The development of membrane-protein based biotechnologies necessitates the identification and design of new membrane materials with enhanced stability. Bolalipids are an interesting class of bipolar lipids that have been proposed for these applications. Archaea are unicellular organisms capable of thriving under extreme conditions, such as high temperature, high salt concentration, or low pH. Archaea often possess significant amounts of bolalipids that contain a hydrolysis resistant ether-linkage, as opposed to the more labile ester-linked monopolar phospholipids of Eukaryota and Bacteria. One distinctive feature of bolalipids is the presence of membrane spanning alkyl chains, which are believed to be responsible for their enhanced physical stability.1 This stability has been utilized to develop planar supported membranes,2 to reconstitute and study integral membrane proteins,3 and as gene and vaccine delivery vehicles.4 Most of these efforts have focused on the use of synthetic archaeal lipid analogues due to the difficulties of obtaining naturally occurring bolalipids in pure form.5,6 Given the growing importance of these analogues, a better understanding of their structural and dynamical properties is needed.
Solid-state 2H NMR spectroscopy is commonly used to probe membrane structure and dynamics.7 2H NMR spectroscopy can probe individual C–2H bonds in a membrane lipid or protein, providing atomically resolved information on the local environment of the deuteron. The observable quadrupolar couplings arise from the interaction of the quadrupole moment of the 2H nucleus with the electric field gradient of the C–2H bond. These couplings can be used to calculate segmental order parameters (SCD), which provide insight into the local membrane organization at the position of the C–2H bond. Here we utilized 2H NMR spectroscopy to analyze three deuterium-labeled variants of a previously synthesized bolalipid analogue, C20BAS-PC.6 The three compounds, [1′,1′,20′,-20′-2H4]C20BAS-PC (1), [2′,2′,19′,19′-2H4]C20BAS-PC (2), and [10′,11′-2H2]C20BAS-PC (3) (Figure 1), were synthesized with deuterium labels on the sn-1 chain (Supporting Information). Residual quadrupolar couplings were calculated from the de-Paked 2H NMR spectra.8 These splittings were used to calculate SCD, the approximate hydrophobic layer thickness for half of the membrane (Dc), and the area per lipid 〈A〉 using a first-order mean-torque model.9 The data were compared to a monopolar lipid, dilauryl phosphatidylcholine (DLPC).9
Figure 1.
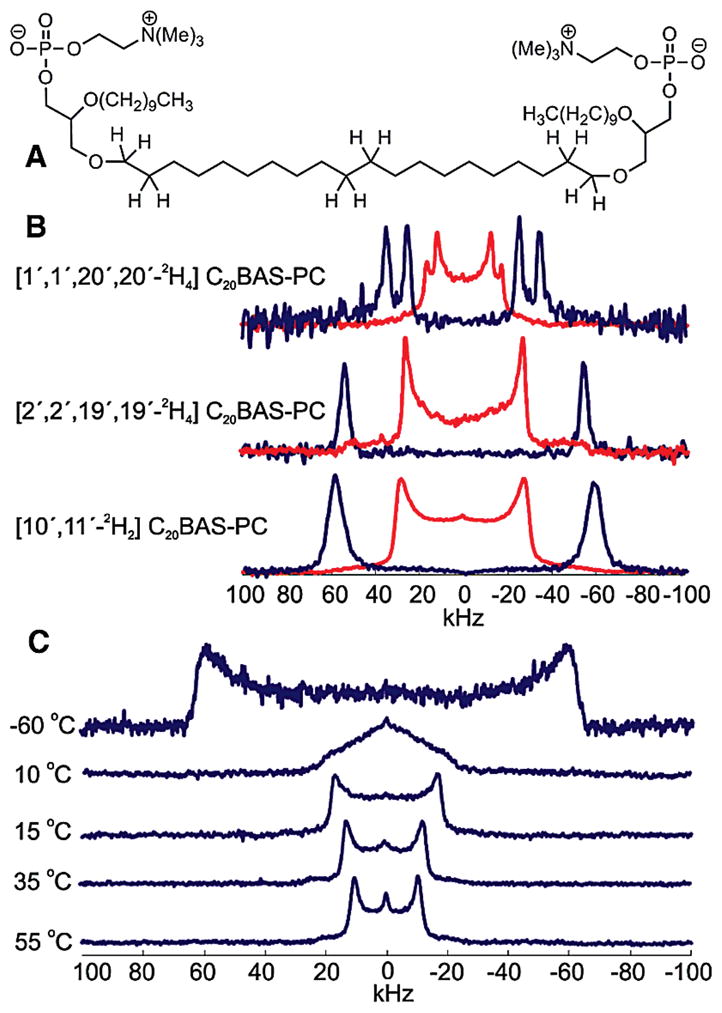
(A) Structure of non-deuterated C20BAS-PC. (B) Powder-type (red) and de-Paked (blue) 2H NMR spectra of [1′,1′,20′,20′-2H4]C20BAS-PC (1), [2′,2′,19′,19′-2H4]C20BAS-PC (2), and [10′,11′-2H2]C20BAS-PC (3) at 25 °C. (C) Powder-type 2H NMR spectra of [10′,11′-2H2]C20BAS-PC at −60, 10, 15, 35, and 55 °C.
