Abstract
Background
There is wide variation in the application of adjuvant chemotherapy in early-stage epithelial ovarian cancer. Our aim was to assess differences in health-related quality of life (hrqol) between patients with early-stage ovarian cancer who did or did not receive chemotherapy as adjuvant treatment.
Methods
All patients diagnosed with early-stage ovarian cancer between 2000 and 2010 within the population-based Eindhoven Cancer Registry (n = 191) were enrolled in this study. Patients were requested to complete questionnaires, including the cancer-specific (qlq-C30) and ovarian cancer-specific (qlq-OV28) quality of life measures from the European Organisation for Research and Treatment of Cancer. Primary outcome measures were the generic-and cancer-specific domain scores for hrqol in ovarian cancer survivors.
Results
Of the 107 patients (56%) who returned the questionnaires, 57 (53.3%) had received adjuvant chemotherapy and 50 (46.7%) had been treated with surgery alone. Significant differences in hrqol between those groups were found in the symptom scales for peripheral neuropathy, attitude toward sickness, and financial situation, with worse scores in the chemotherapy group.
Conclusions
Results of our study show that patients who receive adjuvant chemotherapy have a significantly worse score for 3 aspects of hrqol. Efforts should be made to reduce use of adjuvant chemotherapy in early-stage ovarian cancer. Moreover, preventive strategies to improve long-term quality of life for those who need adjuvant chemotherapy should be explored.
Keywords: Ovarian cancer, early-stage disease, chemotherapy, neuropathy, health-related quality of life, peripheral neuropathy
INTRODUCTION
Ovarian cancer is the most common cause of death among women with gynecologic malignancies1. Most women are diagnosed with advanced-stage disease and have a poor prognosis, with a 20%–60% 5-year survival rate being reported2. Approximately 25%–30% of women are diagnosed with early-stage disease, either confined to the ovary or confined to the pelvis, and experience a significantly better 5-year survival rate of 75%–100%3.
When patients are adequately staged, most patients with International Federation of Gynecology and Obstetrics (figo) stage i–iia ovarian cancer do not need adjuvant chemotherapy, because prognosis is not thereby improved4,5. Yet, if the tumour is clinically confined to the ovary and adequate surgical staging is omitted, chemotherapy has been proved to increase both overall and recurrence-free survival6–8. According to a recently updated Cochrane review, it remains uncertain whether an equal benefit from adjuvant chemotherapy will be achieved for women with low-and intermediate-risk early-stage disease as for women with high-risk disease9. Currently, the percentage of adequate staging in early-stage ovarian cancer varies widely (24%–60%) and is influenced by the type of hospital and collaboration with referral centres10–12. Even in the action trial, only 34% of all patients were optimally staged5. That wide variation in the adequacy of staging procedures will automatically result in broadly varying application of adjuvant chemotherapy for patients with apparent early-stage ovarian cancer.
Side effects of the standard chemotherapy regimen for ovarian cancer, carboplatin–paclitaxel, are both short-term and long-term13. Long-term neuropathy is a frequently reported side effect that can adversely affect hrqol in survivors of gynecologic cancer. Chemotherapy-induced peripheral neuropathy is reported in 38% of patients treated with multiple chemotherapeutic regimens14. We observed neuropathy symptoms in 51% of survivors with ovarian cancer of all stages, even up to 12 years after the end of treatment15. Cognitive impairment is a commonly reported late-term side effect of chemotherapy that can interfere with a cancer survivor’s ability to attain professional and social goals14. Surgery and chemotherapy both cause iatrogenic menopause, with subsequent loss of estrogen that can result in sexual dysfunction. Such changes after treatment can have a great impact on the hrqol of survivors. So far, data concerning the long-term effects of chemotherapy and the impact that those effects have on hrqol in survivors of early-stage ovarian cancer are limited.
The aim of the present study was to assess differences in hrqol between patients with early-stage ovarian cancer who did or did not receive adjuvant chemotherapy as primary treatment.
