Abstract
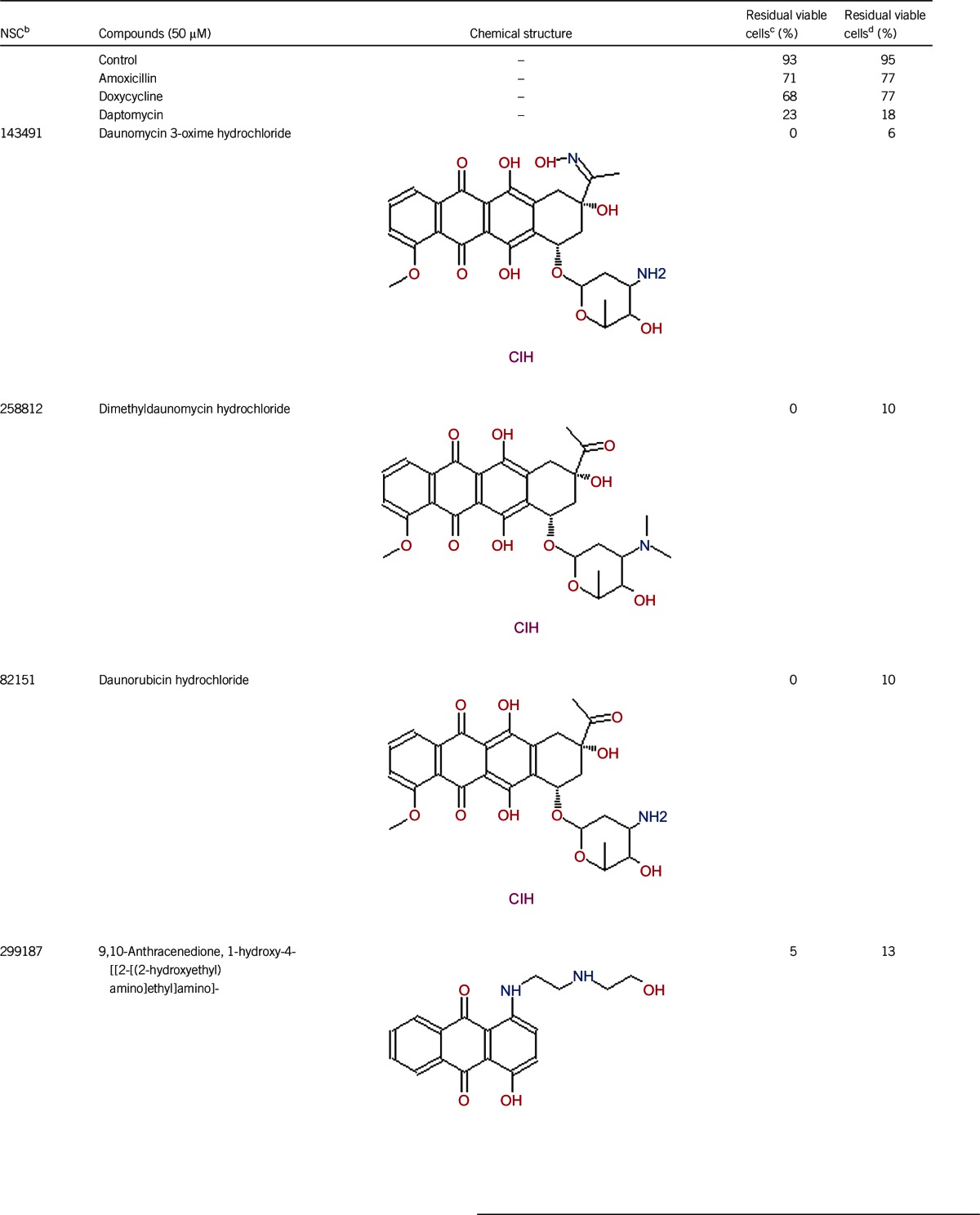
Lyme disease is the leading tick-borne disease in the USA. Whereas the majority of Lyme disease patients with early disease can be cured with standard treatment, some patients suffer from chronic fatigue and joint and muscular pain despite treatment, a syndrome called posttreatment Lyme disease syndrome. Although the cause is unclear, ineffective killing of Borrelia burgdorferi persisters by current Lyme disease antibiotics is one possible explanation. We took advantage of our recently developed high-throughput viability assay and screened the National Cancer Institute compound library collection consisting of 2526 compounds against stationary phase B. burgdorferi. We identified the top 30 new active hits, including the top six anthracycline antibiotics daunomycin 3-oxime, dimethyldaunomycin, daunomycin, NSC299187, NSC363998 and nogalamycin, along with other compounds, including prodigiosin, mitomycin, nanaomycin and dactinomycin, as having excellent activity against B. burgdorferi stationary phase culture. The anthracycline or anthraquinone compounds, which are known to have both anti-cancer and antibacterial activities, also had high activity against growing B. burgdorferi with low minimum inhibitory concentration. Future studies on the structure–activity relationship and mechanisms of action of anthracyclines/anthraquinones are warranted. In addition, drug combination studies with the anthracycline class of compounds and the current Lyme antibiotics to eradicate B. burgdorferi persisters in vitro and in animal models are needed to determine if they improve the treatment of Lyme disease.
Keywords: Borrelia burgdorferi, drug screen, NCI diversity compound library, persisters, treatment
INTRODUCTION
Lyme disease is a multisystem disease caused by the spirochetal bacterium Borrelia burgdorferi and is the leading tick-borne disease in the USA.1 The clinical manifestations of Lyme disease are characterized by an erythema migrans rash and an influenza-like illness, with arthritis and neurological disorders as frequent sequelae of the disease. The infection is transmitted to humans by tick vectors that normally feed on rodents, reptiles, birds, and deer.2 Although the majority of Lyme disease patients with early or early disseminated disease without neurological involvement can be cured with two–four weeks of standard treatment with doxycycline or amoxicillin, approximately 10%–20% of patients treated for Lyme disease have chronic fatigue and joint and muscular pain when assessed six months after treatment, a collection of symptoms called posttreatment Lyme disease syndrome.3 The question of whether B. burgdorferi might persist in some patients after antibiotic therapy and further evade host immune clearance has been raised by some, but it is controversial. In various animal models (mice, dogs, and rhesus macaque monkeys), antibiotic therapy with doxycycline, ceftriaxone, or tigecycline could not eradicate detection of B. burgdorferi as shown by xenodiagnosis and polymerase chain reaction, even though viable organisms could not be cultured in conventional culture medium.4,5,6,7 The findings indicate the continued presence of B. burgdorferi in some form and suggest that current Lyme treatment may not be sufficient to eliminate B. burgdorferi persisters or that the immune system fails to clear persisting organisms or bacterial debris, which may be underlying causes for those who suffer from non-resolving symptoms of Lyme disease. To date, there is currently no effective antibiotic treatment or preventative strategy for those who suffer from persistent symptoms after Lyme disease.
Consistent with the difficulty to eradicate B. burgdorferi in animal models, B. burgdorferi develops various morphological variant forms, such as round bodies and microcolonies, that are refractory or resistant to antibiotics and stresses.8,9,10 For example, it has been demonstrated that whereas the frontline drugs, such as doxycycline and amoxicillin, kill or inhibit the growing spirochetal form of B. burgdorferi effectively, they have little activity in killing non-growing persisters that are enriched in the stationary phase or microcolonies or as biofilm-like aggregates of B. burgdorferi.11,12 There is significant interest in the identification of drugs that target B. burgdorferi persisters.8,12
To identify drugs that can more effectively kill B. burgdorferi persisters, we recently developed a new viability assay using SYBR Green I/propidium iodide (PI) dyes,13 which allowed us to screen an Food and Drug Administration (FDA)-approved drug library against stationary phase B. burgdorferi persisters.12 Using this high-throughput assay, we identified a number of drug candidates, such as daptomycin, clofazimine, cefoperazone, and carbomycin that have excellent activity against in vitro B. burgdorferi persisters.12 In our previous study, we found that daptomycin had the highest activity against B. burgdorferi persisters among all of the candidate drugs. Although daptomycin could almost eradicate B. burgdorferi persisters at 50 μM, this drug concentration is too high for clinical use, and daptomcyin has to be used intravenously, which is not convenient to administer.