In the high-temperature, liquid-crystalline state, 2 and 3 (Figure 1) yielded single quadrupolar splittings due to the equivalence of all deuterium atoms in both compounds, resulting in order parameters of |SCD| = 0.21 and 0.23 at 25 °C, respectively. However, 1 (Figure 1) gave two distinct quadrupolar splittings, indicating the existence of two distinct environments for deuterium in this molecule. These two components were equal in intensity at two different temperatures (25 and 45 °C) in the liquid-crystalline phase. Similar findings have been reported for 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) at the sn-2 C2 position10 and the C1 position of both the sn-1 and sn-2 chains in the ether-linked monopolar phospholipid, 1,2-dihexadecyl-sn-glycero-3-phospho-ethanolamine (DHPE).11 It is believed that the inequivalence of the two deuterium atoms arises from different glycerol H–C1–O–C1′–2H alkyl dihedral orientations of the alkyl chain near the bilayer interface.10
The segmental order parameters of the bolalipid can be used to determine the conformation of the lipid within the membrane. We were specifically interested in whether the bolalipids adopt a transmembrane conformation, with the two polar head groups on opposite sides of the membrane, or a looping conformer, with the polar head groups on the same side of the membrane. The centermost C–2H bonds should exhibit drastically different orientations with respect to the membrane normal between the two conformers. This difference will be observed as a change in the magnitude of the quadrupolar splitting, where the transmembrane conformer generates a much larger splitting than that of the looping conformer.12 A previous study of ester-linked bolaamphiphiles resulted in mostly transmembrane conformers with a small (<10%) percentage of looping conformers.13 The 2H NMR spectrum of 3, however, exhibits a single large quadrupolar splitting. Based on this finding, we infer that C20BAS-PC adopts transmembrane conformers, with no detectable presence of looping conformers.
Powder-type 2H NMR spectra of 3 in the liquid-crystalline phase are well-defined and give a distinct quadrupolar splitting (Figure 1C). As the temperature decreases, the bolalipid motion also decreases upon entering the gel phase (Tm = 15 °C),14 generating a single broad line. This spectrum arises because the trans-gauche isomerization occurs at a rate similar to the NMR time scale, leading to an observed loss of axial symmetry of the lipid with respect to the membrane normal.7 The triangular shape of the 2H NMR spectrum at intermediate temperatures is reminiscent of the η = 1 powder pattern expected for restricted 2-site rotational isomerism. As the temperature is further decreased, the membrane adopts an all trans conformation, and the powder spectrum becomes resolved again due to the reemergence of axial symmetry. The temperature dependence of the quadrupolar splitting of 3 (Figure 2A) displays a large difference in order between the liquid-crystalline and gel phases, the latter of which approaches the 250 MHz rigid lattice limit for the C–2H bond parallel to the B0 field.7,15 Interestingly, there is a large observed increase in segmental order between the C1′(20′) and C2′(19′) positions, and then very little change is observed between the C2′(19′) and C9′(10′) positions. From this we infer that the alkyl chain is highly ordered between C2′ and C19′, in stark contrast to monopolar lipids such as DLPC, where the order is known to decrease drastically as a function of distance from the glycerol backbone (Figure 2B).7,9
Figure 2.
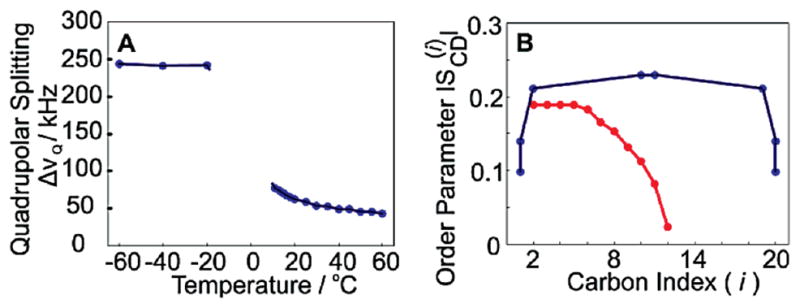
(A) De-Paked quadrupolar splittings (ΔνQ) of [10′,11′-2H2]C20-BAS-PC as a function of temperature. (B) Order parameters of C20-BAS-PC (blue) at 25 °C and DLPC (red) at 30 °C as a function of carbon number.
