Abstract
Purpose
Smoking cessation and increased physical activity (pa) have been linked to better outcomes in cancer survivors. We assessed whether socioeconomic factors influence changes in those behaviours after a cancer diagnosis.
Methods
As part of a cross-sectional study, a diverse group of cancer survivors at the Princess Margaret Cancer Centre (Toronto, ON), completed a questionnaire about past and current lifestyle behaviours and perceptions about the importance of those behaviours with respect to their health. The influence of socioeconomic indicators on smoking status and physical inactivity at 1 year before and after diagnosis were assessed using multivariable logistic regression with adjustment for clinico-demographic factors.
Results
Of 1222 participants, 1192 completed the smoking component. Of those respondents, 15% smoked before diagnosis, and 43% of those smokers continued to smoke after. The proportion of survivors who continued to smoke increased with lower education level (p = 0.03). Of the 1106 participants answering pa questions, 39% reported being physically inactive before diagnosis, of whom 82% remained inactive afterward. Survivors with a lower education level were most likely to remain inactive after diagnosis (p = 0.003). Lower education level, household income, and occupation were associated with the perception that pa had no effect or could worsen fatigue and quality of life (p ≤ 0.0001).
Conclusions
In cancer survivors, education level was a major modifier of smoking and pa behaviours. Lower socioeconomic status was associated with incorrect perceptions about pa. Targeting at-risk survivors by education level should be evaluated as a strategy in cancer survivorship programs.
Keywords: Socioeconomic status, smoking, physical activity, education, survivorship, lifestyle behaviours
INTRODUCTION
Given the growing number of cancer survivors worldwide, the importance of promoting healthy lifestyle behaviours has become increasingly salient. Smoking is an established risk factor for cardiorespiratory disease and a number of cancers1, including leukemia2 and cancers of the urogenital system3. Continued smoking in cancer patients has been associated with negative outcomes, including increased chances of a second malignancy4, reduced quality of life5, poor treatment response6, and increased mortality7. More recently, greater physical activity (pa) in cancer survivors has been linked to improved survival8 and quality of life9 and to less fatigue10; it has also been described as both safe and beneficial during and after cancer treatment11.
A diagnosis of cancer has been described as a “teachable moment” with respect to modification of lifestyle behaviours12. However, studies have suggested that approximately 50%–60% of patients continue to smoke13–15 and close to 80% remain physically inactive16 after diagnosis. A thorough understanding of the factors influencing changes in those behaviours after diagnosis is needed to best inform survivorship program development for a number of cancer groups. Thus far, studies examining smoking cessation have focused primarily on survivors of lung and head-and-neck cancers; those studies have pointed to the level of nicotine dependence17 and psychosocial factors such as social environment18,19 and mental health13,20 as predictors of continued smoking in cancer patients. In contrast, most studies evaluating post-diagnosis pa in cancer patients have involved survivors of breast and colorectal cancers. Those studies suggested that ethnicity21, social support22,23, and cancer-related beliefs24 were influential factors.
Socioeconomic status (ses) has been associated with smoking25,26 and physical inactivity25,27 in general populations. After a diagnosis of cancer, patients might receive more specific counselling and might have greater access to resources and programs. With those possibilities in mind, it is not clear whether the factors that influence health behaviours before diagnosis, particularly those found to be important in healthy general populations, also affect such behaviours after diagnosis. When considering a behavioural change model such as the Health Belief Model28,29 or the Health Action Process Approach28, a higher ses might reflect more resources and fewer barriers in making improvements in lifestyle habits. Evidence suggests that socioeconomic indicators such as occupation, income, and education are not always interchangeable30, and it is thus important to determine which of those factors are the most influential in the context of behavioural change.
The present cross-sectional study focused on ambulatory cancer patients representing various disease sites, disease progression statuses, and stages of treatment. In this group of survivors, we examined whether ses influenced modification of smoking and pa habits, and which socioeconomic indicators were most influential. We further explored how the indicators were associated with perceptions about the effect of smoking and pa on health outcomes.
METHODS
Population and Questionnaire
After approval for the study was granted by the University Health Network Research Ethics Board, participants were approached at outpatient clinics at the Princess Margaret Cancer Centre (Toronto, ON) between May 2012 and August 2013. After providing informed consent, they were asked to complete a one-time questionnaire about their lifestyle behaviours. Recruitment was based on convenience sampling and was directed such that at least 50 participants were recruited from each major cancer grouping (for example, lung cancer, gastrointestinal cancers, and so on). Eligible participants had to meet these criteria:
■ Be more than 18 years of age.
■ Be able to communicate in English
■ Have no significant cognitive impairment
■ Have a histologically confirmed diagnosis of a solid or hematologic malignancy
■ Be expected by their oncologists to live for at least 12 months
■ Have been diagnosed at least 6 months before recruitment
A recruitment goal was to have the median time since diagnosis fall somewhere between 24 months and 30 months (a long enough period to check for substantive behavioural change). To ensure that the goal was met, the median was checked with every additional 200 patients recruited. All procedures accorded with the ethical standards of the institutional or national research committee (or both) and with the 1964 Helsinki Declaration and its later amendments (or comparable ethical standards).
Within the single questionnaire, patients provided information about sociodemographic characteristics, height and weight, patient-reported Eastern Cooperative Oncology Group performance status, and their “health in the last month” (rated as poor, fair, good, very good, or excellent). Survivors were asked to report their smoking and pa habits at that time and also to recall the nature of the same behaviours 1 year before diagnosis. Separate sets of questions elicited patient perceptions of the influence of smoking and pa on their fatigue, quality of life, and chance of survival at 5 years. Clinical data—including date of diagnosis, cancer site, cancer extent at diagnosis (local vs. distant metastatic), and prognosis—were abstracted from each participant’s electronic medical record.
