Abstract
Objective
Head and neck squamous cell carcinoma (HNSCC) patients who smoke are at risk for poor treatment outcomes. This study evaluated symptom burden and clinical, sociodemographic and psychosocial factors associated with smoking in surgical patients to identify potential targets for supportive care services.
Study Design
Cross-sectional survey.
Methods
Individuals with HNSCC of the oral cavity, larynx or pharynx were recruited from two cancer centers and completed questionnaires assessing smoking status (never, former, current/recent), patient characteristics and symptoms before surgery.
Results
Of the 103 patients enrolled, 73% were male, 52% were stage IV, 41% reported current/recent smoking and 37% reported former smoking. Current/recent smokers were less likely than former smokers to have adequate finances (53% vs. 89%, p=.001) and be married/partnered (55% vs. 79%, p=.03). Current/recent smokers were also more likely than both former and never smokers to be unemployed (49% vs. 40% and 13%, respectively, p’s=0.02) and lack health insurance (17% vs. 5% and 13%, respectively, p’s≤.04). Fatalistic beliefs (p=.03) and lower religiosity (p=0.04) were more common in current/recent than never smokers. In models adjusted for sociodemographic/clinical factors, current/recent smokers reported more problems than former and never smokers with swallowing, speech, and cough (p≤.04). Current/recent smokers also reported more problems than never smokers with social contact, feeling ill and weight loss (p≤.02).
Conclusions
HNSCC patients reporting current/recent smoking before surgery have high-risk clinical and sociodemographic features that may predispose them to poor postoperative outcomes. Unique symptoms in HNSCC smokers may be useful targets for patient-centered clinical monitoring and intervention.
Keywords: head and neck cancer, oral cancer, surgery, tobacco, cigarette smoking, symptoms
Introduction
Tobacco use is an established primary risk factor for head and neck squamous cell carcinoma (HNSCC)1–3 and approximately 61,760 new cases of HNSCC and 13,190 deaths are expected in 2016 in the United States.4 The 2014 Surgeon General’s Report concluded that continued smoking by cancer patients causes adverse outcomes including increased overall and cancer-specific mortality and risk for developing a second primary cancer; continued smoking is also strongly associated with cancer treatment toxicity.5 HNSCC patients who use tobacco after diagnosis have decreased survival6–8 and increased risk for recurrence and development of second cancers.9,10 For example, research has demonstrated that continued smoking was associated with decreased survival in 201 p16 positive HNSCC patients treated with laser microdissection11 and that smoking during radiotherapy was associated with increased risk of overall and cancer-specific death.12 Also, as compared with never smokers, former smokers, and HNSCC patients who quit smoking within the past year, current smokers had significantly reduced overall and cancer-specific mortality.13 The adverse effects of smoking on HNSCC outcomes has implications for the clinical care of HNSCC patients and as a consequence, a better understanding of the challenges faced by HNSCC patients who smoke at the time of cancer treatment could assist in tailoring treatment and supportive care plans. To enhance patient-centered HNSCC care, it is imperative to consider the unique needs of patients who smoke.
The optimal approach to addressing the adverse effects of smoking in cancer patients is to provide evidence-based cessation support.14 Patients who smoke and are diagnosed with cancer may be receptive to a “teachable moment” where patients are faced with a life changing diagnosis and may be receptive to making healthy lifestyle changes.15,16 Research has examined factors associated with smoking among cancer patients suggesting that continued smokers tend to be younger, female and unmarried and have lower levels of education, income, and partner support as well as poorer quality of life and more fatalistic beliefs.17–22 However, gaps remain in our understanding of the role of smoking in HNSCC patients’ experiences at the time of surgical treatment planning. Developing a better understanding of symptom burden and other patient challenges at the time of diagnosis may guide identification of appropriate supportive care resources and smoking cessation strategies. The purpose of this study was to evaluate the sociodemographic, clinical, psychosocial and symptomatic factors associated with smoking in HNSCC patients presenting for surgery.
