Summary
Restoration of anti-tumor immunity by blocking PD-L1 signaling using antibodies has proven to be beneficial in cancer therapy. Here we show that BET bromodomain inhibition suppresses PD-L1 expression and limits tumor progression in ovarian cancer. CD274 (encoding PD-L1) is a direct target of BRD4-mediated gene transcription. In mouse models, treatment with the BET inhibitor JQ1 significantly reduced PD-L1 expression on tumor cells and tumor-associated dendritic cells and macrophages, which correlated with an increase in the activity of anti-tumor cytotoxic T cells. The BET inhibitor limited tumor progression in a cytotoxic T cell dependent manner. Together, these data demonstrate a small molecule approach to block PD-L1 signaling. Given the fact that BET inhibitors have been proven safe with manageable reversible toxicity in clinical trials, our findings indicate that pharmacological BET inhibitors represent a treatment strategy for targeting PD-L1 expression.
Introduction
Tumors evade anti-tumor immunity by inhibitory pathways that regulate the function of T lymphocytes, known as immune checkpoints (Topalian et al., 2015). Programmed cell death (PD)-1 protein is predominantly expressed on the surface of T cells, while its ligands such as PD-L1 are expressed on the surface of both cancer cells and immune cells (Zou et al., 2016). Interaction between PD-1 and PD-L1 inhibits T-cell activity, which reduces T-cell mediated cytolysis. Therefore, inhibiting this interaction could result in increased anti-tumor immunity. Indeed, blockade of immune checkpoints by antibodies has demonstrated remarkable activity in several cancer types (Mahoney et al., 2015). For example, antibody-based blockage of PD-1 and PD-L1 signaling is therapeutically beneficial in an expanding list of malignancies (Zou et al., 2016). Despite these anti-tumor benefits, checkpoint blockade using these antibodies is associated with unique adverse effects known as immune-related adverse events (irAEs) due to nonspecific immunologic activation (Naidoo et al., 2015). Prolonged immunosuppression, often required to treat irAEs, predisposes patients to infections.
PD-L1 is associated with prognosis in several cancer types. PD-L1 expression predicts a better prognosis in ovarian cancer (Webb et al., 2016), which remains the most lethal gynecological malignancy in the developed world. Blockade of PD-1/PD-L1 signaling enhances the amplitude of anti-tumor immunity in ovarian cancer (Abiko et al., 2013; Cubillos-Ruiz et al., 2009). PD-L1 expression correlates with clinical response to anti-PD-1/L1 therapy (Zou et al., 2016). Despite the importance of PD-L1 in tumor immunity, the regulation of PD-L1 expression remains poorly understood. DNA hypomethylating agents such as azacytidine increase PD-L1 expression in non-small cell lung cancer (Wrangle et al., 2013). This suggests that chromatin modifiers including writers, readers and erasers (i.e., epigenetic mechanisms) play a critical role in regulating PD-L1 expression. Whether agents that target epigenetic regulators could be used to inhibit PD-L1 signaling remains to be explored.
The bromodomain and extraterminal (BET) protein BRD4 directly binds to acetylated lysine on histone tails and other nuclear proteins to promote gene transcription by RNA polymerase II (Pol II) (Filippakopoulos and Knapp, 2014). Specific BET inhibitors have been developed. Clinical trials in hematopoietic malignancies have demonstrated the anti-tumor activity of BET inhibitors with a manageable toxicity prolife (Filippakopoulos and Knapp, 2014). Here we show that inhibition of BRD4 suppresses PD-L1 expression and increases cytotoxic T cell activity to limit tumor progression in vivo in ovarian cancer models. Our findings establish an immune checkpoint targeting approach by repurposing existing pharmacological BET inhibitors.
