Abstract
Background
Crizotinib was the first agent approved for the treatment of anaplastic lymphoma kinase (ALK)–positive (+) non-small-cell lung cancer (nsclc), followed by ceritinib. However, patients eventually progress or develop resistance to crizotinib. With limited real-world data available, the objective of the present work was to evaluate treatment patterns and survival after crizotinib in patients with locally advanced or metastatic ALK+ nsclc in Canada.
Methods
In this retrospective study at 6 oncology centres across Canada, medical records of patients with locally advanced or metastatic ALK+ nsclc were reviewed. Demographic and clinical characteristics, treatments, and outcomes data were abstracted. Analyses focused on patients who discontinued crizotinib treatment.
Results
Of the 97 patients included, 9 were crizotinib-naïve, and 39 were still receiving crizotinib at study end. The 49 patients who discontinued crizotinib treatment were included in the analysis. Of those 49 patients, 43% received ceritinib at any time, 20% subsequently received systemic chemotherapy only (but never ceritinib), and 37% received no further treatment or died before receiving additional treatment. Median overall survival from crizotinib discontinuation was shorter in patients who did not receive ceritinib than in those who received ceritinib (1.7 months vs. 20.4 months, p < 0.001). In a multivariable analysis, factors associated with poorer survival included lack of additional therapies (particularly ceritinib), male sex, and younger age, but not smoking status; patients of Asian ethnicity showed a nonsignificant trend toward improved survival.
Conclusions
A substantial proportion of patients with ALK+ nsclc received no further treatment or died before receiving additional treatment after crizotinib. Treatment with systemic agents was associated with improved survival, with ceritinib use being associated with the longest survival.
Keywords: Crizotinib, ceritinib, lung cancer, ALK-positive non-small-cell lung cancer, treatment patterns, survival
INTRODUCTION
Patients with lung cancer have a 5-year survival rate of 17%1, which varies with disease stage at diagnosis: 56% for patients diagnosed at stage i and only 2% for those diagnosed at stage iv1. Histologically, about 85% of all lung cancers are non-small-cell lung cancer (nsclc)2. Median survival for patients with advanced nsclc is 4–6 months if untreated and at least twice that if treated2.
Anaplastic lymphoma kinase (ALK) gene rearrangement mutations are found in approximately 4%–7% of nsclc tumours and are associated with a prognosis poorer than that seen in nsclc overall3. Currently approved treatment options in patients with the mutation include the alk inhibitors crizotinib and ceritinib. Crizotinib was approved by Health Canada in April 20124 for the treatment of patients with ALK-positive (ALK+) nsclc. Despite a demonstrated survival benefit for crizotinib compared with chemotherapy5,6, most treated patients will acquire drug resistance or will progress (or both), often within the first year of treatment7.
Ceritinib, a second-generation alk inhibitor, was recently approved in Canada (March 2015) as monotherapy for the treatment of patients with incurable locally advanced or metastatic ALK+ nsclc who have either progressed on, or are intolerant to, crizotinib8. As new therapies become available and are evaluated by payers (sometimes with limited clinical evidence), it is essential to assess real-world treatment patterns and outcomes for patients with ALK+ nsclc who discontinue crizotinib therapy in Canada. Such assessment will provide insights into the unmet burden in such patients.
To date, published real-world data about treatment patterns and outcomes for patients in Canada after crizotinib therapy have been limited. The objectives of the present study were to characterize treatment patterns and to estimate survival for patients in Canada with locally advanced or metastatic ALK+ nsclc in whom crizotinib therapy failed, and to describe the clinico-demographic characteristics of those patients after crizotinib discontinuation. Exploratory objectives were to investigate differences in survival by post–crizotinib treatment status and to identify factors associated with post–crizotinib survival.
METHODS
This retrospective chart review was conducted at 6 Canadian comprehensive cancer centres that had indicated at least 5–10 eligible patients with ALK+ nsclc. The enrolled sites were located in British Columbia (n = 2), Ontario (n = 3), and Quebec (n = 1, Table i). Patients diagnosed with lung cancer between January 2010 and July 2014 were identified, and a registry was created that included all patients 18 years of age and older with incurable locally advanced or metastatic ALK+ nsclc. From that registry, the subset of patients in whom crizotinib treatment had failed were identified (“crizotinib-failure cohort”). Data were collected from the time of primary nsclc diagnosis until death or end of the study period. At 2 sites, the study end date was different: May 2014 because of institutional review board restrictions, and January 2015 because data collection commenced later than at other sites. Starting at the end of the study period, individuals with an incident diagnosis of locally advanced or metastatic ALK+ nsclc were identified from notations in physician charts; charts were then sampled consecutively backward from that date until all eligible patients had been identified. De-identified patient data from pre-existing electronic or paper medical records were entered into an electronic database by study staff at each site. Patients were not contacted for any data collection. Ethics approval was obtained at each study site.
