Abstract
Background
Proton pump inhibitors (ppis) are a commonly used medication. A limited number of studies have identified a weak-to-moderate association between ppi use and colorectal cancer (crc) risk, but none to date have identified an effect of ppi use on crc survival. We therefore postulated that an association between ppi use and crc survival might potentially exist.
Methods
We performed a retrospective chart review of 1304 crc patients diagnosed from January 2005 to December 2011 and treated at the Cancer Centre of Southeastern Ontario. Kaplan–Meier analysis and Cox proportional hazards regression models were used to evaluate overall survival (os).
Results
We identified 117 patients (9.0%) who were taking ppis at the time of oncology consult. Those taking a ppi were also more often taking asa or statins (or both) and had a statistically significantly increased rate of cardiac disease. No identifiable difference in tumour characteristics was evident in the two groups, including tumour location, differentiation, lymph node status, and stage. Univariate analysis identified a statistically nonsignificant difference in survival, with those taking a ppi experiencing lesser 1-year (82.1% vs. 86.7%, p = 0.161), 2-year (70.1% vs. 76.8%, p = 0.111), and 5-year os (55.2% vs. 62.9%, p = 0.165). When controlling for patient demographics and tumour characteristics, multivariate Cox regression analysis identified a statistically significant effect of ppi in our patient population (hazard ratio: 1.343; 95% confidence interval: 1.011 to 1.785; p = 0.042).
Conclusions
Our results suggest a potential adverse effect of ppi use on os in crc patients. These results need further evaluation in prospective analyses.
Keywords: Colorectal neoplasms, proton pump inhibitors, mortality
INTRODUCTION
Colorectal cancer (crc) is the 2nd most common cause of cancer-related death in men and the 3rd leading cause of cancer-related death in women in Canada1. Although incidence and overall mortality rates for crc have been declining since the early 1990s, it is estimated that more than 160,000 new cases and 50,000 deaths occur across North America each year1–3. Changes in rates over time have been specifically influenced by the implementation and use of crc screening tests, the development of novel therapeutic regimens for crc, and the identification of modifiable risk factors associated with crc4.
The identification of modifiable risk factors for crc recurrence and survival has been of increasing interest, because crc survivors constitute one of the largest populations of cancer survivors in North America5. Recently, researchers have looked at the relationships between crc and a number of regularly prescribed medications, including nonsteroidal anti-inflammatories, hormone replacement therapies, histamine receptor antagonists, and proton pump inhibitors (ppis)6,7. Identification of cancer-related risks associated with those medications will allow physicians to more effectively determine the necessity and duration of medication use.
Specifically used for the prevention and management of acid-related conditions including gastroesophageal reflux disease, erosive esophagitis, and peptic ulcer disease, ppis are one of the most widely prescribed medications across the globe8,9. The short-term side effects of these drugs have been well studied, with common side effects being nausea, fatigue, and headaches9–11. The side effects are usually mild, self-limiting, and unrelated to dose or age11.
Since the early 2000s, concern has been growing about the overuse of ppis for fairly benign conditions, with a significant focus on the long-term adverse effects of ppi use. Most recently, long-term use of ppis has been linked to increased risk of respiratory infection, Clostridium difficile infection, bone fractures, and the development of various gastrointestinal cancers10,11. A number of studies have recently investigated the relationship between ppi use and crc specifically.
A significant positive correlation was identified between ppi use, hypergastrinemia, and the development and progression of crc12,13. Unfortunately the association between ppi use and crc in humans is much less clear. Case–control studies have produced inconsistent, conflicting results about the relationship between ppi use and crc risk. In a small number of studies, a weak-to-moderate association was identified between ppi use and crc risk; others have found that the use of ppis might not in fact be associated with an increased risk of developing crc13–16. Overall, studies that have examined the relationship between ppi use and crc have been significantly limited by small sample sizes.
Few studies to date have investigated the relationship between ppi use and overall survival (os) in crc patients. To further elucidate the potential effects of ppi use on crc survival, we performed a retrospective chart review to identify associations between ppi use and clinicopathologic features of crc, including tumour location, differentiation, lymph node status, stage, and patient os.
