Abstract
Introduction
Despite adjuvant systemic therapy in patients with completely resected non-small-cell lung cancer (nsclc), many will subsequently relapse. We investigated treatment choices at relapse and assessed the effect of palliative platinum doublet systemic therapy in this population.
Methods
With research ethics board approval, we performed a retrospective chart review of all patients with resected nsclc who received adjuvant systemic therapy from January 2002 until December 2008 at our institution. The primary outcome was the response rate to first-line palliative systemic therapy among patients who relapsed.
Results
We identified 176 patients who received adjuvant platinum doublet systemic therapy (82% received cisplatin–vinorelbine). In the 85 patients who relapsed (48%), median time to relapse was 18.5 months (95% confidence interval: 15 months to 21.3 months). Palliative systemic therapy was given in 43 patients. Of those 43 patients, 25 (58%) were re-challenged with platinum doublet systemic therapy, with a response rate of 29% compared with 18% in 18 patients who received other systemic therapy (p = 0.48). We observed a trend toward an increased clinical benefit rate (complete response + partial response + stable disease) in patients who were treated with a platinum doublet (67% vs. 41%, p = 0.12). Median overall survival (os) from relapse was 15.3 months in patients receiving palliative systemic therapy and 7.8 months in those receiving best supportive care alone. Compared with patients treated with non-platinum regimens, the platinum-treated group experienced longer survival after relapse (18.4 months vs. 9.7 months, p = 0.041).
Conclusions
In patients previously treated with adjuvant systemic therapy, re-treatment with platinum doublet chemotherapy upon relapse is feasible. Moreover, compared with patients receiving other first-line systemic therapy, patients receiving platinum doublets experienced higher response rates and significantly longer survival.
Keywords: Recurrent non-small-cell lung cancer, adjuvant therapy, first-line therapy, platinum
INTRODUCTION
Lung cancer is the most common cancer worldwide, with an estimated 1,600,000 new cases and 1,380,000 deaths per annum1. Non-small-cell lung cancer (nsclc) accounts for approximately 85% of all cases of lung cancer2.
Most patients with early-stage nsclc undergo surgery (71%); approximately 18% also receive chemotherapy or radiation therapy3. In Canada, the proportion of patients who underwent resection increased to 47% from 27% between 2007 and 20094. Despite optimal surgical management, the 5-year survival rate in resected nsclc ranges from 25% to 73%, according to pathologic stage3.
A meta-analysis of adjuvant chemotherapy trials in resected nsclc published in 1995 reported a 13% reduction in the risk of death, suggesting an absolute benefit of 5% at 5 years with adjuvant chemotherapy3. However the role of adjuvant chemotherapy was not established until the completion of clinical trials utilizing more modern platinum doublets. The landmark studies included the ialt trial, which randomized stages i–iii patients to cisplatin and vinca alkaloid or etoposide compared with observation; the jbr.10 trial, which randomized stages ib–ii patients to cisplatin and vinorelbine compared with observation; and the anita trial, which randomized stages ib–iiia patients to cisplatin and vinorelbine compared with observation5–7.
Adjuvant chemotherapy improves survival in patients with completely resected early-stage nsclc. In that setting, the ialt, jbr.10, and anita trials found, at 5 years, an absolute improvement in os of 4.1%, 15%, and 8.6 % respectively5–7. More recently, the lace meta-analysis confirmed the benefit of adjuvant cisplatin-based chemotherapy, with an improvement in disease-free survival of 5.2% at 5 years (p < 0.0001) and a 5.4% absolute improvement in os at 5 years (p = 0.0043)8. As a result, adjuvant cisplatin–vinorelbine is considered a standard of care in this population9,10.
However, despite adjuvant chemotherapy, 30%–60% of patients will subsequently relapse with advanced incurable disease11. For individuals with a good performance status, palliative chemotherapy is an option, which may involve re-treatment with platinum doublet chemotherapy. To date, there has been no documentation in the literature of the efficacy of chemotherapy at time of relapse in this cohort of nsclc patients previously treated with adjuvant chemotherapy. Specifically, the response rates (rrs) to platinum chemotherapy in the recurrent advanced setting—after prior platinum adjuvant treatment in the adjuvant setting—have not been reported.
We investigated treatment choices on relapse after prior adjuvant chemotherapy, specifically in patients receiving palliative platinum doublet chemotherapy, and their effects on rr and os.