To identify new and more effective drugs than daptomycin in killing B. burgdorferi persisters, we performed new drug screens on stationary phase B. burgdorferi cultures using the chemical repository collection of the National Cancer Institute (NCI) compound library collection. The reason we used B. burgdorferi stationary phase culture is because it is known to enrich persisters and contains more than 70%–80% persisters not killed by the current Lyme antibiotics doxycycline or amoxicillin.12,14 Although B. burgdorferi stationary phase culture is comprised of mainly non-growing cells and some growing cells, it can be considered a convenient close proximate for B. burgdorferi persisters. In addition, our previous study performed on B. burgdorferi stationary phase culture allowed us to identify useful drug candidates, such as daptomycin, clofazimine, and cefoperazone, which are shown to be active against B. burgdorferi persisters not killed by doxycycline and amoxicillin. The NCI compound library collection we used has three compound libraries: the diversity set IV compound library (1593 compounds), the mechanistic set II library (816 compounds), and the natural product set III library (117 compounds), altogether 2526 compounds. These compounds were chosen based on structural diversity from more than 250 000 natural products and synthetic compounds.15 By screening this NCI compound library collection, we identified new compounds active against stationary phase B. burgdorferi that were not found in our previous screens.12 These new active hits could help to develop a new, more effective treatment for Lyme disease.
MATERIALS AND METHODS
Bacterial strain, media, and culture
Borrelia burgdorferi strain B31 (ATCC 35210) was obtained from the American Type Tissue Collection. B. burgdorferi was cultured in BSK-H medium (HiMedia Laboratories Pvt. Ltd.) with 6% rabbit serum (Sigma-Aldrich). All culture media were filter sterilized by 0.2 μM filters. Cultures with 1 × 105 spirochetes/mL initial concentrations of inoculum were incubated in 50 mL closed conical tubes (BD Biosciences, California, USA) at 33°C without antibiotics. Based on our previous study that demonstrated the antibiotic tolerance of the stationary phase cultures,12 we chose seven-day-old stationary phase B. burgdorferi cultures (1 × 107 spirochetes/mL) enriched in persisters for drug screens in 96-well microtiter plates, as previously described.12
Microscopy techniques
Specimens were examined on a Zeiss AxioImager M2 microscope equipped with differential interference contrast (DIC) and epifluorescent illumination and were recorded with a Hamamatsu ORCA-R2 C10600 camera. A cell proliferation assay was performed by direct counting using a bacterial counting chamber (Hausser Scientific Partnership, Horsham, PA, USA) and DIC microscopy. A SYBR Green I/PI assay was performed to assess the viability of B. burgdorferi, as previously described.13 The ratio of live (green) and dead (red) B. burgdorferi was calculated by counting the cells using a bacterial counting chamber and epifluorescence microscopy, as previously described.13 For the aggregated cells, three representative images of each sample were captured for quantitative analysis. Image Pro-Plus software was applied to select the green (including yellow) and red (including orange) areas of different morphological forms to calculate the integrated fluorescence intensity (equal to area × average density or average intensity) of the red and green portions, as previously described.16
Antibiotics and the NCI chemical compound library
Antibiotics, including doxycycline, amoxicillin, and daptomycin, were purchased from Sigma and dissolved in appropriate solvents17 to form stock solutions. All antibiotic stocks were filter sterilized by 0.2 μM filters. Then, the stocks were diluted into 500 μM pre-diluted stocks and stored at −20°C.
The NCI compound library collection, consisting of diversity set V,18 mechanistic diversity set II,19 and the natural products set III,20 was kindly supplied by the National Cancer Institute Developmental Therapeutic Program's Open Compound Repository, NIH. These NCI compound libraries were prepared in 1 mM stock solutions with dimethyl sulfoxide in 96-well plates, leaving the first and the last columns in each plate for controls, which included dimethyl sulfoxide blank controls, doxycycline control, and amoxicillin control. The pre-diluted drug plates were sealed and stored at −20°C.
Screening NCI compound libraries against B. burgdorferi stationary phase persisters
To qualitatively determine the effect of the compounds on B. burgdorferi persisters, each compound (5 μL) from the pre-diluted stocks was added to a seven-day-old B. burgdorferi stationary phase culture (1 × 107 spirochetes/mL) in 96-well microtiter plates. The final volume per well was adjusted to 100 μL to achieve a final drug library concentration of 50 μM. The plates were sealed and placed in a 33°C incubator for seven days, after which the viability of the bacteria was assessed by SYBR Green I/PI assay, as described in our previous study.13 With the excitation wavelength at 485 nm, the fluorescence intensities at 535 nm (green emission) and 615 nm (red emission) were measured for each well of the screening plate using a SpectraMax M2 Microplate Reader (Molecular Devices Inc., USA). Some effective candidates were further confirmed by epifluorescence microscopy, as previously described.13
Minimum inhibitory concentration (MIC) determination
The standard microdilution method was used to determine the MIC that would inhibit visible growth of B. burgdorferi after a 72-h incubation period.11,21,22 B. burgdorferi cells (1 × 105) were inoculated into each well of a 96-well microplate containing 90 μL fresh BSK-H medium per well. Each diluted compound (10 μL) was added to the culture. All experiments were run in triplicate. The 96-well plate was sealed and placed in an incubator at 33°C for five days. Cell proliferation was assessed using the SYBR Green I/PI assay and a bacterial counting chamber after the incubation, as previously described.12
RESULTS
Screening NCI compound library to identify effective drugs active against dormant B. burgdorferi persisters
We used the SYBR Green I/PI assay as a high-throughput screening method for rapid viability assessment for B. burgdorferi after exposure to the compound libraries.12 Based on our previous study, some red-colored compounds caused interference in the SYBR Green I/PI assay, which could make the background red and cause false positive results. Thus, in this study, we used microscopic counting rescreen to examine the hit compounds in the SYBR Green I/PI assay.
To identify effective chemical compounds that have activity against B. burgdorferi persisters, we used the stationary phase B. burgdorferi as a persister model, as it is known to be enriched in persisters.12,14 to screen the NCI compound libraries. Meanwhile, the currently used Lyme disease antibiotics doxycycline and amoxicillin were included as control drugs. Consistent with our previous results,12 the currently used Lyme antibiotics had poor activity against the stationary phase B. burgdorferi persisters, and the bacteria treated with the two antibiotics still had 75% and 76% viable cells remaining, respectively, compared with 93% viable cells in the drug-free control (Table 1).