METHODS
Study Design and Study Population
This retrospective cohort study involved a chart review of patients diagnosed with crc; it was approved by the Queen’s University institutional ethics board. We identified all patients more than 18 years of age with a pathologic diagnosis of TNM stages i–iv crc seen from 1 January 2005 to 31 December 2011 at the Cancer Centre of Southeastern Ontario. In total, 1304 patients were identified using International Classification of Diseases version 10 diagnostic codes. Patients whose tumour pathology cases were reviewed at the Cancer Centre of Southeastern Ontario, but who were never seen there, were excluded from the database (n = 58).
Data Collection and Outcomes Measured
Data were collected from baseline (the time of oncology consultation) to the end of the observation period (that is, the last visit or 6 July 2013). Sociodemographic, tumour, radiographic, and chemotherapy treatment details were abstracted from patient charts. Patient information included age at diagnosis, sex, smoking and alcohol status at diagnosis, comorbidities (cardiac, respiratory, renal, and diabetic complications), body mass index status, medication use (asa, statins, and ppis), and family history of crc. Pathology reports were reviewed for tumour characteristics, including location, cell type, differentiation, lymphovascular and perineural invasion status, and T and N staging, including total number of lymph nodes obtained and the number of positive lymph nodes. Dates of death were obtained via the hospital databases and obituaries. The primary outcome was os duration, calculated from the date of crc pathologic diagnosis (whether biopsy or definitive surgery, whichever date came first) to date of death from any cause or to the last visit if still living.
Statistical Analysis
Data were collected in MS Excel (Microsoft Corporation, Redmond, WA, U.S.A.) and were imported into the IBM SPSS Statistics software package (version 22.0 for Windows: IBM, Armonk, NY, U.S.A.) for statistical analysis. The statistical significance of between-cohort differences in categorical variables was tested by chi-square test. Continuous data were compared using the 2-sample t-test (patients using or not using ppis at the time of oncology consultation). All tests were 2-tailed, with significance accepted at p < 0.05.
Kaplan–Meier curves were constructed to compare patients using and not using ppis for days to death or study end. A multivariate Cox proportional hazards regression analysis was undertaken to assess time to death, while controlling for known risk factors, including age, sex, comorbidities (cardiac, diabetes, renal, and respiratory), stage at diagnosis, differentiation (well, poorly, or moderately differentiated), and pathologically positive lymph nodes.
RESULTS
Patient Demographics and Clinical Characteristics
Table i summarizes patient demographics. Among the identified 1304 crc patients, the prevalence of ppis use at the time of diagnosis was 9.0% (117 patients with a mean age of 73.5 years). Men constituted a slightly larger percentage of the study and control groups at 61.5% and 58.3% respectively. Baseline characteristics—including age at diagnosis; sex; average body mass index; and smoking, drinking, and family history—were not significantly different between the patients who did and did not take ppis.
TABLE I.
Demographics of the study patients
| Variable | Taking a PPI at diagnosis | |
|---|---|---|
|
| ||
| Yes | No | |
| Patients (n) | 117 | 1187 |
| Average age (years) | 73.5 | 70.9 |
| Sex [n (%)] | ||
| Men | 72 (61.5) | 692 (58.3) |
| Women | 45 (38.5) | 495 (41.7) |
| Average BMI | 26.99 | 28.02 |
| Comorbidities [n (%)] | ||
| Cardiac | 92 (78.6)a | 681 (57.4) |
| Respiratory | 21 (17.9) | 168 (14.2) |
| Renal | 3 (0.03) | 70 (0.06) |
| Diabetes | 33 (28.2) | 244 (20.6) |
| Smoking history | ||
| Yes | 66 (56.4) | 608 (51.2) |
| No | 51 (43.6) | 579 (48.8) |
| Alcohol history | ||
| Yes | 75 (64.1) | 739 (62.3) |
| No | 42 (35.9) | 448 (37.7) |
| Family history | ||
| Yes | 18 (15.4) | 251 (21.1) |
| No | 99 (84.6) | 936 (78.9) |
| Medications | ||
| ASA | 45 (38.5)a | 229 (19.3) |
| Statins | 45 (38.5)a | 276 (23.3) |
| PPI | 117 (100) | 1187 (100) |
p < 0.05.
PPI = proton pump inhibitor; BMI = body mass index; ASA = acetylsalicylic acid.