Definitions of Smoking and PA Outcomes
An “ever-smoker” was defined as a patient who reported having smoked more than 100 cigarettes in his or her lifetime. Ever-smokers were further divided into those who reported being or not being smokers 1 year before diagnosis, with the smokers being further subcategorized as either continuing to smoke or having quit at the time of questionnaire completion.
The pa questions were adapted from the Godin Leisure-Time Exercise Questionnaire31. Patients were asked to indicate, for 1 year before diagnosis and at the time of questionnaire completion, the number of times per week they engaged in strenuous, moderate, or mild pa and the number of minutes for each pa session. Occupational activities were not considered pa. “Physically inactive” was defined as a report of zero minutes of any mild, moderate, or strenuous exercise in a typical 7-day period; individuals who were physically inactive at baseline were subcategorized as either physically inactive or physically active at the time of questionnaire completion after diagnosis.
Definitions and Categorization of Other Variables
The ses indicators used in our study were education, household income, and occupation. Education and household income were reported categorically, and patients had the option not to answer those questions. Highest level of education was classified based on the categories of having completed a professional or graduate degree, a university or college undergraduate degree, or a high school degree, or not having completed high school. Household income was classified as high ($100,000), moderate ($60,000–$99,999), low (<$60,000), or “prefer not to answer”—categories that provided an even distribution of participants. For occupation, participants recorded the job at which “they worked the most in their life,” and responses were categorized based on the International Standard Classification of Occupations (isco-08). Body mass index was calculated for each time point (1 year before diagnosis and at the time of questionnaire completion) based on patient-reported height and weight, and was categorized appropriately as underweight, normal, overweight, or obese32.
To assess their perceptions, participants were asked to rate the effects that smoking and pa had on their quality of life, 5-year survival, and fatigue. Those questions used a Likert scale from 1 (make much worse) to 7 (make much better), with 4 being a neutral stance. For smoking, responses were dichotomized as having no effect or making better (4–7) or as making worse (1–3); for pa, responses were dichotomized as having no effect or making worse (1–4) or as making better (5–7). Barriers to pa were assessed with the statement “I find it difficult to spend more time performing physical activity because,” followed by a list that included “I am not sure what to do” and “I have no access to gym or exercise equipment” for which respondents indicated yes or no. The questions were developed for the purpose of the purposes of the present study.
Statistical Analysis
Univariable logistic regression models were used to examine associations of clinical, socioeconomic, and other sociodemographic factors with smoking and physical inactivity 1 year before diagnosis and at the time of questionnaire completion. Subsequent multivariable models used a backward selection algorithm, with entry of all variables significant at p < 0.10 into the univariable analyses. For the multivariable analyses that considered smoking and pa outcomes in addition to the sociodemographic variables (education, household income, and occupation), these additional variables were considered: sex, age at diagnosis, ethnicity, time since diagnosis, body mass index, cancer site, cancer extent at diagnosis, Eastern Cooperative Oncology Group performance status, and health in the preceding month. In the multivariable smoking analyses, years smoked and number of cigarettes per day smoked were also considered; in the multivariable pa analyses, smoking status before diagnosis was also considered. Covariates that resulted in p ≤ 0.05 were retained in the model. All statistical procedures were performed using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.). All tests were 2-sided, and statistical significance was defined at p ≤ 0.05. Records with missing or incomplete predictor or outcomes data were not included in the models.
RESULTS
Of 2185 eligible patients approached, 1456 (67%) consented to participate, of whom 1222 (84%) had complete information for the smoking or pa questions, forming the sample for analysis. Participants were surveyed at a median of 26 months after diagnosis. Table i describes the sociodemographic and clinical characteristics of the study population. Disease site–specific rates of continued smoking and physical inactivity are summarized in Table ii.
TABLE I.
Sociodemographic and clinical characteristics of the study population
| Variable | Value | Missing (n) |
|---|---|---|
| Patients | 1222 | |
| Female sex (%) | 53 | 0 |
| Age at follow-up (years) | ||
| Median | 59 | 8 |
| Interquartile range | 19 | |
| Married or live with partner (%) | 72 | 9 |
| White ethnicity (%) | 82 | 31 |
| Education (%) | ||
| Professional/Masters/PhD | 18 | 18 |
| University or college | 43 | |
| High school | 31 | |
| Less than high school | 8 | |
| Household income (%) | ||
| High (>$100,000) | 28 | 42 |
| Moderate ($60,000–$99,999) | 22 | |
| Low (<$60,000) | 25 | |
| Prefer not to answer | 25 | |
| Occupation (%) | ||
| Professional | 39 | 35 |
| Managers | 11 | |
| Technicians, associate professionals | 17 | |
| Service and sales workers | 12 | |
| Manual occupations | 12 | |
| Not classified | 8 | |
| Months since diagnosis | ||
| Median | 26 | 9 |
| Interquartile range | 56 | |
| Body mass index (%) | ||
| At baseline | ||
| Obese | 22 | 107 |
| Overweight | 38 | |
| Normal | 38 | |
| Underweight | 2 | |
| At follow-up | ||
| Obese | 19 | 93 |
| Overweight | 34 | |
| Normal | 43 | |
| Underweight | 4 | |
| Cancer site | ||
| Breast | 16 | 9 |
| Gastrointestinal | 12 | |
| Genitourinary | 14 | |
| Gynecologic | 9 | |
| Head, neck, and thyroid | 14 | |
| Hematologic | 19 | |
| Lung | 6 | |
| Skin and other cancers | 9 | |
| Unknown | 1 | |
| Cancer extent at diagnosis | ||
| Local (solid tumour) | 69 | 58 |
| Distant metastatic (solid tumour) | 11 | |
| Hematologic | 20 | |
| ECOG performance status | ||
| 0 | 48 | 34 |
| 1 | 37 | |
| 2–4 | 14 | |
| Health in preceding month | ||
| Excellent | 11 | 20 |
| Very good | 25 | |
| Good | 39 | |
| Fair | 22 | |
| Poor | 3 |
TABLE II.
