Abstract
Retinoic acid (RA), the active metabolite of vitamin A, is known to be required for the differentiation of spermatogonia. The first round of spermatogenesis initiates in response to RA and occurs in patches along the length of the seminiferous tubule. However, very little is known about the individual differentiating spermatogonial populations and their progression through the cell cycle due to the heterogeneous nature of the onset of spermatogenesis. In this study, we utilized WIN 18,446 and RA as tools to generate testes enriched with different populations of spermatogonia to further investigate 1) the undifferentiated to differentiating spermatogonial transition, 2) the progression of the differentiating spermatogonia through the cell cycle, and 3) Sertoli cell number in response to altered RA levels. WIN 18,446/RA-treated neonatal mice were used to determine when synchronous S phases occurred in the differentiating spermatogonial population following treatment. Five differentiating spermatogonial S phase windows were identified between spermatogonial differentiation and formation of preleptotene spermatocytes. In addition, a slight increase in Sertoli cell number was observed following RA treatment, possibly implicating a role for RA in Sertoli cell cycle progression. This study has enhanced our understanding of the spermatogonial populations present in the neonatal testis during the onset of spermatogenesis by mapping the cell cycle kinetics of both the undifferentiated and the differentiating spermatogonial populations and identifying the precise timing of when specific individual differentiating spermatogonial populations are enriched within the testis following synchrony, thus providing an essential tool for further study of the differentiating spermatogonia.
Keywords: cell cycle, retinoic acid, spermatogenesis, spermatogonia, testis
INTRODUCTION
Spermatogenesis is the complex morphogenesis of undifferentiated spermatogonia into spermatozoa. The role of the spermatogonial population is to either self-renew to maintain the germ line or expand in number through mitotic divisions. Spermatogonia can be divided into two different subpopulations: 1) undifferentiated (spermatogonial stem cells [SSCs] and transit amplifying progenitor cells) and 2) differentiating (A1, A2, A3, A4, intermediate, and B cells). While there has been extensive investigation of undifferentiated spermatogonia in both adult and juvenile testis [1–3], significantly less is known about the differentiating populations, in part because of the heterogeneous nature of both the onset and the continuation of spermatogenesis.
The onset of spermatogenesis occurs between 3 and 6 days postpartum (dpp), depending on the mouse strain examined, when prospermatogonia either transition directly into differentiating spermatogonia, generating the first round of spermatogenesis, or form the undifferentiated spermatogonia population [4]. The transgenic mouse line neurogenin 3 (Neurog3)/EGFP has been utilized to investigate the characteristics of this decision [5–7], with the undifferentiated versus differentiating fate being directly linked to either the presence or the absence of Neurog3/EGFP expression, respectively [5–7]. Interestingly, the timing of the first round of spermatogenesis in the wild-type neonatal animal is slightly shorter when compared to the subsequent rounds in the adult [4]. One differentiating spermatogonial cell division is skipped during the first round, thus shortening spermatogenesis by approximately 2.5 days [4, 8]. During all subsequent rounds of spermatogenesis, differentiating spermatogonia undergo six mitotic divisions before entering meiosis as preleptotene spermatocytes.
Our current understanding of spermatogonial biology has been shaped mostly by investigations of this cell population within the adult testis. Commonly used markers of the undifferentiated and differentiating spermatogonia have been described following localization studies with adult testis tubules [1, 9–11]. Zinc finger and BTB domain-containing 16 (ZBTB16) has been implicated in playing a role in SSC renewal and maintenance in the adult testis and is commonly used as a marker of the undifferentiated spermatogonial population [12, 13]. In addition, lin-28 homolog A (Caenorhabditis elegans) (LIN28A) was shown to be specifically expressed within the undifferentiated spermatogonia in the adult testis [14]. However, a more recent report found that ZBTB16 was expressed within both the undifferentiated and the differentiating spermatogonial populations in the neonatal testis [15]. The differentiating spermatogonia can be studied by utilizing the retinoic acid (RA)-responsive proteins stimulated by retinoic acid gene 8 (STRA8) and kit oncogene (KIT), which are expressed in A1-A4 and A1-B differentiating spermatogonial populations, respectively [16–21], but there are currently no known markers of the individual subpopulations, and they can be distinguished only via nuclear morphology following electron microscopy or high-resolution light microscopy [22–24]. A more in-depth analysis of differences between spermatogonia during the first and subsequent rounds of spermatogenesis is required to better understand how the differentiating spermatogonial populations progress more quickly during the first round of spermatogenesis compared to subsequent rounds.
Similar to studies performed with common spermatogonial markers, the timing of spermatogonial cell division and cell cycle progression have been studied primarily within the adult testis [25–27]. The cell cycle can be divided into four different phases—G1, S, G2, and mitosis—and cells can exit the cycle toward the end of G1 and enter G0. The duration of the cell cycle in adult spermatogonia was measured to be approximately 28 h [27]; however, the timing during the first round of spermatogenesis has yet to be investigated, and it is unknown whether the skipped differentiating spermatogonial division affects cell cycle progression in any way. The majority of studies that have investigated the cell cycle in spermatogonia have occurred within the adult testis, including investigating the expression of different cyclins that regulate mitosis [28–32]. However, the timing of cell cycle progression for the individual differentiating spermatogonial populations during the first round of spermatogenesis has never been described.
The first differentiation of spermatogonia takes place in response to RA and occurs in patches along the length of the seminiferous tubules in the neonatal testis. This was visualized using a reporter mouse line that expresses β-galactosidase within cells containing an intact RA signaling mechanism [33, 34]. The resulting asynchronous initiation of spermatogenesis induces spermatogonial populations to be at different points in development along the length of the tubule, generating extensive heterogeneity and making studies of individual germ cell populations very difficult. Single cell quantitative RT-PCR and principal component analysis demonstrated that transcript diversity is present even within clusters of germ cells designated as either SSCs, progenitor undifferentiated spermatogonia, or differentiating spermatogonia [35]. In addition, protein expression studies found as many as six different populations of spermatogonia based on colocalization studies using markers for glial cell line-derived neurotrophic factor family receptor alpha 1 (GFRA1), KIT, STRA8, ZBTB16, and cadherin 1 (CDH1) in the 7-dpp mouse testis [36], supporting the extensive heterogeneity. Thus, investigating the onset of spermatogenesis in the neonatal testis has proven to be very difficult due to the various types of germ cells at different points in their differentiation pathway present along the length of the seminiferous tubule.
To overcome the population heterogeneity, our laboratory developed a technique to synchronize the first round of spermatogenesis [21]. WIN 18,446, a potent inhibitor of RA synthesis [37], can be used to block spermatogonial differentiation, generating testis tubules containing only undifferentiated spermatogonia and Sertoli cells and thereby allowing for the investigation of these cell types when the differentiation signal is absent. The block in spermatogenesis can then be released with exogenous RA, driving the majority of spermatogonia to differentiate and generating testes consisting of germ cells undergoing synchronous development [21, 38, 39]. This synchronization protocol allows for the precise investigation of the differentiating spermatogonial populations.
In this study, we have investigated the expression patterns of spermatogonial markers commonly used to study the adult testis within wild-type and synchronized neonatal testes to enhance our understanding of the onset of spermatogenesis. In addition, we utilized WIN 18,446 and RA as tools to generate testes enriched with different populations of spermatogonia to investigate the undifferentiated-to-differentiating spermatogonial transition, cell cycle progression of both the undifferentiated and the differentiating spermatogonial populations, and Sertoli cell number in response to altered RA levels. This study, for the first time, has mapped the maturation of spermatogonia during the onset of spermatogenesis, allowing for future investigation of specific spermatogonial subpopulations following synchronization.
MATERIALS AND METHODS
Animals
All animal experiments were approved by the Washington State University Animal Care and Use Committees and were conducted in accordance with the guided principles for the care and use of research animals of the National Institutes of Health. A C57BL/6-129 mouse colony was maintained in a temperature- and humidity-controlled environment with food and water provided ad libitum. Mice, ranging in age from 0 to 15 dpp, used in these studies were collected from this colony. The animals were euthanized by CO2 asphyxiation followed by decapitation (0–10 dpp) or cervical dissociation (10–15 dpp) and their testes dissected. For immunohistochemical analysis, the testes were fixed in Bouin (M7831-88; EMD Millipore) at room temperature or in 4% paraformaldehyde (P6148; Sigma-Aldrich) at 4°C for 2 h with gentle rotation, washed in a graded series of ethanol, embedded in paraffin, sectioned at 4 μm with at least 20 μm between each cross section, and placed on Superfrost Plus slides (12-5550-15; Fisher Scientific) for immunohistochemical analysis. For whole-mount immunofluorescence, testes were detunicated and the tubules gently dissociated manually with forceps under magnification using a dissecting microscope (SZX-ILLD2-100; Olympus). The dissociated tubules were fixed using 4% paraformaldehyde with gentle rotation for 2 h at 4°C and stored in 1× PBS (137 mM NaCl/2.7 mM KCl/10.1 mM Na2HPO4/1.8 mM KH2PO4) at 4°C until use.
WIN 18,446, RA, and EdU Treatments
Spermatogenesis was synchronized as previously described [21]. Briefly, neonatal mice were pipette fed 100 μg/g body weight WIN 18,446, suspended in 1% gum tragacanth, once every 24 h from 2 through 8 dpp [21]. WIN 18,446 treatments were given at the same time every day during treatment. Twenty-four hours following the final WIN 18,446 treatment, the animals either were euthanized as WIN 18,446-only treated animals or received an i.p. injection of 200 μg of RA, diluted in 10 μl of dimethyl sulfoxide (DMSO), and were left to recover for either 4, 6, 8, 12, or 24 h for whole-mount immunofluorescence or for various intervals between 2 and 148 h for analysis of the cell cycle. In addition, animals treated for cell cycle analysis also received a 20 μl i.p. injection of 5-ethynyl-2′-deoxyuridine (EdU) (A10044; Invitrogen; 10 mM in DMSO) 2 or 12 h prior to euthanization. Vehicle-treated control animals received 1% gum tragacanth once daily from 2 through 8 dpp followed by an i.p. injection of 10 μl of DMSO.