Results
BET inhibitors suppress PD-L1 expression
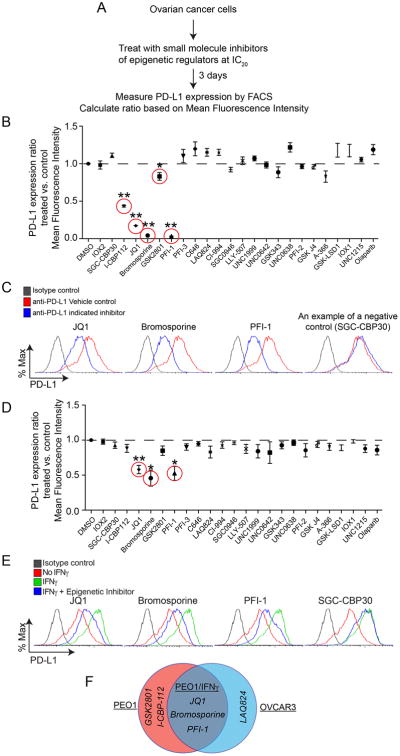
Given the importance of targeting PD-L1 in anti-tumor immunity and the poorly understood nature of its regulation, we evaluated a panel of 24 small molecule inhibitors known to target epigenetic regulators (obtained from The Structure Genomics Consortium) to identify “hits” that suppress the expression of PD-L1. As upregulation of PD-L1 is known to play a critical role in ovarian cancer (Abiko et al., 2013), we focused on epithelial ovarian cancer (EOC) cell lines. To identify suitable cell models for the small molecule screen, we examined PD-L1 expression in a panel of EOC cell lines: PEO1, OVCAR3, OVCAR10, PEO4 and Kuramochi. PEO1 and OVCAR3 cells express high levels of PD-L1 (Figure S1A-B) and were used for the screen. To limit the potential bias introduced by variation in growth inhibition induced by the small molecule inhibitors, we established a growth inhibition curve for each small molecule inhibitor. We used the established IC20 value of each small molecule inhibitor (Table S1). The highest dose tested (20 μM) was used for those inhibitors whose IC20 was not achieved (Figure 1A and Table S1). Using flow cytometric (FACS) analysis, we measured the fold change in PD-L1 expression based on mean fluorescence intensity for each of the 24 inhibitors (Figure 1B). This analysis identified a list of 5 inhibitors that significantly suppressed PD-L1 expression in PEO1 cells. Similar analyses in OVCAR3 cells revealed a list of 4 inhibitors that significantly suppressed PD-L1 expression (Figure S1C). The top three “hits” for reducing PD-L1 expression in both cell lines are BET inhibitors: JQ1, Bromosporine and PFI-1 (Figures 1B and S1C). Inhibition of PD-L1 was specific to BET inhibitors but not bromodomain inhibitors in general because other bromodomain inhibitors such as SGC-CBP30 did not significantly reduce PD-L1 expression (Figure 1C).
Figure 1. BET inhibitors suppress PD-L1 expression in ovarian cancer cells. See also Figure S1 and Table S1.
(A) Flow diagram of experimental design.
(B) Plot of the ratio of PD-L1 expression on PEO1 cells treated with doses of the indicated epigenetic inhibitors or vehicle controls as detailed in Table S1. ** p<0.0001; * p<0.04.
(C) Representative changes in PD-L1 expression determined by FACS on PEO1 cells treated with the indicated BET inhibitors. SGC-CBP30 was used as a negative control.
(D) Same as (B), but for IFNγ stimulated (20ng/L, 24h) PEO1 cells** p<0.002; * p<0.02.
(E) Representative changes in PD-L1 expression determined by FACS on PEO1 cells stimulated with IFNγ (20ng/L, 24h) and treated with the indicated BET inhibitors. SGC-CBP30 was used as a negative control.
(F) Venn diagram of “hits” that suppress PD-L1 expression from the three indicated screens.
Error bars represent S.E.M. of three independent experiments.
IFNγ induces PD-L1 expression. As a secondary screen, we determined the effects of the same panel of epigenetic inhibitors on PD-L1 expression in cells treated with IFNγ (Figure 1D). This screen revealed that only the same three BET inhibitors significantly suppressed PD-L1 expression in the presence of IFNγ stimulation (Figures 1D-F). BET inhibitor-induced suppression of PD-L1 was not due to changes in IFNγ secretion because EOC cell lines did not secrete detectable levels of IFNγ, and JQ1 did not affect the secretion of IFNγ (data not shown). Thus, we identified BET inhibitors as suppressors of PD-L1 expression.