Cancer disease site–specific rates of smoking and physical inactivity before and after diagnosis
| Cancer site | Smoking rate (%) in relation to diagnosis | Physical activity (%) in relation to diagnosis | ||
|---|---|---|---|---|
|
|
|
|||
| 1 Year before (smokers/ever-smokers) | After (still smoking) | 1 Year before (physically inactive/all) | After (still inactive) | |
| Breast | 34 | 42 | 33 | 77 |
| Gastrointestinal | 37 | 53 | 40 | 81 |
| Genitourinary | 15 | 55 | 37 | 82 |
| Gynecologic | 35 | 59 | 44 | 87 |
| Head, neck, and thyroid | 42 | 50 | 39 | 88 |
| Hematologic | 29 | 23 | 37 | 76 |
| Lung | 45 | 27 | 42 | 89 |
| Skin and other cancers | 34 | 47 | 37 | 86 |
Socioeconomic Factors Associated with Smoking Before and After Diagnosis
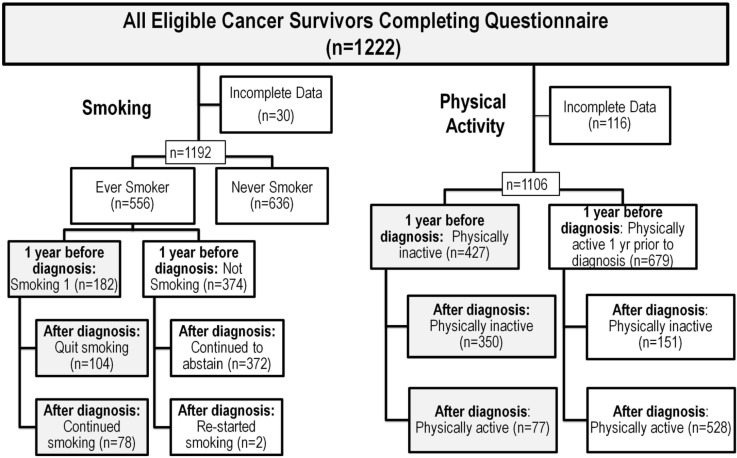
Of 1192 participants analyzed for smoking, 47% were ever-smokers, of whom 32% reported smoking 1 year before diagnosis (Figure 1). Table iii outlines the socioeconomic factors associated with smoking before and after diagnosis. At baseline, the smoking rates among ever-smokers were highest in participants with lung (45%) and head-and-neck and thyroid cancer (42%), and lowest in the participants with genitourinary cancers (15%). Our multivariable model demonstrated that, compared with participants having high household incomes, those with moderate and low household incomes were, respectively, 1.5 and 2.7 times as likely to be smokers (p = 0.006, Table iii). Older age at diagnosis (p < 0.001) and cancer site (p = 0.002) also remained significant in the model, but education and occupation level were not associated with baseline smoking rates (Table iii).
FIGURE 1.
Frequency of the lifestyle behaviours of tobacco smoking and physical activity at 1 year before diagnosis and at questionnaire completion after diagnosis. Subgroup analyses focused on the shaded boxes.
TABLE III.
Socioeconomic factors associated with continued smoking and physical inactivity before and after diagnosis
| Socioeconomic variable | Comparator | 1 Year before diagnosis | After diagnosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Patients (%) | Univariable | Multivariablea | Patients (%) | Univariable | Multivariableb | ||||||||||
|
|
|
|
|
||||||||||||
| OR | 95% CI | p Valuec | aOR | 95% CI | p Valuec | OR | 95% CI | p Valuec | aOR | 95% CI | p Valuec | ||||
| All ever-smokers (n= 556) | Survivors smoking before diagnosis (n=182) | ||||||||||||||
| Associated with smoking | Smoking | Smoking (n=182) vs. not smoking (n=374) | Smoking | Continued smoking (n=78) vs. quit smoking (n=104) | |||||||||||
| Education | |||||||||||||||
| Professional/graduate degree | 20 | Reference | 0.04 | 31 | Reference | 0.03 | Reference | 0.03 | |||||||
| University or college | 34 | 2.09 | 1.1 to 3.9 | 32 | 1.05 | 0.3 to 3.4 | 0.92 | 0.3 to 3.0 | |||||||
| High school | 35 | 2.21 | 1.2 to 4.1 | 49 | 2.14 | 0.7 to 6.8 | 1.99 | 0.6 to 6.4 | |||||||
| <High school | 41 | 2.84 | 1.3 to 6.1 | 64 | 3.85 | 1.0 to 15 | 3.50 | 0.9 to 14 | |||||||
| Household Income | |||||||||||||||
| High | 25 | Reference | 0.005 | Reference | 0.006 | 41 | Reference | 0.64 | |||||||
| Moderate | 29 | 1.24 | 0.7 to 2.2 | 1.50 | 0.8 to 2.7 | 35 | 0.78 | 0.3 to 2.1 | |||||||
| Low | 44 | 2.35 | 1.4 to 3.9 | 2.70 | 1.5 to 4.7 | 47 | 1.27 | 0.6 to 2.9 | |||||||
| Prefer not to answer | 32 | 1.42 | 0.8 to 2.4 | 1.87 | 1.0 to 3.4 | 37 | 0.85 | 0.3 to 2.1 | |||||||
| Occupation | |||||||||||||||
| Professionals | 25 | Reference | 0.003 | 33 | Reference | 0.46 | |||||||||
| Managers | 29 | 1.22 | 0.6 to 2.3 | 29 | 0.83 | 0.3 to 2.8 | |||||||||