METHODS
Design and Setting
This retrospective cohort study was performed between 1 January 2000 and 1 July 2010 in the Gynecological Oncology Centre South (gocs). The gocs is a collaborating organization for the registration and management of oncologic patients in the southern part of the Netherlands. The gocs comprises 10 collaborating hospitals, including 2 referral hospitals. The collaborating hospitals use a Web-based data registration system to register all patients with gynecologic malignancies within the region. The gocs closely collaborates with the Eindhoven Cancer Registry (ecr). Ethics approval for the study was obtained from a certified Medical Ethics Committee at the Elisabeth-TweeSteden hospital (METC/jv/2011.129).
Patient Accrual
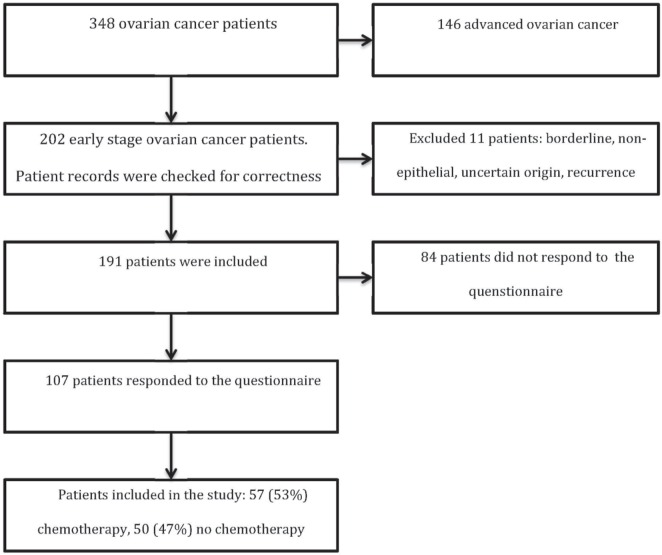
The present study is part of a population-based survey of women with ovarian cancer registered in the ecr. All patients with epithelial ovarian cancer in the gocs region diagnosed between 1 January 2000 and 1 July 2010 were eligible for participation (n = 1147). Deceased patients were excluded by linking the ecr with the Central Bureau for Genealogy. After verification of an early-stage diagnosis (n = 202), 11 more patients were found not to be eligible for the present study. Patients with recurrence (n = 6) and borderline (n = 2) or non-epithelial tumours (n = 2) were excluded, as were patients in whom the origin of the tumour remained uncertain (n = 1). As shown in Figure 1, 191 early-stage ovarian cancer survivors were thus recruited. Those patients were informed about the survey in a letter from their specialist, and 107 responded to the survey questionnaire (56%). Those 107 respondents were included in the study analysis.
FIGURE 1.
Flowchart of data collection.
Data Collection
The ecr collects data for all individuals newly diagnosed with cancer in the southern part of the Netherlands. Standard information was gathered by the Comprehensive Cancer Centre South. The data were collected in 2012 into profiles, a registry for the study of the physical and psychosocial effects of cancer and its treatment, representing a dynamic, growing, population-based cohort of both short-and long-term cancer survivors16. The profiles registry has a large Web-based component (http://www.profilesregistry.nl/) and is linked directly to clinical data from the ecr. Data from the registry are available for non-commercial scientific research, subject to study question, privacy, and confidentiality restrictions and to registration at the Web site.
Detailed information was also collected by one of the investigators (CSB) from patient records and the Web-based data registration system. The information that was extracted included date of diagnosis, patient age, comorbid conditions at diagnosis, dates of recurrence, surgical procedures, findings of the pathologist, and the chemotherapy regimen (the period in which treatment was given, the type of chemotherapy, and the number of cycles). Socioeconomic status was determined by using postal codes to link to socioeconomic status tables from Statistics Netherlands that provide the average household income and housing prices in the particular residential area.
Quality of Life
The survey questionnaires were sent and returned between January 2012 and May 2012. All recruited patients received a cancer-specific questionnaire (qlq-C30) and an ovarian cancer–specific questionnaire (qlq-OV28) from the European Organisation for Research and Treatment of Cancer. The qlq-C30 and the qlq-OV28 module are valid measurements of overall quality of life (qol) for cancer patients and specifically of ovarian cancer patients. All scales are scored in a range from 0 to 100. A high score represents a higher response level. Thus, a high score for a functional scale represents a high or healthy level of functioning, and a high score for the global health status or qol represents high qol; however, a high score on a symptom scale represents a high level of symptomatology or problems.
