Abstract
Adult stem cells are a promising cell source for cartilage regeneration. Unfortunately, due to donor age and ex vivo expansion, stem cell senescence becomes a huge hurdle for these cells to be used clinically. Increasing evidence indicates that environmental preconditioning is a powerful approach in promoting stem cells’ ability to resist a harsh environment post-engraftment, such as hypoxia and inflammation. However, few reports organize and evaluate the literature regarding the rejuvenation effect of environmental preconditioning on stem cell proliferation and chondrogenic differentiation capacity, which are important variables for stem cell based tissue regeneration. This report aims to identify several critical environmental factors such as oxygen concentration, growth factors, and extracellular matrix and to discuss their preconditioning influence on stem cells’ rejuvenation including proliferation and chondrogenic potential as well as underlying molecular mechanisms. We believe that environmental preconditioning based rejuvenation is a simpler and safer strategy to program pre-engraftment stem cells for better survival and enhanced proliferation and differentiation capacity without the undesired effects of some treatments, such as genetic manipulation.
Keywords: Preconditioning, Extracellular matrix, Fibroblast growth factor 2, Hypoxia, Adult stem cell, Chondrogenesis
Introduction
Due to the hostile environment in damaged tissue, such as inflammation, immune compromise, hypoxic stress, and insufficient blood supply [1], the survival rate of transplanted cells in vivo is low, only 1–3% [2,3], which is a huge hurdle for cell-based therapy [4,5]. The concept of “preconditioning-induced protection”, first raised by Murry in 1986, is a process by which myocardial stem cells exposed to a sub-lethal ischemic condition could promote the heart’s tolerance to severe ischemia [6]. Since then, the preconditioning concept has been used as the most effective means of cytoprotection, especially for cell-based treatment of ischemic myocardium and stroke [7]. Despite the fact that cell death in musculoskeletal transplantation, such as cartilage [8] or intervertebral disc (IVD) repair [9], is not as robust as in the heart and brain, it is still crucial for cells to survive before a sufficient repair response is induced.
Common preconditioning approaches include hypoxia, cytokines and growth factors, and genetic manipulation. Genetic manipulation promotes the viability of stem cell engraftment by overexpression of cytoprotective genes. The common overexpressed genes in promoting the survival of mesenchymal stem cells (MSCs) include v-Akt Murine Thymoma Viral Oncogene (AKT) [10], B-cell lymphoma 2 (Bcl-2) [11], heat shock protein 20 (Hsp20) [12], nuclear factor related (erythroid-derived 2)-like 2 (Nrf2) [13], heme oxygenase-1 (HO) [14,15], endothelial nitric oxide synthase (eNOS) [16], connexin 43 (Cx43) [17], and hypoxia inducible factor-1α (HIF-1α) [18]. Other overexpressed genes, such as wild-type p53 inducible phosphatase-1 (WIP-1) [19] and lipocalin 2 (Lcn2) [20], could decrease MSC senescence during the process. However, genetic manipulation of MSCs has limited clinical benefit due to its inherent risks during genetic modification, such as random integration into the host genome inducing mutations [21].
Despite an initial focus on the suppression of inflammatory and immune responses and the promotion of cell survival rate as well as migration and homing of transplanted cells, preconditioning strategies now attract more attention for rejuvenation of regenerative and repair potentials of pre-engraftment cells [22,23]. As expansion is always needed to increase cell numbers for clinical application, it is critical to achieve expansion without compromising differentiation potential. Thanks to the discovery that crosstalk between MSCs and other cells in the native niche modulated MSCs’ properties [24,25], the in vitro establishment of these communications has been demonstrated [26,27]. This review paper focuses on summarizing up-to-date environmental preconditioning strategies during ex vivo expansion and discussing their influence on adult stem cell proliferation and chondrogenic potential, which is important for cartilage tissue engineering and regeneration using autologous stem cells that become prematurely senescent due to donor age and suffer replicative senescence because of extensive expansion. We hypothesize that, from the clinical perspective, environmental preconditioning based rejuvenation is a simpler and safer strategy to program pre-engraftment stem cells for better survival and enhanced proliferation and differentiation capacity without the undesired effects of some treatments, such as genetic manipulation [21].
Hypoxic preconditioning
In native cartilage, cells are exposed to very low oxygen tension – about 7% (53 mmHg) in the superficial zone and 1% (5–8 mmHg) in the deep zone of articular cartilage [28]. There have been many studies investigating the effects of hypoxia on chondrogenic differentiation of MSCs in an attempt to determine the best point in the culture process to expose MSCs to hypoxic conditions. For example, should MSCs be expanded in hypoxia, differentiated in hypoxia, or should both expansion and differentiation take place in hypoxic conditions in order to attain the best results? Increasing evidence suggests that hypoxic pretreatment can not only promote cell survival and migration ability post-engraftment [29,30] but also can benefit cell rejuvenation, in terms of proliferation and differentiation capacity (Table 1) [31].
Table 1.
Hypoxia primed adult stem cells for chondrogenesis.
| MSC | Passage | O2 % | Proliferation | Chondro- | others | References |
|---|---|---|---|---|---|---|
| hASC | 1 | 1,5,10,15 | ↑ CFU-F | ↓ (pellet) | - | 44 |
| hASC | 3 | 2 vs 21 | ↑ stemness/cell number; ↓ PDT |
↑ (pellet) | ↓ osteo- /adipo- |
34 |
| mASC | 2 | 2 vs 21 | ↑ cell number/BrdU; ↓ PDT |
↑ (pellet) | ↓ osteo- | 35 |
| hBMSC | 2 | 3 vs 21 | ↑ number of cell colonies | ↑ (pellet) | - | 42 |
| hBMSC | - | 4 vs 20 | - | ↑ (pellet/gelatin/gelatin+HA) |
- | 41 |
| hBMSC | 2 | 5 vs 21 | ↑ CFU-F/cell number | ↑ (pellet) (ns) | osteo- (nc); ↓ adipo- (ns) |
38 |
| hBMSC | 4 | 1.5 vs 21 | ↑ cell number | ↑ (pellet) | nc | 39 |
| oBMSC | 2 | 5 vs 20 | ↑ CFU-F; ↓β-gal/cell size |
↑ (pellet/collagen I hydrogel) |
- | 37 |
| oBMSC | 2 | 5 vs 20 | ↑ number and size of cell colonies |
↑ (pellet) | - | 36 |
| hIFPSC | 2 | 5 vs 20 | - | ↑ (pellet) | - | 45 |
| gDSC | - | 5 vs 20 | ↓ cell number/CFU-F | ↑ (pellet) | - | 40 |
| pSDSC | 3 | 5 vs 21 | ↑ cell number; ↓ cell size |
nc | - | 43 |
| hUCB CD133(+) |
1 | 1.5 vs 21 | ↑ clonogenic myeloid capacity | - | - | 39 |
Abbreviation: CFU-F: colony forming unit-fibroblast; DSCs: dermis isolated stem cells; g: goat; h: human; HA: hyaluronan; nc: no change; ns: no significant difference; o: ovine/sheep; p: porcine; PDT: population doubling time; stemness markers: REX1, SOX2, OCT4, and NANOG; UCB: umbilical cord blood
Evidence of cell proliferation capacity
Expansion of MSCs in hypoxic conditions has been shown to prevent stem cell senescence and yield higher proliferation rates, enhanced tissue forming capacity, and smaller, more densely organized, spindle-like cells than expansion in normoxic conditions [32,33]. Choi et al. found that hypoxic (2% oxygen) treatment significantly increased stemness markers, reduced expression 1 (REX1), SRY (sex determining region Y)-box 2 (SOX2), octamer-binding protein 4 (OCT4), and NANOG, along with HIF-1α in human adipose stem cells (ASCs) [34]. The proliferation rate of ASCs under hypoxic incubation was also significantly enhanced, evidenced by an increase in cell number but a decrease in the mean population doubling time (PDT) despite no alteration of surface markers, including CD73, CD90, and CD105. Xu et al. found that 2% oxygen treatment yielded a significantly higher cell number and more DNA synthesis as well as shorter PDT in mouse ASCs [35]. They also found that hypoxic treatment significantly reduced the matrix metalloproteinase (MMP) family genes, MMP2, MMP3, MMP8, and MMP13.
