Abstract
Background
Cardiac troponin is an independent predictor of cardiovascular mortality in individuals without symptoms or signs of cardiovascular disease. The mechanisms for this association are uncertain, and a role for troponin testing in the prevention of coronary heart disease has yet to be established.
Objectives
This study sought to determine whether troponin concentration could predict coronary events, be modified by statins, and reflect response to therapy in a primary prevention population.
Methods
WOSCOPS (West of Scotland Coronary Prevention Study) randomized men with raised low-density lipoprotein cholesterol and no history of myocardial infarction to pravastatin 40 mg once daily or placebo for 5 years. Plasma cardiac troponin I concentration was measured with a high-sensitivity assay at baseline and at 1 year in 3,318 participants.
Results
Baseline troponin was an independent predictor of myocardial infarction or death from coronary heart disease (hazard ratio [HR]: 2.3; 95% confidence interval [CI]: 1.4 to 3.7) for the highest (≥5.2 ng/l) versus lowest (≤3.1 ng/l) quarter of troponin (p < 0.001). There was a 5-fold greater reduction in coronary events when troponin concentrations decreased by more than a quarter, rather than increased by more than a quarter, for both placebo (HR: 0.29; 95% CI: 0.12 to 0.72 vs. HR: 1.95; 95% CI: 1.09 to 3.49; p < 0.001 for trend) and pravastatin (HR: 0.23; 95% CI: 0.10 to 0.53 vs. HR: 1.08; 95% CI: 0.53 to 2.21; p < 0.001 for trend). Pravastatin reduced troponin concentration by 13% (10% to 15%; placebo adjusted, p < 0.001) and doubled the number of men whose troponin fell more than a quarter (p < 0.001), which identified them as having the lowest risk for future coronary events (1.4% over 5 years).
Conclusions
Troponin concentration predicts coronary events, is reduced by statin therapy, and change at 1 year is associated with future coronary risk independent of cholesterol lowering. Serial troponin measurements have major potential to assess cardiovascular risk and monitor the impact of therapeutic interventions.
Key Words: cardiac troponin, cardiovascular risk, primary prevention, statins
Abbreviations and Acronyms: CI, confidence interval; HR, hazard ratio; LDL, low-density lipoprotein
Central Illustration
WOSCOPS (West of Scotland Coronary Prevention Study) was a trial of statin-based low-density lipoprotein (LDL) cholesterol-lowering therapy in men ages 45 to 64 years with raised serum cholesterol concentrations. The trial participants had no history of myocardial infarction and were randomized to receive pravastatin or placebo for an average of 5 years. Treatment reduced the risk of a range of cardiovascular endpoints by about one-third (1), and extended follow-up over 15 years revealed that the risk reduction persisted long term (2). This and other similar trials established the benefits of intervention in asymptomatic high-risk subjects 3, 4, but debate continues over the merits of drug therapy in the wider primary prevention setting 5, 6, 7. One approach to resolve this issue has been the search for biomarkers that enhance risk prediction.
Cardiac troponin is a specific marker of myocardial injury and an independent predictor of cardiovascular mortality in patients with and without cardiovascular disease 8, 9, 10, 11, 12, 13, 14. Novel high-sensitivity assays can now accurately measure plasma cardiac troponin I concentrations in everyone. Higher troponin concentrations may reflect subclinical coronary artery disease and identify those at greatest risk who could benefit from targeted preventative therapies. The aims of this study were to determine whether cardiac troponin I concentrations could predict future coronary events, be modified by statins, and assess response to therapy in WOSCOPS.
Methods
Study population
WOSCOPS randomized 6,595 men ages 45 to 64 years with moderate hypercholesterolemia (LDL cholesterol concentrations 152 to 228 mg/dl) and no prior history of myocardial infarction to receive placebo or pravastatin 40 mg/day. The design and conduct of the study have been described elsewhere 1, 15. The study exclusion criteria are reported in full in the Online Appendix. Plasma was obtained before randomization and at 1 year, and was stored at –80°C. For the present analysis, we identified all participants with sufficient stored plasma at both time points. As a result, 3,318 of the 6,595 study participants were included. The research ethics committee of the University of Glasgow and all participating centers approved the trial design, and the participants provided written informed consent.
Troponin assay
Cardiac troponin I concentrations in stored plasma were determined at baseline and at 1 year using the ARCHITECTSTAT high-sensitive troponin I assay (Abbott Laboratories, Abbott Park, Illinois). The limit of detection is 1.2 ng/l, and the interassay coefficient of variation is <10% at 4.7 ng/l. The upper reference limit or 99th-centile value is 34 ng/l in men (16). All samples underwent centrifugation twice (3,000 relative centrifugal force for 10 min) to ensure samples were visibly homogeneous according to the manufacturer’s instructions.
Clinical outcomes and record linkage
The primary outcome of the trial was a composite of nonfatal myocardial infarction (including nonhospitalized silent myocardial infarction) and death from coronary heart disease. During the formal trial period, patients were followed for an average of 4.9 years to determine the occurrence of the primary endpoint and other events, which were adjudicated by an endpoints committee 1, 15. Additional follow-up for up to 15 years after randomization was available through the interrogation of records held by the National Health Service for Scotland (2). Myocardial infarction events during the trial period included silent infarction, whereas those over the 15-year period were based only on hospitalization.