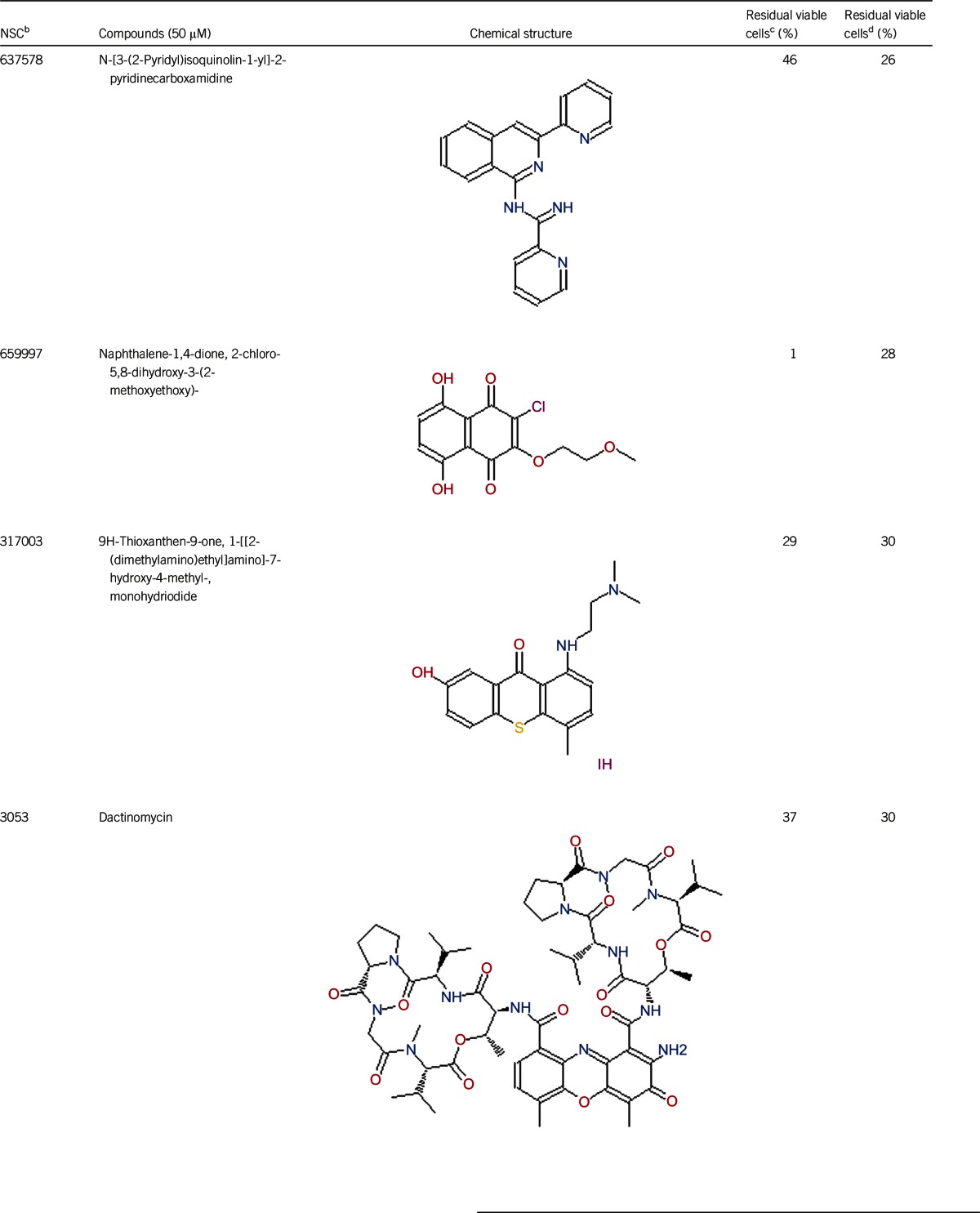
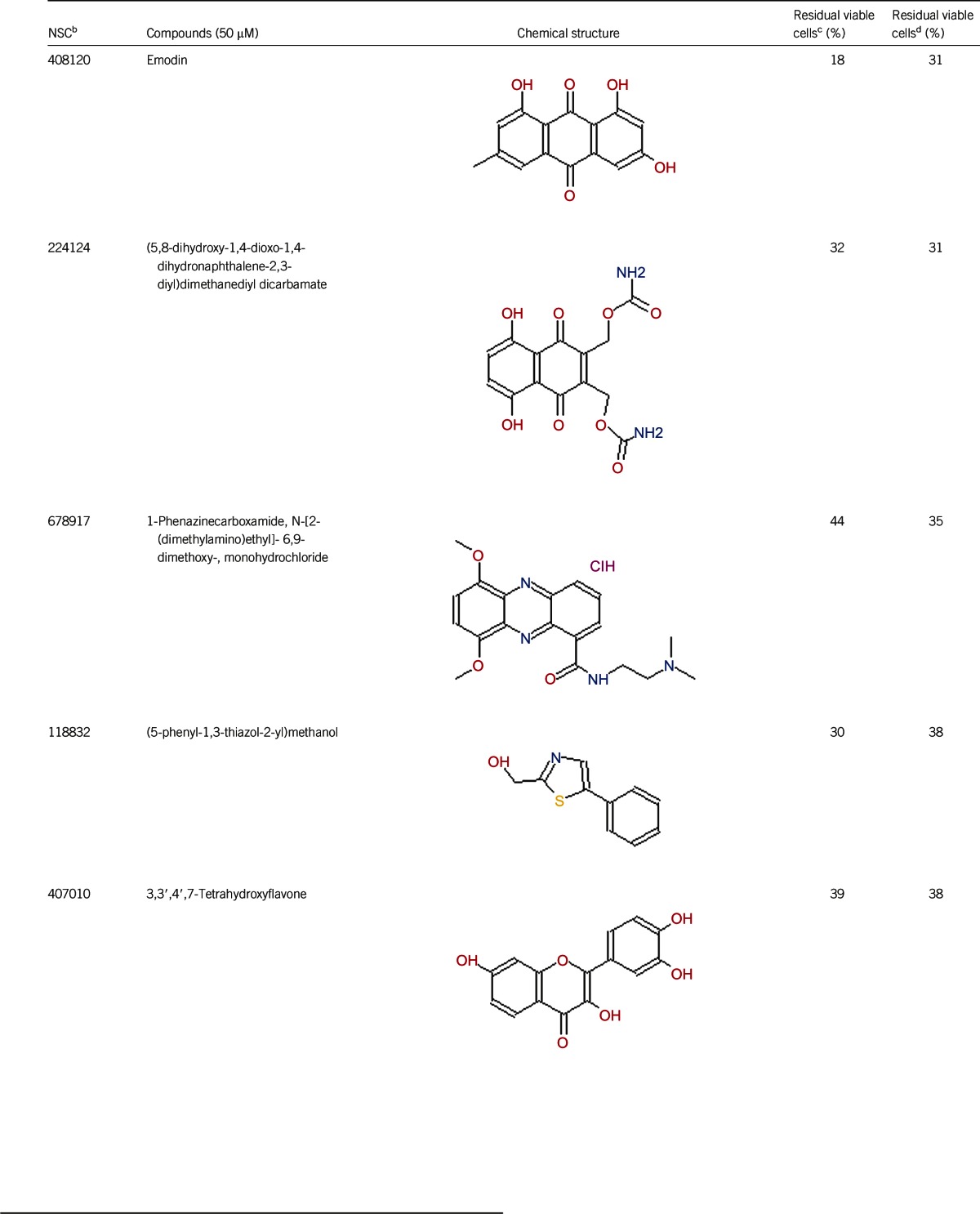
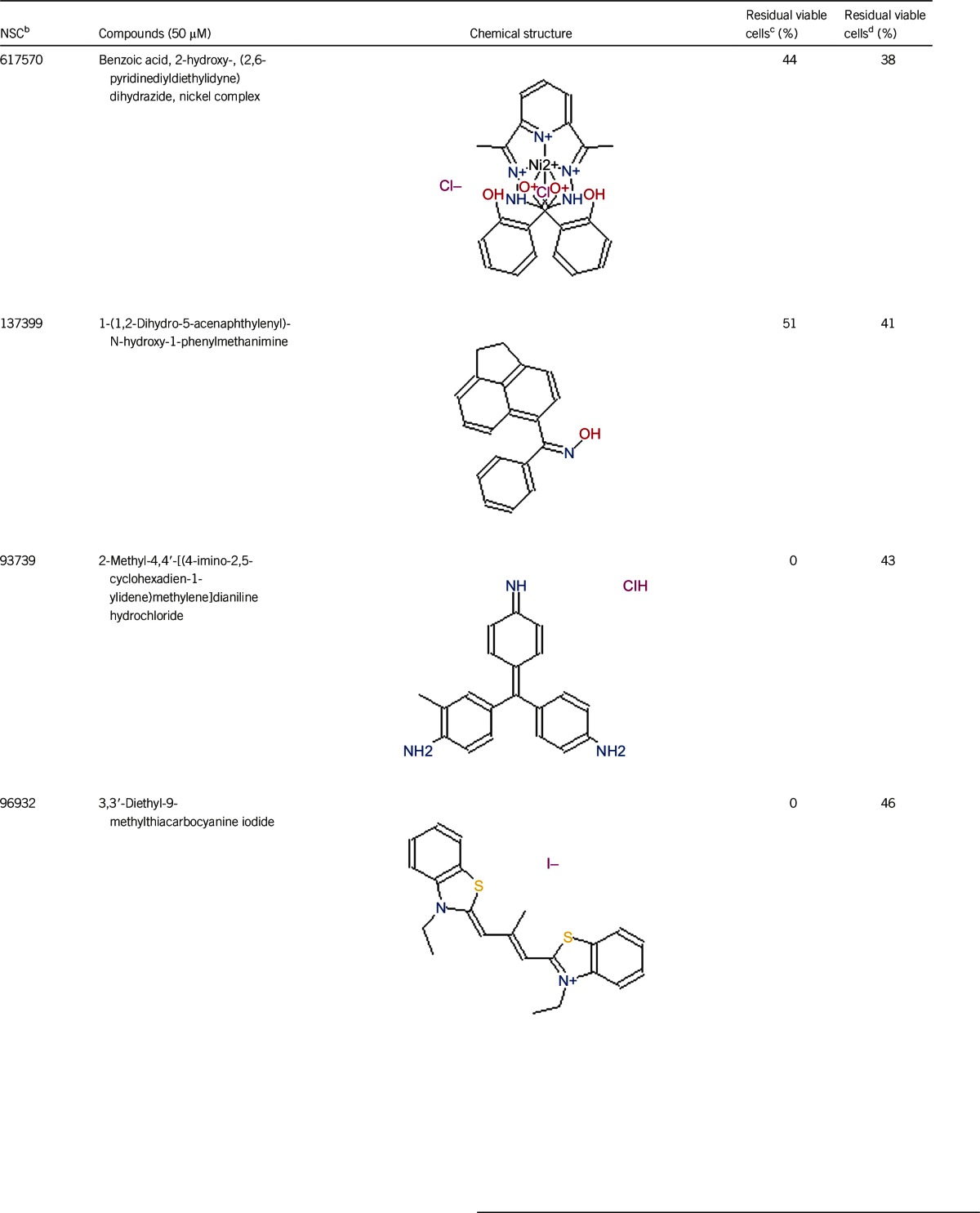
Table 1. Activity of the top 30 active hits that had good activity (better than current clinical drugs) against stationary phase B. burgdorferia.
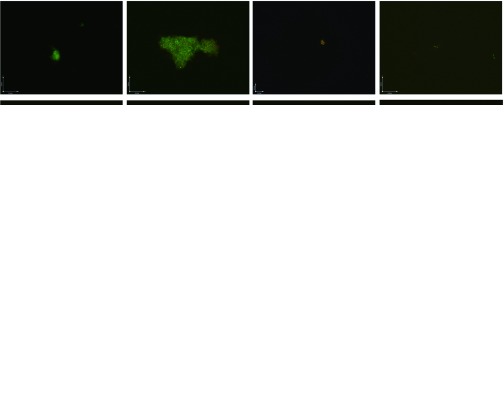
Seven-day-old stationary phase B. burgdorferi culture was treated with drugs or compounds (50 μM) for seven days, at which point the viability of the bacteria was determined, as previously described12.
The NSC number is a numeric identifier for substances submitted to the NCI.
Residual viable B. burgdorferi was calculated according to the regression equation and the ratio of Green/Red fluorescence obtained by the SYBR Green I/PI assay, as previously described12.
Residual viable B. burgdorferi was assayed by epifluorescence microscope counting, as previously described12.
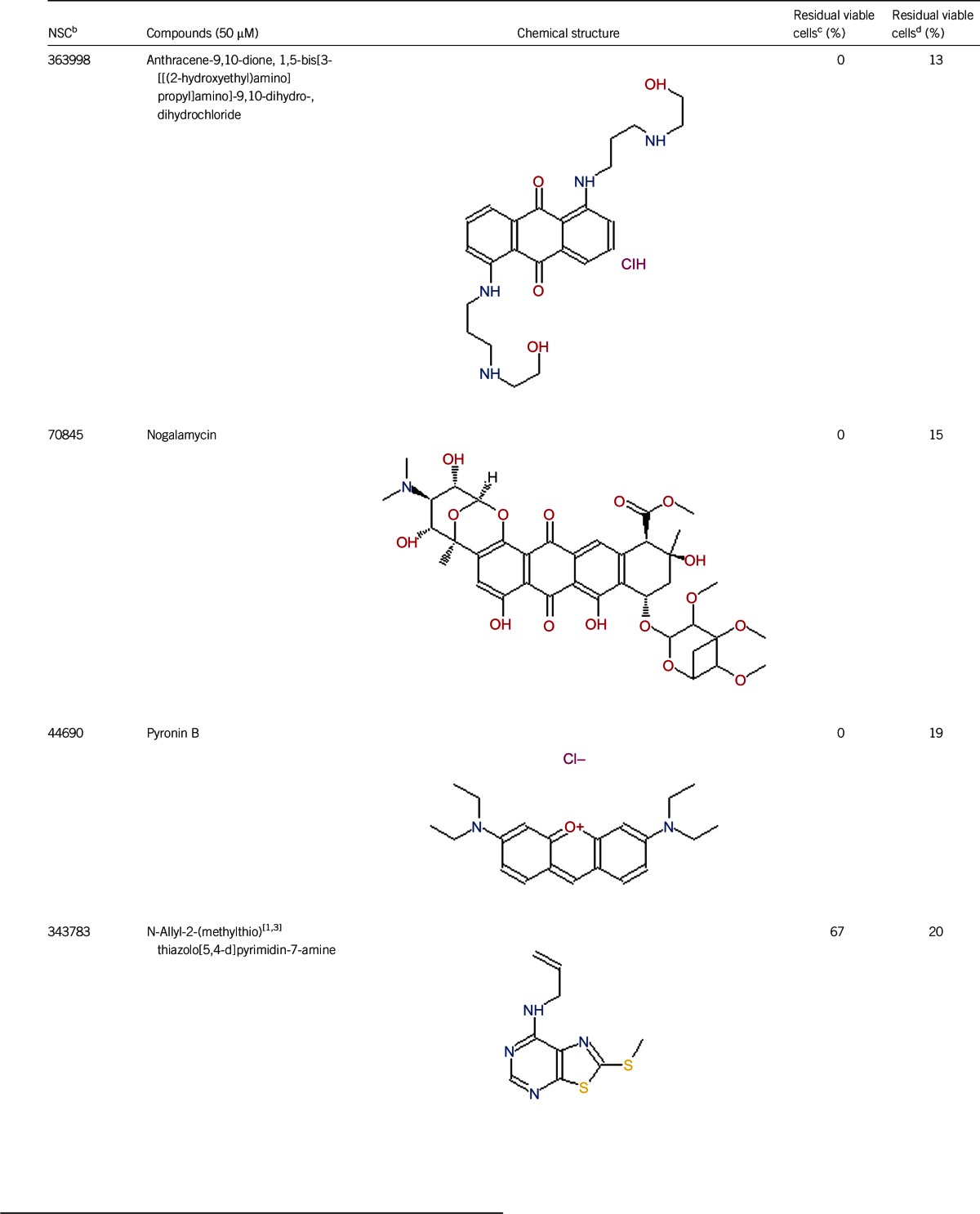
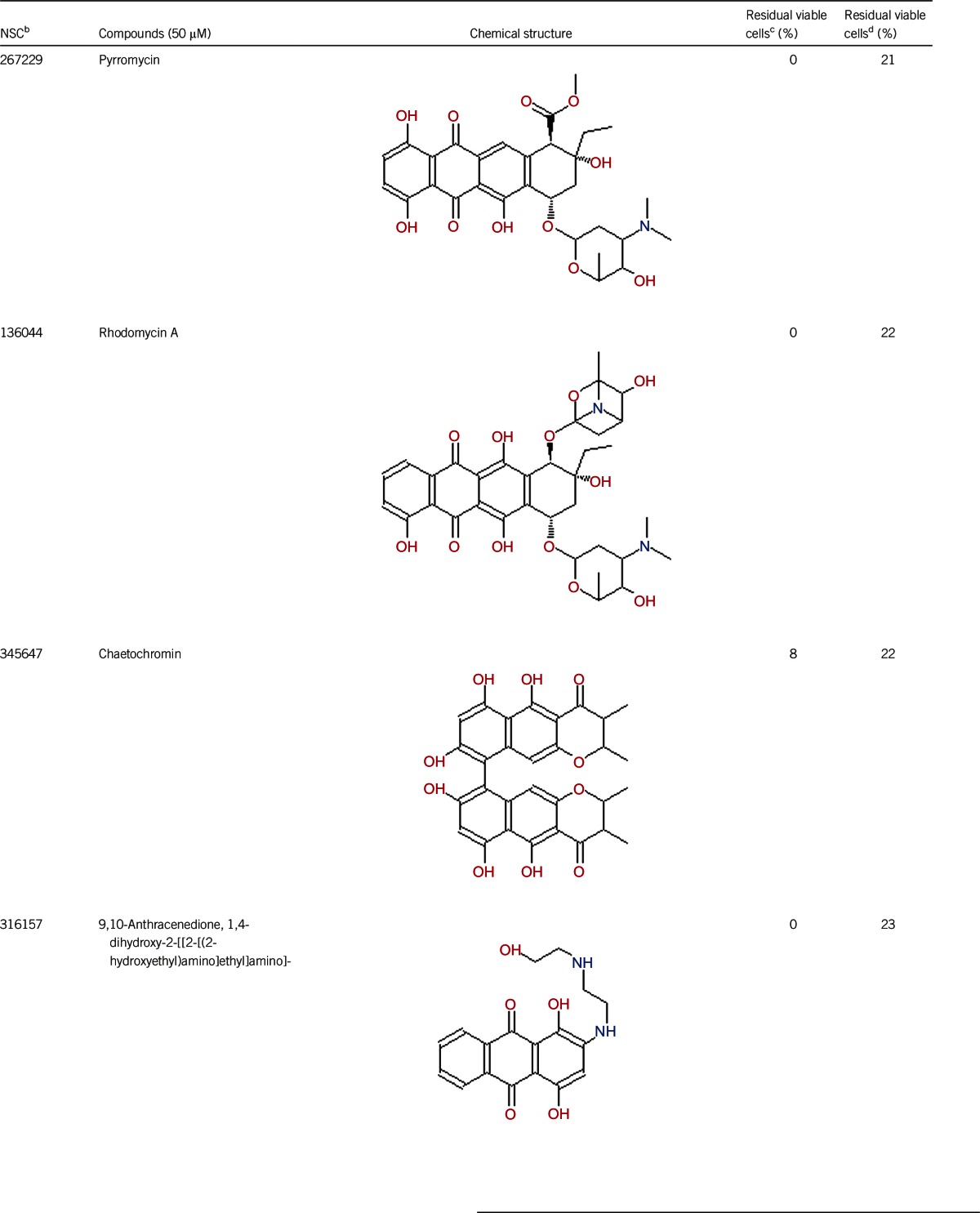
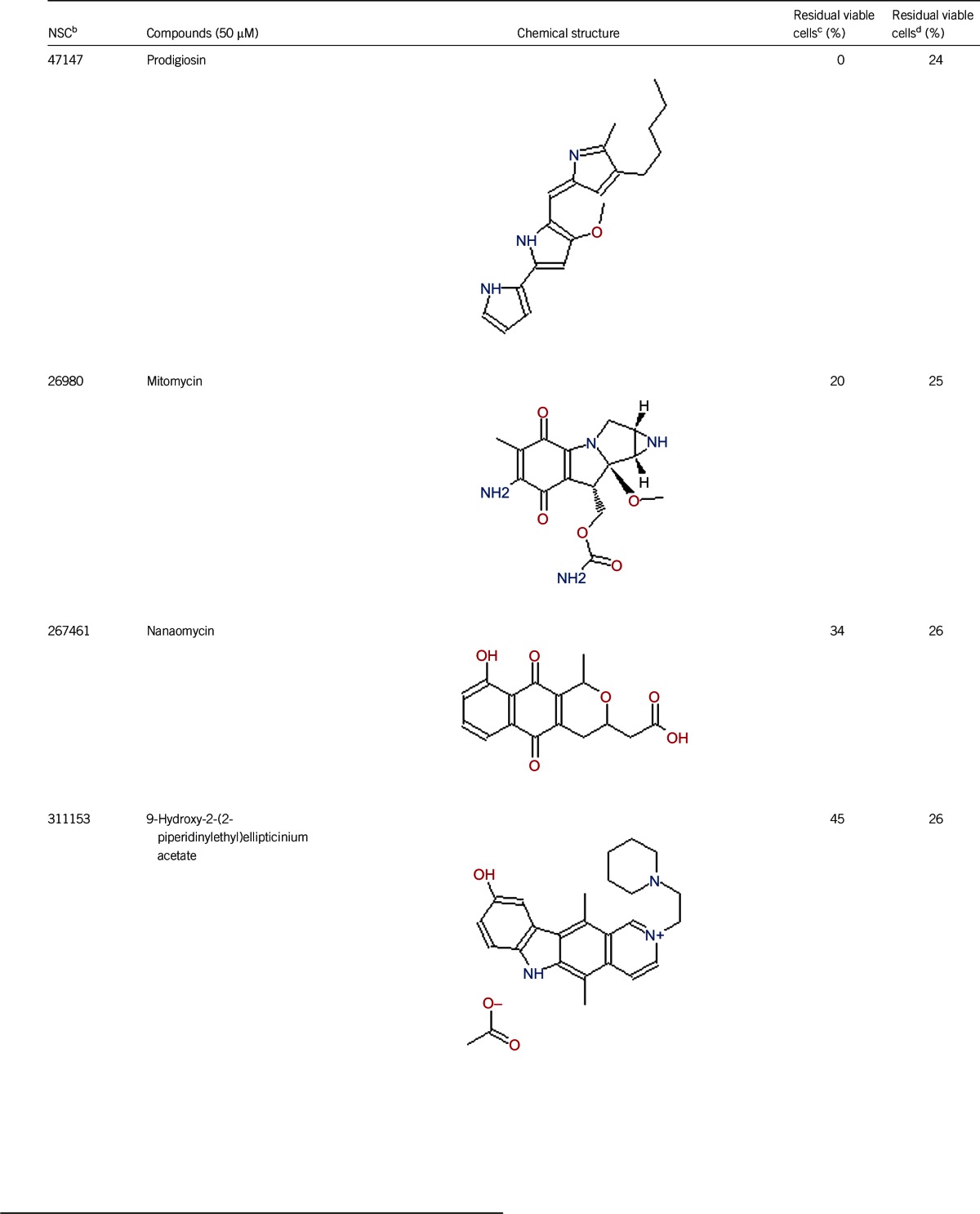
Of the 2526 compounds in the NCI compound library collection tested, 237 were found to have higher activity against B. burgdorferi persisters than doxycycline and amoxicillin in the primary screen. The 237 candidates were rescreened by microscope counting with the SYBR Green I/PI viability assay. After the rescreening by microscopy, we confirmed the top 30 active hits that had less than 50% residual viable cells after treatment (Table 1, Figure 1). Among the 30 active hits, 22 compounds were found in mechanistic set II, nine compounds in diversity set IV, and three compounds in natural product set III. Nanaomycin and dactinomycin were in both mechanistic set II and natural product set III, whereas NSC311153 and NSC637578 were in both mechanistic set II and diversity set IV. These compounds are all aromatic compounds. We identified several clinically used drugs that had excellent activity against stationary phase B. burgdorferi. The activity