Krinner et al. found that hypoxic (5% oxygen) treatment promoted in vitro population growth of ovine bone marrow stromal cells (BMSCs) as demonstrated by significantly larger colonies compared to those under normoxic conditions [36]. Similarly, Zscharnack et al. found that hypoxic treatment (5% oxygen) of ovine BMSCs significantly increased colony numbers and sizes but diminished senescence, as shown by lower levels of granularity and senescence-associated (beta)-galactosidase positive cells [37]. Boyette et al. found that hypoxic treatment (5% oxygen) in human BMSCs enhanced colony formation and proliferation, evidenced by 5-ethynyl-2′-deoxyuridine (EdU) incorporation, but with no change in Ki67 staining [38]. They also found that metabolic activity was increased after 96 h of hypoxic treatment. However, hypoxia was not found to have any impact on cell death and apoptosis rates.
Martin-Rendon et al. found that exposure to hypoxia (1.5% oxygen) for 24 h demonstrated a moderate increase in total colony numbers of umbilical cord blood (UCB) CD133+ cells and a significant increase in viable cell numbers of human BMSCs [39]. In normoxia, there was low expression of endogenous HIF-1α in human BMSCs but not in UCB CD133+ cells; however, exposure to hypoxia for 24 h stabilized/upregulated HIF-1α in both cell populations. Hypoxia likely increased cell proliferation in a cell source-dependent manner. Kalpakci et al. found that hypoxic treatment (5% oxygen) resulted in a significant decrease in number of dermis isolated adult stem cells at days 7, 9, and 11 of culture and a lower colony forming unit – fibroblast (CFU-F) compared with normoxic culture [40].
Evidence of chondrogenic potential
Accumulating evidence indicates that hypoxic preconditioning can promote MSC chondrogenic potential and concurrently inhibit unintended differentiation into the osteogenic lineage. Krinner et al. found that 5% oxygen preconditioning enhanced chondrogenic potential in ovine BMSCs [36]. Similarly, Müller et al. found that 4% oxygen preconditioning enhanced human BMSCs’ chondrogenic differentiation in both micromass and gelatin hydrogel culture systems [41]. Adesida et al. found that hypoxia preconditioned human BMSCs yielded pellets with enhanced chondrogenic capacity in spite of oxygen tension during pellet culture [42]. Also of note, Xu et al. found that hypoxic preconditioning enhanced chondrogenic differentiation but decreased osteogenic differentiation in mouse ASCs [35]. These results indicate that hypoxia during expansion may prepare MSCs specifically for chondrogenesis.
However, conflicting reports exist regarding hypoxic preconditioning, possibly due to varied situations, such as hypoxic extent and donor cell type. Martin-Rendon et al. found that hypoxic preconditioning (1.5% oxygen) did not change the differentiation potential of UCB CD133+ clonogenic myeloid cells but promoted human BMSCs’ chondrogenic potential despite having no effect on adipogenic and osteogenic differentiation [39]. Li and Pei found that hypoxic preconditioning did not promote chondrogenic potential in porcine synovium-derived stem cells (SDSCs) [43]. Pilgaard et al. found that, despite enrichment with CFU-Fs during expansion under hypoxia (5% and 1% oxygen), human ASCs did not exhibit an enhanced chondrogenic differentiation in subsequent chondrogenic induction [44]. Furthermore, Boyette et al. found that hypoxic preconditioning decreased chondrogenesis in human BMSCs in a pellet culture, which worsened if combined with hypoxic treatment during chondrogenic induction; however, human BMSCs preconditioned in 21% oxygen differentiated robustly in pellet culture under both 5% oxygen and 21% oxygen conditions [38].
The effects on MSCs during expansion at normoxic levels and differentiation into chondrocytes at hypoxic levels have also been studied. MSCs under hypoxia for chondrogenic induction showed decreased proliferation, but elevated expression of sulfated glycosaminoglycan (GAG) and chondrogenic genes [45–48]. Interestingly, hypoxic conditions in pellet culture upregulated HIF-2α and downregulated COL10A1 (type X collagen) despite normoxia or hypoxia during cell expansion [42]. Hypoxic treatment also produced a mechanically functional hyaline cartilage-like tissue compared to the cells differentiated in normoxia [49]. Recently, Leijten et al. found that normoxia in pellet culture promoted the expression of the hypertrophic cartilage-enriched gene transcripts of COL10A1, MMP13, and pannexin 3 (PANX3) levels while hypoxia enhanced the articular cartilage-enriched gene transcripts of gremlin 1 (GREM1), frizzled-related protein (FRZB), and Dickkopf Wnt signaling pathway inhibitor 1 (DKK1) which act as inhibitors of hypertrophic differentiation [50]. In addition, they also found that, in a nude mouse model, hypoxia-preconditioned implants retained cartilage; on the other hand, normoxia-preconditioned implants readily underwent endochondral ossification [50]. These studies have shown that differentiation of MSCs in vitro in hypoxic conditions following normoxic expansion improves chondrogenesis when compared to cells differentiated in normoxic conditions.
Conflicting data also exist on the effect of hypoxia on chondrogenic induction. One study has shown that murine ASCs, when differentiated at 2% oxygen, exhibited increased proliferation and fewer chondrogenic markers compared to those differentiated in 21% oxygen [51]. Interestingly, the same investigators later showed that, when murine ASCs were expanded in 2% oxygen, then differentiated at normoxic levels, some chondrogenic markers [GAG content and COL2A1 (type II collagen) expression, but not expression of SOX9 or ACAN (aggrecan)] were increased compared to those expanded in 21% oxygen [52]. The differences between these two reports could have been a result of reactive oxygen species (ROS) accumulation during long-term exposure to hypoxic conditions in differentiation, which would have led to cell damage and decreased chondrogenic potential [35].
Potential mechanisms
The mechanism by which hypoxia exerts its effect on cells is mainly regulated by HIF-1, which is composed of two subunits, α and β [53]. Compared to the presence of the β subunit in the nucleus, the α subunit is constitutively expressed in the cytoplasm, where it is bound by von Hippel-Lindau (vHL) tumor suppressor protein, which is an E3 ubiquitin ligase that targets HIF-1α for degradation by the 26S proteasome. This vHL/HIF-1α interaction is oxygen dependent through a group of prolyl hydroxylases (PHDs), the most important of which is PHD domain-containing protein 2 (PHD2) [54,55]. These enzymes hydroxylate proline residues at Pro402 and Pro564 on HIF-1α [56] and at Pro405 and Pro531 on HIF-2α [57] and require Fe2+ and α-ketoglutarate as co-factors for their catalytic activity [58–60]. As a result of hydroxylation of these proline residues, vHL is able to bind HIF-1α and target it for destruction. However, as oxygen concentration decreases, the overall PHD2 function decreases as well, increasing the amount of HIF-1α that is able to translocate into the nucleus and bind to its counterpart, HIF-1β [59]. This complex binds to the hypoxia response element (HRE) on the genome, which induces expression of hypoxia-regulated genes.
