Adrenocortical carcinomas (ACC) are rare malignant tumors of the adrenal cortex whose prognosis remains ominous. Considerable progress has been made during the last few years in the identification of the molecular mechanisms implicated in their pathogenesis. Overexpression of the transcription factor Steroidogenic Factor-1 (SF-1/NR5A1), a pivotal regulator of adrenocortical development and steroidogenic function, has a stage-independent negative prognostic value in patients with ACC.1 Studies in cell lines and animal models have shown that an increased SF-1 dosage is able to increase adrenocortical cell proliferation and triggers tumorigenesis.2 Those effects are, at least in part, explained by differential regulation by overexpressed SF-1 of a set of genes that are largely distinct from those ones that are regulated by the same transcription factor in conditions of basal expression.3 Understanding of the cellular and molecular function of those SF-1 dosage-dependent target genes is important to shed new light upon the mechanisms of adrenocortical tumorigenesis and to provide potential new tools for therapy.
We have recently characterized the role of one of these genes whose expression is activated in ACC cells following SF-1 overexpression, termed FATE1 (fetal and adult testis expressed).4 FATE1 encodes a cancer-testis antigen, a protein whose expression is restricted to testis within normal tissues, but which is also expressed in some cancer types and against which a humoral immune response can be detected.5 These features make cancer-testis antigens an interesting potential target for immunotherapy. FATE1 transcript and protein are expressed at only very low levels in ACC H295R cells, while they are increased several fold following SF-1 overexpression. We have previously demonstrated that SF-1 binds directly to a consensus binding site in the FATE1 promoter, activating its expression.2 FATE1 encodes a 21 kDa protein which bears similarity in its C-terminal domain (which includes one coiled-coil and one transmembrane regions) to Mff (mitochondrial fission factor), a protein involved in the control of mitochondrial and peroxisomal fission. Similarly to Mff, FATE1 is associated with the outer mitochondrial membrane both endogenously in H295R cells and in transfected HeLa cells. However, surprisingly, a FATE1 mutant bearing a point mutation of a critical leucine (L151) in the coiled-coil region of the C-terminal domain loses mitochondrial localization and is associated to internal membranes (mostly ER). The isolated FATE1 transmembrane domain fused to GFP has the same localization. These data suggest that the FATE1 transmembrane region is inserted into the ER membrane and that the protein interacts with mitochondrial partners through the C-terminal coiled-coil domain. In fact immunoprecipitation followed by mass spectrometry identified both ER and mitochondrial proteins as interacting partners of FATE1. Consistent with this finding, biochemical fractionation studies have shown that a conspicuous fraction of intracellular FATE1 localizes in MAM, the pool of ER membranes that lie in close contact with mitochondria. Among other partners, we identified emerin [EMD, the protein mutated in Emery-Dreifuss muscular dystrophy (EDMD)] as a FATE1 interactor. Most studies have focused on the roles of EMD in the organization of chromatin structure and gene regulation as a component of the nuclear lamina structure, however some previous studies also showed that a sizeable fraction of EMD is localized in the ER. Our finding that EMD interacts with FATE1 and is enriched in MAM suggests a role for this protein also in communication between the ER and mitochondria, with potential consequences on the pathogenesis of EDMD. FATE1, which resides in high molecular weight complexes in H295R cells, was also found to interact with Mic60/mitofilin, a mitochondrial protein that plays a pivotal role in the organization of the MICOS complex regulating mitochondrial morphology and function and which is also found in the MAM fraction. Bases on this finding, it is tempting to speculate that Mic60/mitofilin may have additional structural roles in the organization of multiprotein complexes connecting ER and mitochondria (ER-mitochondria organizing network: ERMIONE).6 We could also show physical uncoupling between ER and mitochondria upon FATE1 expression using different morphological and ultrastructural tools.4 FATE1 localized in MAM has an anti-tethering function between the 2 organelles (Fig. 1), as shown by the finding that FATE1 expression reduced mitochondrial calcium transfer and caspase 3/7 activity triggered by pro-apoptotic stimuli that require ER–mitochondria Ca2+ transfer. Consistent with these data, FATE1 counteracted ACC cell death triggered by mitotane, the most commonly used chemotherapeutic agent in the therapy of ACC. Remarkably, a previous siRNA screening study also identified FATE1 as one of the genes that sensitize a panel of non-small-cell lung cancer cell lines to paclitaxel toxicity.7 Moreover, high FATE1 expression levels were significantly correlated with worse prognosis in a cohort of 141 ACC patients.4 Altogether, these data suggest that FATE1 through its ER-mitochondria uncoupling function has an important role as a dosage-dependent SF-1 target gene in determining the aggressive phenotype of ACC showing SF-1 overexpression. Future studies are warranted to investigate whether an immune response against FATE1 is present in ACC patients, which could be monitored for diagnostic or prognostic purposes, and whether modulation of FATE1 expression and/or function may represent a new therapeutic strategy in ACC.
