Why is our rich and powerful society unable to prevent, defeat, or even contain the yearly onslaught of influenza? A clutch of papers in this issue provides glimpses of the answers.
What is commonly known as influenza or the flu is a syndrome, not a disease. Each year scores of different respiratory viruses (and a few bacteria) cause a mostly benign illness, which cannot be distinguished clinically by causal agent. The syndrome should be referred to as influenza-like illness rather than just influenza. Influenza should be reserved for the illness caused by influenza A or B viruses. Influenza-like illness gives a better idea of the difficulties in diagnosis. Useful pointers may be the seasonality of the epidemic (respiratory syncytial virus and influenza A and B are usually autumnal or winter epidemics) or the age group concerned (respiratory syncytial virus has an affinity for very young people). Another exception may be the presence of a pandemic or a local epidemic in which the causal agent has been identified. However, our knowledge of the annual microbiological breakdown is limited, and the few available studies give static pictures—they tell us what happened in a given population during the period that the study was conducted.1-3 Reliable “real time” information is hard to come by.
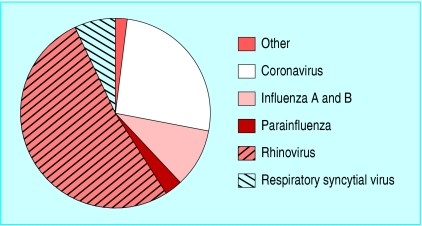
For example, Nicholson et al reported a detailed breakdown of known agents causing respiratory infections in elderly people in Leicestershire in the winters of 1992-4 (figure).1 Such studies, rigorously validated by laboratory identification of agents, can at best provide a rough average of causality of the seasonally complex epidemiology of respiratory tract infections: over several years one third of influenza-like illnesses are of unknown cause, one third are caused by rhinoviruses, and the remainder by a mixed bag of other agents, which include influenza A and B viruses.
Figure 1.
Pathogens isolated from 211 of 497 episodes of influenza-like illness in elderly people in Leicestershire (adapted from Nicholson et al1)
Diagnostic ignorance poses problems in the application of preventive and treatment interventions (vaccines and antivirals), as these are specific against influenza A and B viruses. As the proportion of influenza-like illnesses caused by influenza A and B viruses (the pink slice of the pie in the figure) varies from month to month, the effectiveness of the interventions against influenza will vary. The higher the circulation of influenza A and B, the narrower the gap between efficacy (the capacity of an intervention to prevent influenza) and effectiveness (the capacity of an intervention to prevent acute respiratory infections, or influenza-like illnesses) of vaccines and antivirals will be.
Antigenically well matched inactivated vaccines against influenza A and B have average (that is, spread over several seasons) efficacy of 70% (95% confidence interval 56% to 80%) and effectiveness of around 39% (21% to 53%) in healthy adults.4 However, estimates of efficacy and effectiveness increase and converge whenever circulation of the influenza A or B viruses is high, as shown by Wilkinson et al (p 647).5 As the size of the pink slice of the pie in the figure cannot be predicted, yearly routine vaccination has known costs and variable benefits.
Antiviral compounds may be used in a preventive role. In adults, amantadine is 61% (35% to 76%) efficacious in preventing influenza A cases. Rimantadine has similar efficacy,6 but again their average effectiveness is lower (25%, 13% to 36%).6 The newer neuraminidase inhibitors oseltamivir and zanamivir are 70%-90% efficacious,7,8 but as Harling et al show (p 663),9 routine use of oseltamivir in the presence of influenza-like illness is not likely to be cost effective, given its low average effectiveness. Similarly, antivirals reduce the duration of symptoms by around a day in children, otherwise healthy individuals, and high risk populations.7,10 The possible emergence of resistance further limits the routine use of neuraminidase inhibitors.11
Before committing scarce resources to deal with influenza we need better proof that what we see is influenza and not an influenza-like illness. As Harling et al argue,9 our efforts should be concentrated in strengthening our capability to conduct surveillance.
Reliable real time estimates of the size of the pink slice in the figure would have two benefits. Firstly, timely identification of influenza viruses circulating in a community, swiftly communicated to its general practitioners, should enable rational use of antivirals. Positive predictive value of influenza diagnosis may increase up to 70%-80% during times when influenza is known to be circulating and duly alerted general practitioners use cough and fever as the most predictive symptoms.12 w1 Within half an hour of receiving a sample, rapid testing (immunoassays or viral neuraminidase detection) could be used two to four hours later by antibody staining tests and eventually by viral culture.12 w1 w2 Secondly, cumulative knowledge of which agents circulated during the season would enable apportioning a share of influenza-like illness burden to each, the formulation of better predictions of the effects of vaccines and antivirals, and would help clarify decision making rules. Emerging threats such as those from coronavirus and metapneumovirus would also be quantified and help focus efforts on production of new vaccines and antivirals.w3
In the absence of such knowledge, it is difficult to see how routine, explicit, and rational use of what we have can be made.
Supplementary Material
References
- 1.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ 1997;315: 1060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makela MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimaki M, et al. Viruses and bacteria in the aetiology of the common cold. J Clin Microbiol 1998;36: 539-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambon MC, Stockton JD, Clewley JC, Zambon MC, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet 2001;358: 1410-6. [DOI] [PubMed] [Google Scholar]
- 4.Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2004;3: CD001269. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson P, Pattenden S, Armstrong B, Fletcher A, Kovats R, Mangtani P, et al. Vulnerability to winter mortality in elderly people in Britain: population based study. BMJ 2004;329: 647-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jefferson T, Deeks JJ, Demicheli V, Rivetti D, Rudin M. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev 2004;3: CD001169. [DOI] [PubMed] [Google Scholar]
- 7.Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG, et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003;326: 1235-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson T, Demicheli V, Deeks JJ, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev 2000;(2): CD001265. [DOI] [PubMed]
- 9.Harling R, Hayward A, Watson JM. Implications of the incidence of influenza-like illness in nursing homes for influenza chemoprophylaxis: descriptive study. BMJ 2004;329: 663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matheson NJ, Symmonds-Abrahams M, Sheikh A, Shepperd S, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev 2003;(3): CD002744. [DOI] [PubMed]
- 11.Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364: 759-65. [DOI] [PubMed] [Google Scholar]
- 12.Zambon M, Hays J, Webster A, Newman R, Keene O. Diagnosis of influenza in the community: relationship of clinical diagnosis to confirmed virological, serologic, or molecular detection of influenza. Arch Intern Med 2001;161: 2116-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 Additional references w1-w3 are on
Additional references w1-w3 are on