Abstract
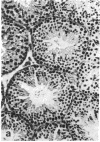
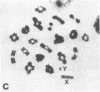
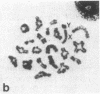
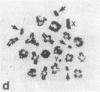
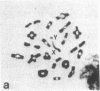
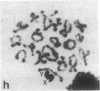
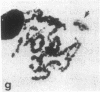
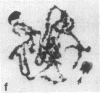
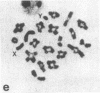
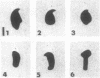
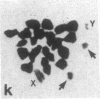
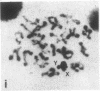
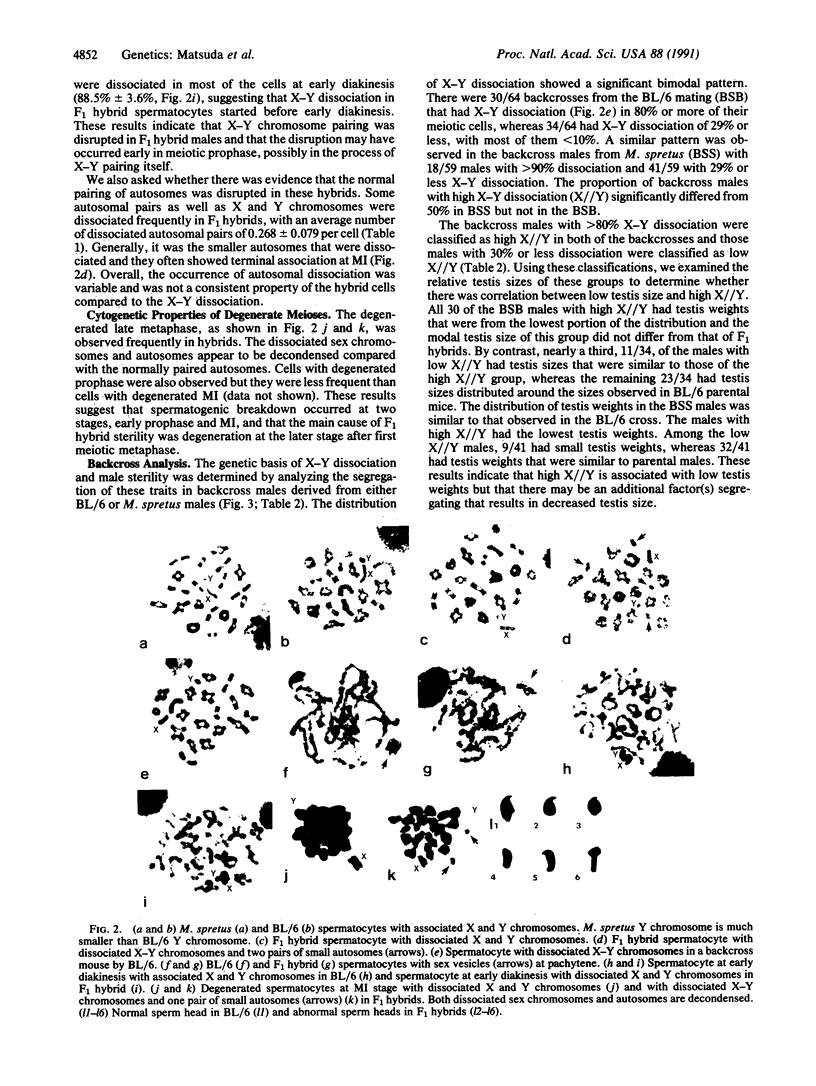
A high frequency of X-Y chromosome dissociation (95%) was found at first meiotic metaphase (MI) in spermatocytes of interspecific hybrids between laboratory mice, C57BL/6J (BL/6) and Mus spretus, compared with an X-Y dissociation frequency of only 3-4% in parental mice. The X-Y dissociation in F1 hybrids occurred before diakinesis rather than as a precocious dissociation at MI. The high X-Y dissociation was accompanied by spermatogenic breakdown after MI, resulting in male sterility. All F1 males were sterile and approximately half of the backcross males from fertile F1 females crossed with either BL/6 or M. spretus males were sterile. Male sterility was highly correlated with X-Y dissociation in both backcrosses. All of the mice with high X-Y dissociation were sterile and all of the males with low X-Y dissociation were fertile or subfertile. This correlation suggested that genetic divergence of the X-Y pairing region could contribute to the male sterile phenotype such that the BL/6 X chromosome would not pair with the M. spretus Y chromosome. The segregation of species-type alleles of amelogenin (Amelb and Amels), a distal X chromosome locus adjacent to the X-Y pairing region, was followed in backcross males that were analyzed for X-Y dissociation and sterility (we have used Amel as the designation for the mouse amelogenin locus; the current designation for this locus is Amg). A 95% concordance between Amelb with fertility and Amels with sterility was observed in backcrosses with BL/6, whereas the converse was observed in the backcross to M. spretus. These results imply that X-Y pairing plays an important role in male fertility and suggest that genetic divergence in X-Y pairing region between Mus species can contribute to the reproductive barriers between species and the process of speciation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avner P., Amar L., Arnaud D., Hanauer A., Cambrou J. Detailed ordering of markers localizing to the Xq26-Xqter region of the human X chromosome by the use of an interspecific Mus spretus mouse cross. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1629–1633. doi: 10.1073/pnas.84.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG J. W. Differential staining of spermatozoa in section of testis. Am J Clin Pathol. 1953 May;23(5):513–515. doi: 10.1093/ajcp/23.5_ts.513. [DOI] [PubMed] [Google Scholar]
- BORUM K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res. 1961 Sep;24:495–507. doi: 10.1016/0014-4827(61)90449-9. [DOI] [PubMed] [Google Scholar]
- Bonhomme F., Catalan J., Britton-Davidian J., Chapman V. M., Moriwaki K., Nevo E., Thaler L. Biochemical diversity and evolution in the genus Mus. Biochem Genet. 1984 Apr;22(3-4):275–303. doi: 10.1007/BF00484229. [DOI] [PubMed] [Google Scholar]