Materials and Methods
Participants and Procedures
In a study of adverse surgical outcomes in HNSCC patients,23 we recruited individuals presenting for surgical management for squamous cell carcinoma of the oral cavity, larynx or pharynx at two academic cancer centers in the southeastern United States. Participants were excluded if they were younger than age 21, not surgical candidates, or had a cognitive impairment that precluded survey completion. Study participants signed informed consent and authorization forms in person following a protocol approved by the Institutional Review Boards of the Medical University of South Carolina and Wake Forest School of Medicine. Participants completed questionnaires by telephone or mail before surgery and received a giftcard. The full details of the questionnaire delivery and collection have been reported previously.23
Measures
Smoking status
Smoking was assessed with questions from the Tobacco Use Supplement to the Current Population Survey.24 Participants were asked about lifetime (ever or never) and current (every day, some days, or not at all) cigarette smoking. Former smokers were asked when they started smoking (age) and when they last smoked cigarettes regularly.
Sociodemographic factors
Gender, age, race, marital status, health insurance, years of education, employment status and financial challenges in the past month were reported.
Clinical factors
Clinical data including cancer site and stage, diagnosis type (new, recurrent/persistent), prior HNSCC treatment, other treatment type (i.e., chemotherapy, radiation), HPV tumor status (positive or negative) and number of comorbid conditions were collected from the electronic medical record at each site using standardized forms.
Psychosocial and behavioral factors
Depression was assessed using the 10-item Center for Epidemiologic Studies Depression Scale,25,26 a widely used instrument with excellent properties. Higher scores represent increased depressive symptoms (range 0–30). Cronbach’s alpha in the study sample was 0.89.
Fatalism was assessed using 5 items (1=Not at all to 5=Extremely) adapted from previous research;27,28 higher scores reflected more fatalistic beliefs (range 1–5; Cronbach’s alpha=0.72).
Religiosity was assessed using the Santa Clara Strength of Religious Faith instrument.29 Participants rated their agreement with 5 statements concerning faith (1=Strongly Disagree to 6=Strongly Agree). Higher scores reflected higher strength of religious faith (range 5–30; Cronbach’s alpha=0.95).
We also assessed alcohol use (current use and binge drinking which was defined as consuming ≥4 or 5 drinks on one occasion for women and men, respectively).
Symptoms
The head and neck cancer module of the European Organization for Research and Treatment of Cancer Core Quality of Life questionnaire (EORTC-QLQ)30,31 was used to assess symptoms. We assessed problems with pain, swallowing, senses (i.e., taste/smell), speech, social eating, social contact (i.e., appearance), teeth, opening mouth, dry mouth, sticky saliva and cough over the past week (1=Not at all to 4=Very much). Also, we examined whether participants felt ill and used pain killers, nutritional supplements or a feeding tube and whether they lost or gained weight in the past week. Scores ranged from 0–100; higher scores reflected worse problems.
Data Analysis
Descriptive statistics were used to summarize sociodemographic and clinical characteristics. Participants were categorized as never smokers if they had not smoked at least 100 cigarettes in their lifetime, former smokers if they had smoked at least 100 cigarettes in their lifetime but successfully stopped smoking at least 6 months prior to enrollment. Patients were defined as current/recent smokers if they reported currently smoking or having quit less than or equal to 6 months prior to enrollment. Current smokers were combined with those who had quit within the past 6 months a priori; this cutoff was set because this group is at high risk of relapse32 and because patients who quit smoking within the past year are at high risk for misrepresenting tobacco use.33,34 Therefore, combining current smoking and recent quitting provides a conservative estimate of the associations between smoking and other variables. We compared sociodemographic (age, gender, race, education, marital status, monthly financial challenges, health insurance type, employment status), clinical (cancer site/stage, diagnosis type (new, recurrent/persistent), prior HNSCC treatment, comorbid conditions), psychosocial and behavioral (depression, fatalism, religiosity, alcohol use) and symptom factors in current/recent, former, and never smokers using Kruskal-Wallis and Fisher’s exact tests for tests for continuous and categorical variables, respectively. Pairwise comparisons (current/recent smokers versus both former and never smokers) followed when overall tests were significant (alpha = 0.10). Multiple linear regression models controlling for sociodemographic and clinical factors were used to further examine symptoms associated with smoking status. Covariates we considered included factors hypothesized a priori to be associated with symptom burden including age, gender, race, health insurance, cancer stage, prior HNC treatment and co-morbid conditions. For each model, these covariates were retained if they were significant (alpha = 0.10) in the presence of other covariates. Model refinement was performed by including all significant covariates in simple models and then successively removing the least significant covariate until all covariates in the model were significant (p ≤.10).
