1 (p41, green) and the A region at the interface between arp2 (yellow) and arp3 (red) and proximal to ARC3 (p21, leaf green). The VCA motif has been chemically crosslinked to these four subunits (Zalevsky et al, 2001). This location is consistent with the rotation model of arp2/3 activation (Robinson et al, 2001), in that the A region is well situated to influence the position of arp2 with respect to arp3 and to allow the two halves of arp2/3 to rotate, enabling arp2 and arp3 to adopt a filament-like orientation. However, Figure 6 also suggests an alternative mechanism, which we shall refer to as the arp2 migration model. Arp2 may be released from its inhibitory interactions with ARC1 and arp3 through competitive interactions with the C and A regions. As such, arp2 will be tethered simply by the flexible N-terminal extension of ARC5 (p16, purple). The A region then may guide arp2 to into a filament-like orientation with arp3.
Figure 6.
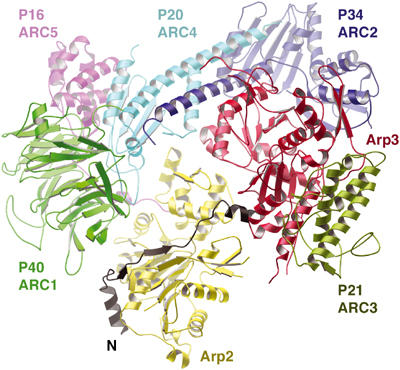
Model of the role of the CA motif in arp2/3 activation. Tβ4 docked onto arp2 within the ATP model of arp2/3 (based on PDB code 1K8K; Robinson et al, 2001). Arp2/3 subunits are colored as follows: Arp3, red; arp2, yellow; ARC1, green; ARC2, royal blue; ARC3, leaf green; ARC4, cyan; and ARC5, purple. The Tβ4:actin model is superimposed on arp2, with only Tβ4 (black) retained in the figure with its N-terminus labeled N. In this figure, Tβ4 residues 1–25 represent the C region of the VCA arp2/3 activators, and residues 26–39 indicate the size and approximate location of the A region. This model is not meant to represent the true structure of the A region, rather to demonstrate that if the A region were to adopt an extended conformation, then it would be of an appropriate size and in the right locale to be able to contact both arp3 (red) and ARC3 (leaf green).
The arp2 migration model, while speculative, has three particularly attractive features. First, all the arp2/3 subunits are implicated in the activation process: Arp2 and arp3 form the nucleus for the daughter filament; ARC1 maintains arp2 in an inactive conformation; ARC5 delivers arp2 to its nucleating position; ARC3 stabilizes arp2 in its nucleating position through direct interaction and through interaction with the A region of VCA; and finally, ARC2 and ARC4 (p20) provide the framework on which the conformational changes and the interaction with the mother filament occur (Gournier et al, 2001). Second, the conformation sensing helices within VCA account for the transient binding of WASp/WAVE proteins during branching. Lastly, the migration model, as the rotation model (Robinson et al, 2001), arose through the superimposition of known structures, and as such, model coordinates are available to be tested against electron microscopy data.
Materials and methods
Protein production and crystallization
The gene segments coding for human gelsolin amino-acid residues 27–152 (G1) and Tβ4 residues 21–43 were obtained by polymerase chain reaction. The G1-Tβ4 hybrid was generated by the incorporation of an Eco721 site on the N-terminus of Tβ4 and cloned into the pHIS-8 vector, which contains eight histidines and a thrombin cleavage site N-terminal of G1-Tβ4. The encoded protein does not contain an amino-acid insertion between G1 and Tβ4. G1+ (gelsolin residues 25–160) and G1–Tβ4 were expressed in Escherichia coli BL21-CodonPlus (DE3) cells and purified essentially as previously described for G1+ (Irobi et al, 2003). Both G1+ and G1-Tβ4 contain an additional, nongelsolin sequence Gly-Ser-His-Gly at their N-termini following thrombin cleavage. G-actin and phalliodin-actin were prepared as described previously (Blanchoin et al, 2000; Irobi et al, 2003). To form G1-Tβ4:actin, purified G1-Tβ4 was added at a 1.5-fold molar excess over G-actin in the presence of 0.1 mM CaCl2. G1-β4:actin complex was purified by Superdex 200 gel filtration chromatography (Amersham Biosciences) in buffer A (2 mM Tris–HCl, 0.2 mM ATP, 0.2 mM CaCl2, 1 mM NaN3, 0.5 mM dithiothreitol, pH 7.6). Fractions were analyzed by SDS–PAGE and those containing the complex were pooled and concentrated to 17 mg/ml.