Statistical analysis
The distribution of troponin I was skewed, and values were log-transformed before analysis. Participants were divided into quarters of the distribution of baseline troponin concentration. Comparisons of baseline characteristics between groups were made using chi-square test for categorical variables and 1-way analysis of variance or the Kruskal-Wallis test for continuous variables. Multivariable Cox proportional hazards regression analyses were used to evaluate the associations between baseline and change in troponin concentration at 1 year with risk of coronary events over 5 and 15 years in the study population and a subgroup with no symptoms or signs of coronary heart disease at enrollment. Treatment effects (pravastatin vs. placebo) are expressed as hazard ratio (HR) with 95% confidence interval (CI). Percent change in troponin was determined from the concentration at 1 year relative to the concentration at baseline, and the association with percent change in LDL cholesterol over the same period was assessed using the Pearson correlation coefficient. An independent-samples Student t test was used to compare the mean change between treatment groups. Participants who had a coronary event before the 1-year visit were excluded for the purpose of this analysis. The effect of change in troponin on coronary events at 5 years was explored further by splitting both treatments into 5 groups based on quintile cutpoints in the placebo group. Determinants of log-transformed baseline troponin I were investigated using stepwise linear regression at p-to-enter of 0.10 and p-to-stay of 0.05. Analyses were performed using SPSS version 20.0.0 (IBM Corp., Armonk, New York), SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, North Carolina), and R version 3.0.0 (R Project for Statistical Computing, Vienna, Austria). Statistical significance was taken as a 2-sided p < 0.05.
Results
Baseline characteristics of the 3,318 participants in this analysis were similar to the full study population (Online Table 1).
Distribution of troponin at baseline
The median troponin concentration at baseline was 4.0 ng/l, with an interquartile range of 3.1 to 5.2 ng/l. Values were above the limit of detection (1.2 ng/l) in 3,311 participants (99.8%) and above the 99th-centile (34 ng/l) in 48 participants (1.5%). For the purpose of this analysis, 7 participants with undetectable troponin concentrations were assigned a value of 1.2 ng/l.
Participants were stratified into quarters of the baseline troponin concentration (Table 1). Compared to the lowest quarter, participants in the upper quarters were older and were more likely to have a history of hypertension, symptoms of angina, and minor abnormalities on the electrocardiogram (p < 0.001 for all). Those in the highest quarter had higher systolic and diastolic blood pressures (139 ± 18 mm Hg vs. 133 ± 17 mm Hg and 85 ± 11 mm Hg vs. 83 ± 11 mm Hg, respectively), and there were small differences in total and LDL cholesterol concentrations across the quarters (4 and 5 mg/dl, respectively). In contrast, there were no significant differences in high-density lipoprotein cholesterol and triglyceride concentrations or the prevalence of diabetes mellitus. In a stepwise linear regression model, higher baseline troponin concentrations were associated with increased age, body mass index, systolic blood pressure, and LDL cholesterol concentrations, and a higher prevalence of anginal symptoms and minor echocardiographic abnormalities (minor ST-segment and T-wave abnormalities) (Online Table 2).
Table 1.
Baseline Characteristics of Participants Stratified by Troponin Concentration Quarters
| All (N = 3,318) |
Quarters of Troponin I Concentration |
p Value Trend | ||||
|---|---|---|---|---|---|---|
| Q1 (n = 748) |
Q2 (n = 889) |
Q3 (n = 843) |
Q4 (n = 838) |
|||
| Troponin concentration, ng/l | ≤3.1 | 3.1–3.9 | 4.0–5.1 | ≥5.2 | ||
| Age, yrs | 55.1 ± 5.5 | 53.8 ± 5.2 | 55.0 ± 5.5 | 55.4 ± 5.5 | 56.2 ± 5.5 | <0.001 |
| Body mass index, kg/m2 | 25.9 ± 3.2 | 25.6 ± 3.2 | 25.9 ± 3.0 | 26.0 ± 3.1 | 26.3 ± 3.3 | <0.001 |