The Hospital Anxiety and Depression Scale is used to determine the levels of anxiety and depression that a patient is experiencing. Higher scores indicate a greater likelihood of depression or anxiety. Recommended cut offs are 8–10, mild symptoms; 11–15, moderate symptoms; and 16 or more, severe symptoms17.
Outcome
Outcome measurements were defined as the generic and cancer-specific domain scores for hrqol in ovarian cancer survivors. The results of the surveys were compared between the early-stage ovarian cancer group treated with surgery alone and the group treated with surgery and adjuvant chemotherapy.
Statistical Analysis
Continuous outcomes are reported as means ± standard deviation, and categorical outcomes are reported as proportions. Missing data are indicated as “unknown” or “missing.” The analysis was performed using the IBM SPSS Statistics software application (version 21: IBM, Armonk, NY, U.S.A.). Fisher exact tests were used to determine group differences in categorical outcomes, and t-tests were used to compare the means for all indices between the patient groups. The significance of all tests was set at p < 0.05.
RESULTS
Baseline Characteristics
Of the 107 early-stage ovarian cancer survivors enrolled in the study who returned the questionnaire, 57 received adjuvant chemotherapy, and 50 were treated with surgery alone. As Table i shows, the percentage of patients receiving adjuvant chemotherapy was slightly higher in the survey responder group than in the non-responder group (56% vs. 44%, p = 0.059). Table ii presents the clinical and tumour characteristics. We observed no significant difference in mean age and mean survival duration from diagnosis, but there was a significant difference in figo stage between the two groups. The group that did not receive chemotherapy consisted mainly of patients with figo stage ia disease (n = 40); the chemotherapy group contained mostly patients with figo stage ic disease (n = 39, p < 0.05). Tumour grade was also significantly different between the two groups, with overrepresentation of high-grade tumours in the chemotherapy group (p < 0.05).
TABLE I.
Selected patient and tumour characteristics, by survey response status
| Characteristic | Survey responder | p Value | |
|---|---|---|---|
|
| |||
| Yes | No | ||
| Patients [n (%)] | 107 (56.0) | 84 (44.0) | |
| Mean age (years) | 64±11.7 | 62±15.5 | 0.341 |
| Mean time since diagnosis (years) | 6.7±3.4 | 7±3.1 | 0.453 |
| FIGO grade [n (%)] | 0.562 | ||
| IA | 53 (49.5) | 48 (57.1) | |
| IB | 4 (3.7) | 3 (3.6) | |
| IC | 48 (44.9) | 30 (35.7) | |
| IIA | 2 (1.9) | 3 (3.6) | |
| Chemotherapy [n (%)] | 0.059 | ||
| Yes | 57 (53.3) | 33 (39.3) | |
| No | 50 (46.7) | 51 (60.7) | |
FIGO = International Federation of Gynecology and Obstetrics.
TABLE II.