Results
One hundred fifty-four patients met inclusion criteria and 120 (78%) consented and were enrolled. One hundred three patients (86% of enrolled) completed questionnaires. Most participants were Caucasian males and over one-half had stage IV cancer (Table 1). Seventeen percent of participants had HPV positive tumors and in addition to surgery, 41% of participants also had chemotherapy and 56% also had radiation. The majority of participants (78%) had a cigarette smoking history. Of these, 27% were current smokers, 14% quit within the past 6 months and 37% quit greater than 6 months ago. Current/recent smokers had smoked an average of 40.2 years (SD=9.5) while former smokers had smoked an average of 31.0 years (SD = 13.1). Among former smokers, the average time since quitting was 14.1 years (SD=10.1 years).
Table 1.
Smoking Status and Sociodemographic, Clinical and Psychosocial Factors in Head and Neck Cancer Surgical Patients
| Characteristic | All N=103 |
Current/recent smokers n=42 |
Former smokers n=38 |
Never smokers n=23 |
Overall test of smoking status p valuea |
|
|---|---|---|---|---|---|---|
| All (%) | 100% | 41% | 37% | 22% | ||
| Sociodemographic factors | ||||||
| Age, Mean [Range] | 59 [24, 80] | 58 [42, 72] | 62 [39, 80] | 57 [24, 77] | 0.26 | |
| Gender (% Female) | 27% | 33% | 18% | 30% | 0.32 | |
| Race (% Caucasian) | 82% | 74% | 89% | 87% | 0.19 | |
| Education (% ≤ high school education) | 50% | 54% | 53% | 39% | 0.55 | |
| Marital status (% partnered) | 68% | 55% | 79% | 74% | 0.06 | |
| Adequate monthly finances (% yes) | 72% | 53% | 89% | 78% | 0.001 | |
| Health insurance | 0.016 | |||||
| None | 12% | 17% | 5% | 13% | … | |
| Private | 56% | 38% | 66% | 74% | … | |
| Public | 32% | 45% | 29% | 13% | … | |
| Employment status | 0.005 | |||||
| Employed | 36% | 31% | 29% | 57% | … | |
| Retired | 32% | 20% | 47% | 30% | … | |
| Unemployed/disability | 31% | 49% | 40% | 13% | … | |
| Clinical factors | ||||||
| Cancer site | 0.28 | |||||
| Hypopharynx/larynx | 27% | 36% | 29% | 9% | … | |
| Oral cavity | 42% | 36% | 37% | 61% | … | |
| Oropharynx | 23% | 21% | 26% | 22% | … | |
| Other | 8% | 7% | 8% | 9% | … | |
| Cancer stage (% stage IV) | 52% | 57% | 47% | 48% | 0.67 | |
| Diagnosis type | 0.007 | |||||
| Newly diagnosed | 59% | 73% | 39% | 65% | … | |
| Recurrent/persistent | 41% | 26% | 61% | 35% | … | |
| Previous treatment (% yes) | 43% | 41% | 53% | 30% | 0.23 | |
| Any comorbid health condition (% yes) | 86% | 79% | 97% | 82% | 0.03 | |
| Psychosocial and Behavioral factors | ||||||
| Depression, Mean, score range 0–30 | 11.4 | 12.3 | 11.3 | 10.1 | 0.58 | |
| Fatalism, Mean, score range 1–5 | 2.2 | 2.3 | 2.2 | 1.9 | 0.08 | |
| Religiosity, Mean, score range 5–30 | 22.0 | 21.1 | 20.8 | 25.5 | 0.05 | |
| Current alcohol use (% yes) | 32% | 33% | 40% | 17% | 0.21 | |
| Current binge drinking (% yes) | 21% | 26% | 29% | 0% | 0.007 | |
Kruskal-Wallis tests were used for continuous variables (age, depression, fatalism and religiosity) and Fisher’s exact tests were used for all other categorical variables to examine the overall association between smoking status (current/recent, former, never smokers) and sociodemographic, clinical, and psychosocial factors.
Smoking Status and Demographic, Clinical and Psychosocial Factors
Smoking status (current/recent, former, never) was significantly associated with employment status, health insurance and financial challenges and marginally associated with marital status (Table 1). Subsequent pairwise comparisons showed that current/recent smokers were less likely than former smokers to be partnered (p=.03) and more likely to lack adequate finances to meet monthly expenses (p=.001). Current/recent smokers were also more likely than both former and never smokers to be unemployed (both p’s=.02) and have no health insurance (p=.04 and .02, respectively).