Figure 1.
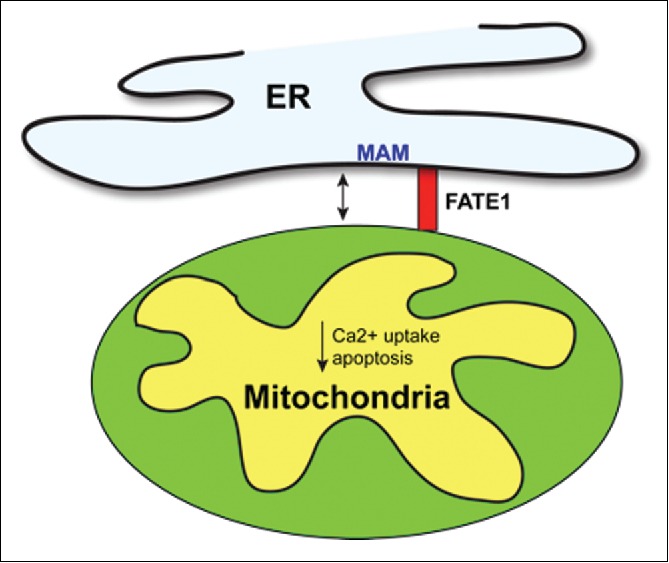
Diagram showing the action of FATE1 to increase ER–mitochondria distance and consequently decrease mitochondrial Ca2+ influx and apoptosis. ER, endoplasmic reticulum; MAM, mitochondria-associated membranes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Sbiera S, Schmull S, Assie G, Voelker H-U, Kraus L, Beyer M, Ragazzon B, Beuschlein F, Willenberg HS, Hahner S, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors.. J Clin Endocrinol Metab 2010; 95:E161-171; PMID:20660055; http://dx.doi.org/ 10.1210/jc.2010-0653 [DOI] [PubMed] [Google Scholar]
- [2].Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer.. Mol Endocrinol 2007; 21:2968-2987; PMID:17761949; http://dx.doi.org/ 10.1210/me.2007-0120 [DOI] [PubMed] [Google Scholar]
- [3].Doghman M, Figueiredo BC, Volante M, Papotti M, Lalli E. Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation.. Nucl Acids Res 2013; 41:8896-8907; PMID:23907384; http://dx.doi.org/ 10.1093/nar/gkt658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Doghman-Bouguerra M, Granatiero V, Sbiera S, Sbiera I, Lacas-Gervais S, Brau F, Fassnacht M, Rizzuto R, Lalli E. FATE1 antagonizes calcium− and drug− induced apoptosis by uncoupling ER and mitochondria. EMBO Rep 2016; PMID:27402544; http://dx.doi.org/ 10.15252/embr.201541504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer.. Nat Rev Cancer 2005; 5:615-625; PMID:16034368; http://dx.doi.org/ 10.1038/nrc1669 [DOI] [PubMed] [Google Scholar]
- [6].van der Laan M, Bohnert M, Wiedemann N, Pfanner N. Role of MINOS in mitochondrial membrane architecture and biogenesis.. Trends Cell Biol 2012; 22:185-192; PMID:22386790; http://dx.doi.org/ 10.1016/j.tcb.2012.01.004 [DOI] [PubMed] [Google Scholar]
- [7].Whitehurst AW, Bodemann BO, Cardenas J, Ferguson D, Girard L, Peyton M, Minna JD, Michnoff C, Hao W, Roth MG, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells.. Nature 2007; 446:815-819; PMID:17429401; http://dx.doi.org/ 10.1038/nature05697 [DOI] [PubMed] [Google Scholar]