Although oxygen concentration is directly associated with the HIF-1 pathway, there are still mechanisms by which oxygen indirectly regulates cells’ response to hypoxia. For example, the AKT/phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways might be important in upstream regulation of HIF-1. When cultured in hypoxic conditions, levels of phosphorylated AKT and p38 MAPK are elevated and, when AKT and p38 MAPK are inhibited, HIF-1α is unable to translocate to the nucleus [47,61]. Recent evidence indicates that the increase of stem cell survival after hypoxic preconditioning is largely through the stabilization of HIF-1α via a hypoxia-induced increase of phosphorylated AKT and p38 MAPK [47], resulting in the upregulation of the glycose-6-phosphate transporter and promotion of MSC survival [62]. Hypoxic preconditioning also modulates the pro-survival and pro-angiogenic factors of MSCs by upregulation of vascular endothelial growth factor (VEGF) and B-cell lymphoma 2 (BcL-2) [30], for example, via the AKT and Extracellular Signal-regulated Kinase (Erk) involved complex pathways [61,63]. Hypoxic preconditioning also plays a critical role in mobilization and homing of MSCs through its capacity to induce HIF-1 mediated expression of stromal cell-derived factor-1 (SDF-1) [64] and its receptor C-X-C chemokine receptor type 4 (CXCR4) [65]. Additionally, CREB-binding protein (CBP) and p300 co-activator encourage transcription of HRE genes by binding the HRE/HIF-1 complex in an oxygen-regulated manner that is independent of the HIF-1 pathway [66].
High oxygen tension has been shown to damage DNA, proteins, and lipids [67] by generation of abnormally high free-radical-derived ROS [68], causing senescence either by the p53-mediated pathway or by accelerating telomere loss [69]. In contrast, low oxygen tension activates HIF-1α, delaying senescence through the activation of macrophage inhibitory factor and inhibition of the p53-mediated pathway [70], regulating cell proliferation [71] and cell differentiation [72–74]. Mouse ASCs with the deletion of HIF-1α exhibited diminished chondrogenic capacity with no significant change in osteogenic differentiation and enhanced adipogenic differentiation [52]. Although HIF-1α has conventionally been thought responsible for the upregulation of chondrogenic gene expression, recent studies have shown that chondrogenesis may be more dependent on signaling through HIF-2 α. For example, MSCs expanded and differentiated within hypoxic conditions demonstrated no marked HIF-1α expression but showed upregulation of HIF-2α[42,45]. Interestingly, human articular chondrocytes with HIF-1α knockdown cultured in hypoxia (1% oxygen) had no effect on chondrogenic gene expression, while knockout of HIF-2α resulted in a marked decrease in expression of chondrogenic genes like SOX9 and COL2A1 [75]. These results indicate that the effects of hypoxia may be carried out by different mechanisms, depending on the level of hypoxia and the cell type.
FGF preconditioning
Growth factors are important in mediating the development and maintenance of hyaline cartilage [76]. As a result, the use of growth factors during MSC ex vivo expansion and chondrogenic induction has been well studied and yielded promising results. In the cytokines and growth factors, transforming growth factor alpha (TGFα) [77], interferon-gramma (IFN-γ) [78], SDF-1 [79,80], epidermal growth factor (EGF) [81], and insulin-like growth factor I (IGF-I) [82] have been extensively studied. Along with hypoxic preconditioning, these approaches are considered environmental manipulation, in which there is a fine-tuned balance between self-renewal and differentiation potential of MSCs [83,84], particularly for basic fibroblast growth factor (FGF-2) mediated preconditioning in MSCs’ chondrogenic and osteogenic potential (Table 2) [85].
Table 2.
Growth factor primed stem cells for chondrogenesis.
| MSC | Passage | Dose (ng/mL) | Proliferation | Chondro- | Adipo- /osteo- |
Reference |
|---|---|---|---|---|---|---|
| mASC | 1 | 5, 10, 50, 100 (F) | ↑ cell number | ↑ (pellet) | - | 86 |
| hBMSC | 2–4 | 10 (F) | ↑ growth index | ↑ (pellet) | No difference | 87 |
| hBMSC | 1–7 | 10 (F) | ↑ PD | ↑ (pellet) | - | 95 |
| hBMSC | 2–4 | 1, 10 (F) | n/a | ↑ (pellet) | - | 165 |
| hBMSC | - | 1, 5, 10 (F) | ↓ cell size & PDT | ↑ (pellet) | - | 92 |
| hBMSC | 2 | 5 (F) | - | ↑ (pellet) | - | 106 |
| hBMSC | - | 1 (F) | ↑ TRF length; ↓ PDT |
↑ (pellet) | - | 89 |
| hBMSC | 1–2 | 10 (F) | - | ↑ (pellet) | 108 | |
| hBMSC | 1 | 1 (F) | ↑ colony size; ↓ colony number |
↑ (pellet) | ↑ osteo- | 85 |
| hBMSC | - | 1 (F) | ↑ PD | ↑ (pellet) | - | 101 |
| hIFPSC | 2 | 10 (F) | ↑ cell number | ↑ (pellet) | - | 88 |
| pIFPSC | 1–2 | 5 (F) | ↓ PDT | ↑ (agarose hydrogel) |
- | 96 |
| bSDSC | 4 | 1 (T) + 5 (F) + 10 (P) | - | ↑ (agarose disc) |
- | 97 |
| dSDSC | 2 | 1 (T) + 5 (F) + 10 (P) | ↓ PDT | ↑ (pellet) | - | 107 |
| hSDSC | 1–2 | 0.1, 1, 10, 100 (F) | ↓ cell size; ↑ cell number |
↑ (pellet) | - | 91 |
| pSDSC | 3 | 10 (T) + 50 (F) + 500 (I) | - | ↑ (pellet) | - | 166 |
| pSDSC | 3 | 10 (F) | ↑ cell number; ↓ cell size |
↑ (pellet); hypertrophy |
- | 43 |
Abbreviation: ASCs: adipose stem cells; BMSCs: bone marrow stromal cells; b: bovine; d: dog; F: basic fibroblast growth factor; h: human; I: insulin-like growth factor I; IFPSCs: infrapatellar fat pad derived stem cells; m: mouse; p: porcine; P: platelet-derived growth factor-BB; PD: population doubling; PDT: population doubling time; SDSCs: synovium-derived stem cells. TRF: telomere restriction fragment; T: transforming growth factor beta 1
Evidence of cell proliferation and chondrogenic potential
FGF treatment could promote MSC proliferation, which is independent of species, such as mouse [86], human [87], and porcine [43], or of varied tissues, such as adipose [86], infrapatellar fat pad [88], bone marrow [89], and synovium [43]. Interestingly, FGF-2 administration during expansion is associated with downregulation of some important surface markers (such as CD49a, CD90, and CD146) but upregulation of chondrogenic potential, indicating that a difference in surface marker distribution does not result in impaired differentiation [87,90,91]. Microarray analysis did not find a clear pattern as to the mitogenic effect of FGF-2 on human BMSCs [92].
Given that supplementation during ex vivo expansion supports a proliferative state, subsequent withdrawal of FGF-2 is proposed to contribute to leaving the cell cycle and coming into a differentiation-competent state. For example, compared to treatment during pellet culture inhibiting chondrogenic differentiation [93,94], FGF-2 pretreated stem cells were reported to promote and retain expanded MSCs’ chondrogenic potential even after 30 population doublings, whereas the non-pretreated stem cells lost chondrogenic differentiation after around 20 population doublings, which is more than 1000-fold difference in the number of cells [95]. Furthermore, FGF-2 pretreated MSCs from porcine infrapatellar fat pad were found to generate the most mechanically functional cartilage tissue [96]. Preconditioning with a specific growth factor cocktail [1 ng/mL TGF-β1, 5 ng/mL FGF-2, and 10 ng/mL platelet-derived growth factor-BB (PDGF-ββ)] in monolayer culture led to remarkable improvement in biomechanical and biochemical properties of bovine SDSC-seeded tissue constructs [97].
Interestingly, during the study on limb development, ten Berge et al. found that FGF-8 and Wnt3a signals worked together to promote limb bud cell proliferation while retaining cells in an undifferentiated condition; once both types of stimulations were withdrawn, the cells switched to chondrogenic differentiation [98]. Inspired by this report, Narcisi et al. observed that combined pretreatment with Wnt3a greatly promoted the effect of FGF2 on human BMSC proliferation by increasing cell doublings from 20 to 30. They also found that co-preconditioned cells acquired enhanced chondrogenic potential; inhibition of Wnt3 signals during differentiation prevented calcification while preserving hyaline cartilage properties following transplantation in a mouse model [99]. In a three-dimensional (3D) pellet model, intriguingly, Centola et al. found that, contrary to their initial hypothesis, Wnt3a treatment induced human BMSCs to a five-fold increase in cell number despite a continuing decrease of total DNA content in the 3D construct; preconditioning with Wnt3a improved cells’ chondrogenic potential, which was antagonized by treatment with FGF2 [100].