BET inhibition reduces PD-L1 expression at the transcriptional level in a dose and time-dependent manner
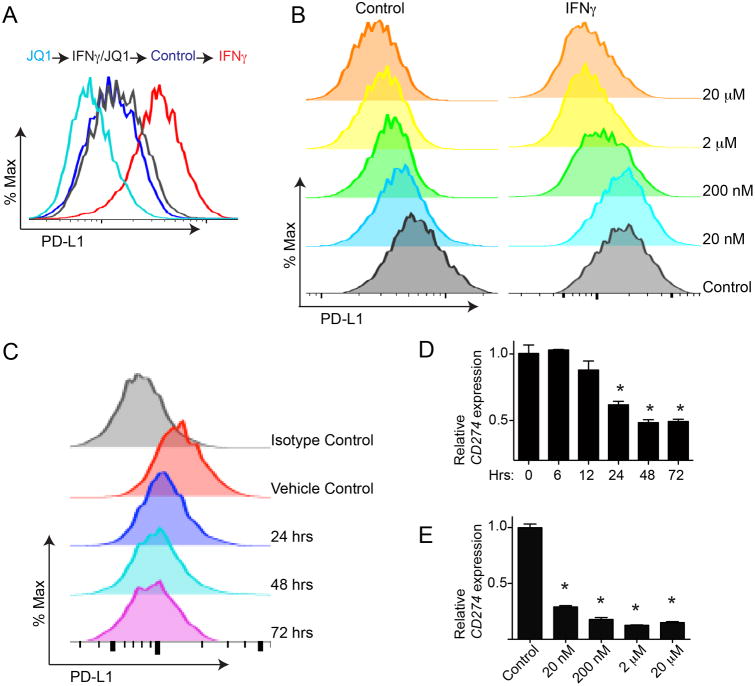
As JQ1 is clinically applicable (known as TEN-010 in clinical trials), we performed further validation on this inhibitor. We demonstrated that JQ1 treatment decreased PD-L1 expression with or without IFNγ stimulation (Figures 2A and S2A). JQ1 reduced PD-L1 expression in a dose-dependent manner (Figure 2B). Similar dose-dependent suppression of PD-L1 expression was also observed in IFNγ stimulated cells (Figure 2B). We also observed a time-dependent suppression of PD-L1 expression by JQ1 where suppression of PD-L1 expression was observed 24 hours post treatment (Figure 2C and S2B). Notably, expression of CD274, the gene encoding PD-L1, was reduced in a dose and time-dependent manner that mirrors PD-L1 downregulation induced by JQ1 (Figure 2D-E and S2C). This indicates that suppression of PD-L1 expression by JQ1 occurs at the transcriptional level. Notably, JQ1 reduced PD-L1 expression at a dose (e.g., 20 nM) that did not affect the growth of treated cells (Figure S2D-E). This suggests that the observed reduction in PD-L1 expression is not a consequence of growth inhibition induced by JQ1 (Figure 2B, 2E and S2D-E). Thus, we conclude that JQ1 reduces PD-L1 expression at the transcriptional level in a dose- and time-dependent manner.
Figure 2. BET inhibitor JQ1 suppresses PD-L1 expression at the transcriptional level. See also Figure S2.
(A) PEO1 cells stimulated with or without IFNγ (20ng/L, 24h) were treated with or without 200 nM JQ1 for 72 hours. PD-L1 expression was determined by FACS.
(B) PEO1 cells with or without IFNγ (20ng/mL, 24h) stimulation were treated with indicated doses of JQ1 for 72 hours. PD-L1 expression was determined by FACS.
(C) PEO1 cells were treated with 200 nM JQ1. PD-L1 expression was determined by FACS at the indicated time points.
(D) PEO1 cells were treated with 200 nM JQ1. mRNA expression of CD274 (encoding PD-L1) was determined by qRT-PCR at the indicated time points.* p<0.05.
(E) PEO1 cells were treated with the indicated doses of JQ1 for 72 hours, and CD274 (encoding PD-L1) mRNA expression was determined by qRT-PCR. * p<0.05.
Error bars represent S.E.M. of three independent experiments.
CD274 is a direct target gene of BRD4
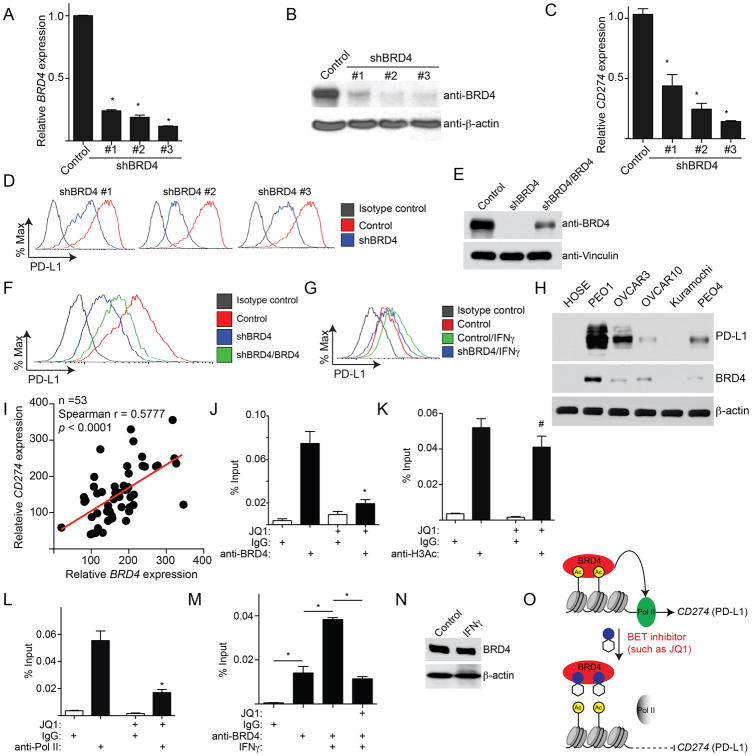
BRD4 is often amplified in ovarian cancer and is a major target of JQ1 (Baratta et al., 2015; Goundiam et al., 2015). To determine whether genetic knockdown of BRD4 directly regulates PD-L1 expression, BRD4 was knocked down using three individual shRNAs (shBRD4s) (Figure 3A-B). All three shBRD4s efficiently knocked down BRD4 expression and decreased PD-L1 expression (Figure 3A-D). Similar results were observed in multiple EOC cell lines (Figure S3A-C). The observed decrease in PD-L1 expression was rescued by the expression of a shBRD4 resistant wild type BRD4 (Figure 3E-F). In addition, BRD4 knockdown also reduced PD-L1 expression in IFNγ stimulated cells (Figure 3G).