of some drugs against stationary phase B. burgdorferi was significantly higher than that of the frontline antibiotics doxycycline or amoxicillin, and even more active than daptomycin, the best antibiotic against B. burgdorferi persisters in our previous study (Table 1, Figure 1). We found that six anthraquinone antibiotics and compounds, daunomycin 3-oxime, dimethyldaunomycin, daunomycin, NSC299187, NSC363998, and nogalamycin showed the highest activities (residual viable cells from 6% to 15%) against stationary phase B. burgdorferi. These six compounds showed higher activity than daptomycin (18% residual viable cells). In addition, another five anthraquinone compounds, pyrromycin, rhodomycin A, NCS316157, emodin, and NSC156516 also had good activity against stationary phase B. burgdorferi (residual viable cells 21%–50%). In addition to the six anthraquinones, pyronin B, a xanthene compound, had good activity (residual viable cells 19%) against stationary phase B. burgdorferi. We found seven nitrogen-containing aromatic compounds, including NSC343783 (residual viable cells 20%) and prodigiosin (24% residual viable cells), NSC637578, NSC 678917, NSC118832, NSC617570, and NSC96932, to be among the 30 most active compounds. Moreover, chaetochromin, a bis-naphtho-γ-pyrone compound, showed good activity, with 22% residual viable cells. Mitomycin, an aziridine-containing benzoquinone antitumor drug, showed reasonably good activity with 25% residual viable cells. We also found three 1,4-naphthoquinones, nanaomycin (residual viable cells 26%), NCS659997 and NCS224124, had relatively good activity against stationary phase B. burgdorferi. A polypeptide antibiotic dactinomycin also had relatively high activity against stationary phase B. burgdorferi (residual viable cells 30%). In addition to 11 clinically used drugs (daunomycin 3-oxime, dimethyldaunomycin, daunomycin, nogalamycin, pyrromycin, chaetochromin, prodigiosin, mitomycin, nanaomycin, and dactinomycin), we found 19 non-medicinal compounds that showed good activity against stationary phase B. burgdorferi to varying levels (Table 1, Figure 1).