Different from hypoxic pretreatment, which inhibits differentiation toward osteogenesis [85], more evidence showed that FGF-2 preconditioning could promote not only type II collagen but also type X collagen [43,91,92], suggesting that FGF-2 expanded MSCs yielded pellets with an enhanced capacity toward endochondral bone formation [93]. This finding indicates that stem cells pretreated with FGF-2 alone would benefit bone tissue engineering rather than cartilage tissue engineering, which is worth noting for future clinical application.
Potential mechanisms
Two potential mechanisms have been proposed for FGF-2 mediated MSC rejuvenation toward chondrogenesis. The first one is that MSCs with inherent chondrogenic potential are preferentially selected by pretreatment with FGF-2 during monolayer culture, in terms of selection mechanism [89]. Investigations found that ex vivo enrichment of MSCs by FGF-2 had long telomeres and could maintain chondrogenic potential for greater numbers of population doublings, despite low or non-detectable expression levels of telomerase activity [89,101]. This finding indicates that long telomeres in FGF-2 pretreated stem cells may be useful genetic markers for chondrogenic progenitor cells. Low expression of telomerase activity might be explained by the findings that expression of ectopic telomerase expands the life expectancy of BMSCs without impacting the proliferation rate and BMSCs transduced with telomerase display a promoted bone formation capability [102,103]; however, telomerase-deficient mice exhibited impaired differentiation of MSCs, indicating that the low levels of telomerase activity may be necessary to maintain the growth of MSCs [104].
Another potential mechanism is that the chondrogenic potential of MSCs may be generally enhanced by pretreatment with FGF-2 [105], possibly by inducing FGF-receptor 2 and N-Cadherin, early mesenchymal condensation markers, and the key transcription factor Sox9 for chondrogenesis [86,106], in terms of priming mechanisms. FGF-2 treatment during expansion caused significant downregulation of chondrogenic genes [92], but showed robust upregulation of these genes in subsequent chondrogenic induction, resulting in greater matrix production per cell [45]. Proteomics analysis indicated that FGF-2 preconditioning mediated an incomplete dedifferentiation of chondrocytes and an incomplete differentiation of SDSCs as observed by the management of a round of extracellular matrix (ECM) related proteins, both of which enabled expansion of cells that have great chondrogenic potential once seeded in three-dimensional (3D) culture [107]. Another example is that, in extensive MSC monolayer cultures, the level of integrin α10, expressed by chondrocytes in cartilage, was downregulated while the level of integrin α11, expressed by subsets of the fibroblastic lineage, was reversed by FGF-2 treatment, thus keeping MSCs more multipotent and also inducing cell proliferation and SOX9 upregulation [108].
Considering that Wnt and MAPK signals play critical roles in cartilage regeneration via crosstalk [109], FGF-2 pretreated stem cells exhibited an upregulation of secreted frizzled-related protein 1 (SFRP1) and downregulation of pregnancy-specific beta-1-glycoprotein 1 (PSG1), two typical Wnt signals, and upregulation of angiopoietin 1 (ANGPT1) and midkine (MDK), two upstream regulators of MAPK signaling [95], indicating that these two signals are also closely associated with the enhancement of chondrogenic potential in FGF-2 expanded cells.
Decellularized ECM (dECM) preconditioning
Increasing evidence indicates that the culture medium of MSCs, which is called secretome or conditioned medium, contains the biological factors secreted by MSCs that could be used in regenerative medicine [110,111]. Furthermore, dECM deposited by stem cells becomes another promising approach to rejuvenate either stem cells or primary cells for chondrogenesis (Table 3) [112].
Table 3.
dECM primed stem cells for chondrogenesis.
| MSC | Passage | dECM | Proliferation | Chondro- | Adipo- /osteo- |
Reference |
|---|---|---|---|---|---|---|
| hBMSC | 2, 5 | Basement membrane-like ECM by PYS-2 cells, endothelial cells |