Smoking status was associated with diagnosis type and comorbid health conditions (Table 1). Pairwise comparisons showed that former smokers were more likely to have recurrent/persistent disease (p=.003) and more comorbid conditions (p=.02) than current/recent smokers.
Depression did not differ by smoking status, but fatalism and religiosity were marginally associated with smoking. Pairwise comparisons showed that current/recent smokers had higher fatalistic beliefs compared with never smokers (p=.03) and never smokers reported stronger religious faith than current/recent smokers (p=.04). Current alcohol use was not associated with smoking status, but binge drinking was with pairwise comparisons showing that this behavior was more prevalent in current/recent smokers compared with never smokers (p=.006).
Smoking Status and Symptoms
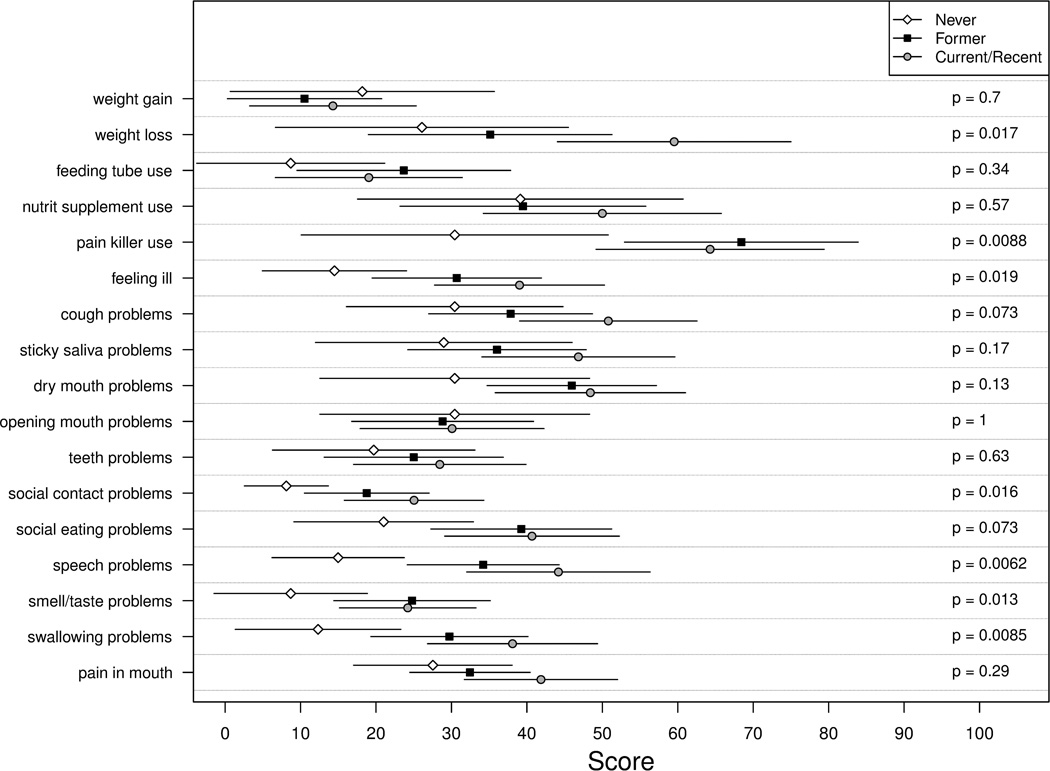
As shown in Figure 1, smoking status was significantly associated with multiple HNC-specific symptoms (7/17 (41%) of the symptoms assessed). For all symptoms except problems opening mouth, current/recent smokers reported worse symptoms than never smokers. Pairwise comparisons showed that current/recent smokers were more likely than never smokers to have used pain killers (p=.01) and report feeling ill (p=.005) over the past week and to have problems with cough (p=.03), social contact (p=.004), social eating (p=.03), speech (p=.005), sense of taste/smell (p=.005), and swallowing (p=.005). Current/recent smokers were also more likely than both former and never smokers to report weight loss over the past week (p=.03 and .01, respectively).
Figure 1. Head and Neck Cancer-Specific Symptoms by Smoking Status.