Selected patient and tumour characteristics for responders, by chemotherapy receipt
| Characteristic | Chemotherapy | p Valuea | |
|---|---|---|---|
|
| |||
| Yes | No | ||
| Patients [n (%)] | 57 (53.3) | 50 (46.7) | |
| Mean age (years) | 65±9.1 | 62±14.3 | 0.303 |
| Mean time since diagnosis (years) | 6.8±3.4 | 6.6±3.4 | 0.722 |
| FIGO stage [n (%)] | <0.001 | ||
| IA | 13 (22.8) | 40 (80.0) | |
| IB | 3 (5.3) | 1 (2.0) | |
| IC | 39 (68.4) | 9 (18.0) | |
| IIA | 2 (3.5) | ||
| Histology | 0.007 | ||
| Serous carcinoma | 18 (31.6) | 13 (26.0) | |
| Mucinous carcinoma | 6 (10.5) | 16 (32.0) | |
| Endometrioid carcinoma | 14 (24.6) | 12 (24.0) | |
| Clear cell carcinoma | 11 (19.3) | 1 (2.0) | |
| Adenocarcinoma | 7 (12.3) | 5 (10.0) | |
| Other | 1 (1.8) | 3 (6.0) | |
| Grade | 0.002 | ||
| I | 7 (12.3) | 19 (38.0) | |
| II | 15 (26.3) | 15 (30.0) | |
| III | 19 (33.3) | 3 (6.0) | |
| Unknown | 16 (28.1) | 13 (26.0) | |
| Comorbidities | 0.772 | ||
| 0 | 17 (29.8) | 15 (30.0) | |
| 1 | 15 (26.3) | 9 (18.0) | |
| 2 | 7 (12.3) | 9 (18.0) | |
| 3 | 11 (19.3) | 7 (14.0) | |
| 4 | 3 (5.3) | 3 (6.0) | |
| ≥5 | 3 (5.3) | 5 (10.0) | |
| Unknown | 1 (1.8) | 2 (4.0) | |
| Socioeconomic status | 0.926 | ||
| Low | 13 (22.8) | 12 (24.0) | |
| Middle | 26 (45.6) | 21 (42.0) | |
| High | 16 (28.1) | 14 (28.0) | |
| Unknown | 2 (3.5) | 3 (6.0) | |
| Marital status | 0.692 | ||
| Married | 22 (38.6) | 17 (34.0) | |
| Not married | 35 (61.4) | 32 (64.0) | |
| Unknown | 1 (2.0) | ||
Statistically significant values appear in boldface type.
Table iii presents the chemotherapy specifications. Most patients received a carboplatin–paclitaxel combination, and most (89.5%) received 6 cycles of platinum-based chemotherapy.
TABLE III.
Chemotherapy characteristics in 57 patients
| Characteristic | Value |
|---|---|
| Chemotherapy type [n (%)] | |
| Carboplatin–paclitaxel | 54 (94.8) |
| Cisplatin–paclitaxel | 1 (1.8) |
| Cisplatin–etoposide | 1 (1.8) |
| Cyclophosphamide–carboplatin | 1 (1.8) |
| Cycles [n (%)] | |
| 3 | 1 (1.8) |
| 4 | 1 (1.8) |
| 5 | 1 (1.8) |
| 6 | 51 (89.5) |
| 7 | 1 (1.8) |
| Unknown | 2 (3.5) |
| Early stop [n (%)] | |
| Yes | 3 (5.3) |
| No | 54 (94.7) |
| Time since first chemotherapy cycle | |
| Mean (years) | 7.9±3.4 |
| Range (years) | 3–13 |
| Missing [n (%)] | 2 (3.1) |
| Time since last chemotherapy cycle | |
| Mean (years) | 7.7±3.4 |
| Range (years) | 2–13 |
| Missing [n (%)] | 29 (51) |
Quality of Life
Table iv shows baseline hrqol scores in the two groups for the selected dimensions of the qlq-C30 and qlq-OV28 instruments. Of the qlq-C30 functional scales, role functioning showed the lowest mean score in both the adjuvant chemotherapy group and the non-chemotherapy group: 79.5 and 76.5 respectively. In both groups, the highest mean scores were recorded for social functioning: 87.6 and 92.5 respectively. On the qlq-C30 symptom scales, most mean scores were very low; high mean scores were recorded for the insomnia, pain, and fatigue scales. Of the qlq-OV28 symptom scales, attitude toward sickness, sexuality, and peripheral neuropathy showed the highest median scores. In most dimensions, including global qol, mean scores were not significantly different between the chemotherapy group and the non-chemotherapy group. The only dimensions that showed a significant difference were financial situation (p = 0.004), attitude towards sickness (p < 0.05), and peripheral neuropathy (p < 0.05).
TABLE IV.