↑cell number | ↑(pellet) | ↑adipo- osteo- |
167 |
| hBMSC | 4 | Chondrocyte-collagen microsphere |
- | ↑(pellet) | - | 168 |
| hBMSC | 2, 5, 10 | Mouse laminin 1, mouse laminin 5, or mouse collagen IV |
↑cell number ↑CFU-F colony number and size |
↑ (pellet) except laminin 5 |
↑adipo- /osteo- |
169 |
| hBMSC | 7, 9 | Human fetal BMSCs | ↑cell number | ↑ | ↑adipo- /osteo- |
119 |
| hBMSC | MC3T3 preosteoblasts | ↑osteo- | 34 | |||
| hBMSC | NIH3T3 fibroblasts | ↑(pellet) | 34 | |||
| hSDSCs | 3 | Human SDSCs | ↑cell number and PI | ↑ (pellet) | - | 116,129,132 |
| hBMSC | 8 | Human USCs | ↑(pellet) | - | 170 | |
| hSDSC | 3 | Human fetal and adult SDSCs | ↑cell number and PI | ↑(pellet) | ↑adipo-, | 118 |
| pSDSC | 2 | Porcine SDSCs | ↑p-cyclin D1 | ↑(pellet) and in vivo resurfacing |
- | 114 |
| pSDSC | 3 | Porcine SDSCs, NPCs, and SDSCs/NPCs (1:1) |
↑cell number | ↑(pellet) | - | 127 |
| pIPFSC | 3 | Porcine SDSCs and IPFSCs | ↑cell number | ↑(pellet) | ↑adipo- | 171 |
| hfSDSC | 7 | Human fetal SDSCs | ↓β-gal | ↑(pellet) | - | 172 |
| hBMSC | 5 | Human BMSCs | ↑cell number | ↑(pellet) | ↑osteo-, ↓adipo- |
115 |
| pSDSC | 3 | Porcine SDSCs | ↑cell number; ↓ cell size |
↑(pellet) | - | 43 |
| pSDSC | 3 | Porcine SDSCs | ↑cell number | ↑(pellet) | ↑adipo-, ↓osteo- |
113 |
Abbreviation: BMSCs: bone marrow stromal cells; CFU-F: colony-forming unit-fibroblast; dECM: decellularized extracellular matrix; f: fetal; h: human; IFPSCs: infrapatellar fat pad derived stem cells; NPC: nucleus pulposus cell; p: porcine; PI: proliferation index; SDSCs: synovium-derived stem cells
Evidence of cell proliferation and chondrogenic potential
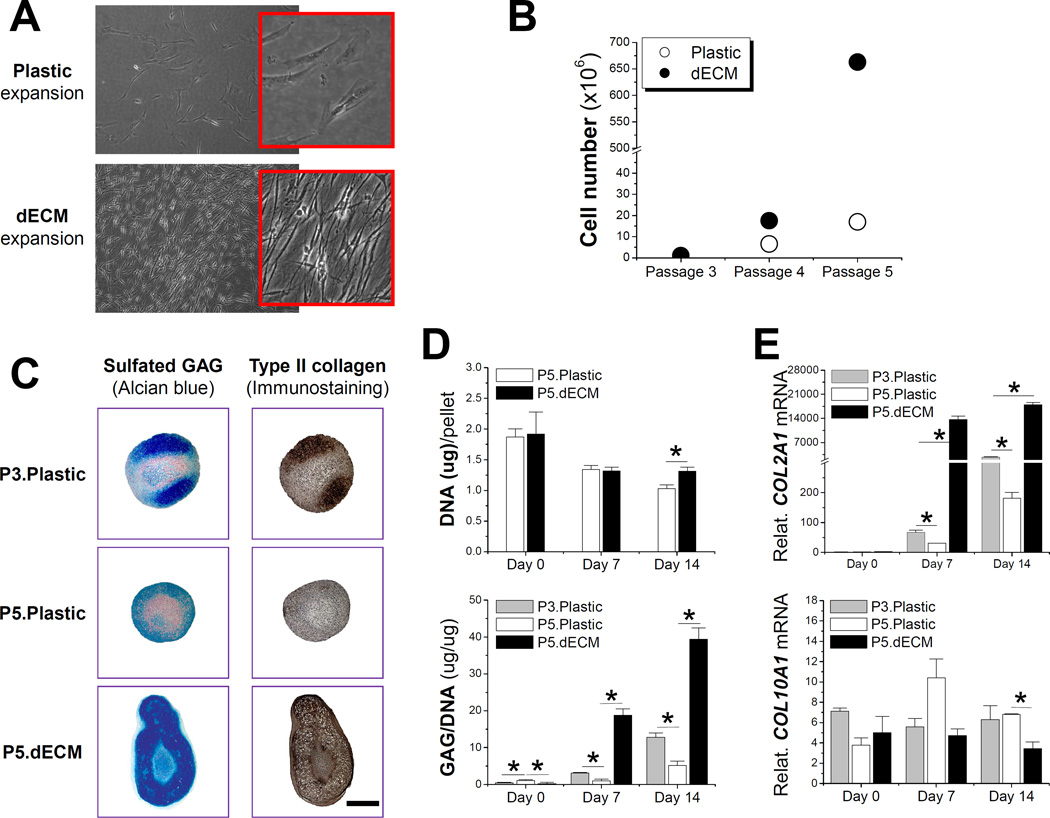
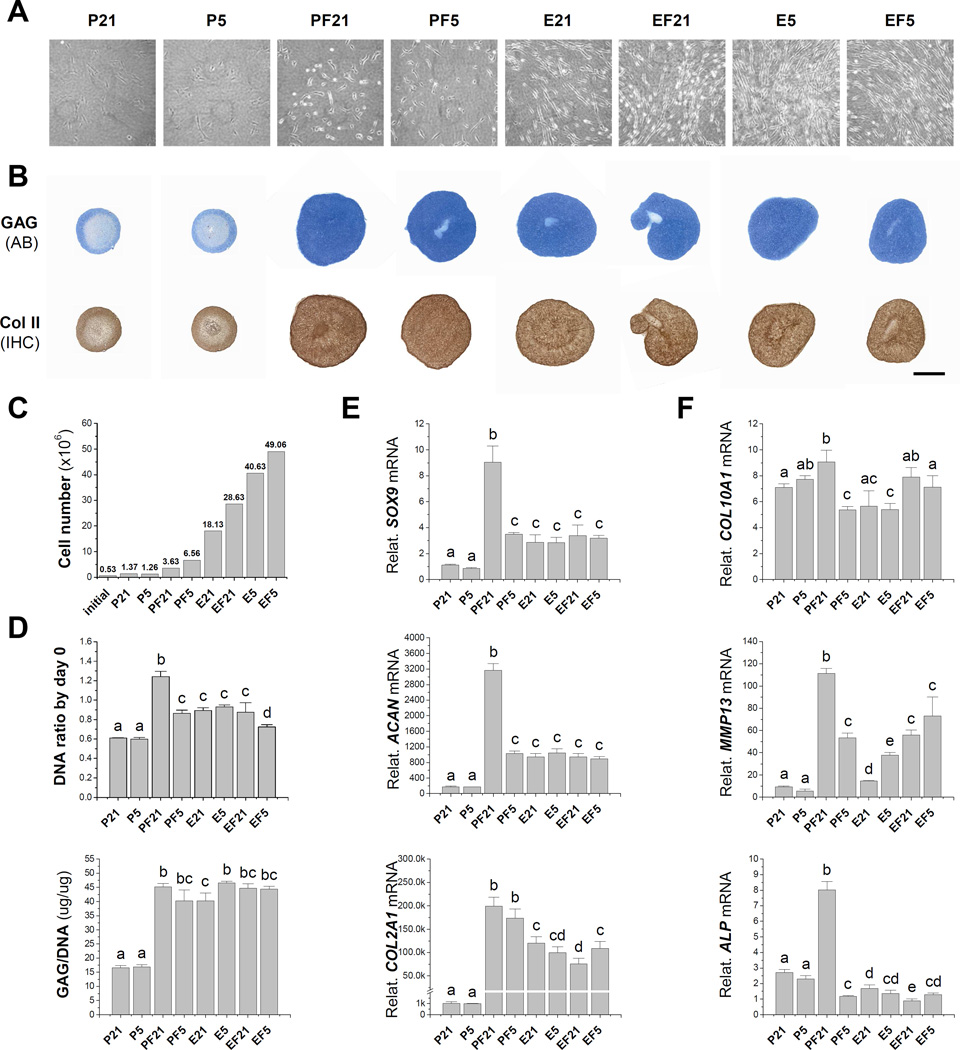
In 2009, we found that dECM deposited by SDSCs dramatically enhanced porcine SDSC expansion (Fig. 1A/B) and subsequent chondrogenic differentiation in a pellet culture system (Fig. 1C/D/E) [113]. Later we reported that, compared to the negligible rejuvenation effect of hypoxic preconditioning alone, FGF-2 and dECM preconditioning greatly enhanced porcine SDSC proliferation and chondrogenic potential (Fig. 2A/B) [43]. Compared to the culture substrate provided by a plastic flask, after six-day expansion, FGF-2 treatment resulted in a 2.65-fold increase in cell number while dECM treatment generated a 13.23-fold increase in cell number. The combined treatment of hypoxia, FGF-2, and dECM produced around a 35.81-fold increase in cell number (Fig. 2C). FGF-2 preconditioning yielded SDSC pellets with higher chondrogenic and hypertrophic differentiation while dECM expanded cells yielded pellets with much lower hypertrophic marker expression (Fig. 2D/E/F). Enhanced chondrogenic potential of SDSCs rejuvenated by dECM preconditioning has also been demonstrated in an in vivo study in which dECM-expanded SDSCs had better performance than plastic flask-expanded SDSCs in resurfacing partial-thickness cartilage defects of a minipig model via boosting type II collagen and sulfated GAG expression [114].
Fig. 1.
Effect of decellularized extracellular matrix (dECM) deposited by synovium-derived stem cells (SDSCs) on porcine SDSCs’ proliferation and chondrogenic differentiation. (A) Cell morphology five days after expansion on dECM and Plastic flasks; (B) Cell proliferation from passage 3 (P3) SDSCs grown on either dECM or Plastic flasks for two consecutive passages; (C) Alcian blue staining for sulfated GAG and immunostaining for type II collagen (scale bar: 800 mm) of 14-day chondrogenically induced SDSCs in a pellet culture system after two passages on dECM (P5.dECM) or Plastic flasks (P5.Plastic) with pre-expansion SDSCs (P3.Plastic) as a control; (D) Biochemical analyses were used to detect DNA content per pellet and ratio of GAG to DNA; (E) TaqMan real-time polymerase chain reaction (PCR) was used to quantitatively assess chondrogenic markers - COL2A1 (type II collagen) and COL10A1 (type X collagen). * indicates a statistical difference (p<0.05). Data are shown as average ± SD for n=6 in biochemical analyses and n=5 in real-time PCR. Reprint with permission from He, F.; Chen, X.; Pei, M. Tissue Eng. Part A 2009, 15, 3809. Copyright (2009) Mary Ann Liebert, Inc. Publications.
Fig. 2.