| Employed | 2,346 (71) | 577 (77) | 637 (72) | 612 (73) | 520 (62) | <0.001 |
| Smoking and alcohol status | ||||||
| Current smoker | 1,393 (42) | 333 (45) | 393 (44) | 343 (41) | 324 (39) | 0.043 |
| Alcohol intake, ≥21 U/week | 546 (17) | 121 (16) | 158 (18) | 138 (16) | 129 (15) | 0.603 |
| Blood pressure and heart rate | ||||||
| Systolic, mm Hg | 136 ± 17 | 133 ± 17 | 134 ± 17 | 136 ± 18 | 139 ± 18 | <0.001 |
| Diastolic, mm Hg | 84 ± 11 | 83 ± 11 | 84 ± 11 | 84 ± 10 | 85 ± 11 | <0.001 |
| Heart rate, beats/min | 65 ± 11 | 67 ± 11 | 66 ± 11 | 65 ± 11 | 65 ± 12 | <0.001 |
| Past medical history | ||||||
| Hypertension | 544 (16) | 87 (12) | 122 (14) | 138 (16) | 197 (24) | <0.001 |
| Diabetes mellitus | 36 (1) | 8 (1) | 9 (1) | 10 (1) | 9 (1) | 0.988 |
| Angina∗ | 168 (5) | 23 (3) | 35 (4) | 48 (6) | 62 (7) | <0.001 |
| Intermittent claudication | 82 (3) | 20 (3) | 16 (2) | 18 (2) | 28 (3) | 0.186 |
| Minor ECG abnormalities∗ | 271 (8) | 36 (5) | 62 (7) | 63 (8) | 110 (13) | <0.001 |
| Family history of CHD death | 191 (6) | 44 (6) | 46 (5) | 42 (5) | 59 (7) | 0.256 |
| Medication | ||||||
| Nitrate | 74 (2) | 8 (1) | 14 (2) | 18 (2) | 34 (4) | <0.001 |
| Beta-blocker | 241 (7) | 38 (5) | 46 (5) | 67 (8) | 90 (11) | <0.001 |
| ACE inhibitor | 36 (1) | 4 (1) | 11 (1) | 8 (1) | 13 (2) | 0.246 |
| Lipid levels | ||||||
| Total cholesterol, mg/dl | 270 ± 22 | 268 ± 22 | 270 ± 22 | 272 ± 22 | 272 ± 22 | <0.001 |
| LDL cholesterol, mg/dl | 191 ± 17 | 188 ± 16 | 190 ± 17 | 193 ± 17 | 193 ± 18 | <0.001 |
| HDL cholesterol, mg/dl | 44 ± 9 | 43 ± 9 | 44 ± 10 | 44 ± 9 | 43 ± 9 | 0.666 |
| Triglycerides, mg/dl | 154 ± 136 | 159 ± 138 | 151 ± 134 | 154 ± 136 | 154 ± 136 | 0.249 |
| Treatment allocation | ||||||
| Placebo | 1,647 (50) | 385 (51) | 456 (51) | 392 (47) | 414 (49) | 0.150 |
| Pravastatin 40 mg | 1,671 (50) | 363 (49) | 433 (49) | 451 (53) | 424 (51) | 0.150 |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; CHD = coronary heart disease; ECG = electrocardiogram; HDL = high-density lipoprotein; LDL = low-density lipoprotein; Q = quarter.
Symptoms consistent with angina on Rose questionnaire and minor ST-segment or T-wave abnormalities on resting 12-lead ECG defined by Minnesota codes (4-2, 4-3, 5-2, 5-3).
Baseline troponin and risk of coronary heart disease events
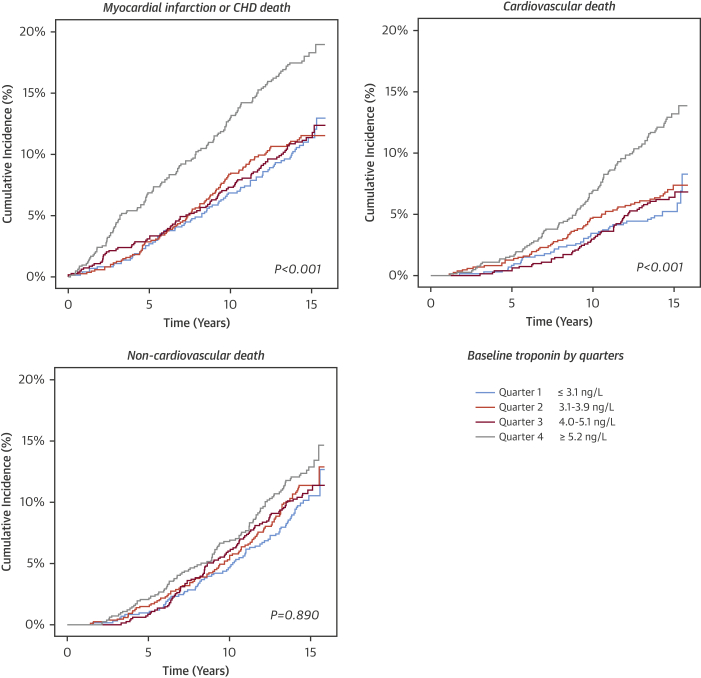
Higher troponin concentrations at baseline were associated with increased risk of coronary heart disease at both 5- and 15-year follow-up (Figure 1, Online Table 3). Compared to the lowest quarter (≤3.1 ng/l), patients in the highest quarter (≥5.2 ng/l) were at the highest risk for nonfatal myocardial infarction or death from coronary heart disease at 5 and 15 years (HR: 2.27; 95% CI: 1.42 to 3.65 and HR: 1.54; 95% CI: 1.16 to 2.05, respectively; p < 0.001 for both). All-cause mortality and cardiovascular death were also associated with baseline troponin concentration, but noncardiovascular death was not. In a sensitivity analysis, similar relationships were observed for the primary endpoint in 2,882 men without symptoms or signs of coronary heart disease at enrollment (Online Figure 1).
Figure 1.
Cumulative Incidence Plot for Primary Outcome of Nonfatal Myocardial Infarction or Death From Coronary Heart Disease, and Secondary Outcomes of Cardiovascular and Noncardiovascular Death Stratified by Quarter of Troponin at Baseline
Higher troponin concentrations at baseline were associated with increased risk of coronary heart disease (CHD) at both 5- and 15-year follow-up. Compared to the lowest quarter (≤3.1 ng/l), patients in the highest quarter (≥5.2 ng/l) were at the highest risk for nonfatal myocardial infarction or death from CHD at 5 and 15 years (hazard ratio: 2.27; 95% confidence interval: 1.42 to 3.65; and hazard ratio: 1.54; 95% confidence interval: 1.16 to 2.05, respectively; p < 0.001 for both). Cardiovascular death was also associated with baseline troponin concentration (p < 0.001), but noncardiovascular death was not (p = 0.890).