Outcome of quality-of-life questionnairesa
| Variable | Mean score with standard deviation | p Valueb | |
|---|---|---|---|
|
| |||
| Chemotherapy (n=57) | No chemotherapy (n=50) | ||
| General quality of life | 76.7±16.9 | 78.2±18.0 | 0.650 |
| EORTC QLQ-C30 function scales | |||
| Physical | 80.3±22.8 | 79.2±24.7 | 0.800 |
| Role | 79.5±27.5 | 76.5±30.0 | 0.603 |
| Emotional | 84.1±22.2 | 85.5±15.6 | 0.715 |
| Cognitive | 85.5±22.7 | 89.3±19.0 | 0.347 |
| Social | 87.6±19.3 | 92.6±14.0 | 0.111 |
| EORTC QLQ-C30 symptom scales | |||
| Fatigue | 24.2±22.6 | 19.5±23.6 | 0.299 |
| Nausea/vomiting | 2.7±7.7 | 3.7±10.4 | 0.569 |
| Pain | 24.7±29.6 | 24.7±30.7 | 0.995 |
| Dyspnea | 10.3±21.2 | 15.6±28.9 | 0.290 |
| Insomnia | 25.5±30.7 | 25.0±28.8 | 0.939 |
| Appetite loss | 5.5±12.4 | 5.4±18.4 | 0.997 |
| Constipation | 13.3±23.7 | 9.5±18.0 | 0.362 |
| Diarrhea | 4.8±16.1 | 3.4±12.3 | 0.631 |
| Financial | 8.5±18.4 | 0.7±4.7 | 0.004 |
| EORTC OV-28 symptom scales or items | |||
| Body image | 16.9±22.5 | 16.7±23.1 | 0.945 |
| Attitude toward sickness | 42.2±24.3 | 28.6±22.5 | 0.004 |
| Sexuality | 21.6±23.1 | 20.3±21.0 | 0.779 |
| Abdominal/gastrointestinal | 14.2±17.5 | 14.6±74.4 | 0.908 |
| Peripheral neuropathy | 26.0±27.6 | 14.1±22.1 | 0.020 |
| Chemotherapy side effects | 20.5±15.1 | 18.5±15.7 | 0.694 |
| Hormonal | 18.6±28.8 | 20.7±27.3 | 0.516 |
| Hair loss | 10.8±20.3 | 6.6±17.8 | 0.271 |
| Hospital Anxiety and Depression Scale | |||
| Anxiety | 5.1±3.7 | 4.5±3.3 | 0.333 |
| Depression | 3.8±3.6 | 3.7±3.5 | 0.329 |
European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-OV28, and the Hospital Anxiety and Depression Scale.
Statistically significant values appear in boldface type.
Table iv also shows scores on the Hospital Anxiety and Depression Scale for both groups. The groups showed no significant differences in depression or anxiety.
DISCUSSION
The present study shows that long-term side effects of chemotherapy in early-stage ovarian cancer negatively affect hrqol, especially with respect to neuropathy, financial situation, and attitude toward sickness. We expected that hrqol would be better for patients who experienced no long-term side effects of chemotherapy, but no significant difference was found in global qol. However, we did observe a significant difference with respect to peripheral neuropathy in women who received adjuvant chemotherapy.
A strength of our study is the high number of ovarian cancer survivors who participated; however, the study is limited by the rate of non-response. Nevertheless, our data show no differences between the responder and non-responder groups in terms of tumour stage and chemotherapy received. Still, selection bias could not be excluded completely. Overall, we succeeded in including about 75% of the patients within the profiles group16. In the present study, the survey materials included an extensive sexuality questionnaire, and partners of the women were also invited to respond, which might have lowered the participation rate.