Optimization of preconditioning strategies to rejuvenate stem cells’ chondrogenesis. (A) Synovium-derived stem cell (SDSC) morphology after five-day expansion on dECM (“E”) or Plastic flasks (“P”) in hypoxia (5% O2, “5”) or normoxia (21% O2, “21”) with or without 10 ng/mL of fibroblast growth factor-2 (“F”). (B) Alcian blue (AB) staining for sulfated GAG and immunohistochemistry (IHC) staining for type II collagen (scale bar: 800 mm) of 14-day chondrogenically induced SDSCs in a pellet culture system after one passaging culture with varied pretreatments. (C) Cell number increase after a six-day expansion with initial cell number as 0.53 × 106 in one 175 cm2 flask. (D) Biochemical analyses after a 14-day chondrogenic induction in a pellet culture system including DNA content per pellet (adjusted by day 0) and ratio of GAG to DNA. TaqMan real-time PCR analyses of chondrogenically induced pellets including chondrogenic markers [SOX9 (SRY (sex determining region Y)-box 9), ACAN (aggrecan), and COL2A1 (type II collagen)] (E) and hypertrophic markers [COL10A1 (type X collagen), MMP13 (matrix metalloproteinase 13), and ALP (alkaline phosphatase)] (F). 18S RNA was used as an internal control. Groups not connected by the same letter are significantly different (p < 0.05). Data are shown as average ± SD for n=4. Reprint with permission from Li, J.; Pei, M. Tissue Eng. Part A 2011, 17, 703. Copyright (2011) Mary Ann Liebert, Inc. Publications.
However, the rejuvenation effect of dECM on human adult stem cells, such as BMSCs [115] and SDSCs [116], is not as powerful as shown above for young porcine SDSCs [113], in terms of cell number increase of 2.51-fold (adult BMSCs) versus 2.35-fold (adult SDSCs) versus 17.5-fold (young SDSCs), respectively. Recent evidence indicates that the “aging” of dECM might influence the rejuvenation effect on adult stem cells [117]. A recent report demonstrated that dECM deposited by fetal SDSCs could better rejuvenate human adult SDSCs in both proliferation and chondrogenic potential [118]. Intriguingly, both fetal and adult dECM expanded SDSCs exhibited a decrease of MSC surface markers CD29, CD90, and CD105 in percentage but heavily at the median; an increase of stage-specific embryonic antigen-4 (SSEA4) both in percentage and at the median; and integrin β5 only in percentage. Consistent with the finding by Li et al. [118], Ng et al. found that adult human BMSCs were more proliferative (~1.6×) on fetal dECM than adult dECM and plastic flasks. However, the average Alcian blue staining for sulfated GAG on both fetal and adult dECMs were similar and higher than the plastic flask group but were not significantly different [119]. The authors stated that the lack of difference between the adult dECM and plastic flask groups in cell proliferation was likely due to more conducive plastic flasks used in this study compared to those used by others in previous literature [120,121]. Similarly, both adult and fetal dECM expanded BMSCs showed a decrease of surface markers CD90, CD105, and CD146 in percentage (not shown at the median) [119]. The correlation of known surface marker downregulation and MSC stemness needs to be further elucidated.
Evidence of anti-dedifferentiation and pro-redifferentiation potential
dECM expansion could rejuvenate not only adult stem cells but also primary chondrocytes. Pei and He found that dECM deposited by porcine SDSCs not only greatly enhanced porcine chondrocyte expansion but also delayed dedifferentiation and enhanced redifferentiation capacity up to passage 6 of expanded chondrocytes compared to expansion on plastic flasks where redifferentiation was retained only in the early passages [122]. Dedifferentiated or aged chondrocytes (from passage 4) [123,124] were found to regain their redifferentiation capacity with the aid of dECM expansion [122]. Similarly, Cha et al. found that the proliferation of rat primary chondrocytes grown on dECM was better than those grown on a plastic coverslip (control) or gelatin [125]. Passaged chondrocytes (passage 4) cultivated on dECM acquired more synthesis of sulfated GAG, which is also reflected in the gene expression level; the dedifferentiating marker, COL1A1, was downregulated whereas the ratio between COL2A1 and COL1A1 and between ACAN and COL1A1, as an indicator of redifferentiation, was greatly boosted. This ex vivo expansion system also works for the rejuvenation of nucleus pulposus (NP) cells, another chondrocyte-like cell. There is increasing evidence to support that porcine NP cells expanded on dECM grew faster with a tiny size compared with those grown on plastic flasks; dECM pretreated NP cells also acquired a robust redifferentiation capacity [126,127].
Evidence of anti-oxidative and anti-inflammatory potential
Cartilage defects usually accompanied with posttraumatic inflammation present a challenge in cartilage repair and the biological constructs for implantation need to be able to survive this harsh environment [128]. Pei et al. found that expansion on dECM promoted the antioxidative and chondrogenic capacities of human adult SDSCs, slowing down the decrease of cell proliferation and the increase in apoptosis, and contributed SDSCs’ resistance to cell-cycle G1 arrest resulting from hydrogen peroxide [116]. Furthermore, dECM preconditioning protected chondrogenically induced human adult SDSCs from interleukin-1 beta (IL-1β) induced inflammatory stress; sb203580 (a p38 MAPK inhibitor) preconditioning promoted dECM rejuvenated human adult SDSCs’ ability against inflammation during chondrogenic induction [129].
Potential mechanisms
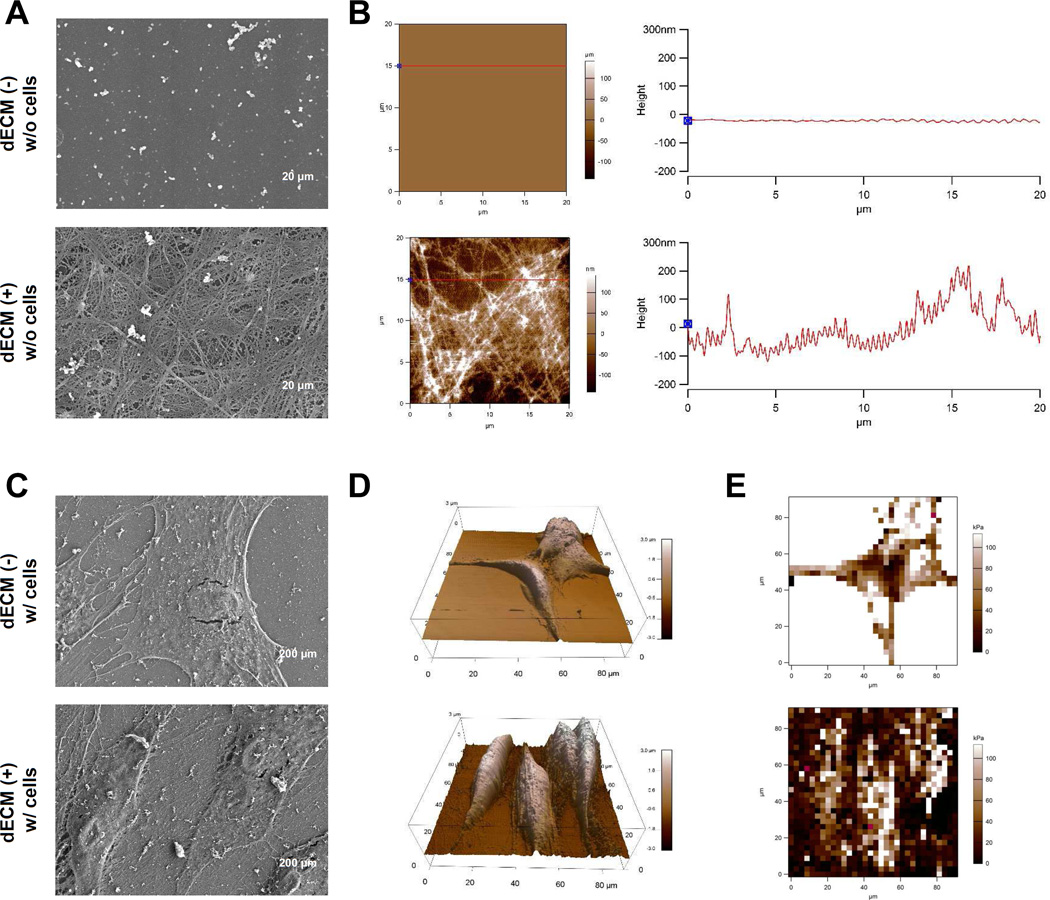
The mechanisms underlying dECM rejuvenation have not been elucidated. There is increasing evidence indicating that both chemical and physical stimulators in dECM play a critical role in the rejuvenation of expanded cells. He et al. found that the dECM deposited by SDSCs exhibited a nanosized 3D fibrillary structure as shown by scanning electron microscope (SEM) (Fig. 3A/C) with type I collagen as one of the essential structural proteins [113], which might contribute to a physical architecture that relays varied mechanical cues to their resident cells [130]. Furthermore, atomic force microscope (AFM) data suggested that SDSCs grown on rough dECM substrate exhibited greater height and more cell volume but lower Young’s moduli compared to those plated on smooth plastic flasks (Fig. 3B/D/E) [131,132]. This finding was in line with another report [118], in which dECM deposited by fetal SDSCs exhibited lower stiffness compared to that from adult SDSCs, which might be associated with the lower expression of elastin in fetal dECM. Interestingly, the stiffness of expanded SDSCs was in line with that of the culture substrate, indicating that lower elasticity in fetal dECM might be associated with enhancement of adult SDSCs’ proliferation and chondrogenic potential. This finding and others [133,134] support that matrix elasticity might contribute to lineage-specific differentiation.