It has been established that Dc, 〈A〉, and thermal expansion coefficients can be calculated from the residual quadrupolar splittings (cf. Supporting Information).9 Our data show that the values of Dc and 〈A〉 of C20BAS-PC at 30 °C are 9.1 Å and 60.5 Å2, respectively (Figure 3). While DLPC has a larger value of Dc than C20BAS-PC (10.5 vs 9.1 Å), the area per lipid is quite similar (62.6 vs 60.5 Å2). Taking into consideration that a DLPC bilayer consists of 22 methylene segments as opposed to 20 in C20BAS-PC, these results demonstrate that the additional mass from the DLPC membrane contributes to a thicker hydrophobic layer while having a much smaller impact on 〈A〉. The isobaric thermal expansion coefficients for Dc and 〈A〉 (α|| and α⊥) are given by the slopes of ln Dc and ln 〈A〉 as a function of temperature. We report α|| and α⊥ for C20BAS-PC as −2.4 × 10−3 and 3.6 × 10−3 K−1, respectively. These data are very similar to those for DLPC (α|| = −3.0 × 10−3 and α⊥ = 4.1 × 10−3 K−1), despite the very different molecular structures and segmental order profiles.
Figure 3.
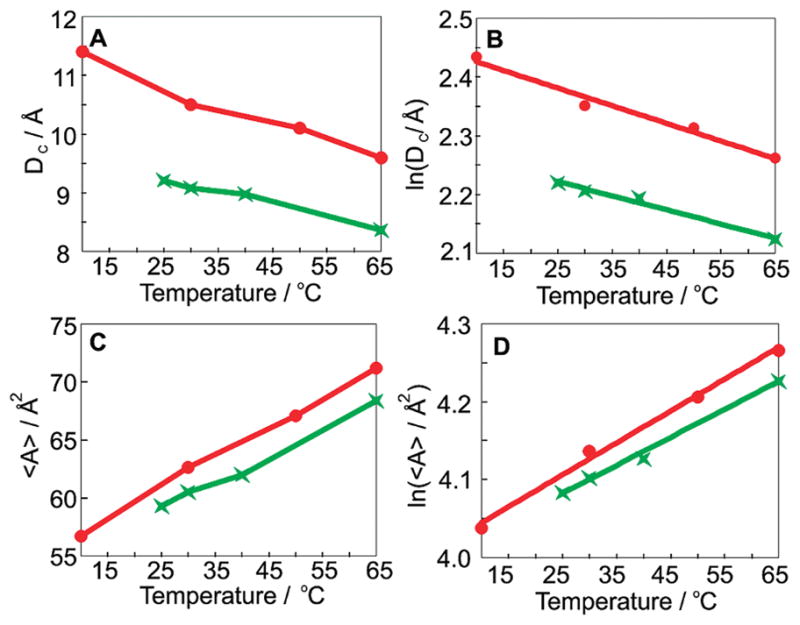
(A) Hydrophobic layer thickness (DC) for [2′,2′,19′,19′-2H4]C20-BAS-PC (green) and DLPC (red) as a function of temperature. (B) Natural logarithm of hydrophobic layer thickness, (C) area per lipid, and (D) natural logarithm of area per lipid as a function of temperature.
These results provide insight into the structural and functional properties of bolalipids and may aid our understanding of their interactions with associated proteins. We are currently utilizing the 2H NMR spectroscopy results reported here to investigate the mixing behavior of bolalipid/monopolar binary membrane systems.
Supplementary Material
Acknowledgments
We thank Victor Constantino for assistance in material preparation and Gabriel Longo, Avigdor Leftin, and Igal Szleifer for helpful discussions. We also gratefully acknowledge the support from the National Institutes of Health Grants CA112427 and EY12049.