Average scores and 95% confidence intervals are presented for each smoking status group. Each measure assessed symptom over the past week on a scale from 0 to 100 with higher scores reflecting worse problems on the European Organization for Research and Treatment of Cancer Head and Neck Module. Kruskal-Wallis tests were used to examine the overall association between smoking status (current/recent, former, never smokers) and symptom factors.
Adjusted Regression Models
Adjusted regression models further examining HNC-specific symptoms significantly associated with smoking status are shown in Table 2. Controlling for potential covariates (age, gender, race, health insurance status, co-morbid health conditions, cancer stage and prior HNC treatment) with p ≤.10, smoking status remained significantly associated with problems with swallowing, speech, appearance, cough, feeling ill, and weight loss (p’s < .05). Specifically, these models highlighted that current/recent smokers reported increased swallowing problems compared to former smokers (p=.04), an association that became even more pronounced compared to never smokers (p<.01). A similar pattern was seen for problems with speech with current/recent smokers reporting more problems than former smokers (p=.03) and even more problems when compared to never smokers (p<.01). Current/recent smokers were also more likely to report elevated levels of problems with cough than both former and never smokers (p<.01 and p=.02, respectively). Lastly, current/recent smokers were more likely to report weight loss (p=.01), feeling ill (p=.02) and problems with social contact (p=.01) when compared to never smokers with the highest difference seen in weight loss.
Table 2.
Associations Between Smoking Status and Symptoms After Adjusting for Sociodemographic and Clinical Factors
| Dependent symptom variablea |
Current/recent smokers versus formerb | Current/recent smokers versus neverb | ||||
|---|---|---|---|---|---|---|
| Unstandardized coefficient |
Standard error |
p value | Unstandardized coefficient |
Standard error |
p value | |
| Swallowing | 14.0 | 6.8 | .04 | 24.0 | 7.7 | <0.01 |
| Smell/taste | −2.9 | 6.4 | .65 | 9.9 | 7.2 | .17 |
| Speech | 16.2 | 7.2 | .03 | 24.6 | 8.2 | <0.01 |
| Social eating | −0.7 | 7.6 | .92 | 12.0 | 8.8 | .18 |
| Social contact | 6.4 | 5.7 | .27 | 16.4 | 6.4 | .01 |
| Cough | 24.0 | 7.5 | <0.01 | 20.0 | 8.2 | .02 |
| Feeling ill | 9.8 | 7.2 | .18 | 19.1 | 8.3 | .02 |
| Pain killer use | −7.0 | 10.8 | .51 | 22.8 | 12.3 | .07 |
| Weight loss | 21.0 | 11.0 | .06 | 32.0 | 12.0 | .01 |
Each measure assessed problem with symptom over the past week on a scale from 0 to 100 with higher scores reflecting worse problems on the European Organization for Research and Treatment of Cancer Head and Neck Module.
All models examined smoking status (former smokers versus current/recent smokers and never smokers versus current/recent smokers) as a predictor of symptoms controlling for age, gender, race, health insurance (any or none), comorbidities (any or none), previous head and neck cancer treatment (yes or no) and cancer stage; a stepwise approach was used with covariates with p ≥0.10 dropped for final models. Bolded findings are significant at the p ≤.05 level.
Discussion
This study highlighted that current and recent smoking HNSCC patients had a higher symptom burden and a profile consistent with fewer social, economic and psychological resources than former and never smokers. Results provide insight into the factors associated with smoking in HNSCC patients presenting for surgical management, a promising smoking cessation opportunity.35 The 2014 Surgeon General’s Report confirmed the adverse effects of smoking on cancer patients and survivors across virtually all cancer disease sites5 and numerous reviews have emphasized the need to address tobacco use in cancer patients to improve clinical treatment outcomes.36,37 Smoking cessation is now advocated as a clinical standard of care by several organizations including the American Society for Clinical Oncology (ASCO)38 and the American Association for Cancer Research (AACR).39 The National Comprehensive Cancer Network (NCCN) created new evidence-based guidelines in 2015 for smoking cessation support for all patients who report smoking within the past 30 days.40 However, what is lacking in many of these guidelines is a tailored approach to the clinical, sociodemographic, psychosocial, and symptomatic factors associated with current smoking in cancer patients. Patient-centered care may be enhanced with provision of evidence-based smoking cessation support in combination with targeting at-risk factors associated with smoking that may affect treatment outcomes.
