Abstract
Context
Poor family functioning affects psychosocial adjustment and the occurrence of morbidity following bereavement in the context of a family’s coping with advanced cancer. Family functioning typologies assist with targeted family-centered assessment and intervention to offset these complications in the palliative care setting.
Objectives
Our objective was to identify the number and nature of potential types in an American palliative care patient sample.
Methods
Data from patients with advanced cancer (N = 1809) screened for eligibility for a larger randomized clinical trial were used. Cluster analyses determined whether patients could be classified into clinically meaningful and coherent groups, based on similarities in their perceptions of family functioning across the cohesiveness, expressiveness, and conflict resolution subscales of the Family Relations Index.
Results
Patients’ reports of perceived family functioning yielded a model containing five meaningful family types.
Conclusion
Cohesiveness, expressiveness, and conflict resolution appear to be useful dimensions by which to classify patient perceptions of family functioning. “At risk” American families may include those we have called hostile, low-communicating, and less-involved. Such families may benefit from adjuvant family-centered psychosocial services, such as family therapy.
Keywords: Family, family therapy, family functioning, psychotherapy, cancer, assessment, bereavement, grief, cluster analysis
Introduction
An advanced cancer diagnosis generates substantial psychosocial distress for both the patient and the family,1,2 including mood disturbance and existential and traumatic stress.3–5 The family is instrumental to assist with coping, including the provision of emotional and active support such as caregiving.6,7 Psychological distress is interdependent among family members in the context of cancer.8 Heightened distress in patients can be deleterious to family members, and the converse is also true. The definition of the family for clinical purposes comprises those members who are psychologically connected with the patient—the psychological family. For some, this is the nuclear family, and for others, this includes the extended family, close friends, or neighbors; in practical terms, each patient defines who they consider their family to be. Moreover, family functioning is a major determinant of patients’ and families’ psychosocial trajectories of adaptation. A family-centered approach to care provision is thus a crucial target for comprehensive treatment in oncology and palliative care.
To this end, Kissane et al.9 developed and refined a family-centered, prophylactic intervention, the primary goal of which is to optimize coping and adaptation in patients and families at risk of heightened distress during palliative care and continuing into bereavement. The intervention was shown effective in ameliorating depression and distress,9 and a further randomized controlled trial to test patient and family outcomes by dose (i.e., number of therapy sessions) proportional to level of family dysfunction is currently underway.
To identify those at risk and warranting such family support, we have screened patients and their carers with the Family Relationships Index (FRI).10 Previous work by Kissane et al. with Australian families identified an empirical classification of perceived family functioning comprising five types.11 This typology is derived from members’ perceptions of their family’s cohesiveness, expressiveness, and conflict resolution, which prove to be the clinically meaningful dimensions of family functioning. Two types proved well functioning with adaptive outcomes: 1) supportive, where cohesion and mutual support are high and 2) conflict resolving, where communication around difficult topics occurs fluidly. Two other types engaged in dysfunctional interactional patterns with lower cohesiveness, decreased expression, and greater interpersonal conflict. Of these, 3) sullen families had muted anger, high rates of depression, and tended to be help-accepting, whereas 4) hostile families were fractured and more help-rejecting; both showed heightened risk for psychosocial morbidity.12 The remaining family type, 5) intermediate, reported moderately reduced cohesiveness11 and also carried high rates of poorer psychosocial outcomes.13 Although families are never labeled as such in the clinical setting, screening for “risk” by identifying more difficult interpersonal relations allows the offer of adjuvant family-centered services, including family therapy.
Cultural differences between classification systems of family functioning have been demonstrated. In one Japanese study, families reported their perceptions of cohesiveness, communication, and conflict resolution, yielding three types: one more functional (supportive), one essentially dysfunctional (conflictive), and one intermediate.14 The number of clusters is not determined a priori as a hypothesis but rather emerges from the comprehensive exploration of the clinical data. We assessed American families within this framework.
Herein, we describe perceptions of family functioning by 1809 American patients diagnosed with advanced cancer, using a cluster analytic methodology to create a typology of family functioning. The aim was to determine whether patients could be classified into clinically meaningful and coherent groups, based on similarities in their patterns of responding across the cohesiveness, expressiveness, and conflict resolution subscales of FRI. These patients were being screened for their eligibility for the dose-response controlled family therapy trial mentioned previously. As no studies to date have identified a typology of family functioning in American patients receiving palliative care for advanced cancer, we also explored whether American culture and values would generate a different classification to those found in other countries, in number of clusters or cluster characteristics.