- Chandley A. C., Goetz P., Hargreave T. B., Joseph A. M., Speed R. M. On the nature and extent of XY pairing at meiotic prophase in man. Cytogenet Cell Genet. 1984;38(4):241–247. doi: 10.1159/000132070. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Keitz B. T., Disteche C. M., Lau E. C., Snead M. L. Linkage of amelogenin (Amel) to the distal portion of the mouse X chromosome. Genomics. 1991 May;10(1):23–28. doi: 10.1016/0888-7543(91)90479-x. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Quarantillo B. A. Electrophoretic variation for X chromosome-linked hypoxanthine phosphoribosyl transferase (HPRT) in wild-derived mice. Genetics. 1983 Apr;103(4):785–795. doi: 10.1093/genetics/103.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A. The genetic basis of Haldane's rule. 1985 Apr 25-May 1Nature. 314(6013):736–738. doi: 10.1038/314736a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gollapudi B. B., Kamra O. P., Blecher S. R. A search for a genetic basis for gonosomal univalency in mice. Cytogenet Cell Genet. 1981;29(4):241–249. doi: 10.1159/000131575. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Cooper J. E., Mace M. L., Jr, Brinkley B. R. Arrangement of centromeres in mouse cells. Chromosoma. 1971;34(1):73–87. doi: 10.1007/BF00285517. [DOI] [PubMed] [Google Scholar]
- Imai H. T., Matsuda Y., Shiroishi T., Moriwaki K. High frequency fo X-Y chromosome dissociation in primary spermatocytes of F1 hybrids between Japanese wild mice (Mus musculus molossinus) and inbred laboratory mice. Cytogenet Cell Genet. 1981;29(3):166–175. doi: 10.1159/000131565. [DOI] [PubMed] [Google Scholar]
- Imai H. T., Wada M. Y., Moriwaki K. The sex chromosome association (Sxa) gene is located on the X-chromosome in mice. Jpn J Genet. 1990 Apr;65(2):65–69. doi: 10.1266/jjg.65.65. [DOI] [PubMed] [Google Scholar]
- Joseph A. M., Chandley A. C. The morphological sequence of XY pairing in the Norway rat Rattus norvegicus. Chromosoma. 1984;89(5):381–386. doi: 10.1007/BF00331256. [DOI] [PubMed] [Google Scholar]
- Kofman-Alfaro S., Chandley A. C. Meiosis in the male mouse. An autoradiographic investigation. Chromosoma. 1970;31(4):404–420. doi: 10.1007/BF00285832. [DOI] [PubMed] [Google Scholar]
- Lifschytz E., Lindsley D. L. The role of X-chromosome inactivation during spermatogenesis (Drosophila-allocycly-chromosome evolution-male sterility-dosage compensation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):182–186. doi: 10.1073/pnas.69.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Imai H. T., Moriwaki K., Kondo K., Bonhomme F. X-Y chromosome dissociation in wild derived Mus musculus subspecies, laboratory mice, and their F1 hybrids. Cytogenet Cell Genet. 1982;34(3):241–252. doi: 10.1159/000131811. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Imai H. T., Moriwaki K., Kondo K. Modes of inheritance of X-Y dissociation in inter-subspecies hybrids between BALB/c mice and Mus musculus molossinus. Cytogenet Cell Genet. 1983;35(3):209–215. doi: 10.1159/000131868. [DOI] [PubMed] [Google Scholar]
- McKee B. D., Karpen G. H. Drosophila ribosomal RNA genes function as an X-Y pairing site during male meiosis. Cell. 1990 Apr 6;61(1):61–72. doi: 10.1016/0092-8674(90)90215-z. [DOI] [PubMed] [Google Scholar]