In this subset of the original study, the HR for the overall relative treatment effect for the primary endpoint at 5 years was 0.45 (95% CI: 0.32 to 0.65; p < 0.001) and for the outcome of coronary death or hospitalized myocardial infarction at 15 years was 0.68 (95% CI: 0.56 to 0.83; p < 0.001). There was no evidence of an interaction between treatment effect and quarters of baseline troponin concentration (p = 0.67 and p = 0.19, respectively, for the 5- and 15-year outcomes) (Online Table 4). At 15 years, the absolute risk reduction in nonfatal myocardial infarction or death from coronary heart disease was greatest in the highest quarter (9.0%) compared to the lower three quarters (2.6% to 3.0%) (Online Figure 2).
Change in troponin and LDL cholesterol with treatment
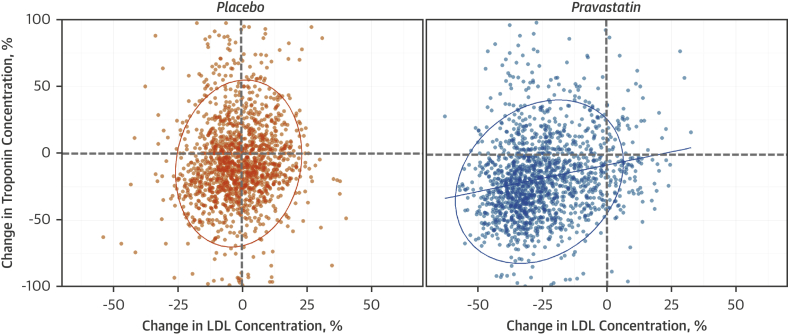
At 1 year, participants taking pravastatin had a greater reduction in troponin concentration (19%) (95% CI: 17% to 20%) than those receiving placebo (6%) (95% CI: 4% to 8%; p < 0.001) (Figure 2). The absolute change in troponin concentration from baseline with pravastatin was 2.0 ng/l (interquartile range: 1.2 to 2.8 ng/l; p < 0.001). Change in troponin concentration on treatment correlated weakly with change in LDL cholesterol (r = 0.20; p < 0.001).
Figure 2.
Percent Change in High-Sensitivity Cardiac Troponin I and LDL Cholesterol Concentrations at 1 Year in Placebo and Pravastatin Groups
Line of fit is a Pearson correlation (pravastatin group r = 0.20; p < 0.001). The ellipses are prediction ellipses with 95% confidence interval under the assumption the samples are bivariate normal to display linear correlation. LDL = low-density lipoprotein.
Change in troponin and coronary heart disease risk reduction
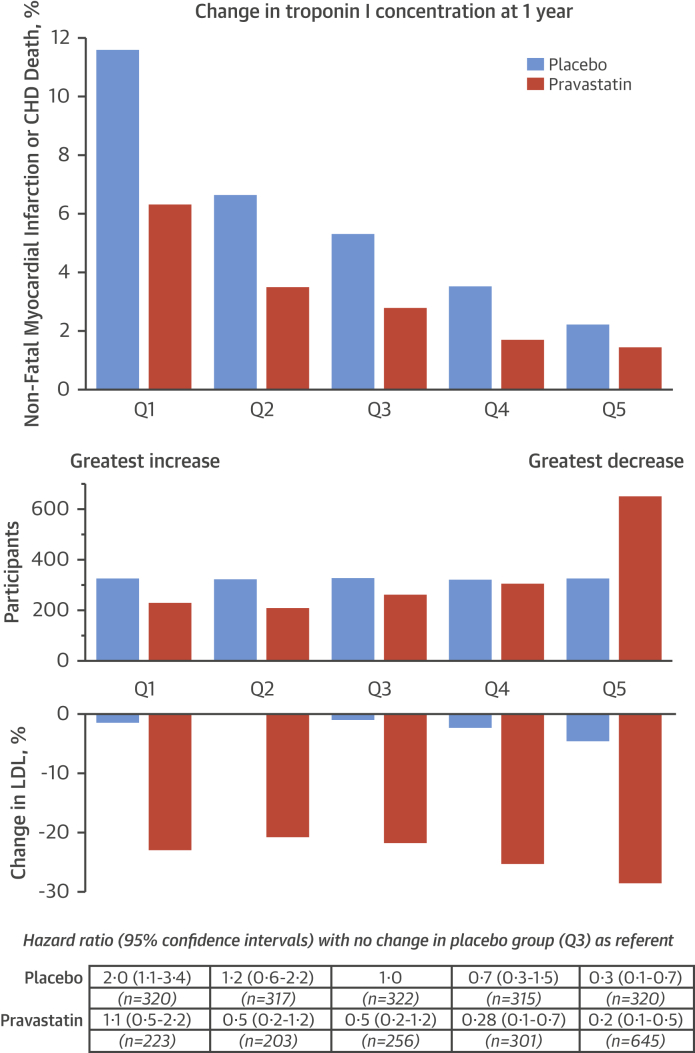
After adjustment for multiple variables (including baseline troponin concentration, and baseline and change in LDL cholesterol), change in troponin concentration at 1 year was an independent predictor of nonfatal myocardial infarction or death from coronary heart disease at both 5 and 15 years in both treatment arms (Table 2). To explore this relationship further, participants were divided into fifths based on the change in troponin concentration in the placebo group. There was a clear gradient of risk across change in troponin. In the placebo group, relative to the referent (middle) fifth, those in the top fifth (>26% increase) had a higher risk of the primary endpoint over 5 years, and those in the bottom fifth (>27% decrease) had a lower risk (p < 0.001 for trend) (Figure 3). In those taking pravastatin, a similar trend was observed, but twice as many men were in the lowest-risk group, with >27% reductions in troponin concentration (645 vs. 320 on placebo; p < 0.001), and 30% fewer were in the highest risk group, with >26% increase in troponin concentration (223 vs. 320 on placebo; p < 0.001). The risk of the primary endpoint in participants on pravastatin was 5-fold lower in those with the greatest reduction in troponin concentration (HR 0.23; 95% CI: 0.10 to 0.53) compared to those with the greatest increase in troponin concentration (HR 1.08; 95% CI: 0.53 to 2.21; p < 0.001 for trend) despite similar reductions in LDL cholesterol concentration (22% to 28%). Compared to placebo, participants taking pravastatin with the greatest reduction in troponin at 1 year (highest quarter: ≥38% reduction vs. lowest quarter: >3% increase) had the largest reduction in cardiovascular events (HR: 0.21. 95% CI: 0.08 to 0.52 vs. HR: 0.82; 95% CI: 0.51 to 1.32, respectively; p = 0.002), whereas the reduction in events was similar across quarters of change in LDL cholesterol (p = 0.823) (Online Figure 3).
