Abstract
In recent years, risk stratification has sparked interest as an innovative approach to disease screening and prevention. The approach effectively personalizes individual risk, opening the way to screening and prevention interventions that are adapted to subpopulations. The international perspective project, which is developing risk stratification for breast cancer, aims to support the integration of its screening approach into clinical practice through comprehensive tool-building. Policies and guidelines for risk stratification—unlike those for population screening programs, which are currently well regulated—are still under development. Indeed, the development of guidelines for risk stratification reflects the translational aspects of perspective.
Here, we describe the risk stratification process that was devised in the context of perspective, and we then explain the consensus-based method used to develop recommendations for breast cancer screening and prevention in a risk-stratification approach. Lastly, we discuss how the recommendations might affect current screening policies.
Keywords: Breast cancer, screening, prevention, recommendations, clinical practice
INTRODUCTION
Breast cancer is the most common malignancy among Western women and affects approximately 1.67 million women annually worldwide1. In Canada, the age-standardized mortality rate in female breast cancer has fallen by 43% since the mid-1980s. That reduction is likely attributable to a combination of increased mammography screening2 and the use of more effective therapies3,4. Early detection by screening, with or without the use of preventive medications, can reduce the burden of disease but has drawbacks, including overdiagnosis, anxiety related to additional testing, and costs associated with screening5–9.
By combining genetic and nongenetic risk factors, risk stratification has the potential to identify individuals at increased risk of breast cancer. Stratification of women according to the risk of developing the disease could improve screening and risk-reduction strategies by targeting those most likely to benefit, leading to a more efficient allocation of clinical resources10–15. In particular, it could increase coverage of the subpopulation of younger women at high risk for the disease who are missed by standard age-based screening. Indeed, approximately 18% of breast cancers are diagnosed in women less than 50 years of age, a substantial proportion of whom have no pertinent family history. Moreover, because breast cancer in younger women correlates with poor survival16, early detection has the potential for improved survival, less-invasive treatment, and a higher quality of life, thus reducing the burden of disease and the costs of treatment.
Breast cancer tends to aggregate in families. The disease is approximately twice as common in the first-degree relatives of breast cancer patients as in the general population17. Twin and family studies indicate that the observed familial risk is largely the result of inherited susceptibility from the combined effects of multiple genetic variants18,19. Three classes of breast cancer susceptibility alleles, with varying levels of risk and prevalence in the population, have been identified: high-, intermediate-, and low-risk alleles20,21. Mutations in genes such as BRCA1, BRCA2, PTEN, TP53, and probably PALB2 confer a high lifetime risk of the disease, but are relatively rare22. Mutations in intermediate- risk alleles—for example, CHEK2, ATM—confer a risk that is increased by a factor of about 2–4. Yet neither the high-risk nor the intermediate-risk alleles are responsible for most of the genetic risk. Large-scale genome-wide association studies, replication, and custom genotyping efforts have systematically identified almost a hundred common low-risk single-nucleotide polymorphisms17,23,24. Those low-risk alleles explain another significant part of the genetic risk.
Although the risk conferred by individual single- nucleotide polymorphisms is not sufficiently large to be useful when used alone in risk prediction, their joined effect has recently been demonstrated to be able to achieve a degree of risk prediction that is useful within a risk-stratification approach25–27. The risk predicted with a genetic profile could be improved by combining family history with lifestyle risk factors, benign breast disease, and breast density28–30.
We can expect that new genomic profiling tests, combined with nongenetic risk factors, will be integrated into risk prediction tools to facilitate the identification of individuals at increased risk for cancer. With several major ongoing initiatives recently launched in Canada, the United States, and Europe to accelerate research for the development of personalized approaches in disease prevention and precision medicine31–33, risk stratification could become part of clinical practice sooner than expected14,20,22. In that context, several studies have recently evaluated the public interest in, and the acceptability of, population-based risk-stratified screening for breast cancer34–36.
In 2013, an international and interdisciplinary research project called perspective [Personalized Risk Stratification for Prevention and Early Detection of Breast Cancer (http://www.genomecanada.ca/medias/pdf/en/Simard.pdf)] was established. A goal of perspective is to develop a comprehensive risk-prediction tool to stratify women into risk levels computed by their individual genomic and other personal risk factors. With the tool, each woman could be assigned a certain risk level (for example, near population risk, intermediate risk, or high risk). As a translational project, perspective also covers the so-called T3 phase of translational genomics, which encompasses the implementation and integration of genomics into routine clinical practice37. That is, the development of policies for the clinical management of women stratified by risk-prediction tools must also be an integral part of risk-stratification research.
Current guidelines address only the management of very high-risk women identified as carriers of a mutation in one of the high-penetrance genes, including BRCA1 and BRCA2, and of women at high risk as identified by a family history38–41. However, to apply the specific exclusions, limits, and frequent updates of those guidelines, a high level of specialized knowledge is required. Use of those tools by general practitioners raises an issue of applicability in the context of routine follow-up. In addition, no guidelines address women at high or intermediate risk as identified using a combination of common and low-frequency genetic variants, personal and family history, and breast density. One of the objectives of perspective is to fill that knowledge gap in the Canadian context.
The integration of risk stratification has the potential to substantially change screening and preventive treatment procedures in clinical practice. Population screening programs in many developed countries generally use age as the primary criterion for screening eligibility: routine mammography is recommended at predetermined intervals, starting between the ages of 40 and 50 years, regardless of potential differences in individual risk. The risk-stratification tools developed in the context of perspective could facilitate the establishment of screening schedules in line with risk levels. For instance, screening schedules for women at high risk could include earlier or more frequent mammography, with the possible use of adjunct technologies such as magnetic resonance imaging (mri).