Figure 3. CD274 is a direct target gene of BRD4. See also Figure S3 and Table S2.
(A) PEO1 cells expressing shBRD4 or control were examined for BRD4 expression by qRT-PCR. * p<0.05.
(B) Same as (A), but examined for the expression of BRD4 protein by immunoblotting.
(C) Same as (A), but examined for CD274 mRNA expression by qRT-PCR. * p<0.05.
(D) Same as (A). PD-L1 expression was determined by FACS.
(E) PEO1 cells expressing shBRD4 (#3) that targets the 3′ UTR region of the human BRD4 gene with or without simultaneous expression of a wild-type BRD4 open reading frame. BRD4 and Vinculin expression was determined by immunoblot.
(F) Same as (E), but examined for PD-L1 expression by FACS.
(G) PEO1 cells expressing shBRD4 (#3) or control with or without IFNγ (20ng/L, 24h) stimulation were examined for PD-L1 expression by FACS.
(H) Expression of BRD4, PD-L1 and β-actin were examined in the indicated ovarian cancer cell lines or normal human ovarian surface epithelial cells by immunoblot.
(I) Correlation between BRD4 and CD274 expression was determined by Spearman statistical analysis in 53 cases of laser capture and microdissected high-grade serous ovarian cancer specimens.
(J-L) PEO1 cells were treated with or without 200 nM JQ1 for 24 hours. The cells were subjected to ChIP analysis using antibodies against: BRD4 (J), H3Ac (K), or Pol II (L). An isotype matched IgG was used as a negative control. The association with the CD274 gene promoter was quantified by qPCR. # p>0.05 and * p<0.05.
(M) Same as (J), but for PEO1 cells with or without IFNγ (20ng/mL, 24h) stimulation were treated with JQ1. * p<0.05.
(N) Same as (M), but examined for BRD4 and β-actin protein expression by immunoblot.
(O) A model for BRD4-mediated regulation of PD-L1 expression. Error bars represent S.E.M. of three independent experiments.
We next profiled the global changes in mRNA expression induced by JQ1 or shBRD4 in PEO1 cells by QuantSeq (Geo Accession Number: GSE81698). Notably, CD274 expression was downregulated ∼4-fold by both JQ1 and shBRD4 in this analysis (Figure S3D). Pathway enrichment analysis on significantly changed genes revealed biological processes including lymphocyte chemotaxis and inflammatory response (Table S2).
We next determined whether BRD4 correlates with PD-L1 expression in EOC. Using a panel of EOC cell lines, we observed a trend towards a positive correlation between BRD4 and PD-L1 expression (Figure 3H). We examined whether BRD4 and CD274 expression is positively correlated in EOC specimens. We used a published database that profiled gene expression in 53 cases of laser capture and microdissected (LCM) high-grade serous EOCs (Mok et al., 2009). Indeed, there was a significant, positive correlation between BRD4 and CD274 in EOCs (Figure 3I, P<0.0001).