Compared with the group that did not take ppis, the group that did take ppis included a larger percentage of individuals with cardiac comorbidities (78.6% vs. 57.4%, p < 0.05). Rates of respiratory, renal, and diabetic comorbidities did not differ significantly between the groups. Use of asa at the time of initial evaluation was significantly higher in the group that took ppis than in the group that did not (38.5% vs. 19.3%, p < 0.05). Similarly, anti-cholesterol therapy at the time of initial evaluation was significantly higher in the group that took ppis than in the group that did not (38.5% vs. 23.3%, p < 0.05).
Tumour characteristics were subsequently compared between the study and control groups (Table ii). We observed no statistically significant differences between the groups with respect to location of the primary tumour, T stage, positive lymph node status, or positivity for lymphovascular or perineural invasion. Additionally, a comparison of the patients taking and not taking ppis showed no differences in any pathologic characteristic analyzed (cell type and differentiation).
TABLE II.
Tumour characteristics in the study cohort
| Variable | Taking a PPI at diagnosis | |
|---|---|---|
|
| ||
| Yes | No | |
| Cell type [n (%)] | ||
| Adenocarcinoma | 99 (86.1) | 1032 (88.7) |
| Mucinous adenocarcinoma | 16 (13.9) | 131 (11.3) |
| Location [n (%)] | ||
| Ascending | 34 (29.6) | 307 (27.3) |
| Transverse | 13 (11.3) | 95 (8.4) |
| Descending | 18 (15.7) | 250 (22.5) |
| Rectal | 50 (43.5) | 473 (42.0) |
| Differentiation [n (%)] | ||
| Well | 5 (4.6) | 43 (3.9) |
| Moderate | 89 (81.7) | 962 (86.7) |
| Poor | 15 (13.8) | 105 (9.5) |
| T Stage [n (%)] | ||
| T1 | 6 (6.5) | 41 (4.4) |
| T2 | 9 (9.8) | 142 (15.4) |
| T3 | 56 (60.9) | 554 (60.0) |
| T4 | 21 (22.8) | 187 (20.2) |
| Staging | ||
| I | 7 (6.0) | 89 (7.5) |
| II | 33 (28.2) | 367 (30.9) |
| III | 52 (44.4) | 432 (36.4) |
| IV | 25 (21.4) | 299 (25.2) |
| Lymph node–positive | 55 (62.5) | 479 (55.1) |
| Lymphovascular invasion–positive | 31 (33.7) | 210 (22.8) |
| Perineural invasion | 6 (6.6) | 74 (8.1) |
PPI = proton pump inhibitor.
PPI Use and Survival Analysis
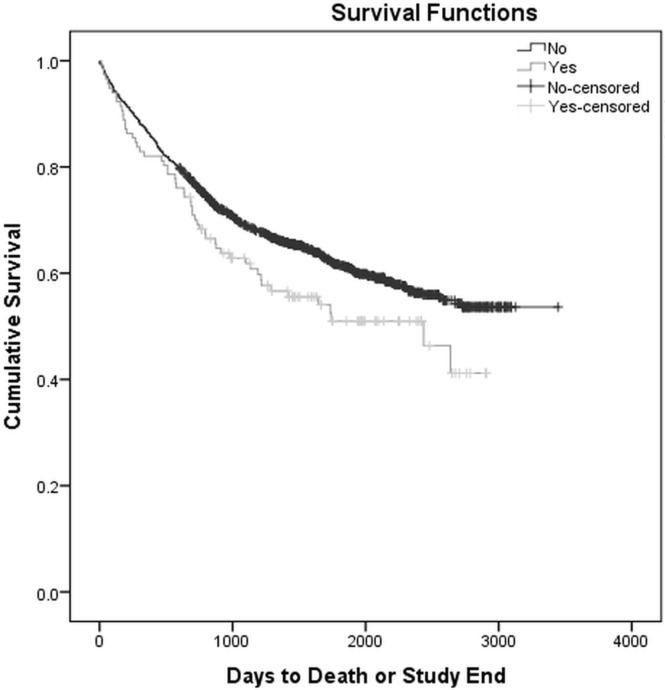
In the overall cohort, 506 deaths (38.8%) occurred. With respect to ppi use, 55 deaths occurred among the 117 patients who took ppis (47.0%) and 451 deaths occurred among the 1187 patients who did not take ppis (38.0%). Univariate analysis (Table iii) comparing patients who were or were not taking ppis at the time of diagnosis showed no statistically significant differences in 1-year os (82.1% vs. 86.7%, p = 0.161), 2-year os (70.1% vs. 76.8%, p = 0.111), or 5-year os (55.2% vs. 62.9%, p = 0.165), although a trend toward increased mortality was evident in the group who took ppis. Kaplan–Meier survival analysis demonstrated that patients taking ppis experienced a significantly shorter cumulative os duration of 1775 days [95% confidence interval (ci): 1557 days to 1993 days]; os duration was 2279 days (95% ci: 2195 days to 2364 days) in patients not taking ppis (p = 0.048, Figure 1).