Here, we describe the rationale and the methodology that led to the formulation of recommendations for breast cancer screening and prevention in the context of a risk-stratification approach to be implemented in the province of Quebec. We also discuss how those recommendations might affect screening policies and directions for breast cancer screening and prevention in the near future.
Risk-Stratification Process
Implementing risk-stratification approaches in clinical practice demands consideration of the organizational features of the health care systems in which clinical practices are embedded. Such features might vary from one health care system to another, but they would generally include the particular health care professionals who will be involved in risk stratification, the populations of women to be stratified, the particular process used to offer genomic profiling, and the integration of the genomic information with other validated risk factors and relevant clinical information needed for an individual risk assessment using a comprehensive risk prediction tool—to name but a few. Currently, few of those features can be determined. Notwithstanding such differences, a number of core components of the risk-stratification approach are already known and can therefore form a basis for the development of clinical policies.
First, the genomic profiling test under development will detect common and low-frequency genetic variants that increase cancer risk slightly (specifically, single-nucleotide polymorphisms) or moderately (ATM and CHEK2, for instance). For the purposes of the recommendations, a provisional hypothesis has been formulated that the genes associated with a substantially increased risk (BRCA1, BRCA2, PTEN, TP53, PALB2, for instance) are not included, taking into consideration the attendant costs, technology, counselling needs, and feasibility issues related to implementation of population screening in a public health context. Active discussion is still underway in the perspective project and in the scientific community about the inclusion of those high-risk genes in population screening42.
Second, risk will be estimated using an updated version of boadicea (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm). The boadicea algorithm, which was designed by researchers at the Centre for Cancer Genetic Epidemiology (Cambridge University, U.K.) is a free online algorithm accessible to health professionals worldwide (http://ccge.medschl.cam.ac.uk/boadicea). The current version of this risk-prediction model is designed to compute mutation carrier probabilities in high- and moderate-risk genes (BRCA1, BRCA2, PALB2, CHEK2, ATM) and age-specific risks of breast and ovarian cancer43–47. The information taken into account in the current version of boadicea is the age of the individuals included in the pedigree (for example, the woman and her biological family); Ashkenazi Jewish ancestry; country of origin; year of birth; family history of breast, ovarian, and pancreatic cancer, as well as breast cancer and prostate cancer in male relatives; breast tumour pathology (estrogen receptor, progesterone receptor, her2, CK5/6 and CK14 cytokeratin status) in affected relatives; and mutation carrier status.
In the near future, an updated version of boadicea developed in the context of the perspective project will also use the results of a genomic profiling test and breast density. Thereafter, selected lifestyle and hormonal factors will be added if the data are sufficiently conclusive for an evidence-based calculation. The latter factors include age at menarche, parity and age at the first child’s birth, age at menopause (including prophylactic oophorectomy), benign breast diseases, breastfeeding, height, and modifiable factors such as body mass index, use of hormone replacement therapy, and alcohol consumption.
The risk-stratification process—designed to facilitate the development of the recommendations—will involve 3 steps:
-
Collection of information
Information about risk factors will be collected from each woman, including medical and familial history, breast density, and results of the genomic profiling test. Note that individual risk can still be estimated even if some risk factors are missing, but the accuracy of the estimation increases with the number of variables used.
-
Risk evaluation and stratification in a risk level
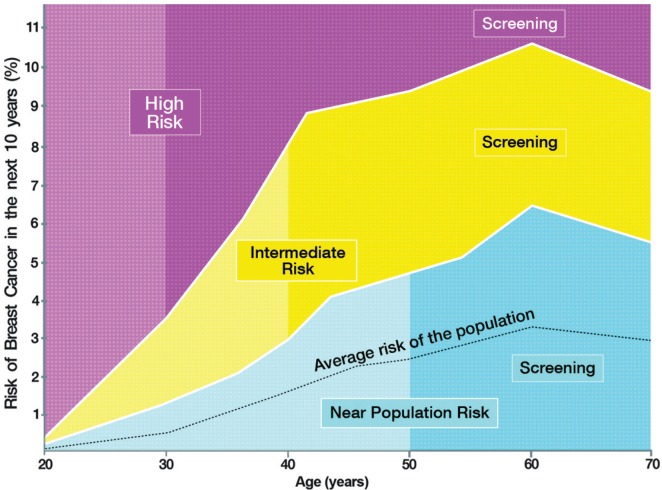
The risk evaluation tool (boadicea) will calculate breast cancer risk for 10 years into the future. The risk stratification curves (Figure 1) will then combine the 10-year risk value with the age of the woman to assign her to 1 of 3 specific risk strata: near population risk, intermediate risk, or high risk.
-
Risk-adapted measures proposal
Screening and preventive measures adapted to individual risk level will be offered in accordance with the applicable recommendations.
FIGURE 1.
Risk stratification curves. All points forming the outlines of the curves are approximate and were used for illustrative purposes only to facilitate the discussions between experts during development of the recommendations. The exact limits determined by each curve will be finalized after completion of the comprehensive cost–benefit analyses currently underway in the context of the interdisciplinary PERSPECTIVE project (http://www.genomecanada.ca/medias/pdf/en/Simard.pdf).
METHODS
Recommendations applying to clinical practice are frequently the product of consensus-building by experts in a given field. When strong evidence is lacking, the most effective alternative is often to resort to consensus-based processes, in which case professional opinion and expertise become necessary48. Such expertise is needed to discuss and to reach agreement about selected policy issues and has already been used in consensus-building in the context of breast cancer screening, prevention, and treatment. Well-known examples of such an approach include the Saint-Paul de Vence recommendations on breast cancer treatment49, the recommendations of the American Society of Clinical Oncology on pharmacologic intervention to reduce breast cancer50, and the St. Gallen recommendations on breast cancer care51.