We next determined whether CD274 is a direct target gene of BRD4. BRD4 chromatin immunoprecipitation followed by next generation sequencing (ChIP-seq) in OVCAR3 cells revealed that BRD4 is enriched at the CD274 gene promoter (Figure S3E). ChIP analysis showed a significant association of BRD4 with the CD274 promoter, which was decreased with JQ1 treatment (Figure 3J and S3F). Notably, JQ1 treatment did not significantly reduce acetylated H3 levels at the CD274 promoter (Figure 3K). The observed JQ1-mediated suppression of PD-L1 correlated with decreased association of RNA Pol II with the CD274 promoter (Figure 3L). We next determined whether upregulation of PD-L1 expression by IFNγ correlates with increased association of BRD4 at the CD274 promoter. Indeed, IFNγ treatment enhanced the association of BRD4 with the CD274 promoter, which was reduced by JQ1 treatment (Figure 3M). This was not due to the upregulation of BRD4 by IFNγ because we did not observe an increase in BRD4 protein expression in cells treated with IFNγ (Figure 3N). Together, these data support the notion that CD274 is a direct target gene of BRD4, which is subject to JQ1-mediated repression at the transcriptional level (Figure 3O).
The BET inhibitor JQ1 limits tumor progression in a cytotoxic T cell dependent manner
Besides tumor cells, PD-L1 is expressed on tumor-associated immune cells such as regulatory dendritic cells (DCs) and macrophages in ovarian cancer (Scarlett et al., 2012). Notably, PD-L1 expression on both DCs and macrophages and the tumor cells is important for evading anti-tumor immunity (Zou et al., 2016). Thus, we determined that JQ1 decreased PD-L1 expression on mouse bone marrow derived DCs (Figure S4A).
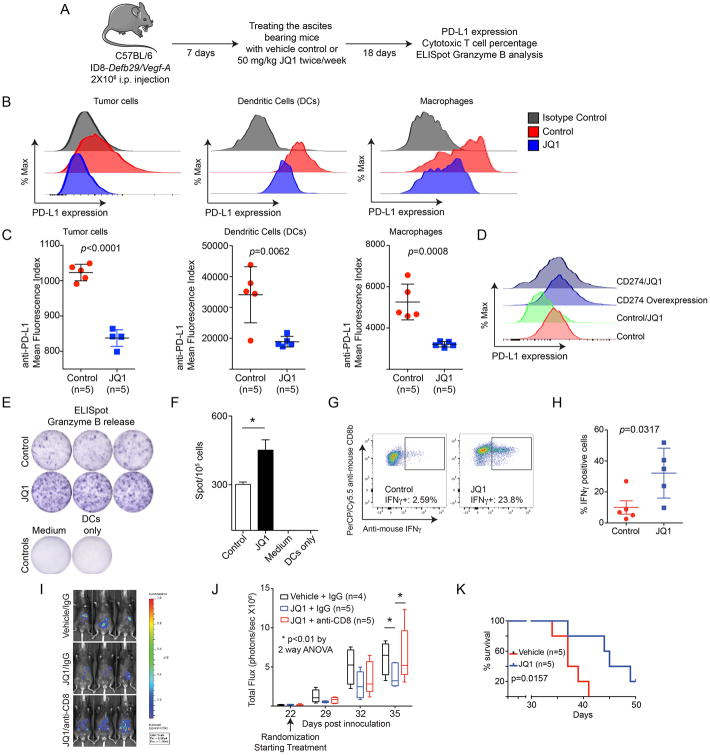
To determine the effects of BET inhibitors on PD-L1 expression and anti-tumor immunity in vivo, we utilized the ID8-Defb29/Vegf-a syngeneic mouse model. This model recapitulates the aggressive inflammatory microenvironment of human ovarian carcinomas, and PD-L1 signaling is critical for cancer progression in this model (Cubillos-Ruiz et al., 2009). For in vivo experiments, the injected mice were allowed to develop ascites for 7 days and treated with 50 mg/kg JQ1 or vehicle control twice weekly for 18 additional days (Figure 4A). PD-L1 expression on both immune and tumor cells from peritoneal washes was examined. Indeed, there was a significant decrease in PD-L1 expression on immune cells such as DCs and macrophages isolated from JQ1 treated mice compared with controls (Figure 4B-C). PD-L1 expression on the tumor cells was also significantly reduced by JQ1 treatment (Figure 4B-C). Notably, the observed JQ1-mediated reduction in PD-L1 expression on tumor cells was overcome by overexpressing CD274 in tumor cells (Figure 4D and S4B). As a control, CD274 overexpression in tumor cells did not affect the suppression of PD-L1 expression by JQ1 in DCs (Figure S4C). This indicates that the observed reduction in PD-L1 by JQ1 in vivo is due to its suppression of endogenous PD-L1 instead of an indirect effect. The dose of JQ1 used was significantly lower than those of previous studies (Filippakopoulos et al., 2010), which did not significantly reduce the percentage of CD8+ cytotoxic T cells (Figure S4D-E). Thus, we can achieve a dose of JQ1 that suppresses PD-L1 expression on both immune cells such as DCs and macrophages and tumor cells without affecting the survival of cytotoxic T cells.