Consistent with prior research in the general population5 and with cancer patients,17,18,21,22 our findings confirm that HNSCC surgical patients who are current or recent smokers can be characterized as a more marginalized group facing increased challenges (e.g., more likely to be unpartnered, unemployed, lacking insurance, and have inadequate finances). The diminished availability of financial resources and the potential implications of educational and support needs for HNC smokers suggests the need for heightened awareness and attention to these challenges prior to treatment and consideration of interventions. Importantly, smokers may also have fewer psychological resources (e.g., lower religious faith41 and higher fatalistic beliefs20) to cope with surgical challenges and recovery and engage more frequently in risky health behaviors such as binge drinking.22
This study demonstrated that HNSCC patients who report current/recent smoking had a higher symptom burden when compared to former and never smokers. Current/recent smokers reported greater severity in 9 of 17 symptoms assessed and in no case did never smokers report significantly greater severity than current/recent smokers. In adjusted models controlling for clinical factors that may impact symptom burden (e.g., cancer stage, previous treatment), current/recent smokers consistently reported more problems with swallowing, speech and cough, with the most severe symptoms reported by current/recent smokers followed by former and then never smokers. In adjusted models, current/recent smokers were also more likely than never smokers to report problems with social contact, feeling ill and weight loss. Consistent with our findings, Jensen and colleagues42 found continued smoking after HNSCC treatment was associated with worse swallowing, cough and weight loss problems and also found that smokers had more problems with other symptoms (pain, sense of smell/taste, dry mouth, use of feeding tube and nutritional supplements). Other studies have also shown that pain43 was worse in smoking HNSCC patients compared to non-smokers. The additional symptom differences found in the Jensen study could be because this study was conducted after treatment when symptoms can escalate.40 Of note, our sample size may have limited our ability to detect differences as we observed that several symptoms, while not significantly different, were higher in smokers than others. Collectively, findings suggest that evaluating these symptoms in HNSCC patients who are current smokers may be important to mitigating adverse outcomes. However, this hypothesis will require future testing, as this study did not evaluate whether treating these symptoms in smokers affected clinical outcomes.
Interestingly, we observed that former smokers presented with more comorbid conditions and greater rates of recurrent/persistent disease than current/recent smokers. This is an intriguing finding because continued smoking predisposes patients to a broad spectrum of adverse health conditions.5 While more research is needed to identify whether this clinical burden among former smokers relates to other tobacco, health or demographic factors, this observation highlights that former smokers may need resources to cope with the challenges of disease recurrence and management of other conditions during treatment.
Results did not support an association between smoking and depression, in contrast to previous research.20,44,45 This could be because the majority of participants had high depression, in contrast to the other studies mostly conducted after treatment when psychological functioning tends to improve. It is critical to consider best practices for monitoring depression during treatment because HNSCC patients demonstrate high levels of depression46 and promising work has emphasized the benefits of addressing smoking and depression simultaneously in HNC.18,47
Overall, this study highlights that HNSCC patients who report current/recent smoking may present with higher symptom burden and several additional sociodemographic, clinical and psychosocial challenges that may place them at an overall disadvantage as compared with former and never smokers. These findings argue for routine assessment of smoking status, symptom burden and other factors at diagnosis within the HNSCC setting. Unfortunately, most oncologists readily assess tobacco use but do not provide cessation support48,49 due to a lack of time, resources, and education.50 The authors are unaware of studies evaluating practice patterns associated with smoking cessation support combined with management of symptoms and other sociodemographic and psychosocial challenges. However, combining smoking cessation with routine screening and supportive care referrals (e.g., social work, counseling) to address financial (e.g., housing, nutrition, transportation) and symptom management needs may benefit these at-risk patients.
Strengths of this study include our exploration of multiple patient characteristics in relation to smoking status to allow a consideration of the whole patient. However, this was also a limitation given our sample size and the statistical significance of any one association needs to be interpreted with caution and instead the focus should be on the consistent pattern of findings observed. For our models, we were limited in the number of covariates we could select due to our modest sample size. Therefore, this provides a preliminary look at the relationships between symptom burden and smoking status and future studies should further examine the role of other clinical and smoking characteristics in symptom burden. This study was also cross-sectional and results should be replicated in the future. Also, while the parent study from which this analysis was conducted23,51 used biochemical verification of smoking on the day of surgery, we used self-reported smoking before surgery. Despite study limitations, strengths include the sample’s demographic and clinical diversity and the use of validated instruments.