Methods
Participants and Procedures
Patients from oncology and palliative care services (outpatient and inpatient) at Memorial Sloan-Kettering Cancer Center with diagnoses of advanced stage cancer and expected survival of 1 year or less according to their oncologist were screened consecutively. A research study assistant asked them to complete FRI. The screening process, as part of the larger randomized controlled trial, received approval from the local institutional review board in accord with an assurance filed with and approved by the Department of Health and Human Services. In addition to completing FRI, patients provided sociodemographic, disease, and treatment information. These data were verified with medical records for accuracy.
A total of 1809 patients were screened. According to medical records, the average patient was middle-aged, married, and self-identified as Caucasian. The sample showed a relatively even gender split. The most prevalent primary cancer diagnoses were pancreatic (n = 302), colorectal (n = 244), upper gastrointestinal (esophageal, gastric; n = 318), breast (n = 157), and melanoma (n = 157). Recruitment focused on these tumor types to capture patients entering palliative care with aggressive disease. Most patients had undergone at least one significant surgical procedure related to the cancer (n = 1651) and been treated with chemotherapy (n = 1634). Detailed sociodemographic, disease, and cancer treatment information for the sample from their medical charts is provided in Table 1.
Table 1.
Patient Sociodemographic, Disease/Prognostic, and Cancer Treatment Variables at Assessment (N = 1809)
| Variables | Mean (SD)/% |
|---|---|
| Sociodemographic | |
| Age at assessment (yrs) | 59.40 (13.95) |
| Gender (% male) | 52.8 |
| Race (% self-reported Caucasian) | 81.8 |
| Marital status (% married) | 65.0 |
| Disease/prognostic | |
| Cancer site (% site)a | |
| Pancreatic | 16.5 |
| Colorectal | 13.3 |
| Other gastrointestinal (excepting pancreatic, colorectal, hepatic) |
17.6 |
| Breast | 8.6 |
| Skin | 8.6 |
| Other site with prevalence rate <8.6% in sample |
46.4 |
| Cancer treatment history at assessment | |
| Significant surgical procedure related to the cancer (% yes) |
90.1 |
| Chemotherapy (% yes) | 89.1 |
| Radiation therapy (% yes) | 39.9 |
Some patients have multiple diagnostic sites; thus, cumulative cancer site percentages add to greater than 100%.
Measure
Perceived Family Functioning
Patients reported perceptions of family functioning on FRI. FRI is a brief (12 items), true-false response scale developed from the Family Environment Scale,10 which has been used extensively, including in patients with heterogeneously staged cancers and bereaved families of patients with advanced cancer.11,13,14 FRI comprises three subscales: 1) cohesiveness (e.g., “There is a feeling of togetherness in our family”), 2) expressiveness (e.g., “We tell each other about our personal problems”), and 3) conflict resolving (e.g., “Family members fight a lot”). The subscales form a global measure of family interaction. Subscale scores range from 0 (low) to 4 (high), and the global score ranges from 0 to 12. Patients’ perceptions of family functioning included individuals who each patient viewed as essential to their family group—spouses, children, siblings, parents, extended family members, and/or friends-like-family. Thus, family compositions varied by patient.
Analytic Strategy
First Phase
Cluster analysis, which comprises various exploratory algorithms aimed at creating useful groupings, was carried out using the statistical software GNU R 2.15.2 using the NbClust package.15,16 Whenever available, patients’ family members also were screened, but their data are not included in the current report. We believe it advantageous to begin with patient data, so that clusters can be first conceptualized with individuals as the unit of analysis.
Guidelines for cluster analysis in health psychology described by Clatworthy et al.17 directed selection of our cluster analysis procedures. In the first step, the well-recognized agglomerative hierarchical clustering approach, employing squared Euclidean distance solutions and Ward’s aggregation schedule, was used.18 Raw FRI subscale scores were scaled and internal indices of model fit were calculated. We used the commonly used Hartigan’s index, which selects the best-fitting model based on maximum differences between hierarchy levels.19
A strong recommendation is to rely on converging sources of evidence from multiple taxometric procedures.20 Thus, in a second step, analysis of variance (ANOVA) with post hoc comparisons was used to contrast clusters across FRI subscales. The statistical software IBM Corp. SPSS 21.021 was used. When ANOVA tests were significant, Scheffé’s post hoc comparisons test was used, as it is a particularly conservative and robust test in the context of unequal group sizes.22 Because of our large sample size, and as we conducted multiple comparisons of FRI characteristics across cluster membership, we set the significance level to a stringent P < 0.01 for all comparisons to attenuate the likelihood of error. All tests were two-tailed.