Crystals of G1-Tβ4:actin were obtained by the microbatch method at 20°C in 2 μl drops containing a 1:1 (v/v) mixture of protein solution and precipitant, 20% (v/v) polyethyleneglycol 8000 (Fluka), 0.1 M sodium acetate and 10 mM CaCl2, pH 6.5. Crystals were soaked (2 h) in 12% polyethyleneglycol 8000, 20% glycerol and 0.1 M sodium acetate, pH 6.5, before flash freezing in liquid nitrogen.
Assays
In the polymerization assay, 4 μM actin (5% pyrene-labeled, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.5 mM dithiothreitol, 0.1 mM CaCl2, 0.2 mM ATP, 3 mM NaN3, 10 mM imidazole, pH 7) was polymerized by the addition of a 1:9 dilution of 10 × KMI (500 mM KCl, 10 mM MgCl2, 100 mM imidazole, pH 7) and followed by fluorescence intensity on safas Xenius and Mos-450 Bio-logic fluorimeters (excitation=366 nm, emission=407 nm).
The elongation assay for actin filament fragmentation assay was carried out in two steps. First, G1+ or G1-Tβ4 were reacted with preformed 1 μM unlabeled actin filaments for 1 min in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.5 mM dithiothreitol, 0.1 mM CaCl2, 0.2 mM ATP, 3 mM NaN3 and 10 mM imidazole, pH 7. Then, the preincubation mixture was used as seeds for the polymerization of 1 μM pyrenyl-actin monomers. Under these conditions, the rate of spontaneous polymerization is much slower than elongation from added filaments. The relative initial rate of elongation was calculated from the initial slope of the polymerization curves.
Microscopy
Actin (4 μM) was polymerized for 1 h and then diluted to 1 μM in the presence of G1+ or G1-Tβ4. After 10 min, 1 μM of rhodamine phalloidin was added. After a further 10 min, actin was diluted to a final concentration of 10 nM in fluorescence buffer (Blanchoin et al, 2000). Images were collected on a Zeiss Axioplan-2 microscope using Hamamatsu digital camera. Filament length was measured manually using Axiovison software.
Structure determination and refinement
Data were collected at 100 K at beamline ID-29 at the European Synchrotron Radiation Facility (ESRF) in Grenoble. Data were indexed and scaled in the programs MOSFLM and SCALA (CCP4, 1994). Structural analysis was initiated by molecular replacement using G1+:actin (Irobi et al, 2003) as the search model in the program AMORE (CCP4, 1994). The solution was unambiguous. Density improvement using wARP led to a high-quality electron density map (CCP4, 1994). The model was refined and a bulk solvent correction applied in REFMAC5, and in the last cycle of refinement TLS refinement was included prior to isotropic B-factor refinement after setting all B-factors to 30 Å2 (CCP4, 1994). The coordinates were refined as six TLS groups: one each for the four subdomains of actin and one each for the G1 and Tβ4 portions of the structure. This treatment improved the Rfree. Omit maps were referred to at each cycle of model building. Water molecules were added and the quality of the final model was assessed in PROCHECK (CCP4, 1994). No nonglycine residues lie in the disallowed or unfavorable regions of a Ramachandran plot.
Model construction
The model of whole Tβ4 bound to actin (Figure 2C) was constructed by superimposing the actin structures from ciboulot: actin structure (Hertzog et al, 2004) and the G1-Tβ4:actin structure. Only ciboulot residues equivalent to Tβ4 residues 1–16 were retained from the first structure and gelsolin residues 27–148 were also removed from the second structure. Finally, the Tβ4 sequence LKKT replaced the homologous gelsolin region FKHV to produce a model of Tβ4 residues 1–39 bound to actin.
The ADP model of the actin filament was constructed by superimposing the structure of rhodamine ADP–actin onto the Holmes model of the filament (Holmes et al, 1990; Otterbein et al, 2001). This provides a structurally robust subdomain 2, otherwise the two filament models are essentially identical. Figure 5C was generated by superimposing two copies of the Tβ4:actin model on two longitudinally related protomers in the filament model, retaining only the two Tβ4 chains.