Conclusions
Current and recent smoking HNSCC patients may be disadvantaged in multiple ways and have a higher symptom burden when compared to former and never smokers before surgery. Limited social, economic, and psychological resources should be considered when planning HNSCC care in patients who are current or recent smokers. Future studies should explore the potential benefits of comprehensively addressing smoking cessation needs as well as symptoms in smokers at clinic presentation to examine whether addressing both can have a meaningful effect on cancer treatment outcomes.
Acknowledgments
This study was funded by the Hollings Cancer Center at the Medical University of South Carolina and the Comprehensive Cancer Center of Wake Forest University (CCCWFU). This project was supported by the Biostatistics Core of the CCCWFU (P30 CA012197). Data management support (REDCap) was provided by the Wake Forest School of Medicine Translational Sciences Institute National Center for Research Resources/National Institutes of Health (NCRR/NIH) grant M01RR007122 and data analysis support was provided in part by the Biostatistics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). Katherine Sterba’s work on this manuscript was supported by a Mentored Research Scholar Grant in Applied and Clinical Research, MRSG-12-221-01-CPPB from the American Cancer Society.
The authors wish to acknowledge recruitment and data collection support from the following individuals: Rebecca Patten, OT and Amy Buchanan, MPH at the Medical University of South Carolina and Kathryn Josephs, MS at Wake Forest School of Medicine.
Footnotes
The authors have no conflicts of interest to disclose.
The authors have no financial disclosures to provide.
Material from this manuscript was presented at the American Society of Preventive Oncology annual meeting, March 11, 2013 in Memphis, TN, USA.
References
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 3.Franceschi S, Talamini R, Barra S, et al. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990;50:6502–6507. [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: 2016. [Google Scholar]
- 5.U.S. Department of Health and Human Services. The Health Consequences of Smoking-- 50 Years of Progress: A Report of the Surgeon General. In: Office on Smoking and Health, editor. Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Atlanta, GA: 2014. [Google Scholar]
- 6.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109:746–749. doi: 10.1001/archotol.1983.00800250040009. [DOI] [PubMed] [Google Scholar]
- 8.Sharp L, McDevitt J, Carsin AE, Brown C, Comber H. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based, study. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2579–2590. doi: 10.1158/1055-9965.EPI-14-0311. [DOI] [PubMed] [Google Scholar]
- 9.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Hiyama T, Sato T, Yoshino K, Tsukuma H, Hanai A, Fujimoto I. Second primary cancer following laryngeal cancer with special reference to smoking habits. Jpn J Cancer Res. 1992;83:334–339. doi: 10.1111/j.1349-7006.1992.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(Suppl 2):S13–S33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 12.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–410. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 14.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15:e568–e580. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 16.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 17.Berg CJ, Thomas AN, Mertens AC, et al. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology. 2013;22:799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy SA, Ronis DL, Valenstein M, et al. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–148. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 19.Ronis DL, Duffy SA, Fowler KE, Khan MJ, Terrell JE. Changes in quality of life over 1 year in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:241–248. doi: 10.1001/archoto.2007.43. [DOI] [PubMed] [Google Scholar]
- 20.Schnoll RA, Malstrom M, James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46:137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 21.Sivasithamparam J, Visk CA, Cohen EE, King AC. Modifiable risk behaviors in patients with head and neck cancer. Cancer. 2013;119:2419–2426. doi: 10.1002/cncr.27993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westmaas JL, Alcaraz KI, Berg CJ, Stein KD. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: Findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2014;23:1783–1792. doi: 10.1158/1055-9965.EPI-14-0046. [DOI] [PubMed] [Google Scholar]
- 23.Hatcher JL, Sterba KR, Tooze JA, et al. Tobacco use and surgical outcomes in head and neck cancer patients. Head Neck. 2014 doi: 10.1002/hed.23944. Epub ahead of print: 17 May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Department of Commerce, Census Bureau. National Cancer Institute-sponsored Tobacco Use Supplement to the Current Population Survey (2010–11) [Accessed November 19, 2014];2012 Availbale at: http://appliedresearch.cancer.gov/tus-cps/