Figure 4. JQ1 decreases PD-L1 expression and limits tumor progression in a cytotoxic T cell dependent manner in vivo. See also Figure S4.
(A) Flow diagram of experimental design. These experiments were repeated three times.
(B) 2×106 total cells from peritoneal wash were subjected to staining using antibodies against CD45.2-PE/Cy7, CD11c-APC/Cy7, F4/80-PerCP/Cy5.5, MHCII-PE and PDL1-APC. PD-L1 expression was determined by FACS. CD45.2-PE/Cy7 negative cells were gated as tumor cells. Among CD45.2-PE/Cy7 positive cells, MHCII and CD11c double positive cells were gated as dendritic cells, while MHCII and F4/80 positive cells were gated as macrophages.
(C) Quantification of PD-L1 expression in indicated cell populations.
(D) C57BL/6 mice were injected i.p. with ID8-Defb29/Vegf-a cells with or without CD274 overexpression. Mice were randomized and treated with 50 mg/kg JQ1 twice every week for 18 days or vehicle controls. Cells from peritoneal wash were subjected to analysis for PD-L1 expression using the same approach as detailed in (B). Shown is PD-L1 expression in tumor cells (CD45.2-PE/Cy7 negative) from the indicated groups. Due to low cell numbers, cells from 5 mice in each of the indicated groups were combined for FACS analysis.
(E) Granzyme B ELISpot assay of total cells from peritoneal washes. 1×105 total cells obtained from peritoneal wash were subjected to analysis. Representative image of positive spot from the indicated groups is shown.
(F) Quantification of (e). * p <0.05.
(G) 2 × 106 Luciferase-expressing ID8 cells were orthotopically transplanted into C57BL/6 mice by i.p. injection. The transplanted tumors were allowed to establish for 22 days. The mice were then randomized and treated with 50 mg/kg JQ1 twice a week with or without an anti-CD8 antibody 500 μg/mice once a week (vehicle controls n=4; JQ1/IgG n=5 and JQ1/anti-CD8 n=5). The percentage of IFNγ CD8 T cells was analyzed by intracellular staining. Shown are representative results of IFNγ+ cells in live CD45+CD3+CD8+ in the indicated groups.
(H) Quantification of (G).
(I) Same as (G). Tumor growth was monitored by luciferase imaging. Shown are representative images taken at the end of the experiment (day 35).
(J) Same as (I). Tumor growth in the indicated groups was monitored by luciferase imaging, and total flux for each of the indicated groups is depicted as a box and whiskers plot that indicates the rage of minimum to maximum total flux of individual mice from each of the indicated groups.
(K) 2 × 106 UPK10 cells were orthotopically-transplanted into C57BL/6 mice by i.p. injection. The tumor bearing mice received 1 × 106 tumor antigen-primed T cells on day 7 and 14. The mice were randomized, treated on day 5 and 12 with or without 50 mg/kg JQ1, and followed for survival (n=5). Kaplan-Meier survival curves of the indicated groups. p value was calculated by Log-rank Mantel-Cox test.
We next determined the effects of JQ1 on CD8+ cytotoxic T cell activity. We observed an increased number of tumor-associated T cells that secreted Granzyme B as determined by ELISpot analysis in JQ1 treated mice compared to controls (Figure 4E-F). Similar results were also obtained with an independent syngeneic mouse model using the UPK10 cell line (Figure S4G-H). Consistently, JQ1 treatment increased the percentage of IFNγ producing CD8+ cytotoxic T cells (Figure 4G-H). Together these findings support the notion that JQ1 treatment suppresses PD-L1 expression on both immune and tumor cells in vivo and increases CD8+ cytotoxic T activity.
We next determined the effects of JQ1 treatment on tumor growth in vivo. JQ1 significantly suppressed tumor growth in an orthotopic ID8-Luciferase syngeneic mouse model (Figure 4I-J and S4I). Significantly, the observed tumor suppressive effects are CD8+ T cell dependent because antibody-mediated depletion of CD8+ T cells abrogated the therapeutic benefit of JQ1 treatment (Figure 4I-J). In addition, JQ1 treatment significantly improved the survival of mice receiving adoptively transferred tumor-reactive T cells in an orthotopic UPK10 syngeneic mouse model (Figure 4k). Together, these data support the notion that BET inhibition limits the progression of ovarian cancer in a CD8+ cytotoxic T cell dependent manner.