TABLE I.
Participating clinical sites in Canada
| Institution | Location |
|---|---|
| Princess Margaret Cancer Centre | Toronto, ON |
| Jewish General Hospital | Montreal, QC |
| Lakeridge Health | Durham Region, ON |
| The Ottawa Hospital | Ottawa, ON |
| BC Cancer Agency | Vancouver, BC |
| Burnaby Hospital | Burnaby, BC |
Study Variables, Assessment of Outcomes, and Statistical Analyses
Patient-level data collected for all registry patients included limited demographic and clinical information. For the crizotinib-failure cohort, data about treatments received, response to treatment, resource utilization, end-of-life care, and status at study end were also collected. Treatment outcomes collected included physician-defined response rates, duration of response, physician-defined progression-free survival [pfs (defined by the physician rather than by the Response Evaluation Criteria in Solid Tumors9)], and overall survival (os).
The statistical analysis was conducted in the R software application (version 3.0.2: The R Foundation, Vienna, Austria). Clinico-demographic characteristics are summarized for all locally advanced or metastatic ALK+ nsclc patients by number and percentage for categorical variables and by mean and standard deviation for continuous variables. Treatment patterns are characterized by the number and percentage of patients receiving different types of treatment before and after crizotinib treatment. Clinical outcomes data are presented as the proportion of patients experiencing the outcomes of interest (progression, survival). Estimates of pfs and os (mean, median, 95% confidence interval, and interquartile range) were evaluated descriptively using Kaplan–Meier methods for censored data. For os, two different definitions were used: first, time from primary diagnosis until death or date last known to be alive; second, time from crizotinib discontinuation until death or date last known to be alive. For pfs, the time from crizotinib discontinuation until failure of the next line of therapy (by progression or death) was determined. Patients were censored based on their last known status in the patient chart; if the patient was last known to be alive, then the study period end date was used for censoring. The os and pfs were compared in a Kaplan–Meier analyses based on these stratifications and the log-rank test:
■ All crizotinib-failure patients classified as stage iv at primary diagnosis, stratified by receipt of ceritinib treatment
■ All crizotinib-failure patients stratified by post-crizotinib treatment (none, no ceritinib treatment, and ceritinib treatment)
For evaluating post-crizotinib survival, Kaplan–Meier curves were derived either from the date of diagnosis (for os analyses) or from the date of crizotinib discontinuation (to account for patients who received no treatment).
An exploratory analysis used a Cox proportional hazards model to identify factors associated with post–crizotinib survival starting with the date of crizotinib discontinuation and using death or date last known to be alive as the censored outcomes. Variables were included in the multivariable analysis based on clinical relevance and significance level in the univariate analysis.
RESULTS
Overall, 97 patients with locally advanced or metastatic ALK+ nsclc were included in the registry. Of those 97 patients, 39 (40%) received ongoing treatment with crizotinib, 9 (9%) were crizotinib-naïve, and 49 (51%) had discontinued crizotinib (crizotinib-failure cohort). Survival analyses were restricted to the last group of patients.
Demographic and Clinical Characteristics of the Crizotinib-Failure Cohort at Primary NSCLC Diagnosis
Table ii summarizes the clinico-demographic characteristics of the crizotinib-failure cohort at primary nsclc diagnosis. Median age was 53 years (range: 28–80 years), and 53% were women. Two thirds were never-smokers (67%), and slightly more had been classified stage iv at primary diagnosis of nsclc (69%). At diagnosis of locally advanced or metastatic nsclc, metastases to bone and liver were most prevalent (33%), and 14% of patients had brain metastases. Nearly all patients had nonresectable disease at diagnosis.
TABLE II.