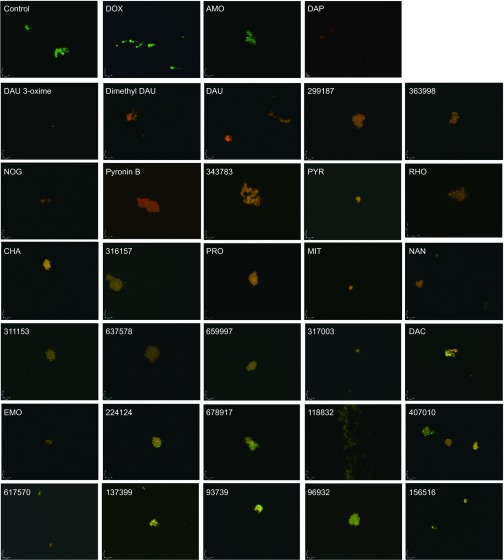
Figure 1.
Representative images at ×100 magnification of stationary phase B. burgdorferi treated with different compounds (50 μM) followed by staining with the SYBR Green I/PI assay. Abbreviations: DOX, doxycycline; AMO, amoxicillin; DAP, daptomycin; DAU, daunomycin; NOG, nogalamycin; PYR, pyrromycin; RHO, Rhodomycin A; CHA, chaetochromin; PRO, prodigiosin; MIT, mitomycin; NAN, nanaomycin; DAC, dactinomycin; EMO, emodin.
Relationship between MIC values and anti-persister activity
We found some compounds that have good activity against the non-growing stationary phase B. burgdorferi (Table 1), but it is necessary to determine the MICs of these compounds against growing B. burgdorferi (Table 2). The standard microdilution method was used to determine the MIC, as described in our previous study.12 We found that three anthracycline antibiotics, daunomycin 3-oxime, daunorubicin, and pyrromycin, in addition to having good activity against stationary phase B. burgdorferi were also highly active against log phase growing B. burgdorferi with low MICs (≤0.36, ≤0.36, 0.36–0.72 μg/mL, respectively). Another anthraquinone compound, NSC299187, had a relatively high MIC (3.26–6.52 μg/mL) although it had excellent activity against stationary phase B. burgdorferi (residual viable cells 13%). We noted that prodigiosin (nitrogen-containing aromatic rings compound), mitomycin (aziridine-containing benzoquinone), nanaomycin (1,4-naphthoquinone), and dactinomycin (polypeptide antibiotic) had good activity against replicating B. burgdorferi with low MICs (≤0.2, ≤0.21, 0.76–1.57, ≤0.78 μg/mL, respectively). On the other hand, pyronin B and chaetochromin were less potent against growing B. burgdorferi, with relatively high MICs (1.8-3.6, 2.74–5.47 μg/mL, respectively), but they had excellent activity against stationary phase B. burgdorferi.