Immunofluorescence
Testis cross sections were washed in xylene (Fisher Chemical) and a graded ethanol series (100%, 95%, and 75%). Antigen retrieval was performed in 0.01 M citrate buffer (2.94 mg/mL sodium citrate dihydrate in ddH20; pH 6) at a rolling boil (>90°C) for 7.5 min. Following two washes in 1× PBS, nonspecific binding was blocked by utilizing 5% goat serum/0.1% bovine serum albumin/PBS for 30 min at room temperature in a humid environment. Tissue sections were incubated with primary antibody, diluted in blocking solution, overnight at 4°C in a humid chamber. The primary antibodies used in this study were rat anti-germ cell nuclear antigen (GCNA) (1:100; kindly supplied by Prof. George Enders, University of Kansas Medical Center), rabbit anti-antigen identified by monoclonal antibody Ki 67 (MKI67) (10 mg/ml, AB15580; Abcam), goat anti-KIT (0.4 μg/ml; AF1356; R&D Systems), rabbit anti-STRA8 (1:2000, made in-house [21]), and rabbit anti-SRY (sex-determining region Y)-box 9 (SOX9) (0.2 mg/ml; AB5535; EMD Millipore). The following morning, three washes with 1× PBS were performed to remove any unbound primary antibody, and the tissue cross sections were incubated in secondary antibody, diluted in blocking solution, for 1 h at room temperature. The secondary antibodies used were biotinylated goat anti-rat (1:500 dilution; AB97178; Abcam), Alexa Fluor 568 donkey anti-goat (4 μg/ml; A11057; Invitrogen), and biotinylated goat anti-rabbit solution (ready-to-use; 956143B; Invitrogen). Incubation of sections for 1 h at room temperature in Alexa Fluor 568 strepavidin conjugate (4 μg/ml; S11226; Invitrogen), diluted in blocking solution, was used to visualize antibody binding within cells. The Click-It EdU Alexa Fluor 488 Imaging Kit (C10337; Invitrogen) was utilized as per the manufacturer's instructions to detect incorporation of EdU in dividing cells. Slides were mounted with fluoroshield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Abcam). Both the total number of EdU-positive Sertoli cells and the total number of Sertoli cells per tubule were counted within at least 200 round tubule cross sections from at least three animals per treatment group. Paired Student t-tests (Microsoft Excel) were performed to determine significance between control and treated samples. A P-value less than 0.05 was deemed significant. Sertoli cells were identified by expression of SOX9. Spermatogonia were identified based on nuclear morphology, STRA8 and KIT expression, and their location on the basement membrane. Preleptotene spermatocytes were identified by their nuclear morphology, condensed chromatin, and STRA8 expression. Cells that incorporated EdU were undergoing or had undergone S phase, and cells that expressed MKI67, a marker of cellular proliferation, were progressing through the cell cycle in either G1, S, G2, or mitosis. Immunofluorescent staining for EdU incorporation or MKI67 and GCNA was performed on three tissue sections, each separated by a minimum of 25 microns, from testes isolated from three mice treated with WIN 18,446-only and received an injection of EdU 12 h prior to the time of euthanization. The number of GCNA and EdU or MKI67 copositive germ cells were quantified to determine the number of germ cells that underwent S phase during the 12 h following the EdU injection or that were progressing through the cell cycle, respectively. This was performed by counting the number of EdU or MKI67 and GCNA copositive cells present in 100 round tubule cross sections analyzed within a minimum of three different cross sections, separated by a minimum of 25 microns per mouse, and these counts were performed on three mice. Immunofluorescent staining for GCNA and MKI67 was performed on a total of at least three tissue sections, each separated by a minimum of 25 microns, from testes isolated from three mice that were allowed to recover for 16, 46, or 72 h post-WIN 18,446/RA treatment. In addition, the time points 4, 6, 8, 10, 12, 14, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 60, and 96 h post-WIN 18,446/RA treatment were investigated for a minimum of N = 2. To determine the percentage of germ cells that incorporate MKI67, the total number of GCNA only-positive and GCNA- and MKI67-copositive spermatogonia per tubule were counted within at least 100 round tubule cross sections analyzed within a minimum of three different cross sections, separated by a minimum of 25 microns per mouse, and these counts were performed on three mice per treatment group. Paired Student t-tests were performed to determine significant differences between the percentages of germ cells that did or did not express MKI67. A P-value less than 0.05 was deemed significant.
Whole-Mount Immunofluorescence
Seminiferous tubules that were stored in 1× PBS for whole-mount immunofluorescence were washed in 0.2% Nonidet P40 Substitute (74385; Sigma-Aldrich)/PBS for 20 min, a graded methanol series in PBS-T (PBS containing 0.1% Tween 20; P1379; Sigma-Aldrich) (25%, 50%, 75%, and 100%) for 5 min each to dehydrate the tubules, and two washes in PBS-T for 5 min each for rehydration. All of the washes were performed while under gentle rotation at 4°C. Following fixation, nonspecific binding was inhibited by immersing the testis tubules in blocking solution (1% bovine serum albumin/10% donkey serum/PBS-T) for 1 h. The tubules were then incubated in primary antibody, diluted in blocking solution, overnight at 4°C with gentle rotation. The primary antibodies used were goat anti-LIN28A (0.5 μg/ml; AF3757; R&D Systems), rabbit anti-ZBTB16 (0.5 μg/ml; sc-22839; Santa Cruz), goat anti-GFRA1 (0.5 μg/ml; AF560; R&D Systems), and rabbit anti-STRA8 (1:1000; made in-house [21]). The tubules were washed in PBS-T 3 times for 5 min. Primary antibody binding was visualized via incubating the tubules in Alexa Fluor reagents donkey anti-rabbit 488 (2 μg/ml; A21206; Invitrogen) and donkey anti-goat 568 (2 μg/ml; A11057; Invitrogen), diluted in PB (1% bovine serum albumin/PBS), for 2 h with gentle rotation at room temperature and from here on were kept light protected. All subsequent washes were performed at room temperature. The tubules were washed in PBS-T three times for 15 min each. Tubules were mounted in fluoroshield mounting medium with DAPI (Abcam) under glass coverslips. Confocal microscopy (TCS SP5 II; Leica) was utilized to image whole seminiferous tubules. Tubules were examined from at least three neonatal animals to ensure consistency. The total number of GFRA1-only, STRA8-only, and GFRA1- and STRA8-copositive spermatogonia were quantified in testis cross sections following WIN 18,446/RA synchronization. At least 100 round tubule cross sections within three sections, separated by 25 microns, from three animals per treatment group were utilized to determine the percentage of cells positive for each marker.
Transmission Electron Microscopy
All chemicals used to process samples for transmission electron microscopy (TEM) were purchased from Electron Microscopy Sciences. Mice were treated with either WIN 18,446-only or WIN 18,446 followed by an injection of RA and allowed 18, 48, 72, 96, 120, or 144 h to recover before euthanization. The testes were collected, detunicated, cut in half, and immersed in 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.2). They were then immediately microwave fixed on ice for 2.5 min using a lab Microwave Processor Pelco 3450. The samples were rinsed with 0.05 M cacodylate buffer (pH 7.2) and held for up to 7 days in the buffer at 4°C. The samples were rinsed two more times in 0.05 M cacodylate buffer (pH 7.2) and postfixed in 1% OsO4 for 24 h. Following postfixation, samples were rinsed with the 0.05 M cacodylate buffer (pH 7.2) three times and dehydrated in a graded ethanol series before transitioning them to acetone. Testes were then infiltrated with a medium hardness recipe Spurr resin (MR [40]) as follows: 1:1 acetone:MR, 24 h; lid removed and the acetone evaporated, 16 h; and 100% MR, 24 h. The samples were embedded in fresh MR and cured for 16 h at 70°C. Ultrathin sections (60–100 nm) were taken with a Reichert Ultracut R ultramicrotome and placed on formvar-coated slot grids. They were stained with a solution of 1% uranyl acetate for 20 min and poststained for 6 min in Reynolds lead citrate [41]. All testis sections were imaged on a FEI Tecnai G2 TEM (FEI Company).
RESULTS
GFRA1 and STRA8 Efficiently Mark the Undifferentiated to Differentiating Spermatogonial Transition in the Neonatal Testis
Previous studies from multiple laboratories have suggested that heterogeneity exists within the spermatogonial populations in the neonatal testis [33–36] and that the onset of spermatogenesis occurs in distinct patches of tubules [33, 34]. To further investigate this, three common markers of undifferentiated spermatogonia (LIN28A, ZBTB16, and GFRA1) and STRA8, a classic marker of RA response and differentiating spermatogonia, were chosen to examine the onset of spermatogenesis in the neonatal testis via whole-mount immunofluorescence. At 10 dpp, LIN28A colocalized with both ZBTB16- (Figure 1A) and STRA8-positive differentiating spermatogonia (Fig. 1B); however, there was a small population of undifferentiated spermatogonia that expressed only LIN28A and ZBTB16. Interestingly, GFRA1 and STRA8 were present in two separate populations of spermatogonia (Fig. 1C). Therefore, GFRA1 and STRA8 were selected to investigate undifferentiated and differentiating spermatogonia at younger ages. At 3 dpp there were two populations of prospermatogonia, GFRA1-positive only and both GFRA1- and STRA8-positive, suggesting that the prospermatogonia positive for both markers were initiating the first round of spermatogenesis (Fig. 2A). By 6 dpp, there were three populations (only two are shown in the picture), GFRA1 only-positive (not shown), GFRA1- and STRA8-copositive, and STRA8 only-positive (Fig. 2B). These data imply that the population of spermatogonia that are colabeled with GFRA1 and STRA8 are undergoing the Aundiff-to-A1 transition. By 10 dpp, there were two populations, GFRA1 only- and STRA8 only-positive spermatogonia (Figs. 1C and 2C).