Discussion
Several BET inhibitors are now in clinical development for a number of cancer types (Filippakopoulos and Knapp, 2014). Although anti-PD-L1 antibody therapy is generally well tolerated, it is known to trigger irAEs, in particular with prolonged treatment (Naidoo et al., 2015). Missing from immunotherapy are traditional small-molecule drugs that may offer several unique advantages (Adams et al., 2015). Our findings raise the possibility of targeting PD-L1 using BET inhibitors. BET inhibitors suppress macrophage inflammatory responses and attenuate systematic inflammatory processes (Belkina et al., 2013; Nicodeme et al., 2010). Thus, it is possible that BET inhibitors may quell the inflammatory response without eliminating the anti-tumor immune response.
PD-L1 positive myeloid cells play a key role in human ovarian cancer (Curiel et al., 2004). BET inhibitors affect PD-L1 expression in both tumor cells and myeloid DCs and macrophages (Figures 4 and S4). Therefore, BET inhibitors suppress PD-L1 expression in both host antigen presenting cells and tumor cells. Compared with immune-associated PD-L1, oncogenic PD-L1 and its role in tumor immunity remain poorly defined (Zou et al., 2016). Interestingly, BET inhibitors suppress PD-L1 expression in tumor cells with or without IFNγ stimulation (Figures 2 and S2). Thus, this mechanism may couple oncogenic and immune-associated PD-L1 regulation.
Identification of potential biomarkers that predict the response to anti-PD-L1 therapy in cancer remains a clinical challenge (Melero et al., 2015). In particular, patients who positively respond to anti-PD-L1 therapy despite a lack of PD-L1 expression highlight the complex regulation of PD-L1 in cancer (Brahmer et al., 2015). Our findings establish that BRD4 is as a critical regulator of PD-L1 expression, as BRD4 blocks the IFNγ-induced upregulation of PD-L1 (Figures 2 and 3). This suggests that BRD4 expression may dictate how well PD-L1 can be induced in response to signals from the tumor microenvironment.
BRD4 is localized to 19p13.1. This BRD4 locus is often amplified in ovarian cancer (Goundiam et al., 2015). In fact, ovarian cancer shows one of the highest BRD4 amplification rates in all cancer types based on the TCGA database (data not shown). BRD4 expression positively correlates with CD274 expression in ovarian cancer specimens (Figure 3I). However, the existence of tumors with high BRD4 expression and low CD274 expression suggests that additional cell intrinsic or extrinsic mechanisms may also regulate CD274 expression. Nonetheless, it will be interesting to correlate the response to anti-PD-L1 blockade therapy with BRD4 amplification or expression. Notably, BRD4 also promotes survival and proliferation of ovarian cancer cells (Baratta et al., 2015). Therefore, BET inhibitors may have dual anti-tumor effects on both tumor cells as well as the tumor-promoting immune-environment.
Our findings demonstrate that BET inhibitors suppress PD-L1 expression, which correlates with an increase in cytotoxic T cell activity. A limitation of the study is that BET inhibition affects the expression of other genes in addition to CD274 (Table S2 and Figure S3). Changes in the expression of other genes could also contribute to the observed anti-tumor effects. However, we previously demonstrated that decreased PD-L1 in myeloid cells, smaller than those elicited by JQ1, are sufficient to have anti-tumor immune responses (Cubillos-Ruiz et al., 2009). Therefore, our data support the notion that downregulation of PD-L1 plays a significant role in the observed anti-tumor immune response induced by JQ1. Given the demonstrated broad applicability of PD-L1 blockade therapy in human cancer, we anticipate our findings to have far-reaching implications for developing future combinatory cancer therapies.
Experimental Procedures
In vivo syngeneic mouse model
All animal protocols described in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at The Wistar Institute. Six to eight-week-old female wild-type C57BL/6 mice were purchased from Charles River. ID8 cells were provided by K. Roby (Department of Anatomy and Cell Biology, University of Kansas, Kansas City, KS) and retrovirally transduced to express Defb29 and Vegf-a (Conejo-Garcia et al., 2004). Mouse ovarian tumor UPK10 cells were described previously (Scarlett et al., 2012). The ID8-Defb29/Vegf-a intraperitoneal tumor model was generated as previously described (Conejo-Garcia et al., 2004). Briefly, 2∼106 ∼70% confluent ID8-Defb29/Vegf-a cells were injected into the peritoneal cavity of mice and allowed to establish tumors. After one week, mice were randomized into two groups and treated twice a week with 50 mg/kg JQ1 or vehicle control by intraperitoneal injection for three weeks.
