Abstract
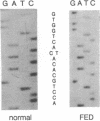
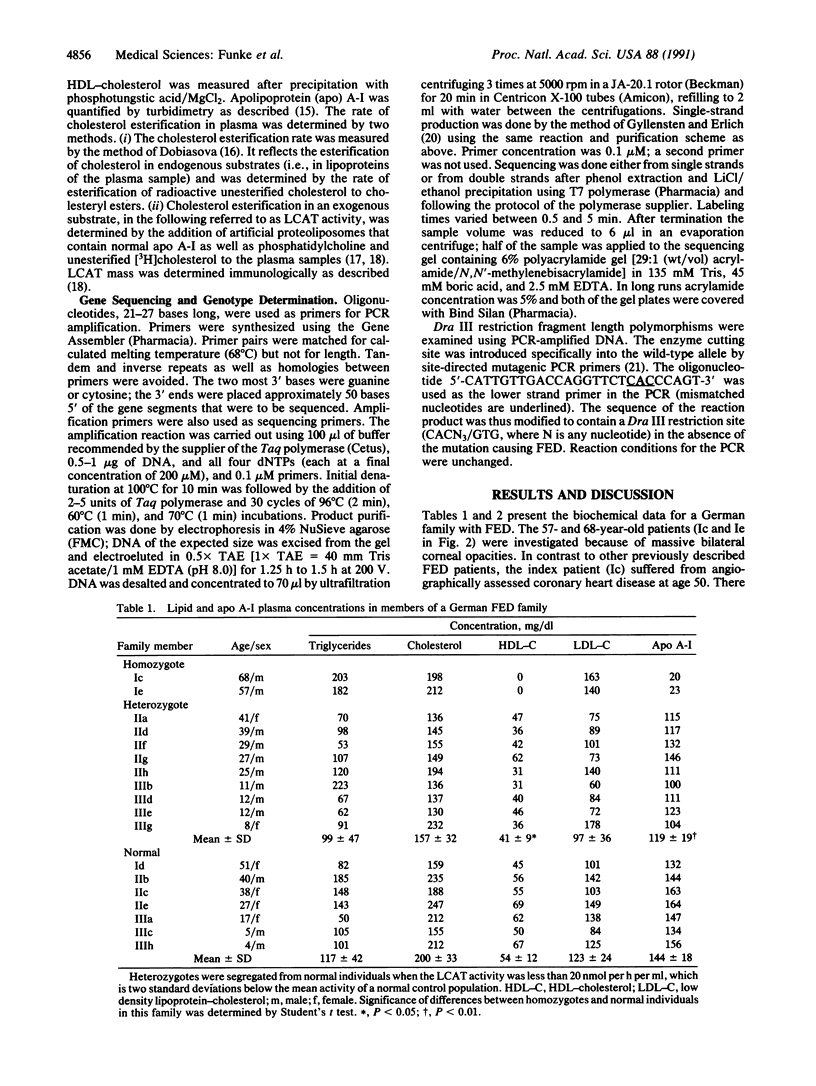
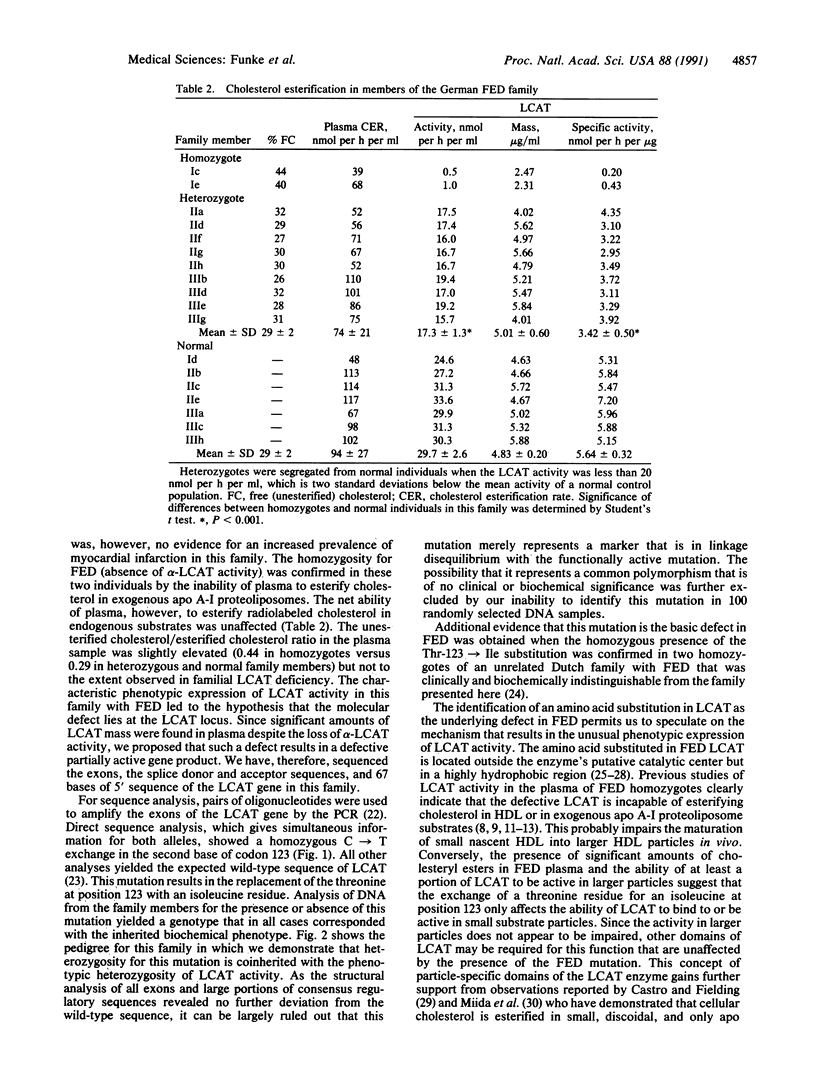
Epidemiological as well as biochemical evidence of recent years has established that a low plasma level of high density lipoprotein-cholesterol is a predictor for the risk of coronary artery disease. However, there is a heterogeneous group of rare familial disorders, characterized by severe high density lipoprotein deficiency, in which the predicted increased risk is not clearly apparent. One such disorder has been called fish eye disease to reflect the massive corneal opacification seen in these patients. In this report, we describe the biochemical and genetic presentation of two German fish eye disease homozygotes and their family members. Vertical transmission of a decrease in the specific activity of lecithin-cholesterol acyltransferase (EC 2.3.1.43) indicated that this enzyme was a candidate gene for harboring the defect responsible for this disorder. Direct sequencing of DNA segments amplified by the polymerase chain reaction (PCR) that encode the exons of the lecithin-cholesterol acyltransferase gene led to the identification of a homozygous mutation resulting in the substitution of threonine at codon 123 for an isoleucine residue in both individuals. Family analysis in an extended pedigree was used to establish a causal relationship between this mutation and the biochemical phenotype for fish eye disease. The homozygous presence of this mutation in two phenotypically homozygous members of an unrelated Dutch family with fish eye disease further supports this finding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Adolphson J. L., Chen C. H. Radioimmunoassay of human plasma lecithin-cholesterol acyltransferase. J Clin Invest. 1981 Jan;67(1):141–148. doi: 10.1172/JCI110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Evidence for the presence in human plasma of lecithin: cholesterol acyltransferase activity (beta-LCAT) specifically esterifying free cholesterol of combined pre-beta- and beta-lipoproteins. Studies of fish eye disease patients and control subjects. Acta Med Scand. 