- Miklos G. L. Sex-chromosome pairing and male fertility. Cytogenet Cell Genet. 1974;13(6):558–577. doi: 10.1159/000130307. [DOI] [PubMed] [Google Scholar]
- Moses M. J., Counce S. J., Paulson D. F. Synaptonemal complex complement of man in spreads of spermatocytes, with details of the sex chromosome pair. Science. 1975 Jan 31;187(4174):363–365. [PubMed] [Google Scholar]
- Moses M. J. Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). II. Morphology of the XY pair in spread preparations. Chromosoma. 1977 Mar 16;60(2):127–137. doi: 10.1007/BF00288460. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Grant S. G., Stephenson D. A., Chapman V. M. Multilocus molecular mapping of the mouse X chromosome. Genomics. 1988 Oct;3(3):187–194. doi: 10.1016/0888-7543(88)90078-x. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Stephenson D. A., Grant S. G., Chapman V. M. Efficient linkage of 10 loci in the proximal region of the mouse X chromosome. Genomics. 1990 May;7(1):19–30. doi: 10.1016/0888-7543(90)90514-u. [DOI] [PubMed] [Google Scholar]
- Pietras D. F., Bennett K. L., Siracusa L. D., Woodworth-Gutai M., Chapman V. M., Gross K. W., Kane-Haas C., Hastie N. D. Construction of a small Mus musculus repetitive DNA library: identification of a new satellite sequence in Mus musculus. Nucleic Acids Res. 1983 Oct 25;11(20):6965–6983. doi: 10.1093/nar/11.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polani P. E. Centromere localization at meiosis and the position of chiasmata in the male and female mouse. Chromosoma. 1972;36(4):343–374. doi: 10.1007/BF00336793. [DOI] [PubMed] [Google Scholar]
- Rapp M., Therman E., Denniston C. Nonpairing of the X and Y chromosomes in the spermtocytes of BDF1 mice. Cytogenet Cell Genet. 1977;19(2-3):85–93. doi: 10.1159/000130800. [DOI] [PubMed] [Google Scholar]
- Schnedl W. End-to-end association of X and Y chromosomes in mouse meiosis. Nat New Biol. 1972 Mar 8;236(62):29–30. doi: 10.1038/newbio236029a0. [DOI] [PubMed] [Google Scholar]
- Snead M. L., Lau E. C., Zeichner-David M., Fincham A. G., Woo S. L., Slavkin H. C. DNA sequence for cloned cDNA for murine amelogenin reveal the amino acid sequence for enamel-specific protein. Biochem Biophys Res Commun. 1985 Jun 28;129(3):812–818. doi: 10.1016/0006-291x(85)91964-3. [DOI] [PubMed] [Google Scholar]
- Snead M. L., Zeichner-David M., Chandra T., Robson K. J., Woo S. L., Slavkin H. C. Construction and identification of mouse amelogenin cDNA clones. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7254–7258. doi: 10.1073/pnas.80.23.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari A. J. The spatial relationship of the X and Y chromosomes during meiotic prophase in mouse spermatocytes. Chromosoma. 1970;29(2):217–236. doi: 10.1007/BF00326080. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tres L. L. Extensive pairing of the XY bivalent in mouse spermatocytes as visualized by whole-mount electron microscopy. J Cell Sci. 1977 Jun;25:1–15. doi: 10.1242/jcs.25.1.1. [DOI] [PubMed] [Google Scholar]
- de Boer P., Nijhoff J. H. Incomplete sex chromosome pairing of oligospermic male hybrids of Mus musculus and M. musculus molossinus in relation to the source of the Y chromosome and the presence or absence of a reciprocal translocation. J Reprod Fertil. 1981 May;62(1):235–243. doi: 10.1530/jrf.0.0620235. [DOI] [PubMed] [Google Scholar]