TABLE III.
Univariate analysis
| Taking a PPI at diagnosis | Deaths [n/N (%)] | Overall survival at ... | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 Year | 2 Years | 5 Years | |||||
|
|
|
|
|||||
| (%) | p Value | (%) | p Value | (%) | p Value | ||
| No | 451/1187 (38.0) | 86.7 | Reference | 76.8 | Reference | 62.9 | Reference |
| Yes | 55/117 (47.0) | 82.1 | 0.161 | 70.1 | 0.111 | 55.2 | 0.165 |
PPI = proton pump inhibitor.
FIGURE 1.
Kaplan–Meier survival curve comparing overall survival for patients with colorectal cancer taking and not taking proton pump inhibitors at the time of diagnosis. Log rank p = 0.048.
Multivariate Cox proportional hazards modelling of all patients (Table iv) further showed that crc patients taking ppis experienced a significantly higher risk of mortality (hr: 1.34; 95% ci: 1.01 to 1.78; p = 0.042). Additionally, the analysis showed that crc patients with comorbidities experienced a higher risk of mortality (cardiac hr: 1.26; 95% ci: 1.03 to 1.55; p = 0.024; renal hr: 1.62; 95% ci: 1.170 to 2.26; p = 0.004; respiratory hr: 1.42; 95% ci: 1.13 to 1.79; p = 0.003). Lastly, tumour stage (hr: 1.97; 95% ci: 1.74 to 2.22; p < 0.001) and tumours with poor cellular differentiation (hr: 1.98; 95% ci: 1.12 to 3.48; p = 0.018) were independent predictive factors for mortality in crc patients.
TABLE IV.
Cox regression model
| Variable | HR | 95% CI | p Valuea |
|---|---|---|---|
| Sex (1=men, 2=women) | 1.01 | 0.85 to 1.20 | 0.927 |
| Age (per year) | 1.01 | 1.00 to 1.02 | 0.034 |
| Comorbidities (0=no, 1=yes) | |||
| Cardiac | 1.25 | 1.02 to 1.53 | 0.029 |
| Renal | 1.63 | 1.18 to 2.27 | 0.004 |
| Respiratory | 1.40 | 1.12 to 1.75 | 0.004 |
| Diabetes | 1.09 | 0.88 to 1.35 | 0.436 |
| Medications (0=no, 1=yes) | |||
| ASA | 0.87 | 0.69 to 1.11 | 0.281 |
| Statins | 0.86 | 0.68 to 1.08 | 0.196 |
| PPI | 1.34 | 1.01 to 1.79 | 0.042 |
| Tumour stage (continuous) | 1.95 | 1.73 to 2.20 | <0.001 |
| Tumour differentiation (reference=well-differentiated) | |||
| Moderate | 1.17 | 0.70 to 1.97 | 0.557 |
| Poor | 1.99 | 1.13 to 3.49 | 0.016 |
| Number of positive lymph nodes (reference=0) | |||
| 1–3 | 0.83 | 0.62 to 1.12 | 0.222 |
| >4 | 1.26 | 0.96 to 1.67 | 0.100 |
Boldface type indicates p < 0.05.
HR = hazard ratio; CI = confidence interval; ASA = acetylsalicylic acid; PPI = proton pump inhibitor.