METHODS
With institutional research ethics board approval, we performed a retrospective chart review of all patients with resected nsclc who received adjuvant chemotherapy from January 2002 until December 2008 at our institution, a tertiary academic cancer centre that is the sole provider of medical oncology services to an urban and rural population of approximately 1.4 million. All patients were assigned a study number, and no personal identifying information was used during the data analysis.
We examined patient demographics, initial nsclc stage and surgical treatment, adjuvant chemotherapy treatment (regimen, number of cycles, dose reductions, dose delays), time-to-event outcomes (date of relapse, date of death), and treatment on relapse (number and type of regimens, response to those regimens). The initial stage was recorded from the resection pathology report using the 6th edition of the American Joint Committee on Cancer TNM staging system. All imaging performed to assess response during follow-up was reviewed and reported using recist (the Response Evaluation Criteria in Solid Tumors), as assessed by the authors. Status of each individual patient was determined from the chart and from all available online obituary sources. If no evidence of death was found, status was reported as “alive,” and the last follow-up date was recorded for censoring. The primary endpoint was the rr to first-line systemic therapy.
Statistical Analysis
The Fisher exact test was used to compare categorical variables, and the Student t-test was used for numeric variables. Multivariate analyses of numeric variables used analysis of variance, and categorical multivariate analyses used logistic regression. Survival curves created by the Kaplan–Meier method were used for the relapse and survival analysis.
RESULTS
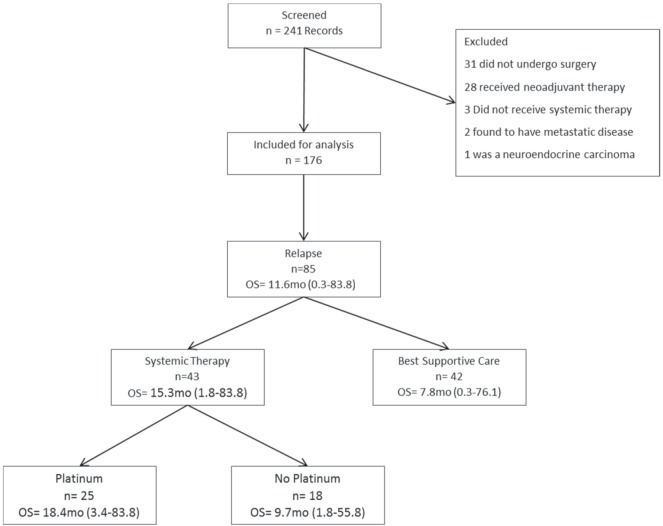
Of the 241 patients initially identified in our institutional database, 65 were excluded (28 had received neoadjuvant treatment, 31 did not undergo surgery, 3 did not receive any type of chemotherapy, 2 had metastatic disease, and 1 had a neuroendocrine carcinoma; Figure 1). The remaining 176 patients who had received adjuvant platinum doublet chemotherapy were therefore included in the analysis, most of whom (82%) had received cisplatin–vinorelbine.
FIGURE 1.
Inclusion process and patient subgroup survival. OS = overall survival [median time from relapse to death or last follow-up in months (mo), with range in parenthesis].
Median age in the cohort was 63 years (range: 25–82 years), and 55% were women. With respect to smoking status, 83 patients were current smokers, 76 were ex-smokers, and 12 were never-smokers; in 5 patients, smoking status was unknown. The primary surgical procedures were lobectomy (79%) and pneumonectomy (15%), followed by segmentectomy and bilobectomy (6% overall). The most common histologic subtypes were adenocarcinoma (53%), squamous cell carcinoma (29%), and large-cell carcinoma (10%), followed by adenosquamous, bronchioalveolar, large-cell neuroendocrine, and undifferentiated carcinomas (7% overall). The pathologic stages recorded after surgery were ia (2%), ib (30%), iia (7%), iib (40%), iiia (15%), and iiib (6%). Table i shows all patient characteristics.
TABLE I.