An ATP-bound model of arp2/3 was constructed. First, subdomains 1 and 2 from the actin and arp3 structures were superimposed. Then, subdomains 3 and 4 from arp3 (with ARC3) were superimposed on the oriented actin structure, providing a model of arp3 with a closed nucleotide-binding cleft. Finally, arp2 was completed by including subdomains 1 and 2 from an actin structure that had been superimposed on arp2, based on subdomains 3 and 4. The model in Figure 6 was created by superimposing the Tβ4:actin model on arp2 within the ATP arp2/3 model, retaining only the Tβ4 portion from the Tβ4:actin model.
Coordinates
The coordinates and merged structure factors of the G1-Tβ4:actin complex have been deposited in the Protein Data Bank under accession code 1T44.
Acknowledgments
We are grateful to the ESRF for the provision of synchrotron radiation. We thank the Heart and Stroke Foundation of BC and Yukon (LDB) and the Swedish Medical Science Research Council (RCR) for financial support. EI thanks Gunnar Johansson of the Biochemistry Department, Uppsala University, for his advice and support.
Competing interests statement The authors declare that they have no competing financial interests.
References
- Ballweber E, Giehl K, Hannappel E, Huff T, Jockusch BM, Mannherz HG (1998) Plant profilin induces actin polymerization from actin:beta-thymosin complexes and competes directly with beta-thymosins and with negative co-operativity with DNase I for binding to actin. FEBS Lett 425: 251–255 [DOI] [PubMed] [Google Scholar]
- Ballweber E, Hannappel E, Huff T, Mannherz HG (1997) Mapping the binding site of thymosin beta4 on actin by competition with G-actin binding proteins indicates negative co-operativity between binding sites located on opposite subdomains of actin. Biochem J 327 (Part 3): 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweber E, Hannappel E, Huff T, Stephan H, Haener M, Taschner N, Stoffler D, Aebi U, Mannherz HG (2002) Polymerisation of chemically cross-linked actin:thymosin beta(4) complex to filamentous actin: alteration in helical parameters and visualisation of thymosin beta(4) binding on F-actin. J Mol Biol 315: 613–625 [DOI] [PubMed] [Google Scholar]
- Bertling E, Hotulainen P, Mattila PK, Matilainen T, Salminen M, Lappalainen P (2004) Cyclase-associated-protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian non-muscle cells. Mol Cell Biol 15: 2324–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD (2000) Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404: 1007–1011 [DOI] [PubMed] [Google Scholar]
- Boquet I, Boujemaa R, Carlier MF, Preat T (2000) Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell 102: 797–808 [DOI] [PubMed] [Google Scholar]
- Bubb MR, Lewis MS, Korn ED (1991) The interaction of monomeric actin with two binding sites on Acanthamoeba actobindin. J Biol Chem 266: 3820–3826 [PubMed] [Google Scholar]
- Carlier MF, Didry D, Erk I, Lepault J, Van Troys ML, Vandekerckhove J, Perelroizen I, Yin H, Doi Y, Pantaloni D (1996) Tbeta 4 is not a simple G-actin sequestering protein and interacts with F-actin at high concentration. J Biol Chem 271: 9231–9239 [DOI] [PubMed] [Google Scholar]
- Carlier MF, Jean C, Rieger KJ, Lenfant M, Pantaloni D (1993) Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: possible implication in the regulation of actin dynamics. Proc Natl Acad Sci USA 90: 5034–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCP4 (1994) Collaborative Computing Project 4, The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 [DOI] [PubMed] [Google Scholar]
- Chik JK, Lindberg U, Schutt CE (1996) The structure of an open state of beta-actin at 2.65 Å resolution. J Mol Biol 263: 607–623 [DOI] [PubMed] [Google Scholar]
- Czisch M, Schleicher M, Horger S, Voelter W, Holak TA (1993) Conformation of thymosin beta 4 in water determined by NMR spectroscopy. Eur J Biochem 218: 335–344 [DOI] [PubMed] [Google Scholar]