Table 2. Comparison of the MIC values and the activities of some compounds against stationary phase B. burgdorferi.
| Antibiotics | MIC (μg/mL) | Activity against stationary phase B. burgdorferi a (%) |
|---|---|---|
| Doxycycline | ≤0.25 | 77 |
| Amoxicillin | ≤0.25 | 77 |
| Daptomycin | 12.5–25 | 18 |
| Daunomycin 3-oxime | ≤0.36 | 6 |
| Daunorubicin | ≤0.35 | 10 |
| NSC299187 | 3.26–6.52 | 13 |
| Pyronin B | 1.8–3.6 | 19 |
| Pyrromycin | 0.37–0.73 | 21 |
| Chaetochromin | 2.74–5.47 | 22 |
| Prodigiosin | ≤0.2 | 24 |
| Mitomycin | ≤0.21 | 25 |
| Nanaomycin | 0.76–1.57 | 26 |
| Dactinomycin | ≤0.78 | 30 |
Shown as the residual viable cell percentage from epifluorescence microscope counting data.
Comparison of anti-persister activity at low drug concentrations
Although we obtained many highly effective hits from the NCI compound library with a 50 μM compound screen, this drug concentration is likely too high for in vivo experiments. Daptomycin at 50 μM showed strong activity against stationary phase B. burgdorferi in our previous study,12 but it could not kill the microcolony form of B. burgdorferi persisters at lower concentration, such as 10 μg/mL.14 To further compare the activity of the hit compounds and daptomycin, we tested the activity against stationary phase B. burgdorferi at a 20 μM drug concentration (approximately 10 μg/mL for most compounds and 32 μg/mL for daptomycin). Most of the residual viable percentages of stationary phase B. burgdorferi increased with the decrease of drug concentration (Table 3, Figure 2), but five anthracyclines: dimethyldaunomycin, NCS363998, nogalamycin, pyrromycin, and Rhodomycin A, at 20 μM still showed as strong an activity against stationary phase B. burgdorferi as at 50 μM (Table 3, Figure 2). Other non-anthracycline compounds showed relatively weaker activity than daptomycin at 20 μM.