Second Phase
The goal of cluster analysis is to create meaningful groups that significantly differ across target constructs, and ANOVA contrasts across clusters are likely to highlight these differences. Thus, Clatworthy et al.17 advise additional contrasts of other characteristics across clusters, such as clinical characteristics. Associations between sociodemographic, disease, and cancer treatment variables from the medical chart and cluster membership were examined. Once again, we set the significance level to P < 0.01 for all tests, which were two-tailed. Chi-square or ANOVA comparisons were used as statistically appropriate. In the case of significant Chi-square tests, groups were examined post hoc for standardized residuals greater or less than 2.58, which is the Z score corresponding with P < 0.01.21 The conservative Scheffé’s post hoc test was used in the case of significant ANOVA tests.
Results
The rate of missing data points for the N = 1809 patients was minimal (2.8% across all 12 raw FRI items). Hartigan’s index19 identified an optimal number of five clusters (Hartigan’s index value = 403.43). In addition to its statistical support, the five-cluster solution was viewed as clinically useful for its parsimony. Thus, the five-cluster solution was selected as the optimal model.
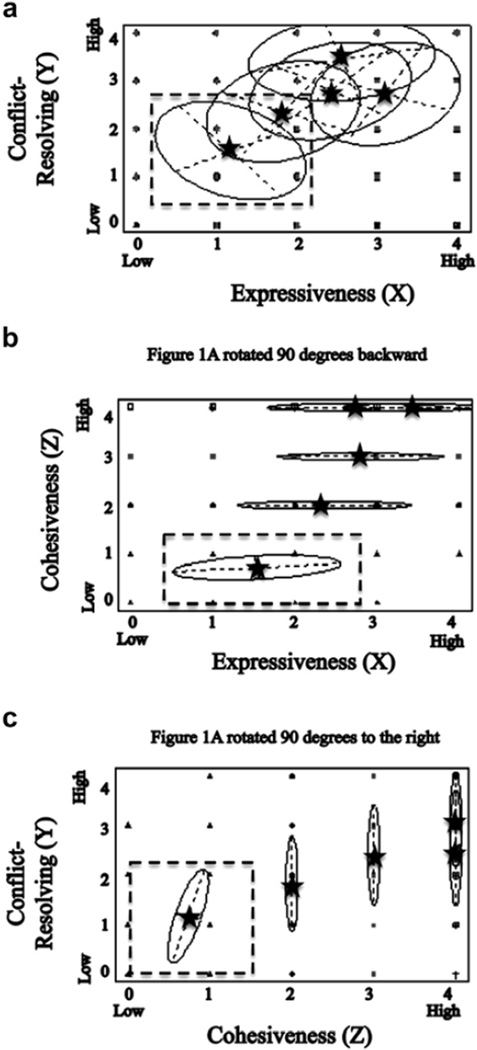
A model-based approach was used in GNU R 2.15.215,16 to extract the five clusters for description and comparison. This procedure showed: 1) 814 (44.9%) of the 1809 patients to be assigned to Cluster 1; 2) 375 (20.7%) to Cluster 2; 3) 418 (23.1%) to Cluster 3; 4) 101 (5.6%) to Cluster 4; 5) and 101 (5.6%) to Cluster 5. Descriptive statistics for FRI subscales across the five clusters are presented in Table 2. All five clusters showed significant differences relative to one another across the three FRI subscales (cohesiveness: F(4, 1794) = 991.30, P < 0.001; expressiveness: F(4, 1794) = 670.40, P < 0.001; conflict resolution: F(4, 1794) = 860.31, P < 0.001). Post hoc tests revealed exceptions to be Cluster 1 vs. Cluster 2 and Cluster 2 vs. Cluster 3 for the cohesiveness subscale (P = 0.016 and 0.035, respectively) and Cluster 3 vs. Cluster 4 for the expressiveness subscale (P = 0.863). Fig. 1 shows the resulting R software figure (Mclust package, plot data command) illustrating three angles of clusters’ positions relative to one another in space across FRI subscales.