Demographic and clinical characteristics of all patients experiencing crizotinib failure at the time of primary diagnosis of non-small-cell lung cancer
| Characteristic | Patient group | ||
|---|---|---|---|
|
| |||
| Overall | Received ceritinib | ||
|
| |||
| Yes | No | ||
| Patients (n) | 49 | 21 | 28 |
| Female sex [n (%)] | 26 (53.1) | 12 (57.1) | 14 (50.0) |
| Race [n (%)] | |||
| White | 26 (53.1) | 14 (66.7) | 12 (42.9) |
| Asian | 11 (22.4) | 4 (19.0) | 7 (25.0) |
| Middle Eastern | 1 (2.0) | 0 (0.0) | 1 (3.6) |
| Other | 2 (4.1) | 1 (4.8) | 1 (3.6) |
| Unknown | 9 (18.4) | 2 (9.5) | 7 (25.0) |
| Mean age at primary diagnosis (years) | 53.2±12.5 | 49.4±11.8 | 55.9±12.4 |
| Prior diagnosis of metastatic cancer or any other solid tumour [n (%)] | 18 (36.7) | 9 (42.9) | 9 (32.1) |
| Smoking history [n (%)] | |||
| Never smoker | 33 (67.3) | 17 (81.0) | 16 (57.1) |
| Current smoker | 2 (4.1) | 0 (0.0) | 2 (7.1) |
| Former smoker | 13 (26.5) | 3 (14.3) | 10 (35.7) |
| Unknown | 1 (2.0) | 1 (4.8) | 0 (0.0) |
| Stage at primary diagnosis [n (%)] | |||
| Stages I–III | 12 (24.5) | 9 (42.9) | 3 (10.7) |
| Stage IV | 34 (69.4) | 11 (52.4) | 23 (82.1) |
| Unknown | 3 (6.1) | 1 (4.8) | 2 (7.1) |
| Family history of lung cancer [n (%)] | 9 (18.4) | 6 (28.6) | 3 (10.7) |
| Cancer histology at primary diagnosis [n (%)] | |||
| Adenocarcinoma | 46 (93.9) | 20 (95.2) | 26 (92.9) |
| Mixed | 1 (2.0) | 0 (0.0) | 1 (3.6) |
| Unknown | 2 (4.1) | 1 (4.8) | 1 (3.6) |
| Metastases at diagnosis of locally advanced or metastatic disease [n (%)] | |||
| Brain | 7 (14.3) | 4 (19.0) | 3 (10.7) |
| Bone | 16 (32.7) | 4 (19.0) | 12 (42.9) |
| Liver | 16 (32.7) | 4 (19.0) | 12 (42.9) |
| Lung | 19 (38.8) | 7 (33.3) | 12 (42.9) |
| Othera | 11 (22.4) | 5 (23.8) | 6 (21.4) |
| Resection of primary tumour [n (%)] | |||
| Complete | 1 (2.0) | 1 (4.8) | 0 (0.0) |
| Partial | 3 (6.1) | 2 (9.5) | 1 (3.6) |
| Not resected | 43 (87.8) | 17 (81.0) | 26 (92.9) |
| Unknown | 2 (4.1) | 1 (4.8) | 1 (3.6) |
Included adrenal glands, pleura, ovary, choroid, and eye.
Compared with patients who did not receive ceritinib after crizotinib discontinuation, those who received ceritinib after crizotinib were younger and more likely to present with earlier-stage disease, and had fewer sites of metastasis.
Treatment Patterns in the Crizotinib-Failure Cohort from Time of Diagnosis of Advanced or Metastatic NSCLC
Crizotinib Therapy
Of the 49 crizotinib-failure patients, 19 (39%) received crizotinib as their first line of treatment, 14 (29%) received crizotinib in the second line, and 16 (33%) received crizotinib in a third line or beyond. Median treatment duration with crizotinib was 7.8 months (interquartile range: 3.3–15.3 months), with 96% of patients requiring early treatment discontinuation. Disease progression (92%) and crizotinib intolerance (37%) were the most common documented reasons for treatment discontinuation (multiple reasons could be selected). Disease progression on crizotinib was determined radiologically in 86% of patients. More than half the patients (n = 26, 53%) remained on crizotinib despite documented progression, but with a short median time from disease progression to treatment discontinuation (1 month; range: 0.0–4.2 months). At the end of crizotinib treatment, 22% of patients had developed new brain metastases, and 18% had developed new liver metastases.
After Crizotinib Therapy
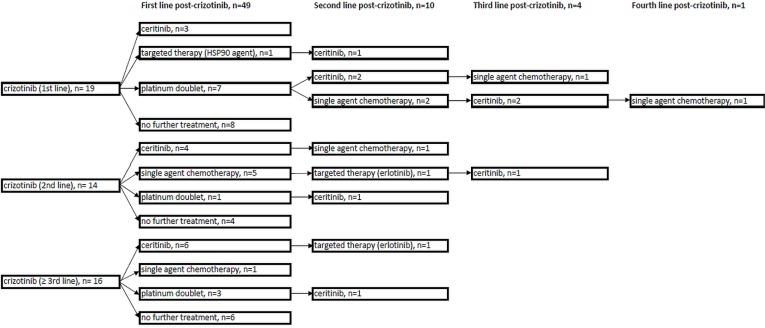
Figure 1 summarizes treatment patterns after crizotinib discontinuation. First-line treatments after crizotinib discontinuation included ceritinib (n = 13, 26%), a platinum doublet (n = 11, 22%), pemetrexed monotherapy (n = 6, 12%), and an investigational agent (n = 1, 2%). In 18 patients (37%), no additional systemic therapy was given.