For anti-CD8 antibody treatment, 2×106 Luciferase-expressing ID8 cells were orthotopically transplanted into C57BL/6 mice by i.p. injection. The transplanted tumors were allowed to establish for 22 days. The mice were then randomized and treated intraperitoneally with 50 mg/kg JQ1 twice a week with or without an anti-CD8 antibody 500 μg/mice once a week. Tumor growth was followed by non-invasive image as previously described (Bitler et al., 2015) using an IVIS Spectrum. Images were analyzed using Live Imaging 4.0 software.
Tumor reactive T cell adoptive immunotherapy
T cells from tumor-free mice were primed with tumor antigen-pulsed bone-marrow dendritic cells (BMDCs) as described (Nesbeth et al., 2009). Briefly, BMDCs were pulsed overnight with γ-irradiated (10,000 rad) and UV-treated (30 min) UPK10 tumor cells at a ratio of 10:1 (dendritic cells:tumor cells). Tumor antigen-pulsed BMDCs were then co-cultured with T cells at a 1:10 (BMDC:T cell) ratio in the presence of IL-2 (10 U/ml) and IL-7 (1 ng/ml) (both from Peprotech) for 7 days. Female C57BL/6 mice (6–8 weeks old) were intraperitoneally injected with 2 × 106 UPK10 tumor cells and treated with JQ1 or vehicle control on day 5 and day 12 intraperitoneally. 1×106 tumor antigen-primed T cells were transferred into tumor bearing mice on day 7 and day 14 post tumor challenge.
Statistical analysis
Graphpad Prism Version 5.0 was used to perform for statistical analyses. The student's t test was used to determine p values of raw data. A p value< 0.05 was considered as significant.
Supplementary Material
Acknowledgments
We thank The Structure Genomics Consortium for providing the panel of small molecule inhibitors that target epigenetic regulators. We thank members of the Zhang lab for discussion and Dr. Katherine Aird for critical comments. This work was supported by US National Institutes of Health/National Cancer Institute R01 grants (CA160331, CA163377 and CA202919 to R.Z.), US Department of Defense (OC140632P1 and OC150446 to R.Z.), an Ovarian Cancer Research Fund (OCRF) program project (to R.Z.) and The Jayne Koskinas & Ted Giovanis Breast Cancer Research Consortium at Wistar (to R. Zhang). H.Z. is an OCRF Ann Schreiber Mentored Investigator. B.G.B. is supported by a US National Institutes of Health/National Cancer Institute grant (K99CA194318). Support of Core Facilities was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Footnotes
Author Contributions: H.Z., F.B., N.S., M.R.R., designed experiments. H.Z., F.B., N.S., M.R.R., B.G.B., M.J.A., Y.Y., A.V.K. conducted experiments, analyzed data. J.E.B. provided key experimental materials. J.R.C. and R.Z. supervised the study. R.Z. conceived the study. H.Z., M.R.R., B.G.B., J.R.C. and R.Z. wrote the manuscript.
Gene Expression Omnibus Accession Number: GSE81698
References
- Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nature reviews Drug discovery. 2015;14:603–622. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- Baratta MG, Schinzel AC, Zwang Y, Bandopadhayay P, Bowman-Colin C, Kutt J, Curtis J, Piao H, Wong LC, Kung AL, et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc Natl Acad Sci U S A. 2015;112:232–237. doi: 10.1073/pnas.1422165112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkina AC, Nikolajczyk BS, Denis GV. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. Journal of immunology. 2013;190:3670–3678. doi: 10.4049/jimmunol.1202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih Ie M, Conejo-Garcia JR, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic anti-tumor immunity. The Journal of clinical investigation. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nature reviews Drug discovery. 2014;13:337–356. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goundiam O, Gestraud P, Popova T, De la Motte Rouge T, Fourchotte V, Gentien D, Hupe P, Becette V, Houdayer C, Roman-Roman S, et al. Histo-genomic stratification reveals the frequent amplification/overexpression of CCNE1 and BRD4 genes in non-BRCAness high grade ovarian carcinoma. Int J Cancer. 2015;137:1890–1900. doi: 10.1002/ijc.29568. [DOI] [PubMed] [Google Scholar]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews Drug discovery. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, Park DC, Hao K, Yip DK, Donninger H, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–532. doi: 10.1016/j.ccr.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbeth Y, Scarlett U, Cubillos-Ruiz J, Martinez D, Engle X, Turk MJ, Conejo-Garcia JR. CCL5-mediated endogenous anti-tumor immunity elicited by adoptively transferred lymphocytes and dendritic cell depletion. Cancer Res. 2009;69:6331–6338. doi: 10.1158/0008-5472.CAN-08-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, Vancriekinge W, Demeyer T, Du Z, Parsana P, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4:2067–2079. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers. and combinations Sci Transl Med. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.