1985;218(2):197–205. doi: 10.1111/j.0954-6820.1985.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Paradoxical esterification of plasma cholesterol in fish eye disease. Acta Med Scand. 1985;217(5):491–499. doi: 10.1111/j.0954-6820.1985.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 1988 Jan 12;27(1):25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Albers J. J. Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. J Lipid Res. 1982 Jul;23(5):680–691. [PubMed] [Google Scholar]
- Dobiásová M. Lecithin: cholesterol acyltransferase and the regulation of endogenous cholesterol transport. Adv Lipid Res. 1983;20:107–194. [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J., Havel R. J., Kane J. P., Tun P. Cholesterol net transport, esterification, and transfer in human hyperlipidemic plasma. J Clin Invest. 1983 Mar;71(3):449–460. doi: 10.1172/JCI110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- Frohlich J., Hoag G., McLeod R., Hayden M., Godin D. V., Wadsworth L. D., Critchley J. D., Pritchard P. H. Hypoalphalipoproteinemia resembling fish eye disease. Acta Med Scand. 1987;221(3):291–298. doi: 10.1111/j.0954-6820.1987.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Karas M., Albers J. J., Assmann G. A frameshift mutation in the human apolipoprotein A-I gene causes high density lipoprotein deficiency, partial lecithin: cholesterol-acyltransferase deficiency, and corneal opacities. J Clin Invest. 1991 Jan;87(1):371–376. doi: 10.1172/JCI114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Rifkind B. M. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989 Nov 9;321(19):1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen M., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. An elucidation of the catalytic mechanism. J Biol Chem. 1986 May 25;261(15):7032–7043. [PubMed] [Google Scholar]
- Jauhiainen M., Stevenson K. J., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. The vicinal nature of cysteine 31 and cysteine 184 in the catalytic site. J Biol Chem. 1988 May 15;263(14):6525–6533. [PubMed] [Google Scholar]