FIGURE 1.
Summary of treatment patterns after crizotinib failure in 49 patients during the study period (2010–2015). In 1 patient, treatment with crizotinib appeared to have been re-instituted; that patient was therefore excluded from the figure. The same patient was also treated with ceritinib after crizotinib. HSP90 = heat shock protein 90.
In the evaluation of all lines of therapy after crizotinib discontinuation, 43% of patients (n = 21) received ceritinib therapy at any time afterwards; 20% (n = 10) received additional systemic therapy, but did not receive ceritinib at any time; and the remaining 37% (n = 18) received no treatment after crizotinib discontinuation. Overall, 35% of patients (n = 17) were documented to have received concurrent specialist palliative care after crizotinib discontinuation. Radiotherapy was received by 33% of patients (n = 16) after crizotinib discontinuation, with 20% receiving radiation to the brain at some time after crizotinib discontinuation.
Survival
From Time of Diagnosis
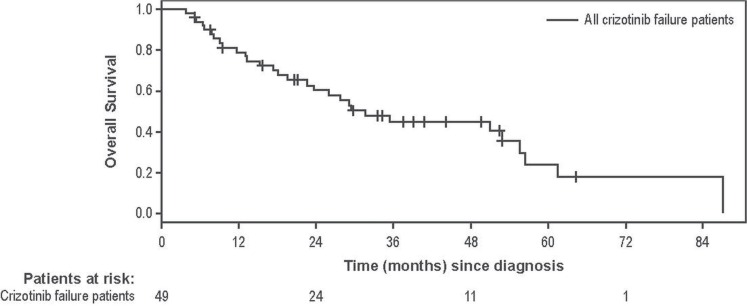
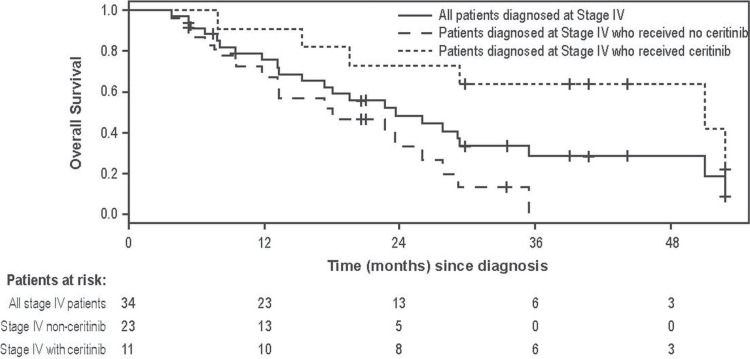
Median follow-up time from nsclc diagnosis was 30.1 months (interquartile range: 17.3–42.1 months). Disease progression was experienced by 40 patients (82%), and 27 patients (55%) died during the post-crizotinib study period. Median os was 31.6 months in all crizotinib-failure patients (Figure 2). Median os was 61.4 and 23.7 months for those diagnosed at stages i–iii and stage iv respectively. As an exploratory analysis in patients with a primary diagnosis of stage iv disease, those who received crizotinib but never ceritinib had a median os of 18.1 months, and those who received crizotinib followed by ceritinib at some point had a median os of 51.0 months (Figure 3).
FIGURE 2.
Overall survival from diagnosis in 49 patients experiencing crizotinib failure. Patients were censored if no further data were collected (that is, the date of last data collection occurred before death). Median overall survival was 31.6 months in patients experiencing crizotinib failure.
FIGURE 3.
Overall survival starting from diagnosis in 34 patients experiencing crizotinib failure who were initially diagnosed with stage IV non-small-cell lung cancer (NSCLC). Also included are overall survival curves for the same patients, depending on whether they did (n = 11) or did not (n = 23) receive ceritinib treatment (p = 0.003). Patients were censored if no further data were collected (that is, the date of last data collection occurred before death). Median overall survival was 23.6 months for all patients in the cohort; 51.0 months for the group that subsequently received ceritinib; and 18.1 months for the group that did not subsequently receive ceritinib.