FIG. 1.
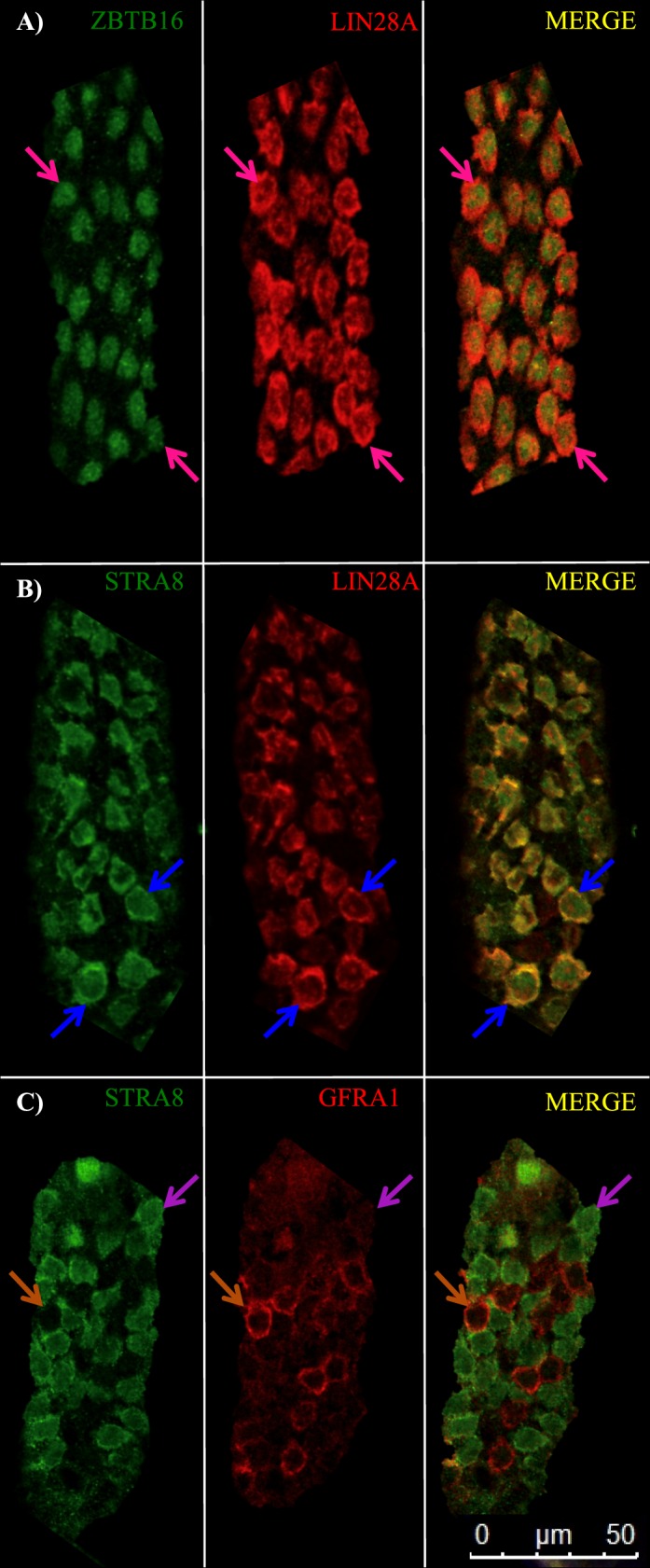
LIN28A and ZBTB16 expression is not restricted to the undifferentiated spermatogonial population in the postnatal testis. Images depict representative sections of whole-mounted tubules from 10-dpp wild-type mouse testes (A–C) stained for ZBTB16 and LIN28A (A), STRA8 and LIN28A (B), and STRA8 and GFRA1 (C). Pink arrows denote ZBTB16 and LIN28A copositive spermatogonia, blue arrows indicate STRA8 and LIN28A copositive spermatogonia, purple arrows designate STRA8 only-positive spermatogonia, and orange arrows depict GFRA1 only-positive spermatogonia. Bar = 50 μm.
FIG. 2.
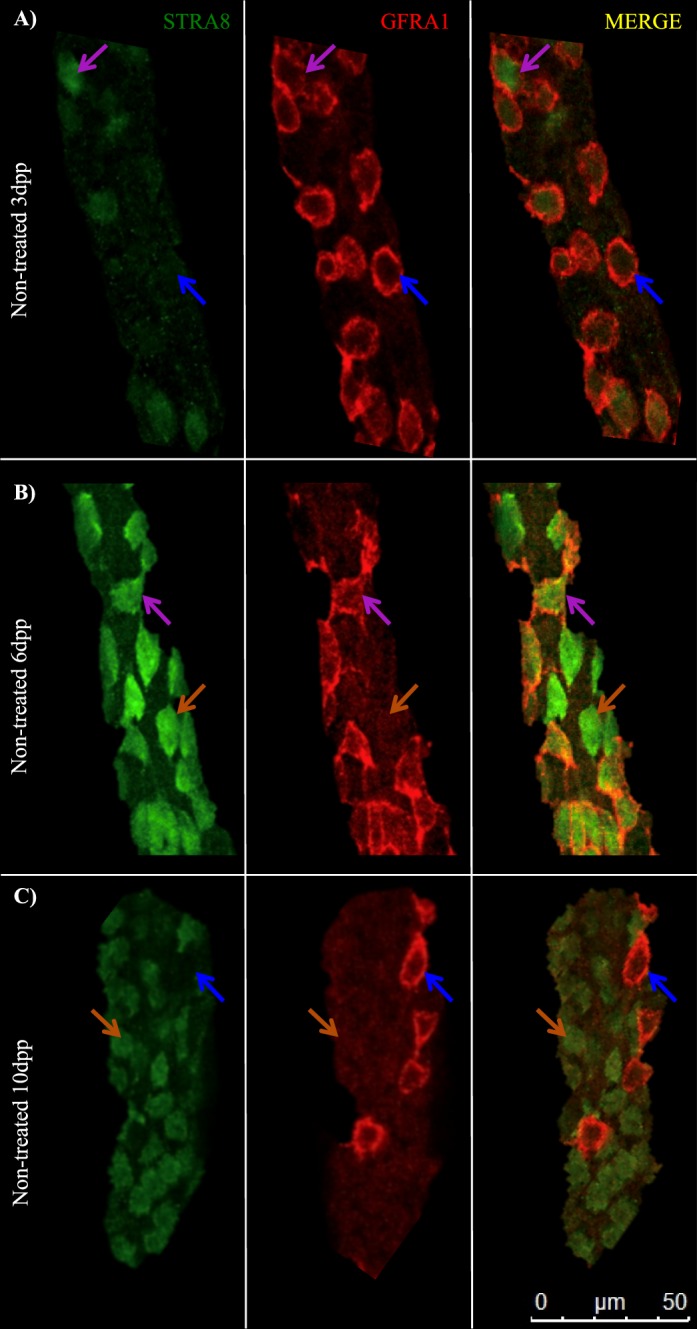
GFRA1 and STRA8 efficiently mark the undifferentiated-to-differentiating spermatogonial transition in the neonatal testis. Images depict representative sections of whole-mounted tubules from 3-dpp (A), 6-dpp (B), and 10-dpp (C) wild-type mouse testes stained for STRA8 and GFRA1. Blue arrows denote GFRA1 only-positive germ cells, purple arrows indicate STRA8 and GFRA1 copositive germ cells, and orange arrows designate STRA8 only-positive spermatogonia. Bar = 50 μm.