| Technicians | 38 | 1.15 | 0.7 to 2.0 | 44 | 1.60 | 0.6 to 4.2 | |||||||||
| Service and sales | 38 | 1.81 | 1.0 to 3.2 | 48 | 1.87 | 0.7 to 4.8 | |||||||||
| Manual occupations | 46 | 2.47 | 1.4 to 4.3 | 46 | 1.70 | 0.7 to 4.1 | |||||||||
| Not classified | 49 | 2.80 | 1.4 to 5.6 | 55 | 2.44 | 0.8 to 7.1 | |||||||||
| All survivors (n=1114) | Survivors physically inactive before diagnosis (n=427) | ||||||||||||||
| Associated with physical inactivity | Inactive | Physically inactive (n=427) vs. active (n=687) | Inactive | Currently physically inactive (n=350) vs. active (n=77) | |||||||||||
| Education | |||||||||||||||
| Professional/graduate degree | 26 | Reference | <0.0001 | Reference | <0.0001 | 75 | Reference | 0.007 | Reference | 0.003 | |||||
| University or college | 33 | 1.43 | 1.0 to 2.1 | 1.37 | 0.9 to 2.0 | 75 | 1.0 | 0.5 to 2.1 | 1.37 | 0.9 to 2.0 | |||||
| High school | 45 | 2.36 | 1.6 to 3.4 | 2.22 | 1.5 to 3.3 | 87 | 2.10 | 1.0 to 4.6 | 2.42 | 1.1 to 5.4 | |||||
| <High School | 63 | 4.92 | 2.8 to 8.5 | 4.05 | 2.3 to 7.2 | 94 | 5.09 | 0.5 to 2.1 | 5.89 | 1.5 to 22 | |||||
| Household Income | |||||||||||||||
| High | 27 | Reference | <0.0001 | 76 | Reference | 0.15 | |||||||||
| Moderate | 38 | 1.66 | 1.2 to 2.4 | 76 | 1.05 | 0.5 to 2.1 | |||||||||
| Low | 40 | 1.86 | 1.3 to 2.6 | 84 | 1.69 | 0.8 to 3.5 | |||||||||
| Prefer not to answer | 48 | 2.49 | 1.8 to 3.5 | 86 | 1.95 | 1.0 to 3.9 | |||||||||
| Occupation | |||||||||||||||
| Professionals | 32 | Reference | <0.0001 | 74 | Reference | 0.08 | |||||||||
| Managers | 27 | 0.77 | 0.5 to 1.2 | 84 | 1.94 | 0.7 to 5.4 | |||||||||
| Technicians | 44 | 1.68 | 1.2 to 2.4 | 87 | 2.45 | 1.2 to 5.1 | |||||||||
| Service and sales | 44 | 1.68 | 1.1 to 2.5 | 81 | 1.57 | 0.7 to 3.4 | |||||||||
| Manual occupations | 53 | 2.36 | 1.6 to 3.6 | 89 | 2.78 | 1.2 to 6.7 | |||||||||
| Not classified | 38 | 1.31 | 0.8 to 2.1 | 85 | 2.09 | 0.8 to 5.8 | |||||||||
For smoking, adjusted for age at diagnosis and cancer site; for physical inactivity, adjusted for age and ethnicity.
For smoking, adjusted for time since diagnosis; for physical inactivity, adjusted for sex. For smoking, other non–socioeconomic status variables considered were sex, age, ethnicity, and body mass index. For physical inactivity, other non–socioeconomic status variables considered were age, ethnicity, time since diagnosis, body mass index, cancer site, cancer extent, Eastern Cooperative Oncology Group performance status, health in the preceding month, smoking status, years smoked, and number of cigarettes smoked 1 year before diagnosis.
Significant values shown in boldface type.
OR = odds ratio; CI = confidence interval; aOR = adjusted odds ratio.
Among participants who were smokers before diagnosis (n = 182), 43% indicated that they were still smoking at the time of questionnaire completion. The highest rates of continued smoking after diagnosis were found for participants with gynecologic (59% of all baseline smokers) and genitourinary (55%) cancers; the lowest rates were found for participants with lung (27%) and hematologic (23%) cancers. After adjusting for time since diagnosis (p = 0.035), the final multivariable model found that education level was the socioeconomic factor most closely associated with continued smoking after a cancer diagnosis. Compared with participants having a graduate-or professional-level education, those with only a high school education and those who did not graduate from high school were, respectively, 2.0 and 3.5 times as likely to continue smoking (p = 0.03, Table iii).