A consensus-based process was considered appropriate for investigating the future implementation challenges and opportunities of the new perspective breast cancer risk-stratification approach with relevant practitioners and stakeholders. To maximize future clinical implementation and adoption of such recommendations, a consensus-building exercise with experts involved in breast cancer care and prevention was planned. Steps toward consensus-building included inviting experts to a consensus meeting to help design the new perspective recommendations, asking them to a vote based on a selection of evidence-based answers, and when necessary, debating a given question among themselves to reach consensus.
At the outset, an expert committee called the Clinical Advisory Committee was created to validate all questions and to adopt the final version of the recommendations. The Committee was composed of 16 health professionals involved in breast cancer prevention and care in the province of Quebec, and 1 observer member from the Quebec Breast Cancer Foundation. A wide range of expertise and health care specialties were represented on the committee: genetic counselling, oncology, general practice, radiology, nursing, medical genetics, public health medicine, surgery, and breast cancer research.
The Clinical Advisory Committee supported the research team during the initial policy development phase and identified the most important topics pertaining to stratified screening and preventive measures. From those general topics, the research team developed 69 multiple- choice questions and answers that were supported by international guidelines and evidence from the literature. The committee reviewed the scientific validity of the proposed questions and assessed their compatibility to ensure the feasibility of implementation of any potential recommendations derived from that exercise. The focus was on intermediate- and high-risk women and so had both to ensure optimal integration of the proposed risk-screening policy into the Quebec health care system and to avoid overlap with the current population-based program. Guidelines for the current breast cancer screening program were outside the purview of the perspective project; that program targets all women 50–69 years of age in the province of Quebec and represents the guidelines in use for the near population risk level. Likewise, recommendations for general prevention topics (for example, alcohol moderation) should be applicable to all women, regardless of risk level. The research team also developed communication and decision aids to facilitate implementation and understanding of the risk-stratification approach.
The exact percentage to be attributed to each risk level (for example, 17%–30% for intermediate risk) was not yet available. As a result, a provisional approximation was used to pursue the work. Determination of the exact percentages will be performed later in the project by incorporating results from ongoing cost–benefit studies (that is, identification of the threshold for balance between the benefits and costs in various screening scenarios) and the recommendations of the Clinical Advisory Committee for each risk level. Thus, provisional and approximate risk percentages were set for all 3 risk levels. The percentages used were those developed by the U.K. National Institute for Health and Care Excellence31, because, to the best of our knowledge, it is the only organization that provides screening and prevention guidelines for 3 risk levels; other sources considered only 2 risk levels. Hence, the exact percentage attributed to each risk level was not part of the consensus reached.
On 29 and 30 March 2014, an additional 29 clinical experts in breast cancer care, together with the Clinical Advisory Committee (for a total of 44 participants), attended a 2-day meeting to answer the 69 questions that had been formulated. That group represented 11 different specialties: oncology (n = 1), public health (n = 4), general practice (n = 8), nursing (n = 2), radiology (n = 7), gynecology (n = 2), surgery (n = 7), breast cancer research (n = 1), genetic counselling (n = 5), medical genetics (n = 5), and pathology (n = 2). After a brief overview of the project, the experts answered the 69 questions using an electronic audience response system (anonymous voting). The results obtained after each question were presented to all the experts in a chart format to evaluate the consensus rate. When a consensual position (≥80% of voter agreement) could not be inferred from the initial vote, a discussion ensued, and a new vote was taken when appropriate. In some cases, questions and answers were modified at the request of the experts to propose an alternative consensus. All discussions were audio- recorded. On site, 3 additional questions were drafted to complement the topics that were presented.
After the meeting, results of the voting and audio records of the meeting were analyzed to identify consensus areas. A consensus was considered reached when agreement exceeded 80%. No question created irreconcilable differences after the vote, and the abstention rate was negligible. After the meeting, 2 complementary questions were sent by e-mail, using the SurveyMonkey platform (Palo Alto, CA, U.S.A.), to all 44 experts. A preliminary version of the recommendations was drafted based on the topics that had garnered consensus; it was reviewed by the Clinical Advisory Committee in conjunction with pie charts of all the vote results. An updated version was sent for validation to all 44 experts who attended the consensus meeting. Minor modifications followed. Lastly, the Clinical Advisory Committee approved the final version of the recommendations in December 2014 (Table i), supported by background literature (see the online supplementary material). All work related to the development of the recommendations occurred in French, and the final version was translated into English.
TABLE I.