Demographic characteristics of the study group
| Characteristic | Value |
|---|---|
| Patients (n) | 176 |
| Age (years) | |
| Median | 63 |
| Range | 25–82 |
| Sex ratio (women:men) | 96:80 (1.2:1) |
| Final pathologic type [n (%)] | |
| Adenocarcinoma | 94 (53.4) |
| Squamous cell carcinoma | 51 (29) |
| Large-cell carcinoma | 18 (10.2) |
| Others | 13 (7.4) |
| Primary surgical procedures [n (%)] | |
| Lobectomy | 137 (79) |
| Pneumonectomy | 27 (15.3) |
| Other | 12 (5.7) |
| Pathologic stage at surgery [n (%)] | |
| IA | 3 (1.7) |
| IB | 53 (30.1) |
| IIA | 12 (6.8) |
| IIB | 71 (40.3) |
| IIIA | 27 (15.3) |
| IIIB | 10 (5.7) |
Median follow-up in this group was 4.2 years. In the 85 patients who relapsed (48%), median time to relapse was 18.5 months (95% confidence interval: 15 to 21.3 months).
Efficacy of Treatment on Relapse
Of the 85 relapsed patients, 43 received palliative chemotherapy, and 42 received best supportive care alone. Of the 43 patients treated, 25 (58%) were rechallenged with platinum doublet chemotherapy, achieving a rr of 29% compared with 18% (p = 0.48) in the 18 patients receiving other systemic therapy, most commonly docetaxel (n = 7, 39%) or erlotinib (n = 4, 22%). The platinum and non-platinum groups showed no significant differences with respect to age, sex, smoking status at diagnosis or pack–years smoked at diagnosis, performance status, tumour size, nodal status, or general pathologic stage. The median time from surgery to relapse for the group re-treated with platinum was 19.8 months; it was 9.5 months for the group re-treated with non-platinum agents (p = 0.002). The platinum doublets commonly used were carboplatin–docetaxel, carboplatin–paclitaxel, cisplatin–gemcitabine, and cisplatin–vinorelbine. Other alternatives were drugs used as monotherapy; they included docetaxel, erlotinib, gefitinib, gemcitabine, pemetrexed, and vinorelbine.
Response was assessed in 41 of the 43 patients treated with first-line systemic therapy after relapse. The overall rr in the assessed patients was 24% [complete response (cr) in 2 patients, partial response (pr) in 8 patients], with stable disease in a further 13 patients (32%) and progressive disease in 18 patients (44%). In the platinum group, the rr was 29% (7 of 24 patients: 1 cr, 6 pr); in the non-platinum group, it was 18% (3 of 17 patients: 1 cr, 2 pr; p = 0.48). We observed a trend toward an increased clinical benefit rate (cr, pr, and stable disease) in patients who were treated with a platinum doublet (67% vs. 41%, p = 0.12). Table ii details the responses.
TABLE II.
Relevant measures of response and survival duration
| Measure | Platinum re-challenge | p Value | |
|---|---|---|---|
|
| |||
| Yes (n=25) | No (n=18) | ||
| Response (n) | |||
| Complete (CR) | 1 | 1 | |
| Partial (PR) | 6 | 2 | 0.43 |
| Stable disease (SD) | 9 | 4 | 0.34 |
| Progressive disease | 8 | 10 | 0.13 |
| Response rate (CR or PR) | 7 | 3 | 0.48 |
| Clinical benefit rate (CR, PR, or SD) | 16 | 7 | 0.12 |
| Missing | 1 | 1 | |
| Median time (months) from ... | |||
| Surgery to relapse | 19.8 | 9.5 | 0.002 |
| Relapse to death or last follow-up | 18.4 | 9.6 | 0.041 |
Only 1 patient was re-exposed to platinum therapy in the second line. At diagnosis she had a stage iiib squamous cell carcinoma of the left main bronchus and underwent pneumonectomy. She relapsed 21.6 months after surgery and experienced disease progression after 2 cycles of first-line docetaxel. After a platinum-free interval of 17.2 months she received 5 cycles of cisplatin–vinorelbine in the second line and achieved stable disease for 8.3 months, very similar to the pattern observed in the patients treated in the first line.