Socioeconomic Factors Associated with Physical Inactivity Before and After Diagnosis
Of 1106 participants analyzed for pa, 39% reported being physically inactive 1 year before diagnosis (Figure 1). Table iii outlines the socioeconomic factors associated with physical inactivity before and after diagnosis. Education was the only socioeconomic variable that remained significant in the multivariable model; compared with participants having a graduate or professional education, those with a high school education or a less than high school education were, respectively, 2.2 and 4.1 times as likely to be inactive (Table iv). Older age (p < 0.0001) and non-white ethnicity (adjusted odds ratio: 1.89; 95% confidence interval: 1.36 to 2.62; p = 0.0002) also remained in the model.
TABLE IV.
Socioeconomic factors associated with perceptions and barriers to physical activity (PA) among cancer survivors
| Factor | Comparator | Perception: “What effect does PA have ...” | Potential barriers: “It is difficult to spend more time performing PA ...” | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||
| On fatigue [no effect or worsens (n=263) vs. lessens (n=830)] | On quality of life [no effect or worsens (n=102) vs. improves (n=1030)] | On 5-year survival [no effect or worsens (n=124) vs. improves (n=971)] | Because of uncertainty about what to do [yes (n=104) vs. no (n=991)] | Because of lack of access to gym or equipment [yes (n=224) vs. no (n=873)] | ||||||||||||
|
|
|
|
|
|
||||||||||||
| OR | 95% CI | p Value a | OR | 95% CI | p Valuea | OR | 95% CI | p Valuea | OR | 95% CI | p Valuea | OR | 95% CI | p Valuea | ||
| Education | ||||||||||||||||
| Professional/Masters/PhD | Reference | 0.0001 | Reference | 0.0001 | Reference | 0.11 | Reference | 0.006 | Reference | 0.92 | ||||||
| University or college | 1.55 | 1.0 to 2.4 | 1.33 | 0.7 to 2.7 | 0.96 | 0.6 to 1.7 | 1.18 | 0.7 to 2.2 | 0.95 | 0.6 to 1.4 | ||||||
| High school | 1.77 | 1.1 to 2.8 | 2.55 | 1.3 to 5.1 | 1.29 | 0.7 to 2.2 | 0.95 | 0.5 to 1.8 | 1.07 | 0.7 to 1.6 | ||||||
| <High school | 3.98 | 2.2 to 7.3 | 4.75 | 2.1 to 11 | 2.10 | 1.0 to 4.5 | 3.02 | 1.4 to 6.4 | 0.93 | 0.5 to 1.8 | ||||||
| Household income | ||||||||||||||||
| High | Reference | <0.0001 | Reference | <0.0001 | Reference | 0.03 | Reference | 0.40 | Reference | 0.29 | ||||||
| Moderate | 1.45 | 0.9 to 2.2 | 2.63 | 1.1 to 6.0 | 0.86 | 0.5 to 1.6 | 1.56 | 0.9 to 2.8 | 0.95 | 0.6 to 1.5 | ||||||
| Low | 2.48 | 1.7 to 3.7 | 5.32 | 2.5 to 9.8 | 1.81 | 1.1 to 3.0 | 1.58 | 0.9 to 2.9 | 1.38 | 0.9 to 2.1 | ||||||
| Prefer not to answer | 1.81 | 1.2 to 2.7 | 4.58 | 2.2 to 9.8 | 1.47 | 0.9 to 2.5 | 1.33 | 0.7 to 2.4 | 1.18 | 0.8 to 1.8 | ||||||
| Occupation | ||||||||||||||||
| Professionals | Reference | 0.002 | Reference | <0.0001 | Reference | 0.16 | Reference | 0.59 | Reference | 0.79 | ||||||
| Managers | 1.03 | 0.6 to 1.7 | 1.17 | 0.5 to 2.8 | 0.67 | 0.3 to 1.5 | 0.85 | 0.4 to 1.8 | 0.78 | 0.5 to 1.3 | ||||||
| Technicians | 1.84 | 1.2 to 2.7 | 2.21 | 1.2 to 4.2 | 1.23 | 0.7 to 2.1 | 0.93 | 0.5 to 1.7 | 0.91 | 0.6 to 1.4 | ||||||
| Service and sales | 1.83 | 1.2 to 2.9 | 2.42 | 1.2 to 4.8 | 1.49 | 0.8 to 2.7 | 1.60 | 0.9 to 2.9 | 1.23 | 0.8 to 2.0 | ||||||
| Manual occupations | 2.17 | 1.4 to 3.4 | 4.44 | 2.4 to 8.3 | 1.76 | 1.0 to 3.2 | 1.16 | 0.6 to 2.3 | 1.05 | 0.6 to 1.7 | ||||||
| Not classified | 1.24 | 0.7 to 2.2 | 1.10 | 0.4 to 3.0 | 0.77 | 0.3 to 1.8 | 1.30 | 0.6 to 2.7 | 1.05 | 0.6 to 1.8 | ||||||
Significant values shown in boldface type.
Although 31% of all patients were physically inactive at the time of questionnaire completion (Figure 1), the percentage of those who remained inactive among those who had been physically inactive before diagnosis (n = 430) was 82%. Lower education level (p = 0.003) and male sex (adjusted odds ratio: 2.39; 95% confidence interval: 1.39 to 4.12; p = 0.002) were factors associated with continued physical inactivity after diagnosis. Compared with participants having a graduate or professional degree, those who had a high school education and who did not complete high school were, respectively, 2.4 and 5.9 times as likely to remain inactive after diagnosis (Table iii).
SES, Barriers, and Perceptions
We explored relationships between the socioeconomic indicators and the perceptions of participants about pa and smoking. Most participants believed that pa could lessen fatigue, improve quality of life, and improve their chance of survival in 5 years. Those with higher education levels, household incomes, and levels of occupation were more likely to indicate that pa would lessen fatigue and improve quality of life (Table iv).