Recommendations of the Clinical Advisory Committee
| 1. Risk Assessment |
| 1.1 Referral to a breast clinic for risk assessment |
| Women who have one of the following risk factors should be referred to a breast clinic to assess their risk: |
|
| The foregoing factors can significantly affect risk level, but are not taken into account by the BOADICEA risk assessment calculation tool. |
| The following risk factors alone do not warrant referral to a breast clinic for risk assessment: |
|
| The foregoing factors affect risk, but do not have enough impact to justify referral to a breast clinic. They are not taken into account by the BOADICEA risk calculation tool. |
| 1.2 Referral to genetic services |
| Although there is a consensus on referring women to genetic services if their risk of having a BRCA mutation surpasses a certain threshold, there is no consensus about the threshold for making such a referral. |
| Referral should be considered when the family history suggests a hereditary predisposition for breast cancer. In addition to mutation of the BRCA1 and BRCA2 genes, other genes might have a significant impact on the risk of developing breast cancer (for example, TP53, PTEN, CDH1, etc.). It could be useful to consult the reference criteria of genetic services. |
| 2. Breast Density |
| It is recommended that mammographic density in four categories be systematically recorded in mammography reports performed outside the Quebec Breast Cancer Screening Program as they are in reports performed within the Program. |
| Mammography is not recommended when the sole objective is to evaluate a woman’s mammographic density for risk calculation purposes. |
| 3. Screening |
| 3.1 Women at near population risk |
| Mammography screening methods for women at near population risk were not discussed, nor were they the subject to recommendations. |
| The Quebec Breast Cancer Screening Program already has measures in place for these women. |
| 3.2 Women at intermediate risk |
| Mammography screening is recommended every 1–2 years for women at intermediate risk. |
| Mammography screening should begin at around age 40. |
| For women who have high breast density (category d under the current BI-RADS classification, or category 4 under the former BI-RADS classification, or >75% dense tissue), |
|
| There is no consensus on the age at which screening should cease for women at intermediate risk. |
| 3.3 Women at high risk |
| Annual mammography screening is recommended for women at high risk. |
| Mammography screening should begin between the ages of 30 and 35. However, if MRI is used as an imaging technique for screening, mammography screening should start at age 35. |
| Mammograms should not be done before age 30. |
| Breast MRI should be considered as an imaging screening technique for women at high risk. |
|
| There is no consensus on the age at which mammography screening should cease for women at high risk. |
| Screening by MRI should not continue beyond age 70. |
| 3.4 Tomosynthesis |
| For screening done outside the Quebec Breast Cancer Screening Program, tomosynthesis could be used instead of mammography when the technology is available. |
| Because of the limited availability of tomosynthesis, priority should be given to |
|
| 3.5 Clinical breast exam |
| Clinical breast exam screening should be offered to all women. |
| For women at near population risk, |
|
| For women at intermediate risk, |
|
| For women at high risk, |
|
| In women with a history of lobular neoplasia or atypical hyperplasia (at-risk lesions), a clinical breast exam should be performed every 6 to 12 months, starting at diagnosis. |
| 3.6 Women with breast implants |
| It is recommended that women with breast implants undergo screening similar to that for other women in their risk category. |
| This recommendation does not pertain to the Eklund technique, which is always recommended for women with breast implants. |
| 4. Prevention |
| 4.1 Habits and lifestyle choices |
| It is recommended that, to lower their risk of breast cancer, women moderate their alcohol consumption. |
| It is recommended that, to lower their risk of breast cancer, women stop smoking. |
| It is relevant to inform women that data seem to indicate that exposure to secondhand smoke increases the risk of breast cancer before menopause. |
| It is recommended to inform women of |
|
| It is recommended that, to lower their risk of breast cancer, women be physically active. Most organizations recommend 150 minutes of moderate physical activity weekly, but any amount of physical activity is potentially beneficial. |
| It is recommended that women maintain a balanced diet in accordance with Canada’s Food Guide. |
| It is recommended to inform women that breastfeeding might lower their risk of breast cancer. |
| It is recommended that women remain aware of changes in their breasts, so that they can consult a health professional should changes occur. |
| 4.2 Pharmacoprevention |
| Pharmacoprevention is a primary prevention strategy to consider for women with diagnosed atypical hyperplasia or lobular neoplasia, regardless of their risk level. |
|
| Pharmacoprevention should not be considered a primary prevention strategy for women at intermediate risk. |
| Pharmacoprevention is a primary prevention strategy to consider for women at high risk. |
|
| 4.3 Preventive surgery |
| Bilateral prophylactic mastectomy, with or without reconstruction, is a possible preventive option for women at high risk. |
| This preventive option should be discussed with women in the high-risk category. |
BI-RADS = Breast Imaging–Reporting and Data System; MRI = magnetic resonance imaging.
RESULTS
A consensus with 80% or more voter agreement was obtained for 32 questions, with each question leading to the development of one or more recommendations reflecting the clear consensus obtained. For questions on which the level of agreement was lower (<80%), recommendations accommodating opposing views and reflecting a “common denominator” on the issue were developed. Overall, 50 recommendations were developed from the answers and discussions recorded at the consensus meeting and were accepted by the Clinical Advisory Committee (Table i).
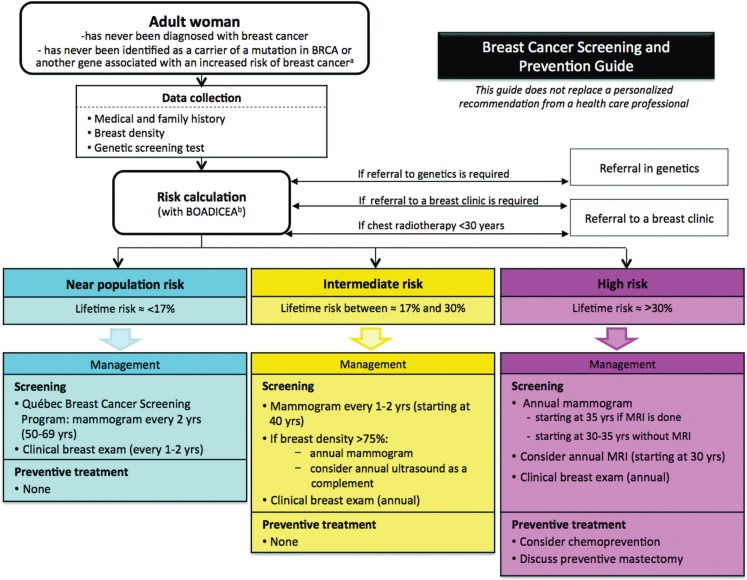
An updated decision aid for health professionals that summarizes the recommendations was also validated by the Clinical Advisory Committee (Figure 2). Recommendation topics included referral to genetics clinics and breast disease clinics, breast density, screening of women at high and intermediate risk, role of tomosynthesis in screening, clinical breast examination, screening of women with breast implants, preventive lifestyle, chemoprevention, and preventive surgery. Imaging technologies (choice and timing) and clinical breast examinations were the topics that elicited the most controversy and were the most-debated issues.