Footnotes
Supporting Information Available: Synthesis and characterization of [1′,1′,20′,20′-2H4]C20BAS-PC (1), [2′,2′,19′,19′-2H4]C20BAS-PC (2), and [10′,11′-2H2]C20BAS-PC (3). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Damste JSS, Schouten S, Hopmans EC, van Duin ACT, Geenevasen JAJ. J Lipid Res. 2002;43:1641–1651. doi: 10.1194/jlr.m200148-jlr200. [DOI] [PubMed] [Google Scholar]; (b) Derosa M, Gambacorta A. Prog Lipid Res. 1988;27:153–175. doi: 10.1016/0163-7827(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cornell BA, Braach-Maksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. Nature. 1997;387:580–583. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]; (b) Fuhrhop AH, Wang TY. Chem Rev. 2004;104:2901–2937. doi: 10.1021/cr030602b. [DOI] [PubMed] [Google Scholar]; (c) Sun XL, Biswas N, Kai T, Dai ZF, Dluhy RA, Chaikof EL. Langmuir. 2006;22:1201–1208. doi: 10.1021/la052125t. [DOI] [PubMed] [Google Scholar]
- 3.(a) Elferink MGL, Dewit JG, Demel R, Driessen AJM, Konings WN. J Biol Chem. 1992;267:1375–1381. [PubMed] [Google Scholar]; (b) Febo-Ayala W, Morera-Felix SL, Hrycyna CA, Thompson DH. Biochemistry. 2006;45:14683–14694. doi: 10.1021/bi061159c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kim JM, Patwardhan A, Bott A, Thompson DH. Biochim Biophys Acta. 2003;1617:10–21. doi: 10.1016/j.bbamem.2003.08.011. [DOI] [PubMed] [Google Scholar]; (d) Veld GI, Elferink MGL, Driessen AJM, Konings WN. Biochemistry. 1992;31:12493–12499. doi: 10.1021/bi00164a028. [DOI] [PubMed] [Google Scholar]
- 4.(a) Patel GB, Sprott GD. Crit Rev Biotechnol. 1999;19:317–357. doi: 10.1080/0738-859991229170. [DOI] [PubMed] [Google Scholar]; (b) Rethore G, Montier T, Le Gall T, Delepine P, Cammas-Marion S, Lemiegre L, Lehn P, Benvegnu T. Chem Commun. 2007:2054–2056. doi: 10.1039/b618568a. [DOI] [PubMed] [Google Scholar]; (c) Denoyelle S, Polidori A, Brunelle M, Vuillaume PY, Laurent S, ElAzhary Y, Pucci B. New J Chem. 2006;30:629–646. [Google Scholar]
- 5.(a) Benvegnu T, Brard M, Plusquellec D. Curr Opin Colloid Interface Sci. 2004;8:469–479. [Google Scholar]; (b) Kai T, Sun XL, Faucher KM, Apkarian RP, Chaikof EL. J Org Chem. 2005;70:2606–2615. doi: 10.1021/jo048167o. [DOI] [PubMed] [Google Scholar]; (c) Patwardhan AP, Thompson DH. Langmuir. 2000;16:10340–10350. [Google Scholar]; (d) Wang GJ, Hollingsworth RI. J Org Chem. 1999;64:4140–4147. doi: 10.1021/jo9818226. [DOI] [PubMed] [Google Scholar]
- 6.Svenson S, Thompson DH. J Org Chem. 1998;63:7180–7182. doi: 10.1021/jo9803176. [DOI] [PubMed] [Google Scholar]
- 7.Brown MF. In: Biological Membranes: A Molecular Perspective from Computation and Experiment. Merz KM Jr, Roux B, editors. Birkhäuser; Basel: 1996. pp. 175–252. [Google Scholar]
- 8.McCabe MA, Wassall SR. J Magn Reson B. 1995;106:80–82. [Google Scholar]
- 9.Petrache HI, Dodd SW, Brown MF. Biophys J. 2000;79:3172–3192. doi: 10.1016/S0006-3495(00)76551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seelig J, Seelig A. Q Rev Biophys. 1980;13:19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- 11.(a) Ruocco MJ, Makriyannis A, Siminovitch DJ, Griffin RG. Biochemistry. 1985;24:4844–4851. doi: 10.1021/bi00339a018. [DOI] [PubMed] [Google Scholar]; (b) Stewart LC, Kates M, Ekiel IH, Smith ICP. Chem Phys Lipids. 1990;54:115–129. [Google Scholar]
- 12.Longo GS, Thompson DH, Szleifer I. Biophys J. 2007;93:2609–2621. doi: 10.1529/biophysj.106.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuccia LA, Morin F, Beck A, Hebert N, Just G, Lennox RB. Chem Eur J. 2000;6:4379–4384. doi: 10.1002/1521-3765(20001201)6:23<4379::aid-chem4379>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Di Meglio C, Rananavare SB, Svenson S, Thompson DH. Langmuir. 2000;16:128–133. [Google Scholar]
- 15.Brown MF, Lope-Piedrafita S, Martinez GV, Petrache HI. In: Modern Magnetic Resonance. Webb GA, editor. Vol. 1. Springer; Dordrecht: 2006. pp. 245–256. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