STRA8 is Expressed in Patches of Seminiferous Tubules During the Initiation of Spermatogenesis
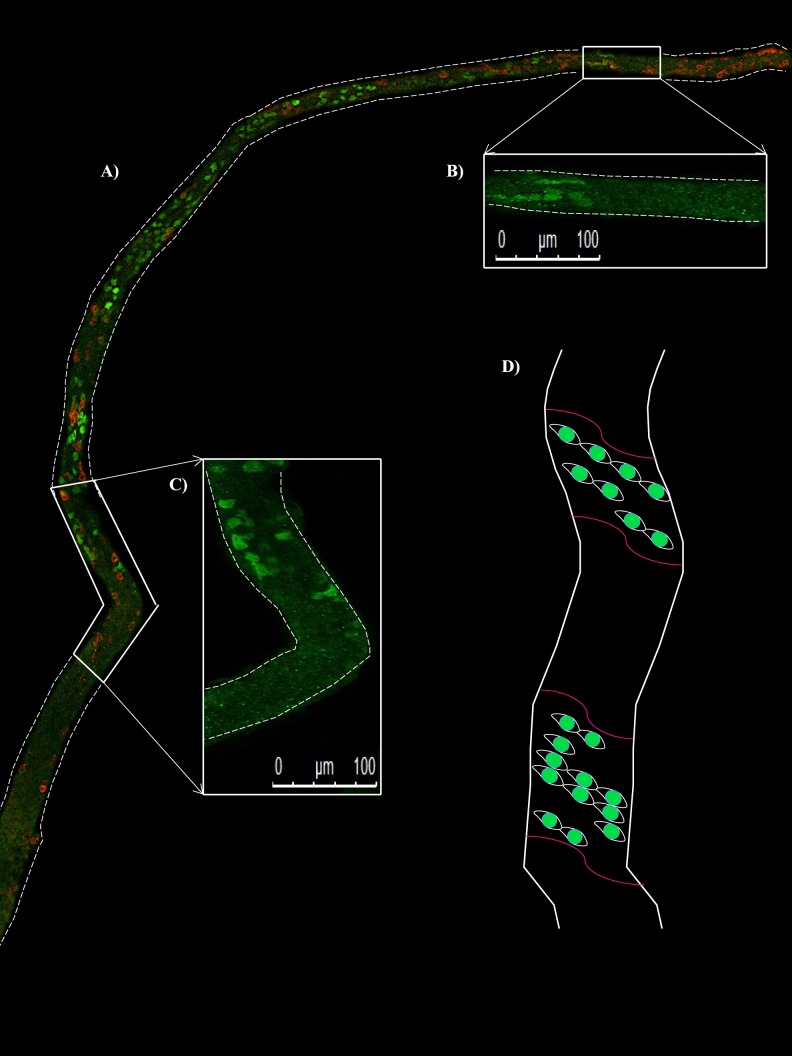
Snyder et al. [33, 34] demonstrated that there are patches of active and inactive RA signaling along the length of neonatal testis tubules during the asynchronous initiation of spermatogenesis by utilizing the Tg (Retinoic Acid Response Element-Hspalb/lacZ)12Jrt (RARE-hsplacZ) reporter transgenic mouse line [42] but only correlated patchy β-galactosidase expression with STRA8 expression in differentiating spermatogonia in testis cross sections. To further investigate the patchy nature of the onset of spermatogenesis, whole-mount immunofluorescence was performed on testis tubules taken from 6-dpp animals. Antibodies for STRA8 and GFRA1 were utilized to examine the differentiating and undifferentiated spermatogonial populations, respectively. STRA8 expression occurred in patches along the length of the seminiferous tubule, denoting the onset of spermatogonial differentiation during the first wave of spermatogenesis, while GFRA1-positive undifferentiated spermatogonia were present throughout the entire tubule (Fig. 3).
FIG. 3.
STRA8 is expressed in patches of seminiferous tubules during the initiation of spermatogenesis. Image depicts a representative section of whole-mounted tubule from a 6-dpp mouse testis stained for STRA8 shown in green and GFRA1 shown in red (A). STRA8-only expression is displayed in the zoomed-in sections of tubule (B, C) to demonstrate where STRA8 expression begins and ends along the length of this tubule. A model for the asynchronous onset of spermatogenesis denotes STRA8 expression in spermatogonia designated by the green cells, and the established RA signaling boundaries are indicated by the pink lines (D). Bars = 100 μm.
The Transition from Undifferentiated to Differentiating Spermatogonia is Fluid Following the RA Trigger
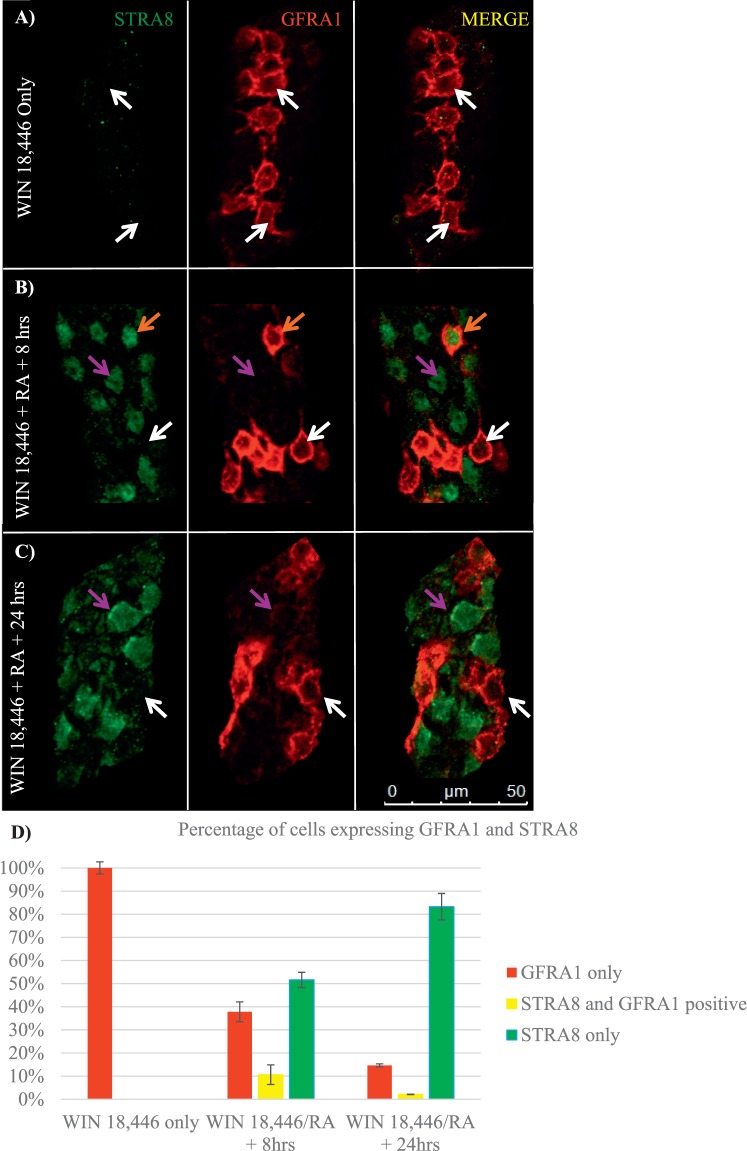
Our laboratory has previously reported a method for enriching the juvenile testis with undifferentiated spermatogonia and then triggering the simultaneous differentiation of the majority of these cells with exogenous RA [21] as a means of investigating the first round of spermatogenesis. However, the timing of this differentiation event and characterization of specific markers of the Aundiff-to-A1 spermatogonial transition following synchronization have yet to be fully examined. GFRA1 and STRA8 were utilized to mark the undifferentiated and differentiating spermatogonial population, respectively, and to determine whether the undifferentiated-to-differentiating spermatogonial transition is fluid following WIN 18,446/RA treatment. In WIN 18,446-only treated animals, the testes were filled with 100% undifferentiated spermatogonia only, denoted by containing only GFRA1-positive spermatogonia (Fig. 4, A and D) and no detectable STRA8-positive cells. By 8 h post-RA injection, there were three different populations of spermatogonia present in the testis: 38% of germ cells were GFRA1 only-positive spermatogonia, 11% were GFRA1- and STRA8-copositive spermatogonia, and 51% were STRA8 only-positive spermatogonia (Fig. 4, B and D). There were primarily two populations of spermatogonia detectable at 24 h post-RA injection: 15% of germ cells were GFRA1 only-positive, and 83% were STRA8 only-positive spermatogonia; however, 2% of germ cells expressed both GFRA1 and STRA8 (Fig. 4, C and D).
FIG. 4.
The transition from undifferentiated to differentiating spermatogonia is fluid following the RA trigger. Images depict representative sections of whole-mounted tubules taken from mice treated with WIN 18,446-only (A) or WIN 18,446 followed by an RA injection and allowed to recover for 8 h (B) and 24 h (C) and stained for STRA8 and GFRA1. White arrows denote GFRA1 only-positive spermatogonia, orange arrows designate STRA8 and GFRA1 copositive spermatogonia, and purple arrows indicate STRA8-positive spermatogonia. Bar = 50 μm. Bar graph depicts the average percentage of GFRA1 only-positive, STRA8 only-positive, and GFRA1- and STRA8-copositive spermatogonia from testes of mice following synchronization, displayed in D. Error bars represent SEM.