FIGURE 2.
Decision aid for breast cancer risk stratification. The percentages used here are approximate; they were used to facilitate discussions between experts during the development of the PERSPECTIVE recommendations. The exact percentages will be finalized after completion of the comprehensive cost–benefit analyses currently underway in the context of the interdisciplinary PERSPECTIVE project (http://www.genomecanada.ca/medias/pdf/en/Simard.pdf). “Lifetime risk” represents risk from age 20.
a For these carriers, refer to other applicable recommendations.
Summary: Key Recommendations
The first important recommendation concerns the collection of breast density data as an important factor for determining disease risk. Participants in the meeting expressed interest in including breast density in the assessment of breast cancer risk and its effect on the detection of breast cancer.
It is well known that dense breasts confer an increased risk of breast cancer52 and reduce the sensitivity of mammography53,54. There is currently strong interest in using breast density to optimize cancer screening. In Ontario, the breast cancer screening program automatically invites women with more than 75% dense tissue to more frequent mammography screenings32. In the United States, approximately 20 states have enacted laws that require women to be informed when their mammogram shows denser breasts55. For example, in the state of California, women showing more than 50% dense tissue in a mammogram must be informed of that observation through a written report56.
Breast density is a parameter that will be used by boad-icea to evaluate the level of risk, and it is assessed by mammography. However, the Clinical Advisory Committee did not recommend that women have a mammogram solely for the purpose of evaluating breast density. Concerns about risks such as radiation or unnecessary biopsies because of false positives were considered potentially more important than the benefit expected from the breast density scores. The recommendations do require, however, that breast density be systematically evaluated when a mammogram is conducted and be documented in the clinical report.
Second, core recommendations focus on screening for women at both the intermediate-risk and high-risk levels:
-
■ Intermediate risk
Annual or biennial mammography is recommended starting at age 40. Women with breast density exceeding 75% should undergo annual mammography, and ultrasonography should also be considered as a complement. Chemoprevention is not recommended for women in this group, with the exception of women having atypical hyperplasia or lobular neoplasia.
-
■ High risk
Annual mammography is recommended starting between the ages of 30 and 35 years. Annual mri should be considered from age 30. Two recommendations address preventive treatments for this group:- ■ For women more than 35 years of age, chemoprevention using one of the two drugs identified for this purpose (tamoxifen for premenopausal and postmenopausal women, and raloxifene for postmenopausal women) should be considered as a primary prevention method.
- ■ Preventive bilateral mastectomy should be discussed with these women.
Third, experts agreed to adopt a position on clinical breast exams for screening purposes. According to the recommendations, clinical breast exams should be offered to women as a screening measure regardless of risk level. Breast exams are recommended to be conducted at intervals between 6 months and 2 years, in accordance with the risk level and current breast pathologies (for example, lobular neoplasia, atypical hyperplasia) as detailed in the full text of the recommendations.
Finally, a set of recommendations to help lower the incidence of breast cancer through specific lifestyle and behaviours was also adopted. Those recommendations include relevant topics such as alcohol consumption, tobacco use, weight, physical activity, nutrition, breastfeeding, and breast awareness.
DISCUSSION AND CONCLUSIONS
Currently, the breast cancer screening program in the province of Quebec offers mammography every 2 years for women between the ages of 50 and 69 years. Physicians can also prescribe mammography for younger or older women on an individual basis; however, no tool is available to evaluate individual risk and to manage screening frequency. Consequently, access to screening can vary from woman to woman, depending on physician preferences and access to a general practitioner. The recommendations developed in the context of perspective propose refinement in the factors currently considered for breast cancer screening (specifically, age and, less commonly, family history) and more individualized metrics for breast cancer risk in screening policies (which are currently adapted to the average risk of the general population). The proposed refinements include
■ adapting screening frequency;
■ initiating screening at an age adapted to individual risk;
■ using emerging technologies (such as tomosynthesis);
■ introducing additional screening exams (mri, ultrasonography); and
■ considering preventive treatment for all women at high risk (chemoprevention and surgery).
The perspective recommendations will create an impetus for enhancing policies related to breast cancer screening and preventive care for women in the province of Quebec, and will hopefully encourage decision-makers to consider implementing the risk stratification approach to optimize resource allocation.
Three recommendation areas provoked noteworthy debates among the experts attending the consensus meeting and highly benefited from the live exchanges between attendees.
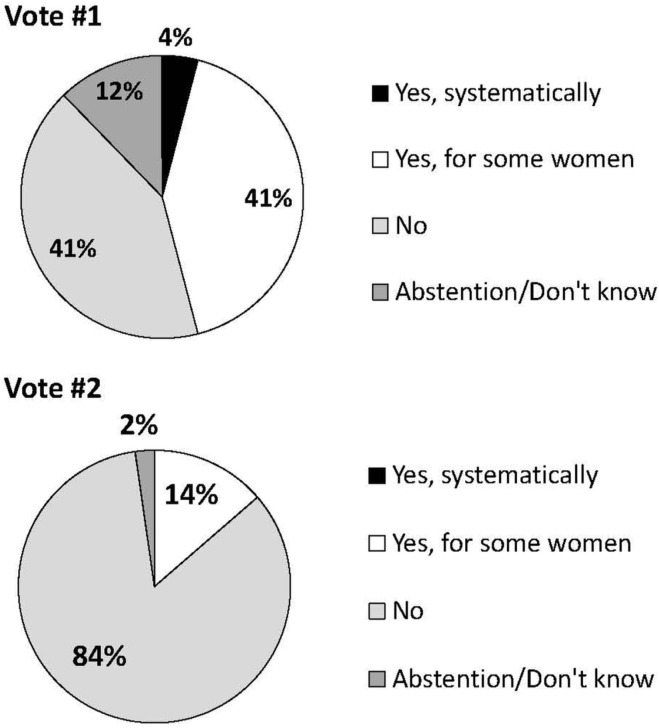
The first pertains to breast density. Concerning the question about whether mammography should be performed solely to estimate risk, many experts expressed concerns that the risks associated with radiation might outweigh the benefits gained for risk estimation. The first vote on this question did not lead to a consensus, and only 41% believed that mammography should not be systematically performed to evaluate the risk of breast cancer. After debate and a second vote on the same question, consensus was reached, with 84% voting that mammography should not be systematically performed only to determine breast density (Figure 3). To the best of our knowledge, no group has ever recommended that mammography be performed solely as a way to assess breast density for risk-evaluation purposes.