We also explored associations between the socioeconomic variables and potential barriers to pa. Specifically, 9% of participants reported that “being unsure of what to do” was a barrier to pa. Patients were more likely to report this barrier if their education level was lower (p = 0.006); however, household income (p = 0.40) and occupation (p = 0.59) were not significant predictors of that perception (Table iv).
A parallel analysis in relation to smoking perceptions was also conducted. No significant relationships were observed between socioeconomic factors and perceptions about smoking and its effect on fatigue, quality of life, or the chance of survival in 5 years (Table v).
TABLE V.
Socioeconomic factors associated with smoking perceptions among cancer survivors
| Factor | Comparator | Perception: “What effect does smoking have ...” | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| On fatigue [has no effect or makes better (n= 171) vs. makes worse (n =377)] | On quality of life [has no effect or makes better (n= 140) vs. makes worse (n= 422)] | On 5-year survival [has no effect or makes better (n=161) vs. makes worse (n= 386)] | ||||||||
|
|
|
|
||||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Education | ||||||||||
| Professional/Masters/PhD | Reference | 0.53 | Reference | 0.68 | Reference | 0.91 | ||||
| University or college | 1.00 | 0.6 to 1.7 | 1.04 | 0.6 to 1.8 | 0.94 | 0.6 to 1.6 | ||||
| High school | 1.33 | 0.8 to 2.2 | 0.83 | 0.3 to 2.1 | 1.11 | 0.7 to 1.9 | ||||
| <High school | 1.02 | 0.4 to 2.4 | 1.04 | 0.6 to 1.8 | 1.01 | 0.4 to 2.4 | ||||
| Household income | ||||||||||
| High | Reference | 0.46 | Reference | 0.66 | Reference | 0.51 | ||||
| Moderate | 0.95 | 0.6 to 1.6 | 0.82 | 0.5 to 1.5 | 1.15 | 0.7 to 2.0 | ||||
| Low | 0.97 | 0.6 to 1.6 | 1.02 | 0.6 to 1.8 | 1.03 | 0.6 to 1.8 | ||||
| Prefer not to answer | 1.36 | 0.8 to 2.2 | 1.19 | 0.7 to 2.0 | 1.42 | 0.9 to 2.4 | ||||
| Occupation | ||||||||||
| Professionals | Reference | 0.66 | Reference | 0.97 | Reference | 0.82 | ||||
| Managers | 0.86 | 0.5 to 1.6 | 0.90 | 0.5 to 1.7 | 0.82 | 0.4 to 1.5 | ||||
| Technicians | 0.99 | 0.6 to 1.& | 1.07 | 0.6 to 1.9 | 0.88 | 0.5 to 1.5 | ||||
| Service and sales | 0.93 | 0.5 to 1.7 | 1.28 | 0.7 to 2.4 | 1.05 | 0.6 to 1.9 | ||||
| Manual occupations | 1.46 | 0.8 to 2.6 | 1.08 | 0.6 to 2.0 | 1.35 | 0.8 to 2.4 | ||||
| Not classified | 1.37 | 0.7 to 2.8 | 1.09 | 0.5 to 2.4 | 0.93 | 0.4 to 2.0 | ||||
DISCUSSION
The promotion of smoking cessation and pa has become a priority for health care practitioners managing cancer survivors. In the present report, we examined a broad cross-section of cancer patients representing various disease sites and cancer progression statuses. The rates of continued smoking (43%) and continued physical inactivity (82%) were similar to those reported in earlier studies in mixed cancer populations13,14,16,33.
Our results suggest that, although other socioeconomic variables such as household income might be associated with lifestyle behaviour at diagnosis, education was the strongest independent predictor of continued smoking and continued physical inactivity after a cancer diagnosis. Those findings are consistent with significant results16,34–36 and nonsignificant trends37 previously reported in studies that did not examine all 3 socioeconomic indicators. This relationship between education and post-diagnosis behaviour modification could also be reflective of a general phenomenon among patients with chronic disease38. Our observations that younger age and lower income are associated with baseline smoking is reflective of known associations in the general population26,39. Similarly, older, more educated, and non-white patients are more likely to be physically inactive39,40.
Although lower education was a strong predictor of unhealthy lifestyle behaviours at follow-up, household income and occupation did not reach significance. Those 3 indicators, albeit related, are not necessarily redundant predictors of key health outcomes; and although most studies examining lifestyle behaviours will adjust for ses, the specific indicator or indicators included in the analyses are not consistent across reports. However, the most influential socioeconomic indicators might vary by country and centre. Canadian patients might experience less of a financial burden related to their cancer than do American patients41, and that difference could partly account for household income appearing, in our study, to be less important in predicting behaviour change after diagnosis.
We found that patients with greater education and household income, and a higher occupation level, were more likely to appreciate the potential benefits of pa on their fatigue and quality of life; however, that association was not apparent for perceptions about smoking. Individuals of a lower ses might engage in detrimental health behaviours because of a lack of knowledge or incorrect beliefs about the associated health risks42, but that relationship might be mediated by other factors as well43. The specific mechanisms are particularly important to determine when developing interventions aimed at promoting smoking cessation and improvement in exercise habits among cancer survivors of various socioeconomic levels. Thus far, several education-based interventions such as telephone counselling44, motivational interviewing45, and an oncologist’s recommendation46 have proved successful for encouraging pa in cancer patients, and further research on those types of approaches should consider examining whether they are equally effective among less educated participants. In contrast, many counselling approaches to smoking cessation have been less effective47,48, suggesting that additional mechanisms could play important roles.