Table 3. Comparison of the activity of some hit compounds at 20 μM and 50 μM against stationary phase B. burgdorferia.
| NSC number | Compounds | Residual viable cells (%) | ||
|---|---|---|---|---|
| 20 μMb | 20 μMc | 50 μMc | ||
| 258812 | Control | 93 | 94 | 95 |
| Amoxicillin | 77 | 77 | 77 | |
| Doxycycline | 76 | 77 | 77 | |
| Daptomycin | 32 | 25 | 18 | |
| Dimethyldaunomycin hydrochloride | 0 | 10 | 10 | |
| 363998 | Anthracene-9,10-dione, 1,5-bis[3-[[(2-hydroxyethyl)amino] propyl]amino]-9,10-dihydro-, dihydrochloride | 22 | 14 | 13 |
| 70845 | Nogalamycin | 3 | 15 | 15 |
| 267229 | Pyrromycin | 6 | 20 | 21 |
| 136044 | Rhodomycin A | 5 | 21 | 21 |
| 345647 | Chaetochromin | 31 | 33 | 22 |
| 47147 | Prodigiosin | 0 | 45 | 24 |
| 267461 | Nanaomycin | 39 | 45 | 26 |
| 659997 | Naphthalene-1,4-dione, 2-chloro-5,8-dihydroxy-3-(2-methoxyethoxy)- | 40 | 50 | 28 |
| 224124 | (5,8-dihydroxy-1,4-dioxo-1,4-dihydronaphthalene-2,3-diyl)dimethanediyl dicarbamate | 54 | 77 | 31 |
| 617570 | Benzoic acid, 2-hydroxy-, (2,6-pyridinediyldiethylidyne) dihydrazide, nickel complex | 66 | 50 | 38 |
| 93739 | 2-Methyl-4,4′-[(4-imino-2,5-cyclohexadien-1-ylidene)methylene]dianiline hydrochloride | 0 | 77 | 43 |
Seven-day-old stationary phase B. burgdorferi culture was treated with drugs for seven days.
Residual viable B. burgdorferi was calculated according to the regression equation and the ratio of Green/Red fluorescence obtained by the SYBR Green I/PI assay.
Residual viable B. burgdorferi was assayed by epifluorescence microscope counting.
Figure 2.
Representative images at ×100 magnification of stationary phase B. burgdorferi strain B31 treated with different compounds (20 μM) followed by staining with the SYBR Green I/PI assay. Abbreviations: DOX, doxycycline; DAP, daptomycin; DAU, daunomycin; NOG, nogalamycin; PYR, pyrromycin; RHO, Rhodomycin A; CHA, chaetochromin; PRO, prodigiosin; NAN, nanaomycin.
DISCUSSION
We recently identified a number of drug candidates that have excellent activity against non-replicating B. burgdorferi stationary phase culture enriched with persisters from an FDA-approved drug library.12 In this study, we attempted to identify new drug candidates or compounds that have high activity against B. burgdorferi stationary phase culture using the NCI compound library collection. From the 2526 compounds in three NCI compound libraries, 237 compounds were found to have higher activity against B. burgdorferi stationary phase culture than doxycycline or amoxicillin, from which the top 30 active hits were confirmed by microscopy rescreening. The use of the mechanistic compound library helped to identify the anthraquinone (anthracycline) class of drugs, which has high activity against stationary phase B. burgdorferi. More than one-third of the 30 most active compounds possess an anthraquinone (also called anthracenedione or dioxoanthracene) structure. The top six active compounds, daunomycin, daunomycin 3-oxime, dimethyldaunomycin, NSC299187, NSC363998, and nogalamycin, are all anthraquinone derivatives, characterized by three aromatic rings linked together with a benzoquinone in the center. Previously, we noted the anti-persister activity of the anthracycline antibiotic doxorubicin,12 but we mistakenly excluded it from the active drugs because it has a red color and interfered with the SYBR Green I/PI staining. However, careful examination by microscopy confirmed the activity of red-colored anthraquinone drugs, including doxorubicin. Not all red-colored anthraquinone compounds have good activity against stationary phase B. burgdorferi. For example, NCS156516 had weak activity and still had 50% residual viable (green) cells (Figure 1). Thus, confirmation by careful microscopic examination is needed to assess compounds that have red color and have activity against stationary phase B. burgdorferi using a low concentration of compounds and subculture studies.
The top six anthraquinone compounds with residual viable cells ranging from 6% to 15% were more active than daptomycin, which had 18% residual viable cells when using the seven-day-old stationary phase culture (Table 1). Meanwhile, these compounds also had very good activity (low MIC) against growing B. burgdorferi (Table 2). Further drug combination and subculture studies are needed to confirm whether the top six anthraquinone compounds are indeed more active than daptomycin against B. burgdorferi persisters in vitro and in vivo.
Anthraquinones are a class of naturally occurring phenolic compounds isolated from Streptomyces and have diverse medical uses, including anti-cancer, antimalarial, and laxative. Anthracycline antibiotics, such as daunomycin, nogalamycin, pyrromycin, and rhodomycin A, are used in chemotherapy for some cancers, especially for several types of leukemia.23 Anthracycline drugs have antibacterial activity against S. aureus, and the MICs of daunomycin and doxorubicin are 8–32 μg/mL and 0.12–0.5 μg/mL, respectively.24 Daunomycin did not show bactericidal activity for gram-negative bacteria Pseudomonas aeruginosa, Klebsiella pneumoniae, and E. coli.18 Our study is the first to demonstrate the activity of this class of anthraquinone compounds against both growing and non-growing forms of B. burgdorferi. However, the mechanisms of action of this class of anthraquinone compounds against B. burgdorferi are unclear and remain to be determined.
Anthracycline antibiotics inhibit DNA and RNA synthesis by inserting base pairs into the DNA/RNA strands.25 Anthracycline antibiotics stabilize the topoisomerase II complex and prevent dissociation of topoisomerase II from its nucleic acid substrate, leading to DNA damage and blocking DNA transcription and replication as well as producing reactive oxygen species, which could damage mitochondria and lead to cardiotoxicity as the main side effect.26,27 The sugar moiety of daunomycin plays a critical role in determining its anticancer activity.24 In this study, we found anthracycline antibiotics with sugar structures and anthraquinone compounds (NCS299187 and NCS363998) without sugar structures both have good activity against stationary phase B. burgdorferi. These findings suggest that the mechanism of action of anthraquinone drugs may not be identical for its anti-cancer activity and its activity in B. burgdorferi. Future studies are needed to identify the mechanism of action of anthracycline antibiotics against B. burgdorferi, to address the structure–activity relationship of this class of compounds and to explore the possibility of utilizing the strong activity of this class of anthracycline compounds without untoward toxicity to host cells.
In addition to the anthracycline antibiotics, we found that some 1,4-naphthoquinones, such as nanaomycin, NCS659997 and NCS224124, showed high activity against stationary phase B. burgdorferi. 1,4-naphthoquinone has an analogous molecular skeleton to anthraquinone. Nanaomycin may interfere with the function of the bacterial cell membrane,28 and such a mode of action may be responsible for its activity against stationary phase B. burgdorferi.
We found that chaetochromin, a bis-naphtho-γ-pyrone produced by several species of chaetomium, also showed high activity against stationary phase B. burgdorferi. Bis-naphtho-γ-pyrones have a broad range of biological activities, such as inhibition of adenosine triphosphate synthesis in mitochondria, cell proliferation inhibition, triacylglycerol synthesis inhibition, and antimicrobial activity.29 Bis-naphtho-γ-pyrones are active against various bacteria, such as S. aureus, E. coli, and M. tuberculosis, with MIC values ranging from 2 μg/mL to 50 μg/mL.29 Inhibition of adenosine triphosphate synthesis can explain the activity of bis-naphtho-γ-pyrone against stationary phase B. burgdorferi. Cephalochromin has been shown to inhibit fatty acid biosynthesis.30 It is possible that fatty acid synthesis plays a role in B. burgdorferi persister formation, and future studies are needed to confirm this possibility.