Table 2.
Multivariate Models for Nonfatal Myocardial Infarction or Coronary Heart Disease Death at 5 and 15 Years According to Treatment Group∗
| Placebo |
Pravastatin |
|||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Over 5 yrs | ||||
| Troponin concentration at baseline† | 1.84 (1.38–2.45) | <0.001 | 1.45 (0.92–2.30) | 0.109 |
| Change in troponin concentration† | 0.66 (0.56–0.78) | <0.001 | 0.63 (0.49–0.80) | <0.001 |
| LDL cholesterol at baseline‡ | 0.97 (0.60–1.56) | 0.885 | 1.24 (0.63–2.42) | 0.535 |
| Change in LDL cholesterol | 1.00 (0.99–1.02) | 0.732 | 1.01 (0.99–1.03) | 0.241 |
| HDL cholesterol at baseline‡ | 0.43 (0.16–1.13) | 0.087 | 0.28 (0.07–1.16) | 0.079 |
| Age, 5 yrs | 1.07 (1.02–1.11) | 0.002 | 1.07 (1.00–1.14) | 0.037 |
| Family history of CHD | 1.65 (0.78–3.49) | 0.190 | 2.17 (0.75–6.30) | 0.154 |
| Systolic blood pressure, 10 mm Hg | 0.99 (0.98–1.01) | 0.326 | 1.01 (0.99–1.03) | 0.439 |
| Diastolic blood pressure, 10 mm Hg | 1.01 (0.98–1.04) | 0.495 | 1.01 (0.97–1.05) | 0.597 |
| Heart rate, 10 beats/min | 1.02 (1.00–1.04) | 0.019 | 1.02 (0.99–1.04) | 0.136 |
| Angina | 1.11 (0.50–2.44) | 0.799 | 2.46 (0.77–7.87) | 0.130 |
| Over 15 yrs | ||||
| Troponin concentration at baseline† | 1.49 (1.21–1.84) | <0.001 | 1.16 (0.88–1.53) | 0.300 |
| Change in troponin concentration† | 0.78 (0.68–0.89) | <0.001 | 0.83 (0.71–0.98) | 0.028 |
| LDL cholesterol at baseline‡ | 0.96 (0.71–1.31) | 0.814 | 1.30 (0.92–1.82) | 0.134 |
| Change in LDL cholesterol | 1.00 (0.99–1.02) | 0.617 | 1.00 (0.99–1.01) | 0.757 |
| HDL cholesterol at baseline‡ | 0.42 (0.22–0.77) | 0.006 | 0.47 (0.23–0.97) | 0.040 |
| Age, 5 yrs | 1.05 (1.02–1.07) | <0.001 | 1.03 (0.99–1.06) | 0.124 |
| Family history of CHD | 1.69 (1.06–2.70) | 0.027 | 1.65 (0.93–2.94) | 0.088 |
| Systolic blood pressure, 10 mm Hg | 0.99 (0.98–1.00) | 0.030 | 1.01 (0.99–1.02) | 0.330 |
| Diastolic blood pressure, 10 mm Hg | 1.02 (1.00–1.03) | 0.083 | 1.01 (0.99–1.03) | 0.242 |
| Heart rate, 10 beats/min | 1.01 (1.00–1.03) | 0.048 | 1.01 (0.99–1.02) | 0.410 |
| Angina | 1.71 (1.03–2.83) | 0.039 | 2.06 (1.07–3.95) | 0.031 |
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
Subjects who experienced a major coronary event in the first year (i.e., before repeat troponin measurement) were excluded from these analyses.
Troponin concentrations were log-transformed. Hazard ratios are reported for a doubling of log troponin concentration at baseline or doubling in the ratio of log troponin concentration from baseline to 1 year.
Increments of 39 mg/dl of LDL and HDL cholesterol. Models also included nitrate consumption, body mass index, smoking status, history of hypertension, diabetes, and ECG abnormalities at baseline: none of these was a significant determinant of the endpoint of nonfatal myocardial infarction/CHD death.
Figure 3.