WIN 18,446 Treatment Halts Undifferentiated Spermatogonia in G0
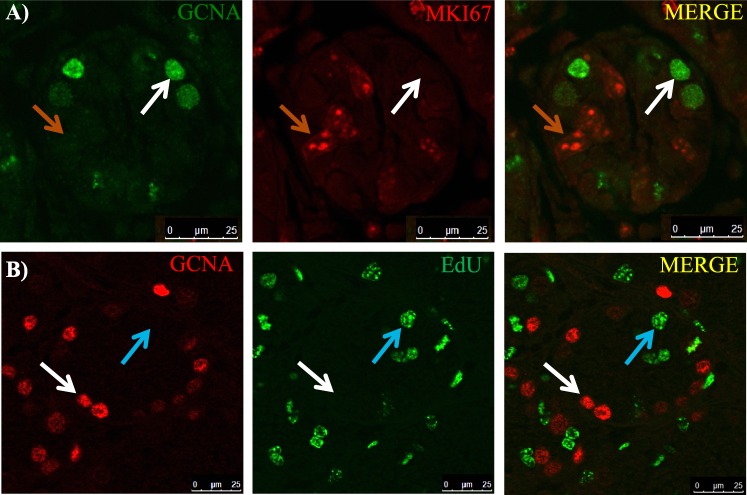
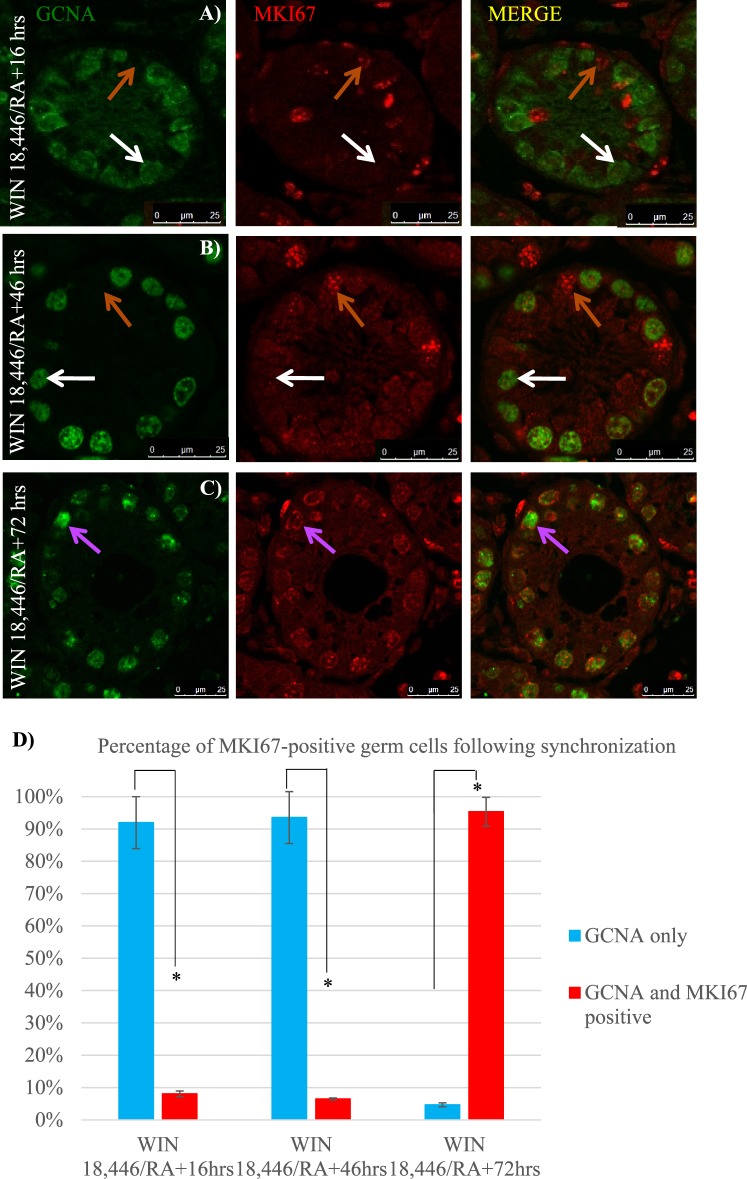
Past studies utilizing adult vitamin A-deficient (VAD) rat testes have investigated the cell cycle kinetics of the undifferentiated spermatogonial population in the absence of RA [25, 26, 43–45]; however, this has yet to be examined within the neonatal testis. Neonatal male mice were treated with WIN 18,446 to investigate whether undifferentiated spermatogonia enter cell cycle arrest in response to RA deficiency at the onset of spermatogenesis. Colocalization of GCNA and MKI67 revealed that very few germ cells were actively within the cell cycle in WIN 18,446-only treated testes (Fig. 5A), with an average of only four dual-positive cells per every 100 round tubules. MKI67-positive Sertoli cells and peritubular myoid cells were present throughout the entire testis cross section (Fig. 5A). As a second measure to investigate the cell cycle status of the undifferentiated spermatogonia, WIN 18,446-only treated mice were injected with EdU 12 h prior to euthanization to examine whether germ cells had undergone S phase during those 12 h. The majority of GCNA-positive spermatogonia had not incorporated EdU (Fig. 5B) with an average of just 32 dual-positive cells per every 100 round tubules. Interestingly, following the RA injection, somatic cells continued to express MKI67, while approximately 93% of germ cells did not (Fig. 6, A, B, and D). This lack of MKI67 expression continued for at least 2 days post-RA treatment. Approximately 95% of germ cells were MKI67-positive 72 h after synchronization (Fig. 6, C and D).
FIG. 5.
WIN 18,446 treatment halts the majority of undifferentiated spermatogonia in G0. Images depict representative cross sections of seminiferous tubules taken from mice treated with WIN 18,446-only and an injection of EdU 12 h prior to euthanization and stained for GCNA and either MKI67 (A) or EdU incorporation (B). White arrows denote GCNA only-positive spermatogonia, orange arrows indicate MKI67 only-positive cells, and blue arrows denote EdU-incorporated cells. Bars = 25 μm.
FIG. 6.
MKI67 is not expressed in spermatogonia immediately following WIN 18,446/RA exposure. Images depicts representative cross sections of seminiferous tubules taken from mice treated with WIN 18,446 followed by an RA injection and allowed to recover for 16 h (A), 46 h (B), and 72 h (C) and stained for GCNA and MKI67. White arrows denote GCNA only-positive spermatogonia, orange arrows indicate MKI67 only-positive cells, and purple arrows depict GCNA- and MKI67-copositive spermatogonia. Bars = 25 μm. Bar graph indicates the average percentage of GCNA only-positive and GCNA- and MKI67-copositive germ cells per round tubule from testes of mice following synchronization, displayed in D. * denotes a P-value < 0.0005. Error bars represent SEM.
Spermatogonial Development Following the WIN 18,446/RA Synchronization Protocol Mimics the Timing of the First Round of Spermatogenesis
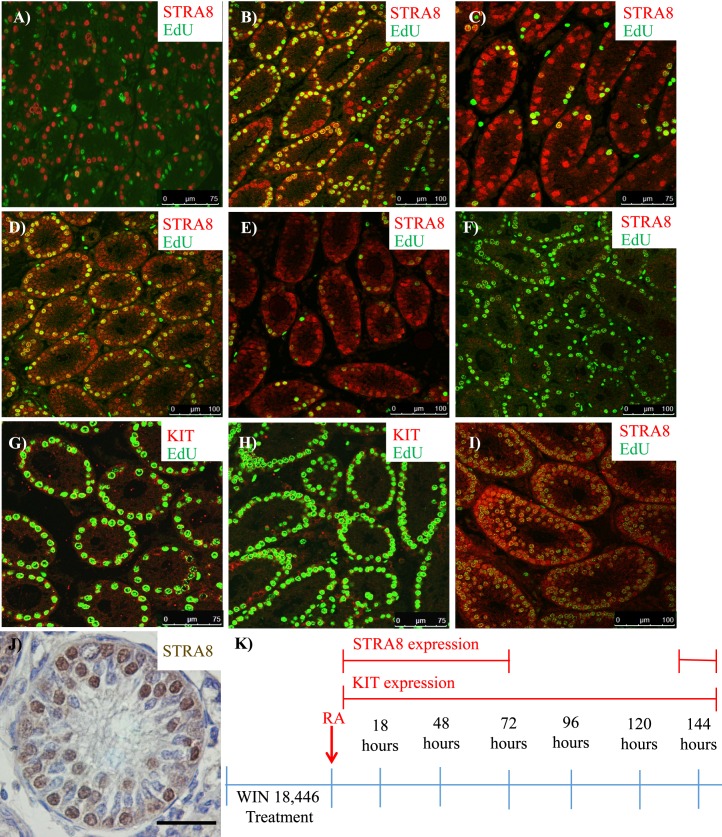
Previous studies have examined the cell cycle kinetics of the first round of spermatogenesis [4, 8] or investigated RA levels and gene expression during a synchronized first round [21, 38], but the cell cycle kinetics of the synchronized onset of spermatogenesis have never been examined. To investigate spermatogonial cell cycle kinetics following WIN 18,446/RA treatment, the number of spermatogonial divisions that occur between the conclusion of treatment and formation of the preleptotene spermatocyte population was mapped. Neonatal male mice were treated with WIN 18,446/RA and allowed to recover for various time points between 2 and 148 h post-RA injection, and EdU was administered to determine whether cells had undergone S phase during the last 2 h of any given recovery window (Fig. 7). During the 2- to 72-h time period, STRA8 and EdU colocalization was detected during three different recovery windows post-RA injection: 1) 16–18 h (Fig. 7B), 2) 46–48 h (Fig. 7D), and 3) 70–72 h (Fig. 7F). However, the intensity of the STRA8 signal appeared to be decreased in the third window in comparison to the first two. The time points in between the previously described windows displayed little to no STRA8 and EdU colocalization (Fig. 7, A, C, and E). Based on the timing of the second and third differentiating spermatogonia S phase windows, it was deduced that subsequent S phases would most likely occur at 24-h intervals; therefore, mice were euthanized every 2 h between 92 and 98 h post-RA injection. The fourth S phase window was detected between 94 and 96 h post-RA injection (Fig. 7G) and the fifth between 118 and 124 h post-RA injection (Fig. 7H). The spermatogonia present during the fourth and fifth windows did not express STRA8 but did express KIT and were colocalized with EdU (Fig. 7, G and H). Following another recovery window of approximately 24 h, samples were collected to determine whether formation of the preleptotene spermatocyte population had occurred. Mice treated with WIN 18,446/RA and given between 144 and 146 h of recovery had testes filled with STRA8-positive preleptotene spermatocytes that had incorporated EdU (Fig. 7, I and J).
FIG. 7.
The onset of spermatogenesis following WIN 18,446/RA synchronization mimics the timing of the first round of spermatogenesis. Images depict representative cross sections of testes from mice treated with WIN 18,446 followed by an RA injection and given 8 h (A), 16–18 h (B), 28 h (C), 46–48 h (D), 56 h (E), 70–72 h (F), 94–96 h (G), 120–122 h (H), and 144–146 h (I, J) of recovery and stained for either STRA8 (A–F, I) or KIT (G, H). Cells positive for EdU incorporation are shown in green (A–I). Five synchronized differentiating spermatogonial S phases are denoted by colocalization of either STRA8 (B, D, F) or KIT (G, H) with EdU. Preleptotene spermatocyte formation occurred 144–146 h post-RA treatment, depicted in fluorescence by colocalization of STRA8 and EdU (I) and by the round nuclear morphology and expression of STRA8 shown by immunohistochemistry (J). Differentiating spermatogonia not undergoing S phase are indicated by STRA8 only-positive expression (A, C, E). A model for STRA8 and KIT expression during the timing of spermatogonial S phases following the onset of synchronized spermatogenesis is diagrammed in K. Black bar = 50 μm.