The foregoing results can also be interpreted in the context of established models for behaviour modification. The Health Action Process Approach and the Health Belief Model emphasize how an individual’s personal barriers and health perceptions influence their ability to modify behaviour28,29, and our data suggest that ses could be an additional secondary mediator from that perspective. However, when considering the broader framework of a socio-ecological model49, our findings could further reflect the importance of community or institutional factors in the cancer population, because those factors can be associated with ses. In addition to considering variables at the individual level, future research should explore whether community factors such a housing, access to facilities, workplaces, and local health resources influence the behaviour patterns of cancer survivors.
Socioeconomic disparities in cancer survival still exist in universal health care systems50,51. Given the present work, it might be worth determining whether health behaviours contribute to those disparities and whether education is a significant predictor of survival. Thus far, most Canadian studies considering cancer survival have used income as the primary ses indicator and have not incorporated smoking or physical inactivity as mediating factors50,52. Education differences in cancer survival have been identified in the United States53 and continental Europe51, but could also exist in Canada and the United Kingdom.
Factors that might limit the generalizability of our work include its single-centre focus, with a sample population skewed toward more socioeconomically advantaged individuals. Ideally, studies examining the importance of ses in health outcomes should incorporate population-level data, which are not currently available in Ontario for the pre-and post-diagnosis smoking and pa outcomes that we examined. To capture a large and broad cross-section of cancer patients at our centre, we opted to administer a single questionnaire that elicited outcomes at both time points. That approach introduces a concern relating to recall bias, and it is unclear whether the reports of smoking and pa outcomes overestimated or underestimated the true values. Studies addressing the issue have found that recall of past pa behaviour up to 30 years into the past is generally reliable, but accuracy of recall for the rigour of the activity can vary54–56. An analogous study examining recall in smoking data indicated that, although 20-year recall was reliable for smoking status, recollection of smoking amounts were less reliable57. That background considered, we attempted to minimize bias in our study by only considering broad outcome measures such as smoking versus not smoking, and physically inactive versus active.
CONCLUSIONS
Our results suggest that, at a comprehensive cancer centre in a universal health care system, the population shows socioeconomic disparities with respect to smoking and levels of physical inactivity. Specifically, cancer patients with less education are more likely to continue smoking and to remain physically inactive after diagnosis. Health care providers should take those factors into consideration when designing survivorship care programs.
ACKNOWLEDGMENTS
This study was funded by Cancer Care Ontario, the Alan Brown Chair, the Lusi Wong Lung Cancer Early Detection Research Fund, the Posluns Family Foundation, the Comprehensive Research Experience for Medical Students (University of Toronto), and the Ontario Patient Reported Outcomes of Symptoms and Toxicity Research Unit.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Secretan B, Straif K, Baan R, et al. on behalf of the who International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–4. doi: 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 2.Chelghoum Y, Danaila C, Belhabri A, et al. Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002;13:1621–7. doi: 10.1093/annonc/mdf269. [DOI] [PubMed] [Google Scholar]
- 3.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2427–35. doi: 10.1158/1055-9965.EPI-09-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. J Natl Cancer Inst. 1997;89:1782–8. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 5.Rowland C, Eiser C, Rowe R, Danson S. The effect of smoking on health-related quality of life in lung cancer patients : a systematic review. BMJ Support Palliat Care. 2012;2:312–18. doi: 10.1136/bmjspcare-2011-000186. [DOI] [PubMed] [Google Scholar]
- 6.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–63. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 7.Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–55. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips SM, McAuley E. Physical activity and quality of life in breast cancer survivors: the role of self-efficacy and health status. Psychooncology. 2014;23:27–34. doi: 10.1002/pon.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson T, Mock V. Exercise as an intervention for cancer-related fatigue. Phys Ther. 2004;84:736–43. [PubMed] [Google Scholar]
- 11.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–32. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–30. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg CJ, Thomas AN, Mertens AC, et al. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology. 2013;22:799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke L, Miller LA, Saad A, Abraham J. Smoking behaviors among cancer survivors: an observational clinical study. J Oncol Pract. 2009;5:6–9. doi: 10.1200/JOP.0912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard CM, Denniston MM, Baker F, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27:246–56. doi: 10.5993/AJHB.27.3.6. [DOI] [PubMed] [Google Scholar]
- 16.Gjerset GM, Fosså SD, Courneya KS, Skovlund E, Thorsen L. Exercise behavior in cancer survivors and associated factors. J Cancer Surviv. 2011;5:35–43. doi: 10.1007/s11764-010-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hymowitz N, Cummings KM, Hyland A, Lynne WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashigar A, Habbous S, Eng L, et al. Social environment, secondary smoking exposure, and smoking cessation among head and neck cancer patients. Cancer. 2013;119:2701–9. doi: 10.1002/cncr.28088. [DOI] [PubMed] [Google Scholar]
- 19.Eng L, Su J, Qiu X, et al. Second-hand smoke as a predictor of smoking cessation among lung cancer survivors. J Clin Oncol. 2014;32:564–70. doi: 10.1200/JCO.2013.50.9695. [DOI] [PubMed] [Google Scholar]