- De La Cruz EM, Ostap EM, Brundage RA, Reddy KS, Sweeney HL, Safer D (2000) Thymosin-beta(4) changes the conformation and dynamics of actin monomers. Biophys J 78: 2516–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski M, Hertzog M, Coutant J, Gutsche-Perelroizen I, Bontems F, Carlier M-F, Guittet E, van Heijenoort C (2004) Coupling of folding and binding of thymosin β4 upon interaction with monomeric actin monitored by nuclear magnetic resonance. J Biol Chem 279: 23637–23645 [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83: 433–473 [DOI] [PubMed] [Google Scholar]
- Eadie JS, Kim SW, Allen PG, Hutchinson LM, Kantor JD, Zetter BR (2000) C-terminal variations in beta-thymosin family members specify functional differences in actin-binding properties. J Cell Biochem 77: 277–287 [DOI] [PubMed] [Google Scholar]
- Feinberg J, Heitz F, Benyamin Y, Roustan C (1996) The N-terminal sequences (5–20) of thymosin beta 4 binds to monomeric actin in an alpha-helical conformation. Biochem Biophys Res Commun 222: 127–132 [DOI] [PubMed] [Google Scholar]
- Gournier H, Goley ED, Niederstrasser H, Trinh T, Welch MD (2001) Reconstitution of human Arp2/3 complex reveals critical roles of individual subunits in complex structure and activity. Mol Cell 8: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Hartwig JH (1992) Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol 118: 1421–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog M, van Heijenoort C, Didry D, Gaudier M, Coutant J, Gigant B, Didelot G, Preat T, Knossow M, Guittet E, Carlier MF (2004) The β-thymosin/WH2 domain: structural basis for the switch from inhibition to promotion of actin assembly. Cell 117: 611–623 [DOI] [PubMed] [Google Scholar]
- Hertzog M, Yarmola EG, Didry D, Bubb MR, Carlier MF (2002) Control of actin dynamics by proteins made of beta-thymosin repeats: the actobindin family. J Biol Chem 277: 14786–14792 [DOI] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W (1990) Atomic model of the actin filament. Nature 347: 44–49 [DOI] [PubMed] [Google Scholar]
- Huff T, Muller CS, Otto AM, Netzker R, Hannappel E (2001) Beta-thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 33: 205–220 [DOI] [PubMed] [Google Scholar]
- Irobi E, Burtnick LD, Urosev D, Narayan K, Robinson RC (2003) From the first to the second domain of gelsolin: a common path on the surface of actin? FEBS Lett 552: 86–90 [DOI] [PubMed] [Google Scholar]
- Jean C, Rieger K, Blanchoin L, Carlier MF, Lenfant M, Pantaloni D (1994) Interaction of G-actin with thymosin beta 4 and its variants thymosin beta 9 and thymosin beta met9. J Muscle Res Cell Motil 15: 278–286 [DOI] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC (1990) Atomic structure of the actin:DNase I complex. Nature 347: 37–44 [DOI] [PubMed] [Google Scholar]
- Kang F, Purich DL, Southwick FS (1999) Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J Biol Chem 274: 36963–36972 [DOI] [PubMed] [Google Scholar]
- Korenbaum E, Nordberg P, Bjorkegren-Sjogren C, Schutt CE, Lindberg U, Karlsson R (1998) The role of profilin in actin polymerization and nucleotide exchange. Biochemistry 37: 9274–9283 [DOI] [PubMed] [Google Scholar]
- Le Clainche C, Didry D, Carlier MF, Pantaloni D (2001) Activation of Arp2/3 complex by Wiskott–Aldrich syndrome protein is linked to enhanced binding of ATP to Arp2. J Biol Chem 276: 46689–46692 [DOI] [PubMed] [Google Scholar]
- Mattila PK, Salminen M, Yamashiro T, Lappalainen P (2003) Mouse MIM, a tissue-specific regulator of cytoskeletal dynamics, interacts with ATP-actin monomers through its C-terminal WH2 domain. J Biol Chem 278: 8452–8459 [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Gouch JT, Mannherz H-G, Weeds AG (1993) Structure of gelsolin segment 1–actin complex and the mechanism of filament severing. Nature 346: 685–692 [DOI] [PubMed] [Google Scholar]