Undifferentiated Spermatogonia Transition Either Directly into A1 or A2 Differentiating Spermatogonia During the Onset of Synchronized Spermatogenesis
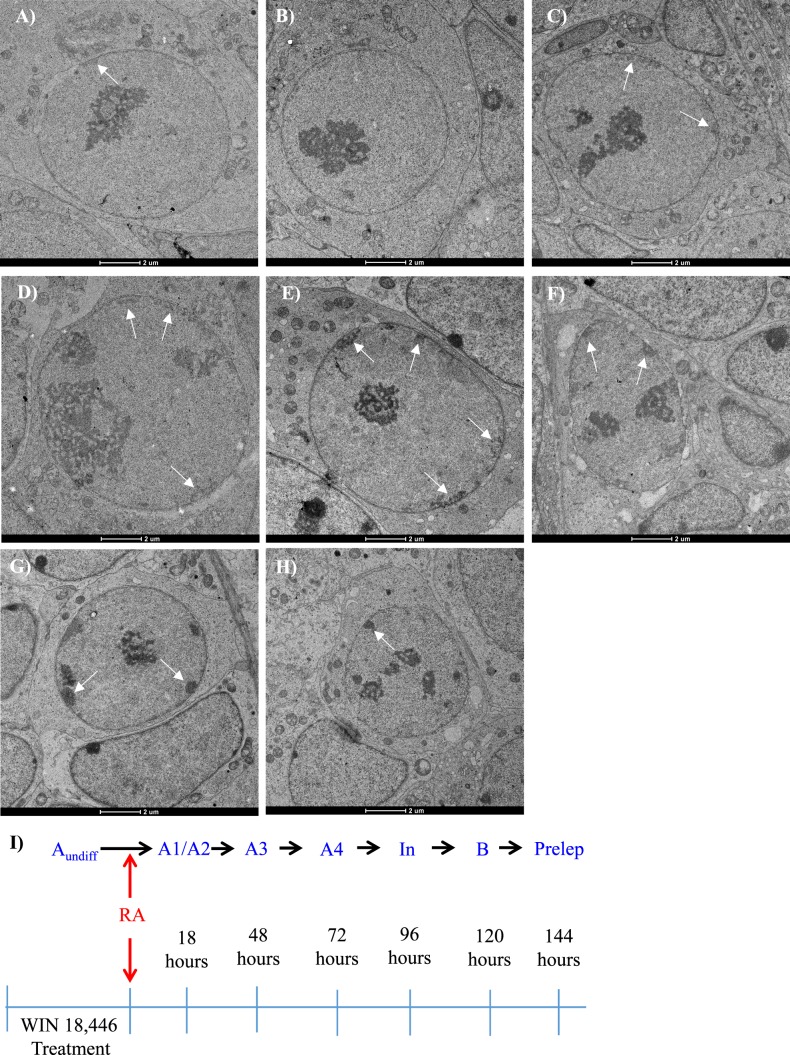
As the EdU analysis demonstrated that the synchronization protocol generates testes full of differentiating spermatogonia moving through their mitoses simultaneously, we decided to use transmission electron microscopy (TEM) to determine which differentiating spermatogonial subtype (A1–B) was present during each of the EdU windows. The most efficient method for distinguishing the various types of differentiating spermatogonia is via TEM, as this technique allows for subtle differences in chromatin compaction and nucleolus number and morphology to be distinguished [23]. Neonatal male mice were treated with either WIN 18,446-only or WIN 18,446/RA and allowed to recover for 18, 48, 72, 96, 120, or 144 h post-RA injection. Testes from WIN 18,446-only treated mice were filled with undifferentiated spermatogonia with very little heterochromatin lining the nuclear envelope and a more compact nucleolus (Fig. 8A). Testes from mice given 18 h of recovery contained 50% A1 differentiating spermatogonia, notable by an absence of heterochromatin along the nuclear envelope (Fig. 8B), and 50% A2 differentiating spermatogonia, distinguished by heterochromatin lining between 4% and 10% of the nuclear envelope and a nucleolus that spread throughout the nucleus (Fig. 8C). In addition to heterochromatin compaction, the number of nucleoli were noted, as an increase of nucleoli occurred with time as the spermatogonia differentiate. A3 and A4 differentiating spermatogonial populations were highly enriched in testes that were collected from mice that were allowed to recover 48 and 72 h post synchronization, respectively (Fig. 8, D and E). A3 spermatogonia were previously reported to possess heterochromatin on 10%–25% of the nuclear envelope, while A4 spermatogonia had heterochromatin along 25%–40% of their nuclear envelope [23]. Testes from mice that were treated with WIN 18,446/RA and recovered for 96 h were highly enriched for intermediate differentiating spermatogonia, distinguished by a thin layer of heterochromatin lining 40%–90% of the nuclear envelope and heterochromatin that starts to appear more compact and protruding further into the nucleus (Fig. 8F). B differentiating spermatogonia and preleptotene spermatocytes were the predominant germ cell subtypes present in testes from mice treated with WIN 18,446/RA and given 120 and 144 h of recovery, respectively (Fig. 8, G and H). B spermatogonia have extremely dark, round, and compact heterochromatin that protrudes more deeply into the nucleus compared to all other populations of spermatogonia [23]. While the chromatin compaction of preleptotene spermatocytes appears very similar to B differentiating spermatogonia, the preleptotene spermatocytes are reduced in size [22]. Undifferentiating spermatogonia were present within each sample examined.
FIG. 8.
Undifferentiated A spermatogonia transition to become either A1 or A2 differentiating spermatogonia during the onset of synchronized spermatogenesis. TEM images depict representative cross sections of testes from mice treated with WIN 18,446-only (A) or with WIN 18,446 followed by an RA injection and given 18 h (B, C), 48 h (D), 72 h (E), 96 h (F), 120 h (G), and 144 h (H) of recovery. White arrows denote heterochromatin present along the nuclear envelope within the undifferentiated and differentiating spermatogonia and preleptotene spermatocytes. A model for the progression of spermatogonial differentiation from undifferentiated spermatogonia following the onset of synchronized spermatogenesis indicating that either the population of A1 or A2 differentiating spermatogonia is skipped is diagrammed in I. Bars = 2 μm.
Excess RA Exposure Slightly Increased the Number of Sertoli Cells in the Postnatal Testis
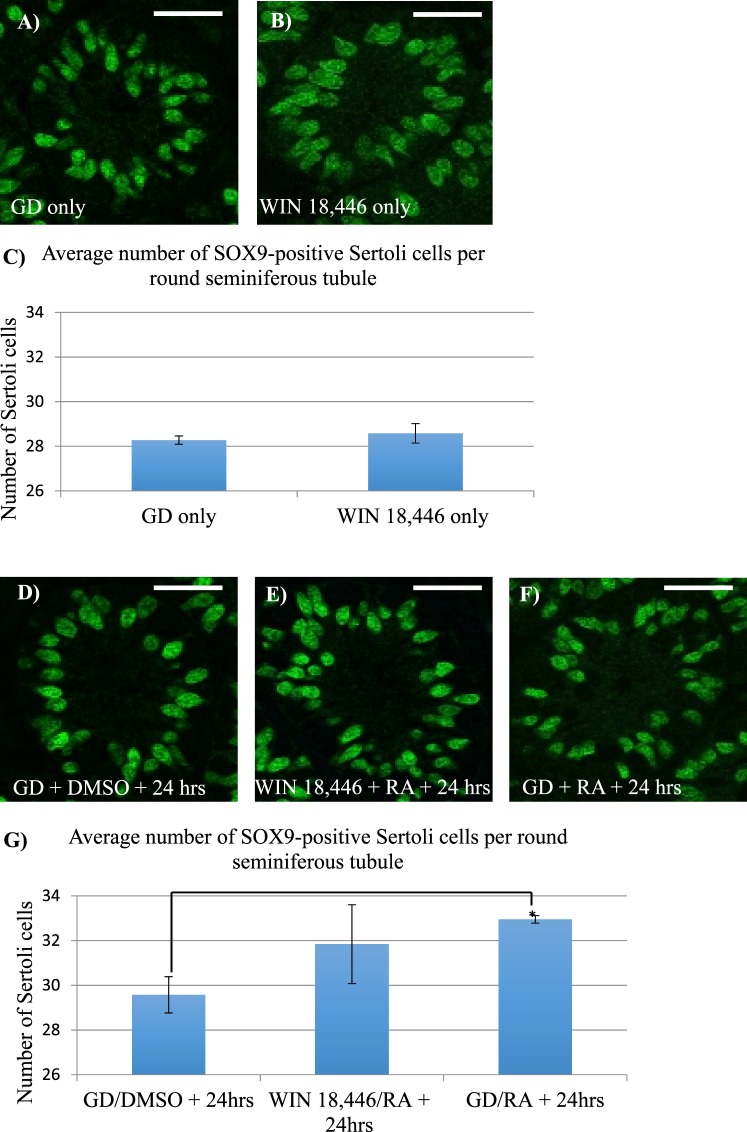
To date, the germ cells have been the primary focus of studies examining the effects of manipulating RA levels in the neonatal testis, with little to no investigation of whether Sertoli cell development is also altered. To determine whether altering RA levels affect Sertoli cell numbers during the onset of spermatogenesis, either WIN 18,446 or RA or a combination of the two treatments was utilized to either lower or raise RA levels within the testis. No difference in the numbers of Sertoli cells per tubule was found following comparison of control versus testes isolated from WIN 18,446-only treated animals (Fig. 9, A–C). In contrast, treatment of male mice with either WIN 18,446/RA or RA alone did appear to result in an increase in the number of Sertoli cells per tubule (Fig. 9, D–G), although due to extensive variation in the WIN 18,446/RA samples, only the RA alone samples reached statistical significance.