DISCUSSION
Our study suggests an adverse effect of ppis on os in crc patients. Specifically, in the patients who were taking a ppi at the time of diagnosis, we observed a trend toward a decreased 1-, 2-, and 5-year os, as well as a statistically significant decrease in cumulative os. Earlier studies looking at ppi use and crc have looked only for correlations between ppi use and the risk of developing crc13–16. The present study is the first to look specifically at a potential relationship between ppis and os in crc patients.
A number of theories currently support a causative relationship between long-term ppi use and the development of a supportive microenvironment in which crc cells would thrive. One theory hypothesizes that ppi use results in hypochlorhydria in the stomach, which causes hypersecretion of the hormone gastrin from the gastric antrum17. In vitro studies have shown that, in turn, hypergastrinemia promotes cell proliferation and cell migration and inhibits apoptosis—several factors that favour the development and progression of neoplasias of the gastrointestinal tract18–22. Animal models have demonstrated that ppis induce hypergastrinemia in mice and rats, enhance proliferation of colonic mucosa cells in those organisms, and promote adenoma progression23–25. A second theory postulates that the use of ppis results in a less acidic environment in the stomach and duodenum, allowing for bacterial overgrowth in the gut, which ultimately results in more toxic bile salt formation26. Specifically, bacterial overgrowth increases gastrointestinal concentrations of carcinogenic bacterial by-products, including nitrites, N-nitroso compounds, and deoxycholic acid, all of which have been associated with high-grade crcs25–27. It therefore follows that, in crc patients, prior ppi use could adversely affect survival by affecting the biology of the disease, specifically alterations in the tumour microenvironment, grade, and stage at presentation.
Previous studies have attempted to identify the impact of ppi use on the development of crc, but none have looked at either cancer-specific or overall mortality in crc patients. Most recently, a meta-analysis of such studies set out to estimate the magnitude of the association between ppi use and increased risk of crc, determining that no statistically significant association between ppi use and crc risk was evident25.
Our study suggests a potential adverse effect of ppis on os in crc patients despite there being no identified differences in the extent or severity of disease. At this point, our analysis cannot account for the cause of the survival difference observed. We attempted to identify many potential comorbidities that can play a role in crc survival, but it is possible that unmeasured comorbidities might be accounting for the survival difference, given that our data also suggest that patients taking ppis tend to be older and might therefore have more medical complications. Additionally, because of the nature of cancer treatment in our region, many patients once treated at our institution are followed in the community, and therefore progression and recurrence could not be captured. We were thus unable to determine whether the survival difference seen in patients taking ppis was in part attributable to a change in cancer progression or recurrence.
Although every attempt was made to minimize errors, our study has several limitations. Firstly, the retrospective nature of the study meant that we were limited to the information available in existing database records. Our multivariate analysis attempted to control for unrecorded information by including a category for “missing data” for each variable, which did not affect the overall results. Second, although our study looked at all-cause mortality, we acknowledge that cancer-specific mortality would have been more appropriate. However, that information was not available because of restrictions in death certificate information, which is not stored in the hospital database. Thus, it remains to be seen whether ppis are associated with cancer-specific death or not. Although our study looked at ppi use, we were not able to determine dose and duration of treatment, and thus could not factor those variables into our analysis. Lastly, patients taking ppis constituted a relatively small percentage of our patient cohort. However, despite the small number, we were still able to identify an adverse survival effect associated with ppis in our crc patient population.
To further elucidate the relationship between crc survival and ppi therapy, prospective studies are necessary. Such studies should specifically focus on high-risk populations such as individuals with precancerous lesions or those with familial polyposis of the colon and would ideally use cancer-specific death as a primary outcome. The results would help to elucidate the specific populations that would benefit from avoiding acid-reducing medications. Overall, additional high-power, prospective randomized controlled trials would be necessary to fully elucidate the relationship between ppi use and crc risk.