- 25.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 26.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 27.Watson M, Greer S, Young J, Inayat Q, Burgess C, Robertson B. Development of a questionnaire measure of adjustment to cancer: the MAC scale. Psychol Med. 1988;18:203–209. doi: 10.1017/s0033291700002026. [DOI] [PubMed] [Google Scholar]
- 28.Schnoll RA, James C, Malstrom M, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25:214–222. doi: 10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- 29.Plante TG, Vallaeys CL, Sherman AC, Watson KA. The development of a brief version of the santa clara strength of religious fFaith questionnaire. Pastoral Psychol. 2002;50:359–368. [Google Scholar]
- 30.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 31.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17:1008–1019. doi: 10.1200/JCO.1999.17.3.1008. [DOI] [PubMed] [Google Scholar]
- 32.Pierce JP, Gilpin EA. A minimum 6-month prolonged abstinence should be required for evaluating smoking cessation trials. Nicotine Tob Res. 2003;5:151–153. doi: 10.1080/0955300031000083427. [DOI] [PubMed] [Google Scholar]
- 33.Morales NA, Romano MA, Cummings KM, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Cause Control. 2013;24:1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nayan S, Gupta MK, Strychowsky JE, Sommer DD. Smoking cessation interventions and cessation rates in the oncology population: an updated systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2013;149:200–211. doi: 10.1177/0194599813490886. [DOI] [PubMed] [Google Scholar]
- 36.Gritz ER, Toll BA, Warren GW. Tobacco use in the oncology setting: advancing clinical practice and research. Cancer Epidemiol Biomarkers Prev. 2014;23:3–9. doi: 10.1158/1055-9965.EPI-13-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren GW, Ward KD. Integration of tobacco cessation services into multidisciplinary lung cancer care: rationale, state of the art, future directions. Transl Lung Cancer Res. 2015;4:339–352. doi: 10.3978/j.issn.2218-6751.2015.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna N, Mulshine J, Wollins DS, Tyne C, Dresler C. Tobacco cessation and control a decade later: American Society of Clinical Oncology policy statement update. J Clin Oncol. 2013;31:3147–3157. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- 39.Toll BA, Brandon TH, Gritz ER, et al. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Comprehensive Cancer Network Practice Guidelines in Oncology: Smoking Cessation V.2.2015. [Accessed March 12, 2016];2015 Avialble at: http://www.nccn.org/professionals/physician_gls/pdf/smoking.pdf. [Google Scholar]
- 41.Thune-Boyle IC, Stygall JA, Keshtgar MR, Newman SP. Do religious/spiritual coping strategies affect illness adjustment in patients with cancer? A systematic review of the literature. Soc Sci Med. 2006;63:151–164. doi: 10.1016/j.socscimed.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 42.Jensen K, Jensen AB, Grau C. Smoking has a negative impact upon health related quality of life after treatment for head and neck cancer. Oral Oncol. 2007;43:187–192. doi: 10.1016/j.oraloncology.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Logan HL, Fillingim RB, Bartoshuk LM, et al. Smoking status and pain level among head and neck cancer patients. J Pain. 2010;11:528–534. doi: 10.1016/j.jpain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berg CJ, Carpenter MJ, Jardin B, Ostroff JS. Harm reduction and cessation efforts and interest in cessation resources among survivors of smoking-related cancers. J Cancer Surviv. 2013;7:44–54. doi: 10.1007/s11764-012-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooley ME, Wang Q, Johnson BE, et al. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer. 2012;76:144–149. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammerlid E, Silander E, Hornestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer--a longitudinal study. Head Neck. 2001;23:113–125. doi: 10.1002/1097-0347(200102)23:2<113::aid-hed1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 48.Warren GW, Marshall JR, Cummings KM, et al. Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol. 2013;8:543–548. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9:258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren GW, Dibaj S, Hutson A, Cummings KM, Dresler C, Marshall JR. Identifying targeted strategies to improve smoking cessation support for cancer patients. J Thorac Oncol. 2015;10:1532–1537. doi: 10.1097/JTO.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 51.Alberg AJ, Worley ML, Tooze JA, et al. The validity of self-reported recent smoking in head and neck cancer surgical patients. Otolaryngol Head Neck Surg. 2015;153(6):990–995. doi: 10.1177/0194599815594385. [DOI] [PMC free article] [PubMed] [Google Scholar]