Change in Troponin Concentration at 1 Year Stratified by Treatment Allocation and Nonfatal Myocardial Infarction or Coronary Heart Disease Death at 5 Years
(Top) Nonfatal myocardial infarction or death from coronary heart disease (CHD) at 5 years in those taking pravastatin (orange) and placebo (blue) stratified into fifths by change in troponin concentration in the placebo group: Q1 = >26% increase; Q2 = 2% to 25% increase; Q3 = 2% increase to 13% decrease; Q4 = 13% to 27% decrease; Q5 = ≥27% decrease. (Middle) Twice as many participants were in the lowest-risk group (Q5), with >27% reductions in troponin concentration (645 vs. 320 on placebo; p < 0.001), and 30% fewer were in the highest-risk group (Q1) with >26% increase in troponin concentration (223 vs. 320 on placebo; p < 0.001). (Bottom) Using participants in the placebo group whose troponin concentrations were unchanged as a referent (Q3), hazard ratios for the primary outcome were determined for each fifth after adjustment for age, body mass index, heart rate, systolic blood pressure, diastolic blood pressure, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, symptoms of angina, diabetes, hypertension, family history of premature coronary heart disease, minor electrocardiographic abnormalities, nitrate use, and smoking status. The risk of the primary endpoint in participants taking pravastatin was 5-fold lower in those with the greatest reduction in troponin concentration (hazard ratio: 0.23; 95% confidence interval: 0.10 to 0.53) compared to those with the greatest increase in troponin concentration (hazard ratio: 1.08; 95% confidence interval: 0.53 to 2.21; p < 0.001 for trend) despite similar reductions in LDL cholesterol concentration (22% to 28%).
Discussion
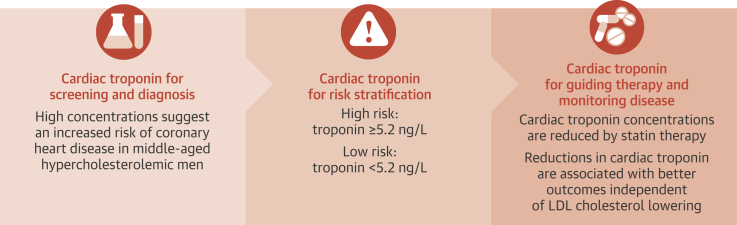
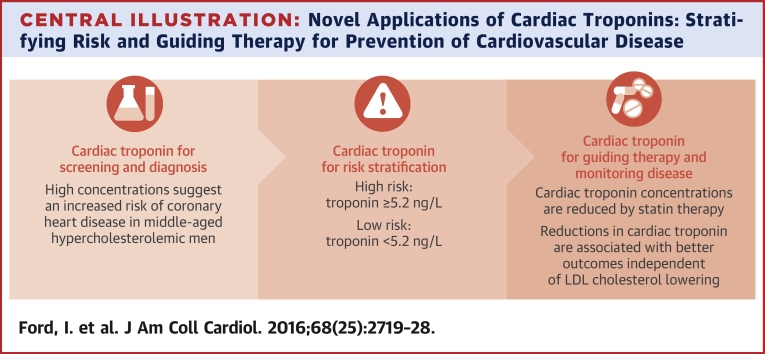
We observed a strong, specific, and independent association between baseline and 1-year change in plasma troponin I concentration and the onset of coronary heart disease over 5 and 15 years in WOSCOPS. Troponin concentrations were reduced by pravastatin therapy, which doubled the number of men whose troponin fell by more than a quarter and were at the lowest risk for future coronary events. Thus, pravastatin treatment caused similar relative risk reductions in each category of troponin change and increased the propensity for troponin concentrations to fall, leading to additive decrements in future risk that appeared to be independent of LDL cholesterol lowering. We conclude that high-sensitivity cardiac troponin assays can be used to predict future risk of coronary heart disease and to assess response to statin therapy (Central Illustration). Therefore, serial troponin measurements appear to have major potential to monitor risk and assess the impact of established or novel therapeutic interventions on future coronary heart disease risk.
Central Illustration.
Novel Applications of Cardiac Troponins: Stratifying Risk and Guiding Therapy for Prevention of Cardiovascular Disease
LDL = low-density lipoprotein.
Our analysis has a number of important and distinctive strengths that distinguish it from previous studies of troponin in coronary heart disease risk prediction. First, we evaluated plasma troponin concentration in a primary prevention setting rather than in populations that included patients with established coronary heart disease. Second, we had access to prolonged robust clinical follow-up of well-characterized participants over a 15-year period. Third, we used the latest-generation high-sensitivity cardiac troponin I assay, which detected troponin in 99.8% of the study population. Finally, in addition to exploring associations with baseline troponin concentration, we were able to critically assess dynamic changes in troponin concentrations as part of a major randomized placebo-controlled trial of statin therapy.
In WOSCOPS, a number of biomarkers have been assessed for cardiovascular risk stratification 17, 18, 19. In previous analyses, C-reactive protein predicted both cardiovascular and noncardiovascular events (19), whereas troponin is a specific biomarker for the prediction of coronary heart disease outcomes. Interestingly, in contrast to recent studies of patients with established coronary heart disease (8), we found that troponin predicted fatal and nonfatal myocardial infarction rather than heart failure events. Similar associations were recently reported in individuals without cardiovascular disease who participated in the JUPITER (Justification for the Use of Statins in Primary Prevention) trial (20). This finding likely reflects differences in the extent of coronary heart disease at enrollment, the use of concomitant therapies, and the low prevalence of heart failure events.