Table 2.
Comparisons Across Sociodemographic, Disease/Prognostic, Cancer Treatment, and Psychosocial Variables for the Five-Cluster Model (N = 1809)
| Cluster | |||||
|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | |
| (n = 814) | (n = 375) | (n = 418) | (n = 101) | (n = 101) | |
| Variables | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% | M (SD)/% |
| FRI subscale | |||||
| Cohesivenessa (0 [low] – 4 [high]) |
3.79 (0.41) b, c, d |
3.69 (0.46) f, g |
3.59 (0.49) b, h, i |
1.91 (0.29) c, f, h, j |
1.30 (0.29) d, g, i, j |
| Expressivenessa (0 [low] – 4 [high]) |
3.52 (0.50) a, b, c, d |
1.57 (0.62) a, e, f, g |
2.16 (0.95) b, e, i |
2.07 (1.11) C, f, j |
1.18 (0.97) c, f, h, j |
| Conflict resolutiona (0 [low] – 4 [high]) |
3.43 (0.69) a, b, c, d |
3.63 (0.48) a, e, f, g |
1.65 (0.55) b, e, h, i |
3.03 (0.82) c, f, h, j |
1.18 (0.75) c, f, h, j |
| Sociodemographic | |||||
| Agea | 59.72 (14.17) | 61.81 (13.16) e |
57.31 (13.38) e |
58.46 (15.72) | 57.34 (14.22) |
| Gendera (% male) | 55 | 58 | 51 | 46 | 32 |
| Race (% Caucasian) | 83 | 82 | 79 | 80 | 83 |
| Marital statusa (% married) | 69 | 68 | 66 | 52 | 34 |
| Disease/treatment | |||||
| Surgery (% yes) | 91 | 93 | 90 | 90 | 88 |
| Chemotherapy (% yes) | 91 | 91 | 90 | 89 | 86 |
| Radiation (% yes) | 40 | 35 | 45 | 42 | 49 |
Bolded numbers indicate which clusters show proportions of a variable significantly different than expected for Chi-square, according to post hoc tests, P < 0.001.
a, b, c, …․j indicate which clusters show significant differences from one another for ANOVA, according to post hoc tests, P < 0.001.
Indicates clusters showing significant differences from one another on a variable (ANOVA tests) or proportions of a variable significantly different than expected (Chi-square tests), P < 0.001.
Fig. 1.
Three views (Panels la, lb, and lc) from a three-dimensional representation of approximate distances between prototypical members from the five clusters across the expressiveness (X axis), conflict-resolving (Y axis), and cohesiveness (Z axis) subscales. Black stars denote cluster centers. The dashed square illustrates where an individual from the “Hostile” cluster, low on the expressiveness, conflict-resolving, and cohesion subscales, might appear for each view.
Subsequent Chi-square and ANOVA comparisons across relevant sociodemographic, disease, and cancer treatment covariates with post hoc pairwise comparisons showed significant differences between clusters. As above, we set the significance level to P < 0.01 for all tests. Clusters differed with respect to: 1) gender, with Cluster 5 containing significantly more women than expected (χ2(4, N = 1809) = 56.75, P < 0.001); 2) age at assessment, with Cluster 2 containing significantly older patients than Cluster 3 (F(4, 1787) = 5.96, P < 0.001); 3) marital status, coded as married vs. not married (i.e., single, divorced, separated, or widowed) (χ2(4, N = 1809) = 56.75, P < 0.001), with Cluster 5 containing significantly more unmarried or isolated patients (n = 67 vs. 34 married). Cluster 5 comprised 34% (n = 34) married, 37% (n = 37) single, 15% (n = 15) divorced, 3% (n = 3) separated, and 12% (n = 12) widowed patients.
Clusters did not differ significantly across race (Caucasian vs. not Caucasian, χ2(12, N = 1809) = 21.31, P = 0.046) or receiving cancer-related surgery (χ2(4, N = 1809) = 3.36, P = 0.503), chemotherapy (χ2(4, N = 1809) = 4.39, P = 0.355), or radiation treatments (χ2(4, N= 1809) = 10.91, P = 0.028). Sociodemographic, disease/prognostic, and cancer treatment information for patients appearing in each of the five clusters is shown in Table 2.