From Time of Crizotinib Discontinuation
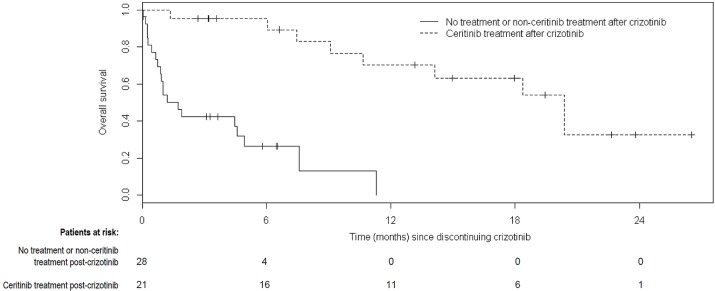
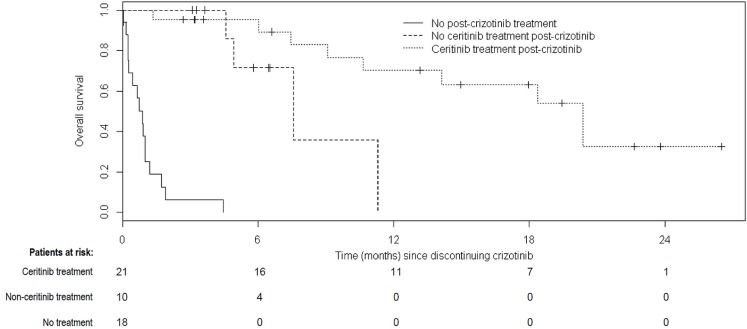
Median os from the time of crizotinib discontinuation was 1.7 months in those who received non-ceritinib treatment or no treatment after crizotinib and 20.4 months in those who received ceritinib after crizotinib (Figure 4, p < 0.0001). Median os after crizotinib discontinuation, stratified by patients who received no further treatment, non-ceritinib treatment, or ceritinib treatment at any time after discontinuation, was 0.9, 7.6, and 20.4 months respectively (Figure 5, p < 0.0001). The 1-year survival rate from the date of crizotinib discontinuation was 70% in patients who received ceritinib and 0% in the patients who never received ceritinib. The corresponding 2-year survival rate was 33% in patients who received ceritinib at any time.
FIGURE 4.
Overall survival after crizotinib discontinuation for all patients experiencing crizotinib failure. Patients were censored if no further data were collected (that is, the date of last data collection occurred before death). Median overall survival was 1.7 months for patients who received no treatment or treatment without ceritinib, and 20.4 months for patients who received ceritinib (p < 0.0001).
FIGURE 5.
Overall survival stratified by treatment received after discontinuation of crizotinib. Patients were censored if no further data were collected (that is, the date of last data collection occurred before death). Median overall survival was 0.9 months in patients who received no treatment after crizotinib, 7.6 months in those who received no ceritinib treatment after crizotinib, and 20.4 months in those who received ceritinib treatment after crizotinib (p < 0.0001).
For the first line of therapy after crizotinib discontinuation, the median physician-defined post-crizotinib pfs estimates were 0.9, 4.7, and 9.6 months for patients receiving no treatment, non-ceritinib treatment, and ceritinib treatment respectively. In subsequent lines of therapy, those durations were 3.8, 4.3, and 4.6 months respectively. Although pfs was longer for patients who received ceritinib after crizotinib than for patients who received non-ceritinib treatment, the trend was not statistically significant (p = 0.12). The comparison of no treatment with ceritinib treatment was statistically significant (p < 0.001).
Factors Associated with Post-Crizotinib Survival
Table iii summarizes the results of the univariate analysis examining potential predictors of os after crizotinib. Liver metastases at diagnosis were associated with poorer os, and compared with nonsmoker status, status as a current or former smoker at diagnosis showed a trend toward poorer os. Longer duration of crizotinib treatment was associated with better os; similarly, compared with no further treatment, ceritinib treatment was also associated with better os.
TABLE III.