- Otterbein LR, Cosio C, Graceffa P, Dominguez R (2002) Crystal structures of the vitamin D-binding protein and its complex with actin: structural basis of the actin-scavenger system. Proc Natl Acad Sci USA 99: 8003–8008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LR, Graceffa P, Dominguez R (2001) The crystal structure of uncomplexed actin in the ADP state. Science 293: 708–711 [DOI] [PubMed] [Google Scholar]
- Panchal SC, Kaiser DA, Torres E, Pollard TD, Rosen MK (2003) A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol 10: 591–598 [DOI] [PubMed] [Google Scholar]
- Pantaloni D, Carlier MF (1993) How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell 75: 1007–1014 [DOI] [PubMed] [Google Scholar]
- Paunola E, Mattila PK, Lappalainen P (2002) WH2 domain: a small, versatile adapter for actin monomers. FEBS Lett 513: 92–97 [DOI] [PubMed] [Google Scholar]
- Robinson RC, Mejillano M, Le VP, Burtnick LD, Yin HL, Choe S (1999) Domain movement in gelsolin: a calcium-activated switch. Science 286: 1939–1942 [DOI] [PubMed] [Google Scholar]
- Robinson RC, Turbedsky K, Kaiser DA, Marchand JB, Higgs HN, Choe S, Pollard TD (2001) Crystal structure of Arp2/3 complex. Science 294: 1679–1684 [DOI] [PubMed] [Google Scholar]
- Safer D, Chowrashi PK (1997) Beta-thymosins from marine invertebrates: primary structure and interaction with actin. Cell Motil Cytoskeleton 38: 163–171 [DOI] [PubMed] [Google Scholar]
- Safer D, Sosnick TR, Elzinga M (1997) Thymosin beta 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry 36: 5806–5816 [DOI] [PubMed] [Google Scholar]
- Schutt CE, Myslik JC, Rozycki MD, Goonesekere NC, Lindberg U (1993) The structure of crystalline profilin–beta-actin. Nature 365: 810–816 [DOI] [PubMed] [Google Scholar]
- Simenel C, Van Troys M, Vandekerckhove J, Ampe C, Delepierre M (2000) Structural requirements for thymosin beta4 in its contact with actin. An NMR-analysis of thymosin beta4 mutants in solution and correlation with their biological activity. Eur J Biochem 267: 3530–3538 [DOI] [PubMed] [Google Scholar]
- Stoll R, Voelter W, Holak TA (1997) Conformation of thymosin beta 9 in water/fluoroalcohol solution determined by NMR spectroscopy. Biopolymers 41: 623–634 [DOI] [PubMed] [Google Scholar]
- Sun HQ, Kwiatkowska K, Yin HL (1996) Beta-thymosins are not simple actin monomer buffering proteins. Insights from overexpression studies. J Biol Chem 271: 9223–9230 [PubMed] [Google Scholar]
- Vaduva G, Martin NC, Hopper AK (1997) Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J Cell Biol 139: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Dewitte D, Goethals M, Vandekerckhove J, Ampe C (1996) Evidence for an actin binding helix in gelsolin segment 2; have homologous sequences in segments 1 and 2 of gelsolin evolved to divergent actin binding functions? FEBS Lett 397: 191–196 [DOI] [PubMed] [Google Scholar]
- Weber A, Nachmias VT, Pennise CR, Pring M, Safer D (1992) Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry 31: 6179–6185 [DOI] [PubMed] [Google Scholar]
- Welch MD, Mullins RD (2002) Cellular control of actin nucleation. Annu Rev Cell Dev Biol 18: 247–288 [DOI] [PubMed] [Google Scholar]
- Yarmola EG, Parikh S, Bubb MR (2001) Formation and implications of a ternary complex of profilin, thymosin beta 4, and actin. J Biol Chem 276: 45555–45563 [DOI] [PubMed] [Google Scholar]
- Zalevsky J, Grigorova I, Mullins RD (2001) Activation of the Arp2/3 complex by the Listeria acta protein. Acta binds two actin monomers and three subunits of the Arp2/3 complex. J Biol Chem 276: 3468–3475 [DOI] [PubMed] [Google Scholar]
- Zarbock J, Oschkinat H, Hannappel E, Kalbacher H, Voelter W, Holak TA (1990) Solution conformation of thymosin beta 4: a nuclear magnetic resonance and simulated annealing study. Biochemistry 29: 7814–7821 [DOI] [PubMed] [Google Scholar]