Discussion
Reports of perceived family functioning on FRI by a large sample of American patients with advanced cancer yielded a model containing five meaningful family types. The first cluster was the largest, with characteristics conveying a high level of mutual support. Cluster 1 families have optimal teamwork, effective communication of both thoughts and feelings, and are free of conflict.
Notably, the second cluster reported a blend of high cohesiveness and ability to resolve or avoid conflict, but noted an absence of open communication. Recognition of blocked communication among otherwise connected relatives is of vital importance to clinical care. Protectiveness, with a desire to limit distress and sustain hope, leads many families in the palliative care setting to fail to communicate directly about end-of-life themes of crucial importance to not only care planning but also coping and adaptation. We term this cluster law-communicating. They have somewhat-similar characteristics to the Australian intermediate cluster,11 but identification of their closed communication creates an immediate therapeutic target.
Cluster 3 reported a high level of cohesiveness with more moderate expressiveness and conflict resolution, similar to the Australian conflict-resolving cluster.11 This descriptive term was selected because such families appear to have the necessary teamwork and adaptive communication to cope with difference of opinion; arguments in these families do not become destructive.
The fourth cluster reported low cohesiveness, which we have termed a less-involved relational pattern. Although not especially conflictual and able to communicate if needed to, these family members live quite independently. This again might constitute a valuable clinical focus: to aim to build collaboration and teamwork in the spirit of greater sharing of the tasks of care provision.
Finally, the fifth cluster identified family characteristics of low cohesiveness, poor communication around difficult topics, and lowest conflict resolution—a fractured or hostile class of dysfunctional families. Interestingly, this cluster comprised, proportionally, more females and fewer married (or potentially isolated) individuals than any other cluster. These distinct sociodemographics may suggest construct validity for this type of functioning in the family.
Potential cultural differences were noted for the current sample compared with prior work in other countries.11,14 Two patterns appear distinctive from the Australian and Japanese cohorts. One is the family that is less communicative (low-communicating, Cluster 2), avoiding open discussion of distress. Such avoidant coping—an essentially stoical stance—was recognized in the family grief literature6 but may have been located among study refusers in studies with smaller samples. The second pattern is a disconnected style (less-involved, Cluster 4), where families lack a strong tradition of mutual support—members are simply less involved with one another. Further studies will be needed to replicate this typology of family functioning and appreciate what is truly determined culturally.
That our data include only patient-reported perceptions of family functioning could present as a potential study limitation. However, patients are generally the most accessible and available individuals in the palliative care setting for assessment. Understanding clinical implications of, specifically, patient response patterns provides a logical starting point for identification of patients and families who may benefit from adjuvant prophylactic psychosocial services. A second limitation may be that a large proportion of our sample was married/partnered. The degree to which these results may extend to patients who are unmarried/unpartnered, who live alone, or who live with other relatives remains uncertain. Low socioeconomic status also may be a moderator of poorer family functioning but also could be an outcome; its relationship is complex although the correlation between the two is clear.
Other possible limitations include those typical of any cluster analysis study (e.g., subjectivity of model selection). However, we endeavored to offset such limitations by using a large sample, multiple analytic techniques with fit indices, and a recommended cluster analysis methodology/reporting protocol for health psychology studies.17 Last, it is possible that the use of a dichotomous instrument restricted response variability compared with use of a continuous instrument; however, for screening purposes with a palliative care population, its brevity and ease of administration is appreciated.
Future directions include: 1) identification of adverse psychosocial sequelae for potentially “at risk” American families; 2) investigation of whether the five-cluster typology is retained when family members’ reports are analyzed; and 3) potential identification of appropriate FRI cut-off scores, informed by these lines of research. The first direction will further establish validity indices of FRI for an American palliative care sample, guiding our understanding of its utility as a screening instrument. The second perspective also will inform screening, where a caregiver or other relatives accompanying the patient receive care. We recognize that not all family members share the same perspective of family life, yet anticipate that several viewpoints may inform clinicians optimally about the family. Ultimately, these will assist in the development/refining of clinically optimal family distress screening tools.
In sum, cohesiveness, expressiveness, and conflict resolution appeared to be useful dimensions by which to classify patient perceptions of family functioning. Although further investigation is warranted, “at risk” American families may include the hostile, low-communicating, and less-involved. Such families may benefit from adjuvant family-centered psychosocial services, such as family therapy.
Acknowledgments
Disclosures and Acknowledgments
This research was supported by a grant from the National Cancer Institute (R01CA115329—David Kissane, Principal Investigator).