FIGURE 3.
Vote results for Question 12: For women not eligible for the Quebec Breast Cancer Screening Program (e.g., <50 years), should mammography be prescribed for the sole objective of obtaining breast density for risk calculation purposes?
Notably, the specific local issue of providing breast density in the mammographic report was discussed. In the province of Quebec, a breast density estimation has to be provided in the report for every mammogram obtained within the screening program. Breast density is classified within 1 of the 4 intervals used by bi-rads (Breast Imaging- Reporting and Data System)57. However, for mammograms performed outside the Quebec screening program, reporting of results is not standardized, and breast density data are not systematically reported. Given that, in 2012, about 27% of Quebec women between 40 and 49 years of age reported having undergone opportunistic mammography in the preceding 2 years (that is, outside the screening program)58, most experts felt that breast density is useful information that should be provided in all mammography reports. A consensus for the systematic provision of breast density information for mammograms performed outside the screening program was obtained with 83%.
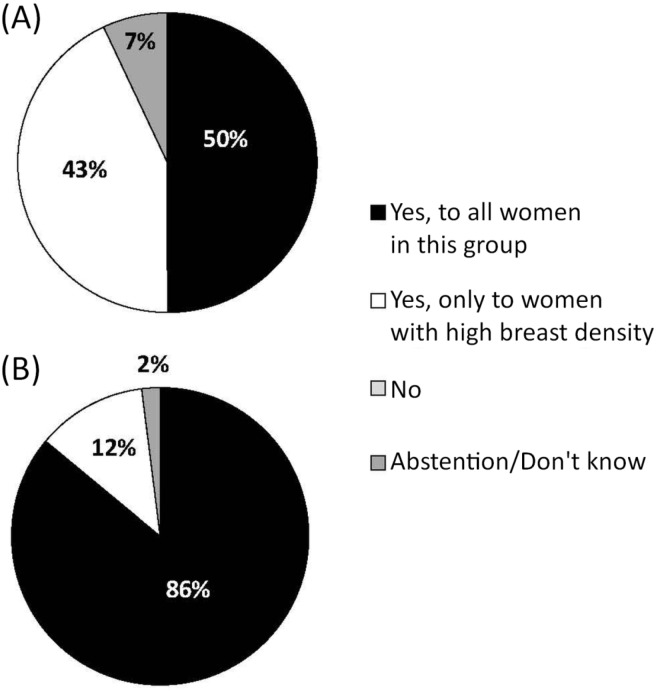
The second topic that provoked considerable debate at the consensus meeting was the issue of additional imaging (for example, ultrasonography or mri) for women at high risk. Approval of only 50% was obtained to “offer” complementary exams to all women at high risk; support increased to 86% when the question was whether such exams should merely be “considered” (Figure 4). The distinction between “offering” and “considering” was thought to be important because the former is perceived to allow less flexibility and to be more constraining for health professionals in a clinical setting. Concerns raised by the members about adding mri to the new set of policies included the longer duration of the test, its costs and availability, false positives, and the absence of data on mortality reduction as a result of its use. The choice to use the verb “consider” can be explained by the absence of exact risk interval limits and the differences between current guidelines. Although some guidelines recommend mri for all women with a lifetime risk of breast cancer exceeding 20%–25%, the guidelines from the National Institute for Health and Care Excellence add a second requirement that these women also have a risk exceeding 30% of carrying a mutation of a highly penetrant gene associated with breast cancer (BRCA1/2 or TP53)30,31,33,59. Since the adoption of the perspective recommendations, the International Agency for Research on Cancer has published its recommendations, in which the Working Group evaluated as “inadequate” the strength of evidence supporting mortality reduction by complementary mri screening for carriers of BRCA1 and BRCA2, illustrating the difficulty of adopting a strong position on this issue9.
FIGURE 4.
Vote results for Questions (A) 17 (Should complementary screening imaging be offered, in addition to mammography, to women at the high-risk level?) and (B) 17.1 (Should complementary screening imaging be considered, in addition to mammography, with women at the high-risk level?) Although those questions about complementary exams did not formally target only magnetic resonance imaging (MRI), discussions focused on MRI as the most appropriate complementary imaging approach. Furthermore, MRI was identified as the complementary imaging technique of choice by 75% of the members.
In the third case, vigorous debate attended the position adopted by members about clinical breast exams, because the position taken strays significantly from some current guidelines. Some agencies do not recommend such exams52, but others still advise them60. The low risk and high clinical utility of detecting tumours by routine breast examination substantiate the recommendation for the use of this technique. Benefits were considered to exceed disadvantages associated with clinical exam. The decision was based on clinical experience, expected risks and benefits9, and current opposing guidelines. Recently, a retrospective study concluded that a significant number of cancers would have been missed if clinical breast exams had not been performed61.