CONCLUSIONS
Our study suggests an adverse effect on os of ppi use in crc patients. This information provides a basis for further evaluating the relationship with prospective studies that will evaluate the association between ppi use, local disease recurrence, disease progression, and cancer-specific mortality.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 2.Kort EJ, Paneth N, Vande Woude GF. The decline in U.S. cancer mortality in people born since 1925. Cancer Res. 2009;69:6500–5. doi: 10.1158/0008-5472.CAN-09-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison EF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 4.Bryant HE, Fekete SV, Major DH. Pan-Canadian initiatives in colorectal cancer screening: adopting knowledge translation tools to accelerate uptake and impact. Curr Oncol. 2011;18:111–18. doi: 10.3747/co.v18i3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson CC, Jankowski M, Rolnick S, Yood MU, Alford SH. Influence of nsaid use among colorectal cancer survivors on cancer outcomes. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000164. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–43. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flossmann E, Rothwell PM, on behalf of the British Doctors Aspirin Trial and the uk-tia Aspirin Trial Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone Survey. JAMA. 2002;287:337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Targownik LE, Metge C, Roos L, Leung S. The prevalence of and the clinical and demographic characteristics associated with high-intensity proton pump inhibitor use. Am J Gasteroenterol. 2007;102:942–50. doi: 10.1111/j.1572-0241.2007.01106.x. [DOI] [PubMed] [Google Scholar]
- 10.Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014:213–18. doi: 10.1097/MED.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 11.Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896–903. doi: 10.1016/j.amjmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Watson SA, Morris TM, McWilliams DF, et al. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567–73. doi: 10.1038/sj.bjc.6600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sorensen HT. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case–control study. Gastroenterology. 2007;133:755–60. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Yang YX, Hennessy S, Propert K, Hwang WT, Sedarat A, Lewis JD. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748–54. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Chubak J, Boudreau DM, Rulyak SJ, Mandelson MT. Colorectal cancer risk in relation to use of acid suppressive medications. Pharmacoepidemiol Drug Saf. 2009;18:540–4. doi: 10.1002/pds.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai SW, Liao KF, Lai HC, Lin CL, Sung FC. Use of proton pump inhibitors correlates with increased risk of colorectal cancer in Taiwan. Asia Pac J Clin Oncol. 2013;9:192–3. doi: 10.1111/ajco.12054. [DOI] [PubMed] [Google Scholar]
- 17.Han YM, Hahm KB, Park JM, Hong SP, Kim EH. Paradoxically augmented anti-tumorigenic action of proton pump inhibitor and GastrininAPCMin/+ intestinal polyposis model. Neoplasia. 2014;16:73–83. doi: 10.1593/neo.131510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JP, Wood JG, Solomon TE. Elevated gastrin levels in patients with colon cancer or adenomatous polyps. Dig Dis Sci. 1989;34:171–4. doi: 10.1007/BF01536047. [DOI] [PubMed] [Google Scholar]
- 19.Wong K, Beardshall K, Waters CM, Calam J, Poston GJ. Postprandial hypergastrinemia in patients with colorectal cancer. Gut. 1991;32:1352–4. doi: 10.1136/gut.32.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winsett OE, Townsend CM, Jr, Glass EJ, Thompson JC. Gastrin stimulates growth of colon cancer. Surgery. 1986;99:302–7. [PubMed] [Google Scholar]
- 21.Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via cck-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338–48. doi: 10.1038/sj.bjp.0706053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdalla SI, Lao-Sirieix P, Novelli MR, Loval LB, Sanderson IR, Fitzgerald RC. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4783–92. doi: 10.1158/1078-0432.CCR-04-0015. [DOI] [PubMed] [Google Scholar]
- 23.Pawlikowski M, Wajs E, Lewinski A, Szkudlinski M, Rybicka I, Sewerynek E. Effect of omeprazole-induced hypergastrinaemia on the proliferation of colonic mucosal epithelial cells in the rat. Exp Clin Endocrinol. 1991;97:50–4. doi: 10.1055/s-0029-1211038. [DOI] [PubMed] [Google Scholar]
- 24.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn JS, Park SM, Eom CS, Kim S, Myung SK. Use of proton pump inhibitor and risk of colorectal cancer: a meta-analysis of observational studies. Korean J Fam Med. 2012;33:272–9. doi: 10.4082/kjfm.2012.33.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Soest EM, van Rossum LG, Dieleman JP, et al. Proton pump inhibitors and the risk of colorectal cancer. Am J Gastroenterol. 2008;103:966–73. doi: 10.1111/j.1572-0241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 27.Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651–68. doi: 10.1046/j.1365-2036.2000.00768.x. [DOI] [PubMed] [Google Scholar]