Factors associated with overall survival after crizotinib discontinuation: univariable and multivariable analysis
| Factor | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Pts (n) | HR | 95% CI | p Value | Pts (n) | HR | 95% CI | p Value | |
| Sex | ||||||||
| Women | 26 | Reference | 26 | Reference | ||||
| Men | 23 | 0.84 | 0.40 to 1.75 | 0.643 | 23 | 3.07 | 1.06 to 8.90 | 0.039 |
| Age at diagnosis (per 5-year increase) | 49 | 1.02 | 0.88 to 1.20 | 0.762 | 49 | 0.76 | 0.61 to 0.95 | 0.015 |
| Smoking status | ||||||||
| Never smoker | 33 | Reference | 33 | Reference | ||||
| Current or former smoker | 16 | 2.17 | 0.99 to 4.75 | 0.052 | 16 | 1.42 | 0.44 to 4.53 | 0.556 |
| Race | ||||||||
| White | 26 | Reference | 26 | Reference | ||||
| Asian | 11 | 0.57 | 0.19 to 1.69 | 0.307 | 11 | 0.26 | 0.05 to 1.21 | 0.086 |
| Other | 12 | 1.29 | 0.55 to 3.01 | 0.553 | 12 | 0.30 | 0.09 to 1.01 | 0.051 |
| Disease characteristics at diagnosis | ||||||||
| ECOG performance statusa | 49 | 1.03 | 0.55 to 1.92 | 0.938 | ||||
| Comorbidity indexa | 49 | 0.98 | 0.75 to 1.29 | 0.891 | ||||
| Stage at diagnosis | ||||||||
| Stage IV | 34 | Reference | ||||||
| Other (stage I–III or unknown) | 15 | 0.56 | 0.24 to 1.33 | 0.187 | ||||
| Stage at diagnosis (alternate classification) | ||||||||
| Stage IV without liver metastases | 22 | Reference | 22 | Reference | ||||
| Stage IV with liver metastases | 12 | 2.56 | 1.03 to 6.34 | 0.042 | 15 | 2.98 | 0.92 to 9.72 | 0.068 |
| Other (stages I–III or unknown) | 15 | 0.73 | 0.28 to 1.83 | 0.498 | 12 | 4.49 | 0.88 to 22.89 | 0.070 |
| Metastases at time of diagnosis of locally advanced or metastatic NSCLC | ||||||||
| Brainb | 7 | 1.10 | 0.42 to 2.88 | 0.854 | ||||
| Liverb | 16 | 2.52 | 1.18 to 5.39 | 0.017 | ||||
| Lungb | 16 | 1.27 | 0.60 to 2.65 | 0.533 | ||||
| Boneb | 19 | 1.79 | 0.85 to 3.77 | 0.123 | ||||
| Treatment up to stopping crizotinib | ||||||||
| Time on crizotinib (per month increase) | 49 | 0.94 | 0.89 to 0.99 | 0.016 | 49 | 0.89 | 0.81 to 0.97 | 0.012 |
| Number of treatments before crizotiniba | 49 | 1.18 | 0.90 to 1.56 | 0.234 | ||||
| Treatment immediately after crizotinib | ||||||||
| Systemic treatment | 31 | Reference | 31 | Reference | ||||
| No further treatment | 18 | 9.25 | 4.06 to 21.1 | <0.001 | 18 | 39.5 | 7.45 to 210 | <0.001 |
| Ceritinib | ||||||||
| No ceritinib at any time after crizotinib | 28 | Reference | 28 | Reference | ||||
| Ceritinib at any time after crizotinib | 21 | 0.10 | 0.04 to 0.29 | <0.001 | 21 | 0.06 | 0.01 to 0.31 | <0.001 |
| Number of treatments before ceritiniba | 21 | 1.27 | 0.54 to 2.99 | 0.592 | ||||
| Time on ceritinib (per month increase) | 21 | 0.91 | 0.79 to 1.07 | 0.249 | ||||
Per point increase, treated as a continuous variable.
Each metastasis was analyzed in a separate model; comparisons were for metastasis versus no metastasis (for example, brain metastases vs. no brain metastases, or liver metastases vs. no liver metastases).
Pts = patients; HR = hazard ratio; CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; NSCLC=non-small cell lung cancer.
In the multivariable analysis (Table iii), statistically significantly higher hazard ratios for death were associated with male sex and younger age at diagnosis than with female sex and older age. Results indicated a non-significant trend for improved survival in patients of nonwhite ethnicity than in those of white ethnicity. Longer duration on crizotinib and treatment with any systemic therapy (and specifically ceritinib treatment) were both independently and significantly associated with longer os after crizotinib discontinuation.
DISCUSSION
In most Canadian provinces, crizotinib is approved and funded as a first-line monotherapy for patients with ALK+ nsclc and as a second-line monotherapy for those who have received prior chemotherapy10. Nearly all patients treated with crizotinib will acquire drug resistance or experience disease progression (or both), often within the first year of treatment7.
Ceritinib, a new alk inhibitor, has shown beneficial results in clinical trials for patients with ALK+ locally advanced or metastatic nsclc who have progressed on, or shown intolerance to, crizotinib. Published data about treatment and outcomes in such patients after crizotinib discontinuation are limited.