The association between troponin and coronary heart disease risk is nonlinear, with an apparent threshold at 5.2 ng/l that identifies those 2 to 3 times more likely to have a coronary event over 15 years. This is consistent with findings from other cohorts using the same assay (21), in which receiver-operating curve analysis identified a threshold of 6 ng/l in a randomized trial population with established coronary heart disease (14) and 7 ng/l in men who participated in the Scottish Heart Health Study (12). Interestingly, troponin concentrations <5 ng/l also identify patients as low risk in the emergency department (21), and increasingly this approach is being used to rule out myocardial infarction in clinical practice. Primary prevention guidelines recommend statins for the prevention of cardiovascular disease in any individual with a 10-year estimated risk of a cardiovascular event >7.5% (22). This approach has been widely debated, with the benefits and risks for the individual more evenly balanced 5, 6, 7. We believe cardiac troponin may help to better stratify those healthy individuals at risk for coronary heart disease who would benefit most from statin therapies and that this approach should be formally addressed in prospective trials.
We hypothesized that statin therapy would reduce cardiac troponin concentrations. However, the observations that a large majority of participants on pravastatin had lower troponin concentrations at 1 year and that this reduction correlated with the decrease in LDL cholesterol were unexpected. White et al. (23) reported that cardiac troponin concentrations were reduced with pravastatin in patients with coronary heart disease. However, troponin concentrations were measured by a contemporary sensitive assay and were undetectable in more than one-third of patients and were below the limit of analytical precision in the majority. As such, the assay had insufficient sensitivity to evaluate the relationship among pravastatin therapy, troponin concentration, and LDL cholesterol. In contrast, we used a novel high-sensitivity assay that was able to measure cardiac troponin in >99% of our study population. This allowed us to explore associations among change in troponin, LDL cholesterol, and coronary events.
We observed that change in troponin predicted risk with an approximately 5-fold range in HRs when comparing men with the greatest increases and decreases in troponin concentration. We observed the lowest rate of coronary heart disease events (1.4% over 5 years) in men treated with pravastatin whose troponin concentration fell by more than a quarter and the highest in those taking placebo with an increase in troponin of more than a quarter (11.6% over 5 years). Although LDL cholesterol level is currently used to select patients for statin therapy and to monitor treatment response, it was notable that neither baseline nor change in LDL cholesterol predicted future coronary events. Importantly, pravastatin more than doubled the likelihood of a reduction in troponin concentration and this appeared to be independent of LDL cholesterol lowering. In addition to reducing serum cholesterol concentrations, statins reduce the levels of other oxidized proteins, improve endothelial nitric oxide bioavailability, and slow progression of atherosclerosis (24). Our observations suggest the preventative effects of statin therapy are mediated in part by these other mechanisms. It was surprising that change in troponin concentration at 1 year was an independent predictor of risk in the placebo group, in which LDL cholesterol concentrations were unchanged. It is possible that cardiac troponin concentrations are modulated by a minor fraction, such as oxidized LDL, that is not reflected when measuring total LDL concentration. Such oxidized lipoproteins may influence troponin concentration directly through a toxic effect on cardiomyocytes or indirectly through their effects on nitric oxide bioavailability and myocardial perfusion. We speculate that recruitment to WOSCOPS may have increased awareness of cardiovascular risk in some trial participants, thereby inducing lifestyle modifications that both lower cardiac troponin concentration and improve outcomes.
A biomarker that can dynamically track the risk of coronary heart disease over time would be a major step forward. Indeed, previous studies have failed to demonstrate that changes in inflammatory, hemostatic, or lipid biomarkers can predict future cardiovascular risk 17, 18, 19. This unique property of change of troponin with time has major ramifications and suggests that serial high-sensitivity troponin measurements could represent a major surrogate biomarker to help manage our patients and evaluate coronary heart disease risk with established or novel cardiovascular and noncardiovascular therapies.
Study limitations
The trial was conducted in middle-aged men with raised cholesterol levels, and the findings may not be applicable to the wider population including women. This is an important point, as the threshold of 5.2 ng/l that identified men at increased risk in our study population may not apply to women or to other groups (16). We were able to obtain results from only 3,318 participants, and the requirement for a 1-year sample restricted the study population to those who were compliant with the protocol. This is the most likely explanation for the apparently greater treatment effect in this study compared to the intention-to-treat risk reduction reported previously (8). Our analysis was performed on samples stored for 20 years, and it is possible we underestimated the association between troponin and coronary heart disease events because of interference from the formation of fibrin microparticulates during storage. Although the effect of pravastatin on troponin concentrations was highly consistent across the study population, the absolute and relative reductions were modest at 2 ng/l and 19%, respectively. Although assay precision is very good at concentrations below our median concentration of 4.1 ng/l (∼10% coefficient of variation) (25) and even smaller changes in cardiac troponin concentration were strongly associated with coronary heart disease events across the study population, we acknowledge it will be more challenging to monitor changes accurately in individual patients. Changes in troponin concentration of 2 ng/l after initiation of statin therapy may be masked by other aspects of biological or analytical variability (26). However, there are methodological approaches to reduce the former, for example, performing duplicate measurements 2 weeks apart, and novel technologies that enhance assay precision through single molecular counting that will minimize the latter (27). Before this approach can be applied in clinical practice, prospective studies are needed to determine whether we can consistently quantify small changes in cardiac troponin I concentration in response to treatment in individuals.