FIG. 9.
Excess RA exposure slightly increased the number of Sertoli cells in the postnatal testis. Immunofluorescent images depict representative cross sections of testes from mice treated with the WIN 18,446 vehicle control only (GD) (A), WIN 18,446-only (B), GD followed by RA vehicle (DMSO) (D), WIN 18,446 followed by an injection of RA (E), and GD followed by an injection of RA (F) stained for SOX9 expression. Bar graphs indicate the average number of SOX9-positive Sertoli cells per round tubule from testes of mice treated with either WIN 18,446-only compared to GD-only, displayed in C, or from mice treated with either WIN 18,446 followed by an injection of RA or GD followed by an injection of RA compared to GD followed by an injection of DMSO, displayed in G. * denotes a P-value = 0.01. Error bars represent SEM. White bars = 25 μm.
DISCUSSION
This study has identified, for the first time, the individual differentiating spermatogonial populations following the onset of synchronized spermatogenesis in the mouse testis. In addition, we have thoroughly examined the cell cycle kinetics of both undifferentiated and differentiating spermatogonia using the WIN 18,446/RA synchronization protocol and shown that in the absence of RA, the undifferentiated spermatogonia arrest in G0. Interestingly, germ cell-specific MKI67 expression appears to not be present immediately following synchronization in our system even though germ cells have re-entered the cell cycle. Finally, we have provided evidence that exposure to RA may increase the number of Sertoli cells in the postnatal testis.
Recently published studies have begun to characterize the heterogeneity that exists within the spermatogonial population of the juvenile testis. Niedenberger et al. [36] demonstrated that at 4 dpp, the majority of spermatogonia were either GFRA1- or KIT-positive, with a small percentage of germ cells positive for both, and determined that all populations of spermatogonia were ZBTB16-positive. The data presented here are in complete agreement with these previous observations and suggest, based on the colocalization patterns for STRA8, LIN28A, and ZBTB16, that the latter two of these three markers are not completely restricted to the undifferentiated spermatogonial population and therefore should no longer be considered as only present within these cells in a juvenile testis. However, GFRA1 does appear to be a robust and reliable marker of undifferentiated spermatogonia in both juvenile and adult testis. We mostly observed two clear spermatogonial populations: one that was only STRA8-positive, presumably the differentiating spermatogonia, and one that was only positive for GFRA1, likely the undifferentiated spermatogonia. These data also agree with the clear populations of spermatogonia distinguished in the testis in the Niedenberger et al. [36] study. Taken together. these studies have helped highlight the differences between spermatogonia present in the neonate versus the adult testis and emphasized the need for analysis of cell markers at different ages.
It is clear that RA is required for the undifferentiated-to-differentiating spermatogonial transition, but whether this step is a clear “on/off” switch or occurs as a more fluid change is not yet understood. We aimed to further investigate this transition by utilizing GFRA1 and STRA8 in neonatal wild-type animals and following a synchronized onset of spermatogenesis. Within the testes of the neonatal wild-type animals, STRA8 expression was present in patches along the length of the tubule, mimicking the segmental staining seen in the RARE-hsplacZ mouse [33, 34]. Due to the heterogeneous nature of the onset of spermatogenesis in the testes of wild-type neonatal animals, animals that were treated with WIN 18,446/RA were utilized to examine spermatogenesis during the synchronized onset. At 8 h post-WIN 18,446/RA treatment, there were three different populations of spermatogonia present in the testis: 1) GFRA1 only-positive, 2) GFRA1- and STRA8-copositive, and 3) STRA8 only-positive spermatogonia. The first group likely represents the SSC population that is protected from a RA stimulus via an unknown mechanism, while the third population is likely the newly formed differentiating spermatogonia. We hypothesize that the second group are spermatogonia undergoing the transition to the differentiating form of this cell type. As a result, our data suggest that the undifferentiated-to-differentiating spermatogonial transition is fluid and that it takes time for the gene products marking the undifferentiated fate to be degraded following the RA trigger.
Data generated by previous studies have provided differing opinions with regard to the effects of vitamin A deficiency on the rat spermatogonial cell cycle [25, 26, 43, 44]. Ismail et al. [44] injected VAD rats with 3H-thymidine and found no incorporation of the labeled nucleotide within the undifferentiated spermatogonia, indicating mitotic arrest in the absence of RA. The same assay was then performed in VAD rats following an injection of retinol, and mitosis in A1 spermatogonia was induced within 3 h. As a result, the authors concluded that the undifferentiated spermatogonial population was arrested in G2 [44]. In contrast, Wang and Kim [43] analyzed the level of H1 histone kinase activity associated with the p34/cyclin B complex and the number of condensed chromosome figures following deficiency and immediately after RA/retinol exposure in rats and concluded that the undifferentiated spermatogonial population was arrested in S phase. Contrary to these two studies, Van Pelt and De Rooij [26] utilized 5-bromode-oxyuridine to investigate DNA replication in rats following vitamin A withdrawal and replacement. Based on the timing of the first S phase occurring at 24 h posttreatment, the authors concluded that the undifferentiated spermatogonia in the VAD rat testis were arrested during G1 [26]. In addition, a DNA content of 2n has been reported for the undifferentiated spermatogonial population in a VAD rat testis, providing further support for G1 arrest [25]. We aimed to help clarify whether spermatogonia undergo cell cycle arrest by examining the expression of MKI67, a reportedly global marker of cells actively within the cell cycle, within a WIN 18,446-treated testis. WIN 18,446 treatment creates testes full of undifferentiated spermatogonia [21] and therefore provided the perfect platform for revisiting spermatogonial cell cycle progression in the absence of vitamin A. The near complete lack of dual GCNA- and MKI67-positive germ cells, only four cells for every 100 tubules, and very few EdU-positive germ cells following 12 h of exposure in the WIN 18,446-only treated testes are strongly suggestive of undifferentiated spermatogonia entering G0 in response to RA deficiency.
We then extended this study to investigate when spermatogonia re-enter the cell cycle following RA exposure. Surprisingly, we did not see robust MKI67 expression in germ cells until approximately 72 h post-RA treatment. It is not clear why in our synchronization system MKI67 expression is not present when we know that the differentiating spermatogonial populations are actively replicating and dividing. Gerdes et al. [46] investigated the expression pattern of MKI67 through the duration of the cell cycle and found that cells transitioning from G0 to G1 were MKI67-negative and only became positive toward the end of G1 before entering S phase. Although this does not explain why in our 16-h post-RA samples, during which the differentiating spermatogonia are undergoing an S phase, the germ cells remain MKI67-negative. Further work will need to be performed to clearly understand the role of MKI67 during the progression of the cell cycle in spermatogonia. However, our data do suggest that in response to RA, the undifferentiated spermatogonia transition into differentiating spermatogonia prior to the cells re-entering the cell cycle and that RA exposure does not appear to accelerate the re-entry of the germ cells into the cell cycle.
In the wild-type neonatal testis, spermatogonia differentiate asynchronously along the length of the seminiferous tubule, making it almost impossible to study a single type of differentiating spermatogonia. This is reflected by a near complete lack of published information regarding whether there are specific markers of the different populations of differentiating spermatogonia and no understanding of whether these cells are functionally distinct. Utilizing our WIN 18,446/RA synchronization protocol, we were able to precisely detect all of the differentiating spermatogonial S phases following the onset of spermatogenesis, thereby accurately mapping when testes are full of each different type of differentiating spermatogonia. As a result, this study will now allow for novel and precise investigations of each type of differentiating spermatogonia, a population of cells that has remained almost completely unstudied. In addition, we believe that this study builds on our previously published microarray analyses of testes collected on different days following WIN 18,446/RA treatment of neonatal mice [38], as the exact timing and cell identification from this study will now allow for that data set to be mined with specific cell types in mind. However, due to the very precise timing of our study, we believe that a follow-up RNA deep sequencing experiment utilizing our exact timing after synchronization would provide a more accurate data set.
The first round of spermatogenesis is shortened by approximately 2.5 days when compared to subsequent rounds of spermatogenesis [8]. However, there are currently conflicting hypotheses surrounding why this is the case. De Rooij et al. [4] concluded that prospermatogonia transition directly into A2 spermatogonia, skipping the A1 phase, yet a more recent publication reported that prospermatogonia transition into A1 spermatogonia that then divide and form A3 and A4 spermatogonia 24 h later [8]. Surprisingly, only five spermatogonial S phases were detected following synchronization in the present study, indicating that one differentiating spermatogonial division was skipped. This suggests that the WIN 18,446/RA protocol drives spermatogonial development that mimics the timing of the first round of spermatogenesis. TEM confirmed that A undifferentiated spermatogonia directly transition into either A1 or A2 differentiating spermatogonia using this synchronization protocol (Fig. 8I), while STRA8-positive A3 differentiating spermatogonia were exclusively present 48 h following synchronization. The differentiating spermatogonia present 72 h following synchronization displayed dim STRA8 expression; therefore, it is likely that these germ cells were A4 spermatogonia, as the A4 population comprises the most advanced differentiating spermatogonia to express STRA8 (Fig. 7K). In addition, the spermatogonia present during the fourth and fifth windows of S phase did not express STRA8 but did express KIT, a marker expressed in all differentiating spermatogonia, and were colocalized with EdU. Based on this localization pattern and their TEM appearance, we determined that the intermediate spermatogonia were enriched during the fourth window [16, 17, 20] (Fig. 7K) and B spermatogonia during the fifth window (Fig. 7K). These data suggest that following synchronization, there is some plasticity regarding which differentiating spermatogonial subtype is skipped, with either undifferentiated A spermatogonia advancing directly to A2 or A1 spermatogonia dividing to become A3.