The authors thank the patients, families, and research professionals and trainees of the Family Focused Grief Therapy team.
Footnotes
The authors have no disclosures or conflicts of interest to report.
References
- 1.Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psychooncology. 2004;13:562–576. doi: 10.1002/pon.773. [DOI] [PubMed] [Google Scholar]
- 2.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 3.Andersen BL, Shapiro CL, Farrar WB, Crespin TR, Wells-Di Gregorio SM. Psychological responses to cancer recurrence: a controlled, prospective study. Cancer. 2005;104:1540–1547. doi: 10.1002/cncr.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarenmalm K, Ohlén J, Odén A, Gaston-Johansson F. Experience and predictors of symptoms, distress, and health-related quality of life over time in postmenopausal women with recurrent breast cancer. Psychooncology. 2008;17:497–505. doi: 10.1002/pon.1258. [DOI] [PubMed] [Google Scholar]
- 5.Voogt E, van der Heide A, van Leeuwen AF, et al. Positive and negative affect after diagnosis of advanced cancer. Psychooncology. 2005;14:262–273. doi: 10.1002/pon.842. [DOI] [PubMed] [Google Scholar]
- 6.Kissane D, Bloch S. Family grief. Brit J Psychiatry. 1994;164:728–740. doi: 10.1192/bjp.164.6.728. [DOI] [PubMed] [Google Scholar]
- 7.Northouse L, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30:1227–1234. doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- 8.Dorros SM, Card NA, Segrin C, Badger TA. Interdependence in women with breast cancer and their partners: an interindividual model of distress. J Consult Clin Psych. 2010;78:121–125. doi: 10.1037/a0017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissane D, McKenzie M, Bloch S, et al. Family-focused grief therapy: a randomized, controlled trial in palliative care and bereavement. Am J Psychiat. 2006;163:1208–1218. doi: 10.1176/ajp.2006.163.7.1208. [DOI] [PubMed] [Google Scholar]
- 10.Moos RH, Moos BS. Family Environment Scale manual. Stanford, CA: Consulting Psychologists Press; 1981. [Google Scholar]
- 11.Kissane D, Bloch S, Dowe D, et al. The Melbourne Family Grief Study I: perceptions of family functioning in bereavement. Am J Psychiat. 1996;153:650–658. doi: 10.1176/ajp.153.5.650. [DOI] [PubMed] [Google Scholar]
- 12.Kissane D, McKenzie M, McKenzie D, et al. Psychosocial morbidity associated with patterns of family functioning in palliative care: baseline data from the family focused grief therapy controlled trial. Palliat Med. 2003;17:527–537. doi: 10.1191/0269216303pm808oa. [DOI] [PubMed] [Google Scholar]
- 13.Kissane D, Bloch S, Onghena P, et al. The Melbourne Family Grief Study II: psychosocial morbidity and grief in bereaved families. Am J Psychiat. 1996;153:659–666. doi: 10.1176/ajp.153.5.659. [DOI] [PubMed] [Google Scholar]
- 14.Ozono S, Saeki T, Inoue S, et al. Family functioning and psychological distress among Japanese breast cancer patients and families. Support Care Cancer. 2005;13:1044–1050. doi: 10.1007/s00520-005-0816-5. [DOI] [PubMed] [Google Scholar]
- 15.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 16.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NBClust: An examination of indices for determining the number of clusters, NbClust Package Version 1.3. Quebec, Canada: Université Laval; 2013. [Google Scholar]
- 17.Clatworthy J, Buick D, Hankins M, Weinman J, Home R. The use and reporting of cluster analysis in health psychology: a review. Brit J Health Psychol. 2005;10:329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- 18.Everitt BS, Hothorne T. A handbook of statistical analyses using R. 2nd. Boca Raton, FL: Chapman & Hall/CRC; 2009. [Google Scholar]
- 19.Hartigan JA, Wong MA. Algorithm AS 136: a k-means clustering algorithm. J Roy Stat Soc C-App. 1979;28:100–108. [Google Scholar]
- 20.Lubke G, Tueller S. Latent class detection and class assignment: a comparison of the MAXEIG taxometric procedure and factor mixture modeling approaches. Struct Equ Modeling. 2010;17:605–628. doi: 10.1080/10705511.2010.510050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IBM Corp. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 22.Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison. 2nd. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]