Lung cancer often affects individuals of older age with a smoking history. However, patients with ALK+ nsclc are generally younger and often nonsmokers, as reported in the present study. Overall, the demographic characteristics reported here are consistent with those reported elsewhere for patients with ALK+ nsclc11–13.
Despite the small sample size in the present study, treatment patterns after crizotinib were heterogeneous during the study period, reflective of the varying availability of ceritinib, which was available primarily on an investigational basis during the study period. Nonetheless, most patients received ceritinib at some time after crizotinib failure, through their involvement in clinical trials or on a compassionate basis.
Although exploratory, a notable finding in our study is how long patients with incurable stage iv ALK+ disease lived if they received both crizotinib and ceritinib, regardless of whether the date of diagnosis or the date of crizotinib discontinuation was used as the starting point of the survival analysis. In either case, the survival estimate is substantially longer than estimates in historical data from patients with non-ALK non-EGFR stage iv nsclc. When crizotinib and ceritinib were given sequentially, both drugs were associated with a median pfs lasting 9.6 months, suggesting a potential benefit in the subset of patients who received both drugs. Our data also suggest that starting ceritinib as the next line of therapy upon discontinuation of crizotinib, rather than as a later line of therapy, is associated with substantial improvements in pfs and os. However, our observational study cannot determine whether the ALK+ crizotinib-failure patients who did not receive ceritinib would have also benefited in both pfs and os had they been offered ceritinib immediately after crizotinib failure and discontinuation. Further, given that post-crizotinib treatment decisions were made in real-world practice and that ceritinib was available only through clinical trials or for compassionate use during the study period, the patients who received ceritinib, non-ceritinib treatment, or no further treatment after crizotinib discontinuation might have had systematic differences that would independently affect survival. Indeed, compared with patients who did not receive ceritinib in the present study, those who received post-crizotinib ceritinib were more likely to be diagnosed at an earlier disease stage, and had fewer sites of metastasis. Although those circumstances might have led to an overestimate of the survival benefits associated with ceritinib treatment after crizotinib failure, the considerable differences in survival observed between the treatment groups indicate that, compared with non-ceritinib treatment or no further treatment, ceritinib treatment after crizotinib discontinuation is associated with improvements in survival.
In this observational study, patients who received no further treatment after crizotinib did extremely poorly, a finding that accords with results reported in other recently published studies11–13. Anecdotally, tumour “flare” upon withdrawal of crizotinib has been reported in some patients. Data from the present study support the hypothesis that, upon discontinuation of crizotinib, rapid initiation of subsequent treatment should be considered. In at least 3 patients in the present observational analysis, a mandatory washout period of several weeks to be eligible for trial-based ceritinib led to a rapid decline in performance status and death before the end of the washout period.
Comparative observational os data for patients with incurable ALK+ nsclc after crizotinib from other jurisdictions are becoming available. A recent study of patients in the United States with metastatic ALK+ nsclc (n = 119) reported that, at crizotinib failure, the 42% of patients who received no further treatment experienced a median survival of 0.56 months compared with 5.9 months in the 42% of patients who received post-crizotinib chemotherapy—numbers consistent with our data11. In a combined analysis of patients in Europe, South Korea, and Latin America with incurable ALK+ nsclc after crizotinib failure (n = 158), 47% received no further treatment and 22% received standard chemotherapy; median os was 4.9 months12.
The multivariable analysis also identified other factors associated with post-crizotinib os. Male sex, younger age at diagnosis, and receipt of no further therapy after crizotinib discontinuation were each associated with poorer os. In contrast, greater time on crizotinib treatment and receipt of ceritinib immediately after crizotinib failure were significantly associated with improved os after crizotinib failure. Although exploratory, those results accord with a similar trend reported in a recent French study13. Other significant variables in the French study13, such as performance status and smoking status at crizotinib initiation, could not be tested in the present analysis because of the small sample size.
Our retrospective analysis was conducted to evaluate real-world data for patients with locally advanced or metastatic ALK+ nsclc after crizotinib failure. Although the study data reflect clinical practice, it is important to note that, given the study period start date in 2010, access to crizotinib was not universal for all ALK+ patients in Canada during the study (crizotinib was approved in early 2012). Therefore, during the 2 years before crizotinib approval, patients would have had access to crizotinib only in clinical trials or compassionate use programs. However, the study’s early start date was meant to allow for as many patients as possible to be included, thus providing early real-world data. Still, that choice might have introduced selection bias into the study.