Fig. 3.
Morphological characterization of culture substrates and expanded cells. Surface topography of the two substrates, Plastic (flasks) and dECM, was characterized using scanning electron microscope (SEM) (A) and atomic force microscope (AFM) (B). Scale bar for SEM (A): 20 µm. Expanded SDSCs on either Plastic or dECM after fixation in glutaraldehyde were characterized using SEM (C) and AFM (D) for morphology and using AFM (E) for elasticity. Scale bar for SEM (C): 200 µm. Reprint with permission from Zhang, Y.; Li, J., Davis, M.E., et al. Acta Biomater 2015, 20, 39. Copyright (2015) Elsevier Publications.
Li et al. found that not only biomechanical impact but also chemical composition of dECM might play a role in promoting cell proliferation and differentiation potential [118]. Proteomics data (Table 4) showed that fetal dECM had advantageous expression of fibrilin-2, tenascin C, and clusterin over adult dECM. Both fibrillin-2 and tenascin C are actively involved in tissue regeneration [135–138] while a fair amount of clusterin found in fetal dECM might be responsible for less apoptosis detected in fetal dECM expanded adult SDSCs since clusterin could inhibit apoptosis by interfacing with activated Bax [139]. On the other hand, adult dECM had more biglycan, decorin, dermatopontin, elastin, periotin, thrombospondin-1, and TGF-β1 than fetal dECM. Biglycan, decorin, and thrombospondin-1 were reported to inhibit cell proliferation [140,141] while dermatopontin, periotin, and TGF-β1 promoted cell differentiation [142–144], indicating that, compared to the action of fetal dECM on cell expansion, adult dECM contained extra matrix components preferring cell differentiation.
Table 4.
Select ECM and ECM interacting proteins identified in fetal and adult ECMs.
| Swiss-Prot Accession |
Select ECM Proteins | Assigned Spectra |
% of protein in the insoluble pellet |
||
|---|---|---|---|---|---|
| FE | AE | FE | AE | ||
| P98160 | Perlecan | 91 | 95 | 22% | 51% |
| P21810 | Biglycan | 37 | 95 | 14% | 53% |
| P07585 | Decorin | 40 | 95 | 65% | 54% |
| Q07507 | Dermatopontin | 16 | 35 | 100% | 66% |
| P15502 | Elastin | 2 | 18 | 100% | 89% |
| Q9Y6C2 | EMILIN-1 | 131 | 113 | 1% | 25% |
| Q9BXX0 | EMILIN-2 | 35 | 46 | 46% | 43% |
| P35555 | Fibrillin-1 | 394 | 529 | 39% | 53% |
| P35556 | Fibrillin-2 | 76 | 2 | 0% | 0% |
| P02751 | Fibronectin | 767 | 873 | 29% | 43% |
| P23142 | Fibulin-1 | 5 | 4 | 0% | 50% |
| P98095 | Fibulin-2 | 149 | 150 | 68% | 63% |
| Q96RW7 | Fibulin-6 (hemicentin-1) | 1 | 12 | 0% | 0% |
| P09382 | Galectin-1 | 22 | 12 | 0% | 0% |
| P55001 | Microfibrillar-assoc. prot. 2 | 39 | 47 | 85% | 81% |
| Q13361 | Microfibrillar-assoc. prot. 5 | 28 | 37 | 86% | 68% |
| P20774 | Mimecan | 16 | 6 | 0% | 17% |
| Q14112 | Nidogen-2 | 7 | 6 | 0% | 0% |
| Q15063 | Periostin | 125 | 400 | 15% | 57% |
| P24821 | Tenascin | 259 | 120 | 3% | 36% |
| P22105 | Tenascin-X | 59 | 49 | 10% | 0% |
| P07996 | Thrombospondin-1 | 6 | 79 | 33% | 68% |
| Q15582 | TGFBI | 164 | 258 | 45% | 44% |
| P13611 | Versican core protein | 58 | 22 | 74% | 50% |
| Swiss-Prot Accession |
Collagens | Assigned Spectra |
% of protein in the insoluble pellet |
||
|---|---|---|---|---|---|
| FE | AE | FE | AE | ||
| P02452 | Collagen alpha-1(I) chain | 250 | 494 | 11% | 86% |
| P08123 | Collagen alpha-2(I) chain | 206 | 339 | 6% | 59% |
| P02461 | Collagen alpha-1(III) chain | 41 | 31 | 0% | 81% |
| P20908 | Collagen alpha-1(V) chain | 18 | 36 | 28% | 47% |
| P05997 | Collagen alpha-2(V) chain | 7 | 12 | 0% | 75% |
| P12109 | Collagen alpha-1(VI) chain | 602 | 799 | 79% | 68% |
| P12110 | Collagen alpha-2(VI) chain | 634 | 633 | 87% | 73% |
| P12111 | Collagen alpha-3(VI) chain | 3019 | 3542 | 67% | 56% |
| Q99715 | Collagen alpha-1(XII) chain | 798 | 1563 | 30% | 64% |
| Q05707 | Collagen alpha-1(XIV) chain | 163 | 47 | 17% | 34% |
| Additional Proteins | |||||
| Q6UY14 | ADAMTS-like protein 4 | 8 | 18 | 0% | 0% |
| P10909 | Clusterin | 5 | 0 | 0% | |
| Q08397 | Lysyl oxidase homolog 1 | 22 | 23 | 36% | 35% |
| P21980 | Transglutaminase 2 | 18 | 44 | 28% | 30% |
As critical pathways for chondrogenesis, non-typical changes of both MAPK and Wnt signals were reported in dECM mediated stem cell chondrogenesis [118]. The data showed that dECM preconditioning resulted in p-Erk downregulation in the cell expansion phase, upregulation in the condensation phase, and downregulation after 10-day chondrogenic induction. This trend was evident in those cells expanded using fetal dECM. The early downregulation of p-Erk expression was associated with a decline in cell senescence [145] but the later downregulation facilitated better chondrogenic differentiation because p-Erk signals during induction promoted chondrogenic differentiation at the early stage but inhibited it at the later stage [146]. The adaptation of p-Erk expression in expanded stem cells following dECM preconditioning and subsequent removal might be responsible for the rejuvenation in cell proliferation and chondrogenic differentiation. dECM pretreatment was also reported to downregulate Wnt3a, a typical canonical Wnt signal, while upregulating Wnt5a and Wnt11, two typical noncanonical signals, in expanded SDSCs [118]. This finding is contradictory to previous reports, in which Wnt3a stimulated MSC proliferation [147] while Wnt5a and Wnt11 mainly promoted cell migration and differentiation [148,149]. The contribution of dECM on stem cell proliferation and chondrogenic potential needs to be further elucidated.