Conclusions
Cardiac troponin I is an independent predictor of coronary heart disease events in middle-aged hypercholesterolemic men without prior myocardial infarction. Troponin concentrations are reduced by statin therapy, and reductions in troponin concentrations are associated with better outcomes independent of LDL cholesterol lowering. These findings suggest that high-sensitivity cardiac troponin has major potential to identify those at greatest risk and to assess their response to interventions for the prevention of coronary heart disease. Finally, serial high-sensitivity troponin concentrations may represent a new paradigm in the assessment of the efficacy and safety of novel cardiovascular and noncardiovascular therapies.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In hypercholesterolemic men, plasma cardiac troponin I concentrations correlate with the onset of coronary heart disease over 5 and 15 years and are reduced by statin therapy. Reductions in troponin concentrations are associated with better outcomes independent of LDL cholesterol lowering.
TRANSLATIONAL OUTLOOK: Further studies are needed to clarify the predictive value of high-sensitivity cardiac troponin I assays to identify persons at increased risk and assess whether other interventions that lower plasma troponin levels reduce the risk of coronary heart disease.
Acknowledgments
The authors thank Edwin Carter and Mary Stoddart in the Department of Clinical Biochemistry at the Royal Infirmary of Edinburgh and Philip Stewart at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow for assistance.
Footnotes
This research was supported by a Special Project Grant from the British Heart Foundation (SP/12/10/29922) and by an investigator initiated research grant from Abbott Laboratories. Drs. Mills and Newby are supported by the Butler Senior Clinical Research Fellowship (FS/16/14/32023) and Chair (CH/09/002) awards from the British Heart Foundation. Dr. Shah has acted as a consultant for Abbott Laboratories. Dr. McAllister has been a paid lecturer for Roche; and has served as an advisor for the GSK-COPD trial and for Galecto. Dr. Packard has received grants from Merck Sharp & Dohme and Roche; and has received honoraria from Merck Sharp & Dohme, Pfizer, and Sanofi. Dr. Mills has acted as a consultant for Abbott Laboratories, Beckman-Coulter, Roche Diagnostics, and Singulex. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
For exclusion criteria for participation in WOSCOPS as well as supplemental tables and figures, please see the online version of this article.
Appendix
References
- 1.Shepherd J., Cobbe S.M., Ford I. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.Ford I., Murray H., Packard C.J., Shepherd J., Macfarlane P.W., Cobbe S.M. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 3.Downs J.R., Clearfield M., Weis S. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P.M., Danielson E., Fonseca F.A. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 5.Redberg R.F., Katz M.H. Healthy men should not take statins. JAMA. 2012;307:1491–1492. doi: 10.1001/jama.2012.423. [DOI] [PubMed] [Google Scholar]
- 6.Abramson J.D., Rosenberg H.D., Jewell N., Wright J.M. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347:f6123. doi: 10.1136/bmj.f6123. [DOI] [PubMed] [Google Scholar]
- 7.Pencina M.J., Navar-Boggan A.M., D'Agostino R.B., Sr. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 8.Omland T., de Lemos J.A., Sabatine M.S. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lemos J.A., Drazner M.H., Omland T. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deFilippi C.R., de Lemos J.A., Christenson R.H. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggers K.M., Venge P., Lindahl B., Lind L. Cardiac troponin I levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol. 2013;61:1906–1913. doi: 10.1016/j.jacc.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Zeller T., Tunstall-Pedoe H., Saarela O. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- 13.Omland T., Pfeffer M.A., Solomon S.D. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Kavsak P.A., Xu L., Yusuf S., McQueen M.J. High-sensitivity cardiac troponin I measurement for risk stratification in a stable high-risk population. Clin Chem. 2011;57:1146–1153. doi: 10.1373/clinchem.2011.164574. [DOI] [PubMed] [Google Scholar]
- 15.The West of Scotland Coronary Prevention Study Group A coronary primary prevention study of Scottish men aged 45-64 years: trial design. J Clin Epidemiol. 1992;45:849–860. doi: 10.1016/0895-4356(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 16.Shah A.S., Griffiths M., Lee K.K. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packard C.J., O'Reilly D.S., Caslake M.J. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 18.Lowe G., Rumley A., Norrie J. Blood rheology, cardiovascular risk factors, and cardiovascular disease: the West of Scotland Coronary Prevention Study. Thromb Haemost. 2000;84:553–558. [PubMed] [Google Scholar]
- 19.Sattar N., Gaw A., Scherbakova O. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 20.Everett B.M., Zeller T., Glynn R.J., Ridker P.M., Blankenberg S. High-sensitivity cardiac troponin I and B-type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–1860. doi: 10.1161/CIRCULATIONAHA.114.014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A.S., Anand A., Sandoval Y. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone N.J., Robinson J.G., Lichtenstein A.H. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 23.White H.D., Tonkin A., Simes J. Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long-term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (Long-Term Intervention With Pravastatin in Ischaemic Disease) J Am Coll Cardiol. 2014;63:345–354. doi: 10.1016/j.jacc.2013.08.1643. [DOI] [PubMed] [Google Scholar]
- 24.Tousoulis D., Psarros C., Demosthenous M. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol. 2014;63:2491–2502. doi: 10.1016/j.jacc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Chin C.W., Shah A.S., McAllister D.A. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu A.H.D. Biological and analytical variation of clinical biomarker testing: implications for biomarker-guided therapy. Curr Heart Fail Rep. 2013;10:434–440. doi: 10.1007/s11897-013-0156-6. [DOI] [PubMed] [Google Scholar]
- 27.Todd J., Freese B., Lu A. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007;53:1990–1995. doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.