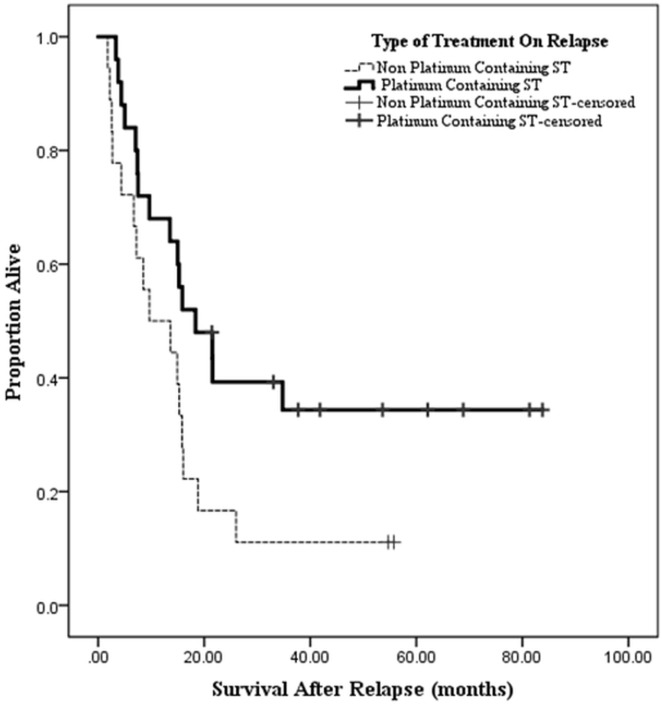
For all patients, median time from date of surgery to last follow-up or death was 51.8 months. In patients who relapsed, that time was 36.5 months, with a median time from relapse to death or last follow-up of 11.6 months (Figure 2). In patients receiving any chemotherapy after relapse (n = 43), median time from relapse to last follow-up or death was 15.3 months (range: 1.8–83.8 months); it was 7.8 months (range: 0.3–76.1 months) in those receiving best supportive care alone (p = 0.017).
FIGURE 2.
Survival according to type of systemic therapy after relapse.
Patients in the platinum-treated group experienced longer survival after relapse than did patients treated with non-platinum regimens [18.4 months (range: 3.4–83.8 months) vs. 9.7 months (range: 1.8–55.8 months), p = 0.03]. Table ii compares patients treated with platinum and non-platinum regimens.
DISCUSSION
In the present study, we report on the efficacy of platinum chemotherapy as palliative treatment in patients who previously received adjuvant platinum chemotherapy. Given the significant number of patients who relapse despite adjuvant therapy, determining the efficacy of platinum re-treatment is clearly of clinical relevance. We found a relevant rr difference of 11 percentage points favouring a platinum doublet re-challenge compared with other systemic therapies. Further, we observed a significant median benefit in time from relapse to last follow-up or death of 8.7 months in the platinum doublet group (18.4 months vs. 9.7 months, p = 0.03). After adjuvant therapy with platinum, platinum resistance could be expected on second exposure for relapse. However, a response of 29% in our platinum group (compared with 18% in the non-platinum group, p = 0.48) is very much in line with the first-line response rates to platinum chemotherapy as reported in the well-known phase iii studies investigating platinum doublets12,13.
We question whether our analysis supports re-challenging all patients with a platinum doublet on recurrence of disease after platinum-based adjuvant chemotherapy in anticipation that the response will be similar to that seen in chemonaïve patients. Rather, we suggest that re-challenging patients with platinum is a reasonable option in selected patients. A retrospective report by Imai et al.14 that included 16 patients who received complete resection followed by an adjuvant platinum doublet and who were re-treated with a platinum doublet upon relapse found a response rate of 31.2%, which clearly supports our findings.
We moderate our conclusions because the primary limitation of our study is an inability to address the issue of patient selection. We fully recognize that differences in relapse-free survival after adjuvant chemotherapy for those who were subsequently re-treated with platinum (19.8 months) and those who received non-platinum therapy (9.5 months) reflects underlying differences in tumour biology. It is quite plausible that the features of a cancer that led to an earlier relapse are the same factors that led to platinum resistance. Alternatively, clinicians could have chosen the fittest patients to receive platinum therapy and that fitness could have predisposed the patients to longer survival. Clinical judgment could have thus led to the selection of platinum-sensitive patients.
A recent pooled analysis of platinum re-challenge in the second line for metastatic nsclc that included retrospective and prospective trials demonstrated a rr of 26.9% for cisplatin combinations. That rate is similar to the rr that we report here. If proven prospectively, those results could signify a benefit that is constantly available at various points in the disease course despite previous use of the drug15.