- Karathanasis S. K., Ferris E., Haddad I. A. DNA inversion within the apolipoproteins AI/CIII/AIV-encoding gene cluster of certain patients with premature atherosclerosis. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7198–7202. doi: 10.1073/pnas.84.20.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Dunn L. L. Designed diagnostic restriction fragment length polymorphisms for the detection of point mutations in ras oncogenes. Oncogene Res. 1989;4(3):235–241. [PubMed] [Google Scholar]
- McLean J., Wion K., Drayna D., Fielding C., Lawn R. Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression. Nucleic Acids Res. 1986 Dec 9;14(23):9397–9406. doi: 10.1093/nar/14.23.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miida T., Fielding C. J., Fielding P. E. Mechanism of transfer of LDL-derived free cholesterol to HDL subfractions in human plasma. Biochemistry. 1990 Nov 20;29(46):10469–10474. doi: 10.1021/bi00498a007. [DOI] [PubMed] [Google Scholar]
- Miller J. J., Gaffney P. R., Rees J. A., Symes M. O. Lymphocyte reactivity in patients with carcinoma of the breast and large bowel. Br J Cancer. 1975 Jul;32(1):16–20. doi: 10.1038/bjc.1975.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Muller C. A., Lubbe W. F. Cyclic A.M.P. and arrhythmias revisited. Lancet. 1978 Oct 28;2(8096):921–923. doi: 10.1016/s0140-6736(78)91634-3. [DOI] [PubMed] [Google Scholar]
- Ordovas J. M., Cassidy D. K., Civeira F., Bisgaier C. L., Schaefer E. J. Familial apolipoprotein A-I, C-III, and A-IV deficiency and premature atherosclerosis due to deletion of a gene complex on chromosome 11. J Biol Chem. 1989 Oct 5;264(28):16339–16342. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sandkamp M., Tambyrajah B., Schriewer H., Assmann G. Simplified turbidimetric determination of apolipoproteins A-I, A-II and B using a microtitre method. J Clin Chem Clin Biochem. 1988 Nov;26(11):685–688. doi: 10.1515/cclm.1988.26.11.685. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Manoogian D., Pao Q., Lee F. S., Knapp R. D., Gotto A. M., Jr, Pownall H. J. Lecithin:cholesterol acyltransferase. Functional regions and a structural model of the enzyme. J Biol Chem. 1987 Mar 5;262(7):3086–3091. [PubMed] [Google Scholar]