In this study, we found that some antibiotic compounds, such as prodigiosin, mitomycin, and dactinomycin, had partial activity against stationary phase B. burgdorferi, although their activities (24%–30% residual viable cells) are not as strong as daptomycin (18% residual viable cells) (Table 1). Prodigiosin is a secondary metabolite of Serratia marcescens and has antibacterial, antifungal, antiprotozoal, antimalarial, immunosuppressive, and anticancer activities.31 Mitomycin shows its activity as a DNA cross-linker through its aziridine functional group and cross-links the complementary strands of the DNA double helix to cause the death of a bacterial cell.32,33 The activity of mitomycin against stationary phase B. burgdorferi may be due to its DNA cross-linking activity. Dactinomycin is a polypeptide antitumor antibiotic isolated from the soil bacteria Streptomyces,34 and it binds DNA and interferes with DNA replication34 and also inhibits RNA transcription.35
Persisters are heterogeneous and include persisters in the stationary phase, which can grow after antibiotic exposure and those that survive antibiotic treatment in vivo but cannot grow (viable but non-culturable) in a continuum.36 Thus, the use of stationary phase cultures as a surrogate of persisters in this study has limitations because they cannot represent the viable but non-culturable persisters that have been found in vivo in different animal models after antibiotic treatment.5,6,7 Nevertheless, the discovery of the drug candidates with activity against B. burgdorferi stationary phase cultures from this study and our previous study12,14 offers the opportunity to assess whether such drugs are useful for eradicating persistent infection in vivo in animal models and in patients in future studies.
In summary, we identified the anthracycline class of compounds including daunomycin, daunomycin 3-oxime, dimethyldaunomycin, NSC299187, NSC363998, and nogalamycin along with some other compounds, including prodigiosin, mitomycin, nanaomycin, and dactinomycin, as having excellent activity against both the non-growing stationary phase and growing B. burgdorferi cultures. The structure–activity relationship and mechanisms of action of the anthracycline/anthraquinone class of compounds against B. burgdorferi persisters should be addressed in future studies. In addition, drug combination studies with the anthracycline class of compounds and the current Lyme antibiotics are required to assess whether they improve treatment of Lyme disease in animal models and in patients.
Acknowledgments
We thank the NCI Developmental Therapeutic Program/NIH for providing the NCI compound library collection. This work was supported by the Global Lyme Alliance (formerly Lyme Research Alliance). Ying Zhang was supported in part by Global Lyme Alliance and NIH grant numbers AI099512 and AI108535.
References
- Centers for Disease Control and Prevention. Lyme Disease. Atlanta: CDC, 2014. Available at: http://www.cdc.gov/lyme/(accessed 13 September 2014) .
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 2012; 10: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Post-treatment Lyme Disease Syndrome. Atlanta: CDC, 2014. Available at : http://www.cdc.gov/lyme/postLDS/index.html (accessed 13 September 2014).
- Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother 2010; 54: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers ME, Barthold SW, Borda JT et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 2012; 7: e29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 2014; 9: e86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol 1997; 35: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson O, Brorson SH, Scythes J, MacAllister J, Wier A, Margulis L. Destruction of spirochete Borrelia burgdorferi round-body propagules (RBs) by the antibiotic tigecycline. Proc Natl Acad Sci USA 2009; 106: 18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapi E, Bastian SL, Mpoy CM et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One 2012; 7: e48277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diterich I, Rauter C, Kirschning CJ, Hartung T. Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression. Infect Immun 2003; 71: 3979–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapi E, Kaur N, Anyanwu S et al. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect Drug Resist 2011; 4: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang T, Shi W et al. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microb Infect 2014; 3: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang T, Zhang S, Shi W, Zhang Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS One 2014; 9: e111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Auwaerter PG, Zhang Y. Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS One 2015; 10: e0117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developmental Therapeutics Program NCI/NIH. Open Repository Collection of Synthetics and Pure Natural Products. Shady Grove: DTP, 2014. Available at : http://dtp.nci.nih.gov/branches/dscb/repo_open.html (accessed 15 March 2015).
- Shopov A, Williams SC, Verity PG. Improvements in image analysis and fluorescence microscopy to discriminate and enumerate bacteria and viruses in aquatic samples. Aquat Microb Ecol 2000; 22:103–110. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement. CLSI document M100-S17 2007; 27: 154–161. Wayne: CLSI, 2007. Available at : http://www.microbilab-bg.com/CLSI.pdf (accessed 11 January 2015).
- Moody MR, Morris MJ, Young VM, Moye LA 3rd, Schimpff SC, Wiernik PH. Effect of two cancer chemotherapeutic agents on the antibacterial activity of three antimicrobial agents. Antimicrob Agents Chemother 1978; 14: 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developmental Therapeutics Program NCI/NIH. Mechanistic Set Information. Shady Grove: DTP, 2015. Available at : http://dtp.nci.nih.gov/branches/dscb/mechanistic_explanation.html (accessed 11 January 2015).
- Developmental Therapeutics Program NCI/NIH. Natural Products Set III. Shady Grove: DTP, 2015. Available at : http://dtp.nci.nih.gov/branches/dscb/natprod_explanation2.html (accessed 11 January 2015).
- Dever LL, Jorgensen JH, Barbour AG. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol 1992; 30: 2692–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner J, Failing K, Wittenbrink MM. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentralbl Bakteriol 1995; 283: 49–60. [DOI] [PubMed] [Google Scholar]
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 1967; 20: 333–353. [DOI] [PubMed] [Google Scholar]
- Zhu L, Cao X, Chen W, Zhang G, Sun D, Wang PG. Syntheses and biological activities of daunorubicin analogs with uncommon sugars. Bioorg Med Chem 2005; 13: 6381–6387. [DOI] [PubMed] [Google Scholar]
- Mizuno NS, Zakis B, Decker RW. Binding of daunomycin to DNA and the inhibition of RNA and DNA synthesis. Cancer Res 1975; 35: 1542–1546. [PubMed] [Google Scholar]
- Jensen PB, Sorensen BS, Sehested M et al. Different modes of anthracycline interaction with topoisomerase II. Separate structures critical for DNA-cleavage, and for overcoming topoisomerase II-related drug resistance. Biochem Pharmacol 1993; 45: 2025–2035. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 2010; 17: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Unemoto T, Minami-Kakinuma S, Tanaka H, Omura S. The mode of action of nanaomycins D and A on a gram-negative marine bacterium Vibrio alginolyticus. J Antibiot 1982; 35: 1078–1085. [DOI] [PubMed] [Google Scholar]
- Lu S, Tian J, Sun W et al. Bis-naphtho-gamma-pyrones from fungi and their bioactivities. Molecules 2014; 19: 7169–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JW, Cronan JEJr. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu Rev Microbiol 2001; 55: 305–332. [DOI] [PubMed] [Google Scholar]
- Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2007; 2: 605–618. [DOI] [PubMed] [Google Scholar]
- Szybalski W, Iyer VN. Crosslinking of DNA by enzymatically or chemically activated mitomycins and porfiromycins, bifunctionally “alkylating” antibiotics. Fed Proc 1964; 23: 946–957. [PubMed] [Google Scholar]
- Tomasz M. Mitomycin C: small, fast and deadly (but very selective). Chem Biol 1995; 2: 575–579. [DOI] [PubMed] [Google Scholar]
- Hollstein U. Actinomycin. Chemistry and mechanism of action. Chem Rev 1974; 74: 625–652. [Google Scholar]
- Sobell HM. Actinomycin and DNA transcription. Proc Natl Acad Sci USA 1985; 82: 5328–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Persisters, persistent infections and the Yin-Yang model. Emerg Microb Infect 2014; 3: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]