- 20.Simmons VN, Litvin EB, Jacobsen PB, et al. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119:1420–7. doi: 10.1002/cncr.27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120:2174–82. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11:389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 23.Park CL, Edmondson D, Fenster JR, Blank TO. Positive and negative health behavior changes in cancer survivors: a stress and coping perspective. J Health Psychol. 2008;13:1198–206. doi: 10.1177/1359105308095978. [DOI] [PubMed] [Google Scholar]
- 24.Rabin C, Pinto B. Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psychooncology. 2006;15:701–12. doi: 10.1002/pon.1000. [DOI] [PubMed] [Google Scholar]
- 25.Smith SK, Herndon JE, Lyerly HK, et al. Correlates of quality of life–related outcomes in breast cancer patients participating in the Pathfinders pilot study. Psychooncology. 2011;564:559–64. doi: 10.1002/pon.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corsi DJ, Subramanian SV, Lear SA, Chow CK, Teo KK, Boyle MH. Co-variation in dimensions of smoking behavior: a multivariate analysis of individuals and communities in Canada. Health Place. 2013;22:29–37. doi: 10.1016/j.healthplace.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Merritt RK, Heath GW, et al. Physical activity behaviors in lower and higher socioeconomic status populations. Am J Epidemiol. 1991;133:1246–56. doi: 10.1093/oxfordjournals.aje.a115836. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (hapa) Rehabil Psychol. 2011;56:161–70. doi: 10.1037/a0024509. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–83. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 30.Geyer S, Hemström O, Peter R, Vågerö D. Education, income, and occupational class cannot be used interchangeably in social epidemiology. Empirical evidence against a common practice. J Epidemiol Community Health. 2006;60:804–10. doi: 10.1136/jech.2005.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 32.Obesity: preventing and managing the global epidemic. Report of a who consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 33.Blanchard CM, Courneya KS, Stein K, on behalf of the American Cancer Society ’s scs-ii Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s scs-ii. J Clin Oncol. 2008;26:2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 34.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol. 2002;20:1608–16. doi: 10.1200/JCO.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 35.Allison P. Factors associated with smoking and alcohol consumption following treatment for head and neck cancer. Oral Oncol. 2001;37:513–20. doi: 10.1016/S1368-8375(01)00015-X. [DOI] [PubMed] [Google Scholar]
- 36.Hong S, Bardwell WA, Natarajan L, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (whel) Study. Breast Cancer Res Treat. 2007;101:225–32. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 37.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–8. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 38.Margolis R. Educational differences in healthy behavior changes and adherence among middle-aged Americans. J Health Soc Behav. 2013;54:353–68. doi: 10.1177/0022146513489312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matheson FI, Dunn JR, Smith KL, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. 2012;103(suppl 2):S12–16. doi: 10.1007/BF03403823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith P, Frank J, Mustard C. Trends in educational inequalities in smoking and physical activity in Canada: 1974–2005. J Epidemiol Community Health. 2009;63:317–23. doi: 10.1136/jech.2008.078204. [DOI] [PubMed] [Google Scholar]
- 41.Longo CJ, Deber R, Fitch M, Williams AP, D’Souza D. An examination of cancer patients’ monthly “out-of-pocket” costs in Ontario, Canada. Eur J Cancer Care (Engl) 2007;16:500–7. doi: 10.1111/j.1365-2354.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 42.Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Heal. 2003;57:440–3. doi: 10.1136/jech.57.6.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–70. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Heal Psychol. 2013;32:616–26. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 45.Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56:18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Jones LW, Courneya KS, Fairey AS, Mackey JR. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28:105–13. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 47.Schnoll RA, Zhang B, Rue M, et al. Brief physician-initiated quit-smoking strategies for clinical oncology settings: a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21:355–65. doi: 10.1200/JCO.2003.04.122. [DOI] [PubMed] [Google Scholar]
- 48.Nayan S, Gupta MK, Strychowsky JE, Sommer DD. Smoking cessation interventions and cessation rates in the oncology population: an updated systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2013;149:200–11. doi: 10.1177/0194599813490886. [DOI] [PubMed] [Google Scholar]
- 49.Crosby RA, Salazar LF, Diclemente RJ. Ecological approaches in the new public health. In: Diclemente RJ, Salazar LF, Crosby RA, editors. Health Behavior Theory for Public Health: Principles, Foundations, and Applications. Burlington, MA: Jones and Bartlett Learning; 2013. pp. 231–52. [Google Scholar]
- 50.Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15:1680–9. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]
- 51.Van der Heyden JH, Schaap MM, Kunst AE, et al. Socioeconomic inequalities in lung cancer mortality in 16 European populations. Lung Cancer. 2009;63:322–30. doi: 10.1016/j.lungcan.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer. 2010;116:4160–7. doi: 10.1002/cncr.25427. [DOI] [PubMed] [Google Scholar]
- 53.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 54.Blair SN, Dowda M, Pate RR, et al. Reliability of long-term recall of participation in physical activity by middle-aged men and women. Am J Epidemiol. 1991;133:266–75. doi: 10.1093/oxfordjournals.aje.a115871. [DOI] [PubMed] [Google Scholar]
- 55.Falkner K, Trevisan M, McCann S. Reliability of recall of physical activity in the distant past. Am J Epidemiol. 1999;150:195–205. doi: 10.1093/oxfordjournals.aje.a009980. [DOI] [PubMed] [Google Scholar]
- 56.Slattery ML, Jacobs DR., Jr Assessment of ability to recall physical activity of several years ago. Ann Epidemiol. 1995;5:292–6. doi: 10.1016/1047-2797(94)00095-B. [DOI] [PubMed] [Google Scholar]
- 57.Krall EA, Valadian I, Dwyer JT, Gardner J. Accuracy of recalled smoking data. Am J Public Health. 1989;79:200–2. doi: 10.2105/AJPH.79.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]