The duration of spermatogonia cell cycles in the adult testis has been estimated to be approximately 28 h [27], but the timing of the individual differentiating spermatogonial cell cycles has not been thoroughly investigated during the first round of spermatogenesis. This study identified 24-h cell cycle durations for most of the differentiating spermatogonia. Interestingly, there was a large difference in cell cycle duration between the first and second S phase windows when compared to the timing between all other S phases: 30 h compared to 24 h. It is unknown why there is such a difference in the timing of cell cycles, but we hypothesize that it may be a response to the germ cells being hit with a bolus of RA. Two previous studies have detected a delay in germ cell development following RA exposure in neonatal mice [15, 33], and a slightly extended cell cycle may partially account for this delay.
During the onset of spermatogenesis, Sertoli cells proliferate to enable their population size to increase so that the spermatogenic process can be supported in the adult; however, few studies have investigated whether there is an effect on Sertoli cells following manipulation of RA levels in the postnatal testis. Evidence derived from Sertoli cell cultures suggests that retinol but perhaps not RA can increase Sertoli cell proliferation [47, 48]. We aimed to build on these in vitro studies and investigate whether manipulating RA levels in vivo had any effect on the number of Sertoli cells in the postnatal testis. There was no effect on Sertoli cell number in the absence of RA (WIN 18,446-only); however, there appeared to be some effect in the presence of excess RA. Mice injected with RA at 9 dpp and given 24 h of recovery displayed a slight but significantly higher number of Sertoli cells per testis tubule. As a result, our present study is in agreement with previous publications and implies that Sertoli cell number may be sensitive to excess RA during juvenile development and may implicate RA as playing a role in initiating progression into the cell cycle.
Taken together, our study has thoroughly examined the asynchronous initiation and synchronous onset of spermatogenesis. For the first time, the cell cycle progression of the differentiating spermatogonial populations has been identified, now allowing for the study of the individual differentiating spermatogonial populations and further characterization of novel markers for each subtype. In addition, we have provided evidence to suggest that Sertoli cell development can be influenced by RA and provided additional marker and cell cycle kinetics characterization within the undifferentiated spermatogonial population and in germ cells following synchronized spermatogenesis. Overall, this study has enhanced our understanding of the spermatogonial populations present in the neonatal testis during the onset of spermatogenesis and provided an essential tool for further study of the differentiating spermatogonia.
ACKNOWLEDGMENT
The authors thank Dr. John Amory for providing the WIN 18,446 and the Franceschi Microscopy and Imaging Center, specifically Dr. Daniel L. Mullendore, for processing and his assistance in imaging tissue for TEM.
Footnotes
Supported by NIH Grant R01 HD10808 to M.D.G. Presented in part at the 47th Annual Meeting of the Society for the Study of Reproduction, July 19–23, 2014, Grand Rapids, Michigan.
REFERENCES
- de Rooij DG, Griswold MD. Questions about spermatogonia posed and answered since 2000. J Androl. 2012;33:1085–1095. doi: 10.2164/jandrol.112.016832. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MD, Oatley JM. Concise review: defining characteristics of mammalian spermatogenic stem cells. Stem Cells. 2013;31:8–11. doi: 10.1002/stem.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluin PM, de Rooij DG. A comparison between the morphology and cell kinetics of gonocytes and adult type undifferentiated spermatogonia in the mouse. Int J Androl. 1981;4:475–493. doi: 10.1111/j.1365-2605.1981.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction. 2011;142:145–155. doi: 10.1530/REP-10-0431. [DOI] [PubMed] [Google Scholar]
- Jan SZ, Hamer G, Repping S, de Rooij DG, van Pelt AM, Vormer TL. Molecular control of rodent spermatogenesis. Biochim Biophys Acta. 2012;1822:1838–1850. doi: 10.1016/j.bbadis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Zhou Q, Griswold MD. Regulation of spermatogonia In: StemBook [Internet] Cambridge (MA) Harvard Stem Cell Institute; 2008. http://www.stembook.org/node/457 [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Zheng K, Wu X, Kaestner KH, Wang PJ. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Kaye EP, Renegar RH, Geyer CB. Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biol Reprod. 2014;90:64. doi: 10.1095/biolreprod.113.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Snyder E, Kent T, Mitchell D, Small C, Amory JK, Griswold MD. Suppression of Stra8 expression in the mouse gonad by WIN 18,446. Biol Reprod. 2011;84:957–965. doi: 10.1095/biolreprod.110.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- Oulad-Abdelghani M, Bouillet P, Decimo D, Gansmuller A, Heyberger S, Dolle P, Bronner S, Lutz Y, Chambon P. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Evanoff R, Mitchell D, Kent T, Small C, Amory JK, Griswold MD. Turning a spermatogenic wave into a tsunami: synchronizing murine spermatogenesis using WIN 18,446. Biol Reprod. 2013;88:40. doi: 10.1095/biolreprod.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Chiarini-Garcia H, Russell LD. Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction. 2002;123:567–577. doi: 10.1530/rep.0.1230567. [DOI] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Russell LD. High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–1178. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, van Dissel-Emiliani FM, Gaemers IC, van der Burg MJ, Tanke HJ, de Rooij DG. Characteristics of A spermatogonia and preleptotene spermatocytes in the vitamin A-deficient rat testis. Biol Reprod. 1995;53:570–578. doi: 10.1095/biolreprod53.3.570. [DOI] [PubMed] [Google Scholar]
- Van Pelt AM, De Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod. 1990;42:677–682. doi: 10.1095/biolreprod42.4.677. [DOI] [PubMed] [Google Scholar]
- Monesi V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J Cell Biol. 1962;14:1–18. doi: 10.1083/jcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee K, Wolgemuth DJ. Cdk family genes are expressed not only in dividing but also in terminally differentiated mouse germ cells, suggesting their possible function during both cell division and differentiation. Dev Dyn. 1995;204:406–420. doi: 10.1002/aja.1002040407. [DOI] [PubMed] [Google Scholar]
- Wolgemuth DJ. Function of cyclins in regulating the mitotic and meiotic cell cycles in male germ cells. Cell Cycle. 2008;7:3509–3513. doi: 10.4161/cc.7.22.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth DJ, Laurion E, Lele KM. Regulation of the mitotic and meiotic cell cycles in the male germ line. Recent Prog Horm Res. 2002;57:75–101. doi: 10.1210/rp.57.1.75. [DOI] [PubMed] [Google Scholar]
- Wolgemuth DJ, Manterola M, Vasileva A. Role of cyclins in controlling progression of mammalian spermatogenesis. Int J Dev Biol. 2013;57:159–168. doi: 10.1387/ijdb.130047av. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth DJ, Roberts SS. Regulating mitosis and meiosis in the male germ line: critical functions for cyclins. Philos Trans R Soc Lond B Biol Sci. 2010;365:1653–1662. doi: 10.1098/rstb.2009.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84:886–893. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod. 2010;83:783–790. doi: 10.1095/biolreprod.110.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Mutoji KN, Velte EK, Ko D, Oatley JM, Geyer CB, McCarrey JR. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol Reprod. 2015;92:54. doi: 10.1095/biolreprod.114.125757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenberger BA, Busada JT, Geyer CB. Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction. 2015;149:329–338. doi: 10.1530/REP-14-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SL, Kent T, Hogarth CA, Griswold MD, Amory JK, Isoherranen N. Pharmacological inhibition of ALDH1A in mice decreases all-trans retinoic acid concentrations in a tissue specific manner. Biochem Pharmacol. 2015;95:177–192. doi: 10.1016/j.bcp.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Hogarth C, Mitchell D, Griswold M. Riding the spermatogenic wave: profiling gene expression within neonatal germ and Sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol Reprod. 2014;90:108. doi: 10.1095/biolreprod.114.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth CA, Arnold S, Kent T, Mitchell D, Isoherranen N, Griswold MD. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol Reprod. 2015;92:37. doi: 10.1095/biolreprod.114.126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron Microscopy: Principles and Techniques for Biologists. Sudbury, MA: Jones & Bartlett Learning; 1999. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kim KH. Vitamin A-deficient testis germ cells are arrested at the end of S phase of the cell cycle: a molecular study of the origin of synchronous spermatogenesis in regenerated seminiferous tubules. Biol Reprod. 1993;48:1157–1165. doi: 10.1095/biolreprod48.5.1157. [DOI] [PubMed] [Google Scholar]
- Ismail N, Morales C, Clermont Y. Role of spermatogonia in the stage-synchronization of the seminiferous epithelium in vitamin-A-deficient rats. Am J Anat. 1990;188:57–63. doi: 10.1002/aja.1001880107. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, Morena AR, van Dissel-Emiliani FM, Boitani C, Gaemers IC, de Rooij DG, Stefanini M. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55:439–444. doi: 10.1095/biolreprod55.2.439. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Zanotto-Filho A, Schroder R, Moreira JC. Differential effects of retinol and retinoic acid on cell proliferation: a role for reactive species and redox-dependent mechanisms in retinol supplementation. Free Radic Res. 2008;42:778–788. doi: 10.1080/10715760802385702. [DOI] [PubMed] [Google Scholar]
- Klamt F, Dal-Pizzol F, Roehrs R, de Oliveira RB, Dalmolin R, Henriques JA, de Andrades HH. de Paula Ramos AL, Saffi J, Moreira JC. Genotoxicity, recombinogenicity and cellular preneoplasic transformation induced by vitamin A supplementation. Mutat Res. 2003;539:117–125. doi: 10.1016/s1383-5718(03)00155-4. [DOI] [PubMed] [Google Scholar]