Similarly, although the study was not designed to determine ceritinib uptake, patients receiving post-crizotinib ceritinib through clinical trials or compassionate use were included in the study. Because patients treated with crizotinib or ceritinib as part of a clinical trial might be healthier than patients who were not so treated, comparisons of survival by treatment status could be confounded. Additional treatment received after crizotinib discontinuation might be closely related to the prognosis of the patient at that time, a situation that would potentially lead to an overestimation of the survival benefits associated with post-crizotinib treatment. Results should thus be interpreted with caution. Additional limitations include the relatively small sample size considered here. Further, given that 6 centres across Canada were included, study results might not be representative of other centres in Canada. However, because of the rare nature of ALK+ nsclc, other sites in Canada would likely have contributed very few patients each. Determination of os and physician-defined pfs made use of modified definitions, given that one of the starting times for censored data measurement was the date of crizotinib discontinuation and not the date of treatment initiation as is typical in clinical trial results.
CONCLUSIONS
The present study provides valuable real-world evidence about treatments received and outcomes experienced by patients with locally advanced or metastatic ALK+ nsclc who have discontinued crizotinib. Although the results of the study indicate improved survival with a longer duration of crizotinib treatment and with post-crizotinib ceritinib treatment, there is a need for continued examination of real-world treatment patterns and outcomes as more treatments with longer follow-up become available.
ACKNOWLEDGMENTS
This study was funded by Novartis Pharmaceuticals Canada. We thank the contributing sites and the study coordinators at each of the sites for participating in this study and conducting data collection. We also thank Medha Sasane, who provided support in the design of the study, and Sujata Swaminathan, for preparation and editing of the manuscript. Both are employees of Novartis.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SK, JW, and KO are employees of icon plc, a company contracted by Novartis Pharmaceuticals Canada for this study. MH and CK are employees of Novartis Pharmaceuticals Canada, and JZ is an employee of Novartis Pharmaceuticals Corporation. PWP has received fees from Novartis Pharmaceuticals Canada for participation in an advisory board meeting. JR has received fees from Pfizer, Novartis, Bristol–Myers Squibb, Eli Lilly, and Boehringer Ingelheim for ad hoc advisory and speaking engagements. BM, VC, and GL have no disclosures to make. No co-author received a fee for publication of this study.
REFERENCES
- 1.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (seer) program. Oncologist. 2003;8:541–52. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 3.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Canada . Notice of Compliance–Authorization with Conditions for Xalkori [Web page] Ottawa, ON: Health Canada; 2015. [Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices-avis/conditions/index-eng.php; cited 16 March 2016. [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Solomon BJ, Mok T. First-line crizotinib in ALK-positive lung cancer. N Engl J Med. 2015;372:782. doi: 10.1056/NEJMc1415973. [DOI] [PubMed] [Google Scholar]
- 7.Forde PM, Rudin CM. Crizotinib in the treatment of non-small-cell lung cancer. Expert Opin Pharmacother. 2012;13:1195–201. doi: 10.1517/14656566.2012.688029. [DOI] [PubMed] [Google Scholar]
- 8.Novartis Pharmaceuticals Canada . Zykadia: Ceritinib Capsules, 150 mg Capsules, Protein Kinase Inhibitor [product monograph] Dorval, QC: Novartis Pharmaceuticals Canada; 2016. [Downloadable from: https://www.ask.novartispharma.ca/resources.htm?letter=Z; cited 16 March 2016] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Provincial Funding Summary: Crizotinib (Xalkori) for Advanced Non-Small Cell Lung Cancer. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2013. Pan-Canadian Oncology Drug Review. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/pcodr-provfund_xalkorire-nsclc.pdf; cited 16 March 2016. [Google Scholar]
- 11.Guerin A, Sasane M, Wakelee H, et al. Treatment, overall survival, and costs in patients with ALK-positive non-small-cell lung cancer after crizotinib monotherapy. Curr Med Res Opin. 2015;31:1587–97. doi: 10.1185/03007995.2015.1057115. [DOI] [PubMed] [Google Scholar]
- 12.Park K, Cadranel J, Arrieta O, et al. Treatment patterns and survival among ALK+ non-small cell lung cancer (nsclc) patients: a chart review study [abstract 3108] Eur J Cancer. 2015;51(suppl 3):S638. doi: 10.1016/S0959-8049(16)31749-X. [DOI] [PubMed] [Google Scholar]
- 13.Duruisseaux M, Besse B, Cadranel J, et al. Crizotinib outcome and post-progression management in ALK+ nsclc: ifct-1302 clinalk [abstract Oral 33.01] J Thorac Oncol. 2015;10(suppl 2):S237. doi: 10.1097/JTO.0000000000000412. [DOI] [Google Scholar]