As illustrated, consensus-based recommendations for breast cancer screening and prevention can be facilitated by the active involvement of an advisory committee and the collaboration of external clinical experts. The recommendations thus adopted are essential to the subsequent implementation phases of the risk-stratification approach under development. Risk-based management could optimize resources in the health system by targeting women who can benefit the most from those resources. Moreover, the involvement of health professionals in the development of the recommendations presented here will likely foster their adoption into clinical practice. These recommendations supporting management of risk-stratified women could also facilitate the adoption and implementation of the risk-stratification approach by decision-makers.
ACKNOWLEDGMENTS
The development of these recommendations was funded by the Quebec Breast Cancer Foundation. The perspective project is also funded by the Government of Canada (via Genome Canada and the Canadian Institutes of Health Research) and by the Ministère de l’Économie, de la Science et de l’Innovation (via Genome Quebec).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: LL and IT are radiologists at the clinic Léger et associés. ND is member of the medical team at the Clinique radiologie Audet and is also a consultant and shareholder at SonoCiné Inc. The other authors declare that they have no conflicts to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al., editors. Estimated cancer incidence, mortality and prevalence worldwide in 2012 [Web resource] Lyon, France: International Agency for Research on Cancer; 2013. globocan 2012. [Available at: http://globocan.iarc.fr; cited 8 June 2015] [Google Scholar]
- 2.Shields M, Wilkins K. An update on mammography use in Canada. Health Rep. 2009;20:7–19. [Available online at: http://www.statcan.gc.ca/pub/82-003-x/2009003/article/10873-eng.pdf; cited 8 June 2015] [PubMed] [Google Scholar]
- 3.Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst. 2002;94:1626–34. doi: 10.1093/jnci/94.21.1626. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status for cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 5.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 6.Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–86. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Bonnanni B, et al. on behalf of the serm Chemoprevention of Breast Cancer Overview Group Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381:1827–34. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer. J Natl Cancer Inst. 2014;106:dju261. doi: 10.1093/jnci/dju261. pii: [DOI] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B, Scoccianti C, Loomis D, et al. on behalf of the International Agency for Research on Cancer Handbook Working Group Breast-cancer screening—viewpoint of the iarc Working Group. N Engl J Med. 2015;372:2353–8. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 10.Howell A, Astley S, Warwick J, et al. Prevention of breast cancer in the context of a national breast screening programme. J Intern Med. 2012;271:321–30. doi: 10.1111/j.1365-2796.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- 11.Pashayan N, Duffy SW, Chowdhury S, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104:1656–63. doi: 10.1038/bjc.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall P, Easton D. Breast cancer screening: time to target women at risk. Br J Cancer. 2013;108:2202–4. doi: 10.1038/bjc.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton H, Chowdhury S, Dent T, Hall A, Pashayan N, Pharoah P. Public health implications from cogs and potential for risk stratification and screening. Nat Genet. 2013;45:349–51. doi: 10.1038/ng.2582. [DOI] [PubMed] [Google Scholar]
- 14.Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120:2955–64. doi: 10.1002/cncr.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell A, Anderson AS, Clarke RB, et al. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014;16:446. doi: 10.1186/s13058-014-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PloS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet. 2001;358:1389–99. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 18.Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nat Genet. 2000;26:411–14. doi: 10.1038/82533. [DOI] [PubMed] [Google Scholar]
- 19.Mucci LA, Hjelmborg JB, Harris JR, et al. on behalf of the Nordic Twin Study of Cancer (NorTwinCan) Collaboration Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M. Genetic susceptibility to breast cancer. Mol Oncol. 2010;4:174–91. doi: 10.1016/j.molonc.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fachal L, Dunning AM. From candidate gene studies to gwas and post-gwas analyses in breast cancer. Curr Opin Genet Dev. 2015;30:32–41. doi: 10.1016/j.gde.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Easton D, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast cancer risk. N Engl J Med. 2015;372:2243–57. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–61. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michailidou K, Beesley J, Lindstrom S, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–80. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mavaddat N, Pharoah PD, Michailidou K, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107:djv036. doi: 10.1093/jnci/djv036. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pharoah PD, Antoniou AC, Easton DF, Ponder BA. Polygenes, risk prediction, and targeted prevention of breast cancer. N Engl J Med. 2008;358:2796–803. doi: 10.1056/NEJMsa0708739. [DOI] [PubMed] [Google Scholar]
- 27.phg Foundation . Stratified Screening for Cancer: Recommendations and Analysis from the COGS Project. Cambridge, UK: PHG foundation; 2014. [Available online at: http://www.phgfoundation.org/file/15380; cited 8 June 2015] [Google Scholar]
- 28.Darabi H, Czene K, Zhao W, Liu J, Hall P, Humphreys K. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 2012;14:R25. doi: 10.1186/bcr3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Closas M, Gunsoy NB, Chatterjee N. Combined associations of genetic and environmental risk factors: implications for prevention of breast cancer. J Natl Cancer Inst. 2014;106:dju305. doi: 10.1093/jnci/dju305. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer–Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2016;17:147. doi: 10.1186/s13058-015-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genome Canada . Large-Scale Applied Research Project Competition [Web page] Ottawa, ON; 2012. n.d. [Available at: https://www.genomecanada.ca/en/programs/large-scale-science/past-competitions/large-scale-research-project-competitions/2012-large; cited 8 June 2015] [Google Scholar]