Re-challenging with prior chemotherapy regimens, including platinum, has proved effective and appropriate in other settings. One is second-line therapy for small-cell lung cancer, where reports (although retrospective) have found significantly higher rr, progression-free survival, and os on re-challenge in platinum-sensitive patients16–20.
Another issue is the ability to predict response in patients for whom re-exposure is feasible. Unfortunately, given our data and the most current evidence, we cannot say to what extent a long relapse-free interval after adjuvant platinum therapy can predict response. Likewise, determining mechanisms of drug resistance was not the goal of our study, and that issue would be best addressed by biopsy at relapse for genomic and proteomic evaluation.
Ultimately, it will take a randomized study of platinum compared with non-platinum chemotherapy in this group of patients to definitively answer the foregoing questions. However, an alternative would be to analyze response and survival in patients entered into modern first-line platinum chemotherapy trials and to retrospectively analyze according to the presence or absence of prior adjuvant chemotherapy. Our group is planning such an analysis.
Further limitations include the number of patients in the cohort and the retrospective nature of the data. Nonetheless, our patient population is representative of patients with non-small-cell lung cancer who undergo adjuvant systemic therapy: median age 63 years, 40%–50% adenocarcinoma, 30%–40% squamous cell carcinoma, and 60%–65% men5–7. Our relapse rate was 48%, which is approximately 10% higher than that observed in earlier prospective trials in which only patients with a very good performance status were enrolled5–7.
CONCLUSIONS
In patients previously treated with adjuvant chemotherapy, re-treatment with a platinum doublet upon relapse is feasible and is associated with a higher rr and significantly longer survival than are seen in patients receiving other first-line systemic therapy. However, our data cannot answer the issue of patient selection, and so further investigation with prospectively gathered data is planned.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Erratum in: CA Cancer J Clin 2011;61:134] [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non–small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. doi: 10.1136/bmj.311.7010.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Partnership Against Cancer (cpac). The 2012 Cancer System Performance Report. Toronto, ON: CPAC; 2012. [Google Scholar]
- 5.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, on behalf of the International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage ib–iiia non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 7.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 8.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the lace Collaborative Group. J Clin Oncol. 2008;26:3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger D, Johnson B. Update: nccn small cell and non–small cell lung cancer clinical practice guidelines. J Natl Compr Canc Netw. 2005;3(suppl 1):S17–21. [PubMed] [Google Scholar]
- 10.Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages i–iiia resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 11.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:3852–9. doi: 10.1200/JCO.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 12.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase iii trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–18. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH, Harrington D, Belani CP, et al. on behalf of the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 14.Imai H, Shukuya T, Yoshino R, et al. Efficacy and safety of platinum combination chemotherapy re-challenge for relapsed patients with non-small-cell lung cancer after postoperative adjuvant chemotherapy of cisplatin plus vinorelbine. Chemotherapy. 2013;59:307–13. doi: 10.1159/000356155. [DOI] [PubMed] [Google Scholar]
- 15.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Ardine M, Barni S. Platinum rechallenge in patients with advanced nsclc: a pooled analysis. Lung Cancer. 2013;81:337–42. doi: 10.1016/j.lungcan.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Garassino MC, Torri V, Michetti G, et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer. 2011;72:378–83. doi: 10.1016/j.lungcan.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Korkmaz T, Seber S, Kefeli U, et al. Comparison of second-line treatment outcomes between sensitive and refractory small cell lung cancer patients: a retrospective analysis. Clin Transl Oncol. 2013;15:535–40. doi: 10.1007/s12094-012-0960-6. [DOI] [PubMed] [Google Scholar]
- 18.Postmus PE, Berendsen HH, van Zandwijk N, Splinter TA, Burghouts JT, Bakker W. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol. 1987;23:1409–11. doi: 10.1016/0277-5379(87)90128-3. [DOI] [PubMed] [Google Scholar]
- 19.Shao L, Su D, Song ZB, et al. Analysis of efficacy and survival of patients receiving second-line treatment for sensitive recurrent small-cell lung cancer. Tumor. 2012;32:892–8. [Google Scholar]
- 20.Giaccone G. Second line chemotherapy in small cell lung cancer. Lung Cancer. 1989;5:207–13. doi: 10.1016/0169-5002(89)90169-4. [DOI] [Google Scholar]