The findings of the study are supported by Guidozzi18, who observed that the most significant contributing factors to deterioration of qol were peripheral neuropathy, impaired hearing, lack of energy, and financial hardship. Equally, in our own prior study that included all stages of ovarian cancer, we observed that symptoms of neuropathy were experienced by 51% of women and seriously affected their hrqol15. Interesting data from Mirabeau-Beale et al.19 showed a significant difference in fear of cancer recurrence between early-stage and advanced-stage survivors. In addition, those authors observed a significant correlation of lower qol with increased fear of recurrence for early-stage survivors. The findings with respect to financial situation seem to be consistent with results in other studies that observed an equal concern for financial situation in ovarian cancer survivors20. Decreased cognitive and sexual functioning has been reported by others, but could not be confirmed in our data14. That discrepancy might be explained by differences in age and figo stage. Although sexual dysfunction is commonly reported as a long-term side effect of cancer according to earlier studies, responses to questions about sexuality were unfortunately missing in most cases in the present study (85%). Because patients could choose to respond “I don’t want to answer this question,” it is unclear why so many data points are missing. A slight preponderance of patients (55.8%) reported not being sexually active at that moment, which might be reflected in a reduced interest in answering the questions.
Given the known toxicity of some chemotherapeutic agents, it is likely that peripheral neuropathy is significantly more common in patients who receive chemotherapy than in those who do not. The observed differences in attitude toward sickness on the part of patients who received adjuvant chemotherapy might be explained by the different course of treatment. Chemotherapy reinforces the awareness of cancer. Additionally, extended therapy—in terms of both length and intensity—might influence the attitude toward sickness. Finally, the significant difference in financial situation scores is probably related to that extended duration of therapy, which could have hampered a return to work for the patients. Extended sick leave might often result in the woman losing her job. Although the social system in the Netherlands is well organized, such factors could at least partly explain the observed results.
The current findings of the effects of chemotherapy warrant a search for alternative treatment options that mitigate the long-term negative effects of adjuvant chemotherapy. A switch of chemotherapy regimens from carboplatin–paclitaxel to pegylated liposomal doxorubicin–carboplatin might reduce the toxicity21. Additionally, a reduction in the number of adjuvant chemotherapy cycles from 6 to 3 in patients with high-risk epithelial ovarian cancer did not result in a higher recurrence rate and was associated with reduced toxicity6. Alternatively, carboplatin monotherapy might be an attractive alternative to reduce the long-term burden of toxicity22.
Proper staging of presumed early-stage ovarian cancer is important starting point, because adjuvant chemotherapy could be withheld in many patients with early-stage disease21,23. Hence, adequate staging, specifically of patients with figo ia and ib disease, could avoid long-term side effects when chemotherapy is withheld in those patients. With respect to patients who, according to the evidence, would benefit from chemotherapy, we have an obligation to increase knowledge about the pathogenesis, prevention, and treatment of peripheral neuropathy and other long-term side effect of chemotherapy.
At the present moment, no agents are recommended for the prevention of chemotherapy-induced peripheral neuropathy. With respect to the treatment of existing chemotherapy-induced peripheral neuropathy, current data support treatment with duloxetine24. Although the evidence to support other treatments is insufficient, a therapeutic trial of gabapentin–pregabalin or a tricyclic antidepressant (nortriptyline or desipramine) seems reasonable, given the limited therapeutic options and the demonstrated efficacy of those drugs for other neuropathic pain conditions22. Patients who receive chemotherapy might need additional psychosocial support to improve their attitude toward sickness. Paying attention to the negative influences and effects of therapies on hrqol, and increasing knowledge in this area, will help clinicians to optimize patient management.
CONCLUSIONS
The present study demonstrates that use of chemotherapy in patients with early-stage ovarian cancer decreases 3 aspects of hrqol. Proper staging of all patients with early-stage ovarian cancer to reduce the use of adjuvant chemotherapy is the first step in mitigating those effects. Preventive and treatment strategies for the negative influences of chemotherapy should be explored to improve long-term qol.