- 32.European Commission . Towards an International Consortium for Personalised Medicine. Brussels, Belgium: European Commission; 2016. [Available online at: http://ec.europa.eu/research/conferences/2016/permed2016/pdf/towards_ic_permed.pdf; cited 9 October 2016] [Google Scholar]
- 33.United States, The White House . Fact Sheet: President Obama’s Precision Medicine Initiative [media release] Washington, DC: Office of the Press Secretary; 2015. [Available online at: http://www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative; cited 8 June 2015] [Google Scholar]
- 34.Koitsalu M, Sprangers MA, Eklund M, et al. Public interest and acceptability of the prospect of risk-stratified screening for breast and prostate cancer. Acta Oncol. 2016;55:45–51. doi: 10.3109/0284186X.2015.1043024. [DOI] [PubMed] [Google Scholar]
- 35.Meisel SF, Pashayan N, Rahman B, et al. Adjusting the frequency of mammography screening on the basis of genetic risk: attitudes among women in the UK. Breast. 2015;24:237–41. doi: 10.1016/j.breast.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans DG, Donnelly LS, Harkness EF, et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br J Cancer. 2016;114:1045–52. doi: 10.1038/bjc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schully SD, Khoury MJ. What is translational genomics? An expanded research agenda for improving individual and population health. Appl Transl Genom. 2014;3:82–3. doi: 10.1016/j.atg.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Risk Reduction. Fort Washington, PA: NCCN; 2014. Ver 1.2014. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf (free registration required); cited 8 June 2015] [Google Scholar]
- 39.U.K. National Institute for Health and Care Excellence (nice) Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. London, U.K.: NICE; 2013. [PubMed] [Google Scholar]
- 40.Cancer Care Ontario (cco) Information for Healthcare Providers on the Ontario Breast Screening Program (OBSP) Toronto, ON: CCO; 2013. [Google Scholar]
- 41.National Breast and Ovarian Cancer Centre . Advice About Familial Aspects of Breast Cancer and Epithelial Ovarian Cancer. 2nd ed. Surry Hills, Australia: Cancer Australia; 2010. [Current version available online at: https://canceraustralia.gov.au/system/tdf/publications/advice-about-familial-aspects-breast-cancer-and-epithelial-ovarian-cancer/pdf/2015_bog_familial_aspects_int.pdf?file=1&type=node&id=2878; cited 8 June 2015] [Google Scholar]
- 42.Foulkes WD, Knoppers BM, Turnbull C. Population genetic testing for cancer susceptibility: founder mutations to genomes. Nat Rev Clin Oncol. 2016;13:41–54. doi: 10.1038/nrclinonc.2015.173. [DOI] [PubMed] [Google Scholar]
- 43.Mavaddat N, Rebbeck TR, Lakhani SR, Easton DF, Antoniou AC. Incorporating tumour pathology information into breast cancer risk prediction algorithms. Breast Cancer Res. 2010;12:R28. doi: 10.1186/bcr2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham AP, Antoniou AC, Easton DF. Clinical software development for the Web: lessons learned from the boadicea project. BMC Med Inform Decis Mak. 2012;12:30. doi: 10.1186/1472-6947-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacInnis RJ, Bickerstaffe A, Apicella C, et al. Prospective validation of the breast cancer risk prediction model boadicea and a batch-mode version boadiceacentre. Br J Cancer. 2013;109:1296–301. doi: 10.1038/bjc.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC, on behalf of the Consortium of Investigators of Modifiers of BRCA1/2 and the Breast Cancer Association Consortium boadicea breast cancer risk prediction model: updates to cancer incidences, tumour pathology and Web interface. Br J Cancer. 2014;110:535–45. doi: 10.1038/bjc.2013.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee AJ, Cunningham AP, Tischkowitz, et al. Incorporating truncating variants in PALB2, CHEK2, and ATM into the boadicea breast cancer risk model. Genet Med. 2016 doi: 10.1038/gim.2016.31. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loblaw DA, Prestrud AA, Somerfield MR, et al. American Society of Clinical Oncology clinical practice guidelines: formal systematic review-based consensus methodology. J Clin Oncol. 2012;30:3136–40. doi: 10.1200/JCO.2012.42.0489. [DOI] [PubMed] [Google Scholar]
- 49.Gligorov J, Namer M. Juste dix ans: 5es recommandations francophones de Saint-Paul-de-Vence [editorial, French] Oncologie. 2013;15:565–6. doi: 10.1007/s10269-013-2358-7. [DOI] [Google Scholar]
- 50.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology practice guideline. J Clin Oncol. 2013;31:2942–62. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 51.Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 2013;8:102–9. doi: 10.1159/000351193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156:635–48. doi: 10.7326/0003-4819-156-9-201205010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 54.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081–7. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 55.D.E.N.S.E. State Efforts [Web page] Woodbury, CT: Are You Dense Advocacy; Are You DENSE? n.d. [Current version available online at: http://areyoudenseadvocacy.org/dense; cited 8 June 2015] [Google Scholar]
- 56.California State Senate . Senate Bill 1538: An Act to Add and Repeal Section 123222.3 of the Health and Safety Code, Relating to Mammograms. Sacramento, CA: California Legislative Information; 2012. [Available online at: http://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201120120SB1538; cited 8 June 2015] [Google Scholar]
- 57.D’Orsi CJ, Bassett LW, Berg WA, et al. bi-rads: mammography. In: D’Orsi CJ, Mendelson EB, Ikeda DM, et al., editors. Breast Imaging Reporting and Data System: ACR BI-RADS—Breast Imaging Atlas. 5th edition. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 58.Canadian Partnership Against Cancer (cpac) Breast Cancer Control in Canada: A System Performance Special Focus Report. Toronto, ON: CPAC; 2012. [Available online at: https://content.cancerview.ca/download/cv/quality_and_planning/system_performance/documents/breastcancercontrolreppdf?attachment=0; cited 8 June 2015] [Google Scholar]
- 59.Saslow D, Boetes C, Burke W, et al. on behalf of the American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with mri as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [Erratum in: CA Cancer J Clin 2007;57:185] [DOI] [PubMed] [Google Scholar]
- 60.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis. Fort Washington, PA: NCCN; 2015. Ver 1.2015. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf; cited 8 June 2015] [DOI] [PubMed] [Google Scholar]
- 61.Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol. 2016;23:e332–9. doi: 10.3747/co.23.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.