ACKNOWLEDGMENTS
We thank all patients and their doctors for their participation in this study. We also thank the following hospitals for their cooperation: Amphia Hospital, Breda; Catharina Hospital, Eind-hoven; Elkerliek Hospital, Helmond and Deurne; Jeroen Bosch Hospital, Hertogenbosch; Maxima Medical Centre, Eindhoven and Veldhoven; St. Anna Hospital, Geldrop; Elisabeth-TweeSteden Hospital, Tilburg and Waalwijk; VieCuri Hospital, Venlo and Venray; and Instituut Verbeeten, Tilburg.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Sant M, Chirlaque Lopez, MD, Agresti R, et al. on behalf of the eurocare-5 Working Group Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the eurocare-5 study. Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.07.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Oncoline. Epithelial ovarian cancer [Web resource] Integral Cancer Institute.; Utrecht, Netherlands: 2012. Integraal Kankercentrum Nederland. [Available at: http://www.oncoline.nl/ovariumcarcinoom; cited 9 October 2016] [Google Scholar]
- 3.Baldwin LA, Huang B, Miller RW, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012 Sep;120:612–18. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 4.Mutch DG, Prat J. 2014 figo staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133:401–4. doi: 10.1016/j.ygyno.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Trimbos JB, Vergote I, Bolis G, et al. on behalf of the eortc-action collaborators Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer–Adjuvant Chemotherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–25. doi: 10.1093/jnci/95.2.113. [DOI] [PubMed] [Google Scholar]
- 6.Bell J, Brady MF, Young RC, et al. on behalf of the Gynecologic Oncology Group Randomized phase iii trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;102:432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Colombo N, Guthrie D, Chiari S, et al. on behalf of the International Collaborative Ovarian Neoplasm (icon) collaborators International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst. 2003;95:125–32. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]
- 8.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant Chemotherapy in Ovarian Neoplasm trial: two parallel randomized phase iii trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95:105–12. doi: 10.1093/jnci/95.2.105. [DOI] [PubMed] [Google Scholar]
- 9.Lawrie TA, Winter-Roach BA, Heus P, Kitchener HC. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev. 2015:CD004706. doi: 10.1002/14651858.CD004706.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernooij F, Heintz AP, Coebergh JW, Massuger LF, Witteveen PO, van der Graaf Y. Specialized and high-volume care leads to better outcomes of ovarian cancer treatment in the Netherlands. Gynecol Oncol. 2009;112:455–61. doi: 10.1016/j.ygyno.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006;103:383–90. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 12.van Altena AM, van den Akker PA, de Hullu JA, et al. Efficacy of a regional network for ovarian cancer care. Obstet Gynecol. 2013;122:668–75. doi: 10.1097/AOG.0b013e3182a054ee. [DOI] [PubMed] [Google Scholar]
- 13.Parmar MK, Ledermann JA, Colombo N, et al. on behalf of the icon and ago collaborators Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the icon4/ ago-ovar-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/S0140-6736(03)13718-X. [DOI] [PubMed] [Google Scholar]
- 14.Andrews S, von Gruenigen VE. Management of the late effects of treatments for gynecological cancer. Curr Opin Oncol. 2013;25:566–70. doi: 10.1097/CCO.0b013e328363e11a. [DOI] [PubMed] [Google Scholar]
- 15.Ezendam NP, Pijlman B, Bhugwandass C, et al. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based profiles registry. Gynecol Oncol. 2014;135:510–17. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 16.van de Poll-Franse LV, Horevoorts N, van Eenbergen M, et al. The Patient Reported Outcomes Following Initial Treatment and Long Term Evaluation of Survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47:2188–94. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Guidozzi F. Living with ovarian cancer. Gynecol Oncol. 1993;50:202–7. doi: 10.1006/gyno.1993.1193. [DOI] [PubMed] [Google Scholar]
- 19.Mirabeau-Beale KL, Kornblith AB, Penson RT, et al. Comparison of the quality of life of early and advanced stage ovarian cancer survivors. Gynecol Oncol. 2009;114:353–9. doi: 10.1016/j.ygyno.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Howell D, Fitch MI, Deane KA. Impact of ovarian cancer perceived by women. Cancer Nurs. 2003;26:1–9. doi: 10.1097/00002820-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Collinson F, Qian W, Fossati R, et al. on behalf of the icon1 collaborators Optimal treatment of early-stage ovarian cancer. Ann Oncol. 2014;25:1165–71. doi: 10.1093/annonc/mdu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 23.Winter-Roach BA, Kitchener HC, Lawrie TA. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database Syst Rev. 2012:CD004706. doi: 10.1002/14651858.CD004706.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–67. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]