Other factors for preconditioning
Two dimensional culture conditions for MSC expansion, including plating density and culture media and term, play a critical role in governing the chondrogenic potential of MSCs [150–152]. Low seeding density or formulation of the base medium also could promote progenitor cells’ chondrogenic potential [85,89,153]. For instance, Li et al. found that expansion at a low seeding density (30 cells/cm2) yielded human adult SDSCs with enhanced proliferation and chondrogenic differentiation capacity compared to those grown at a high seeding density (3000 cells/cm2); downregulation of Erk1/2 and c-Jun N-terminal kinases (Jnk) expression and upregulation of p38 MAPK level might be associated with the retained stemness in the cells expanded at low density [151]. However, there also exists a conflicting report. Neuhuber et al. found that the initial seeding density was not critical for retaining a well-defined, multipotent MSC population despite the fact that a plating density of 200 cells/cm2 favored rat BMSC growth compared to either 20 or 2000 cells/cm2 [154]. They also found that cell expansion from all seeding densities developed an increased proportion of flat cells over passaging.
Conclusion and Perspectives
Finding straightforward and efficient strategies for promoting in vivo survival and benefiting differentiation of transplanted stem cells is important for the success of stem cell based tissue regeneration. Adult stem cells are promising sources for tissue regeneration but present challenges by becoming senescent during ex vivo expansion as well as having a harsh environment for transplantation. An increasing number of studies on preconditioning strategies to refine ex vivo expansion microenvironment for promoting engraftment [155], homing [156], and viability [157–159] after stem cell transplantation, particularly for cardiac tissue regeneration [155,159], have been published. Different from the above-mentioned strategies for cell preconditioning, this paper, for the first time, summarizes and assesses current efforts at altering the ex vivo microenvironment via hypoxia, soluble factors, and/or dECM to improve stem cell survival and chondrogenic potential post-transplantation.
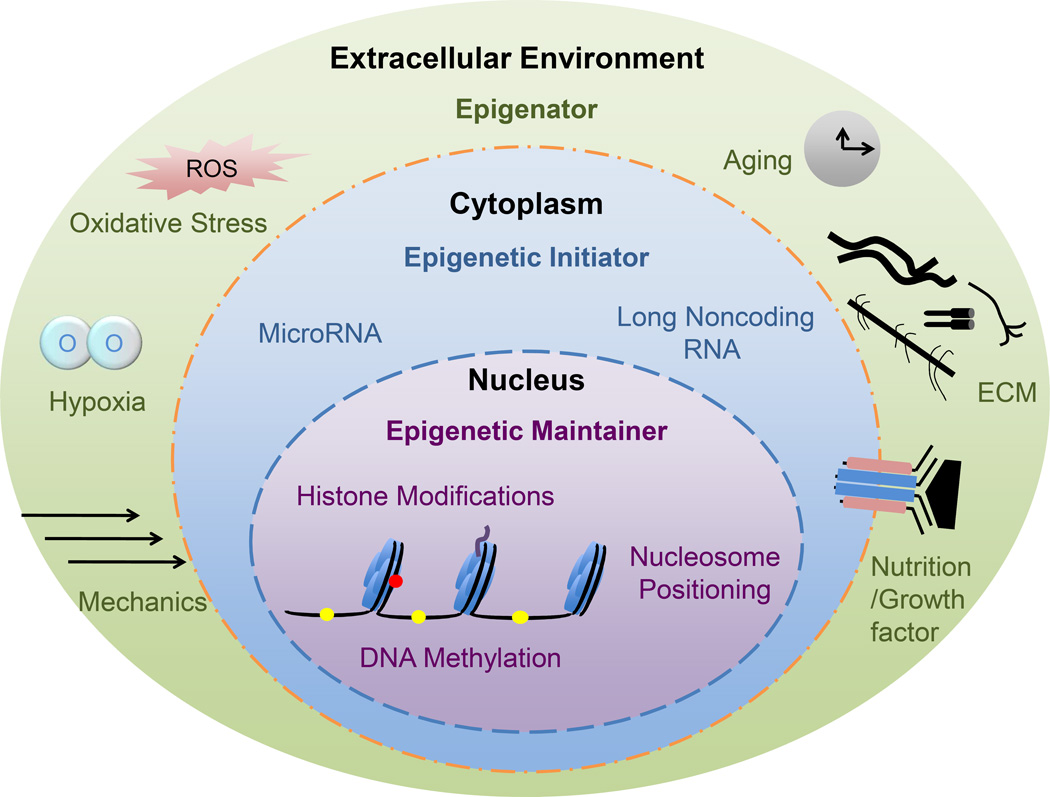
Besides the potential mechanisms discussed in each section, epigenetic changes are also proposed to play a critical role in this microenvironment mediated cell rejuvenation rather than genetic manipulation (Fig. 4) [160]. For instance, hypoxic stress often results in changes of gene expression that are affiliated with adaptations in chromatin structure by histone modifying and chromatin remodeling complexes [161,162]. Hypoxia also triggers microRNAs in the regulation of vascular endothelial growth factor (VEGF) for angiogenesis, some of which are downstream effectors of HIFs [163]; induction of HIF-1α in hypoxic preconditioned stem cells caused upregulation of miR-210 and this cytoprotective effect of hypoxic preconditioning could be negated by inhibition of HIF-1α or miR-210 [164]. This evidence indicates that hypoxic stress can cause epigenetic adaptation that, in adult stem cells, is dedicated not only to maintain cell stemness but also to drive cell differentiation [160]. Unfortunately, there are few reports to elucidate the rejuvenation effect of environmental preconditioning on adult stem cell proliferation and chondrogenic potential. Still in its infancy, the study of epigenetic effect on stem cells’ rejuvenation deserves further in-depth investigation.
Fig. 4.
The landscape view of epigenetic events during cartilage regeneration. Reprint with permission from Li, J., Ohliger, J., Pei, M. Stem Cell Dev. 2014, 23, 1178. Copyright (2014) Mary Ann Liebert, Inc. Publications.
Supplementary Material
Acknowledgments
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R03AR062763-01A1 & 1R01AR067747-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
No competing financial interests exist.
References
- 1.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Roche ET, Hastings CL, Lewin SA, Shvartsman DE, Brudno Y, Vasilyev NV, O'Brien FJ, Walsh CJ, Duffy GP, Mooney DJ. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35:6850–6858. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 4.Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010;1:e22. doi: 10.1038/cddis.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 6.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 7.Shafiq M, Jung Y, Kim SH. Insight on stem cell preconditioning and instructive biomaterials to enhance cell adhesion, retention, and engraftment for tissue repair. Biomaterials. 2016;90:85–115. doi: 10.1016/j.biomaterials.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 9.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM, Roudkenar MH. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012;17:553–565. doi: 10.1007/s12192-012-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamedi-Asl P, Halabian R, Bahmani P, Mohammadipour M, Mohammadzadeh M, Roushandeh AM, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones. 2012;17:181–190. doi: 10.1007/s12192-011-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 16.Mees B, Récalde A, Loinard C, Tempel D, Godinho M, Vilar J, van Haperen R, Lévy B, de Crom R, Silvestre JS. Endothelial nitric oxide synthase overexpression restores the efficiency of bone marrow mononuclear cell-based therapy. Am J Pathol. 2011;178:55–60. doi: 10.1016/j.ajpath.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Haider HKh, Porollo A, Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovasc Res. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiani AA, Abdi J, Halabian R, Roudkenar MH, Amirizadeh N, Soleiman Soltanpour M, Kazemi A. Over expression of HIF-1α in human mesenchymal stem cells increases their supportive functions for hematopoietic stem cells in an experimental co-culture model. Hematology. 2014;19:85–98. doi: 10.1179/1607845413Y.0000000093. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Lee MO, Moon BH, Shim SH, Fornace AJ, Jr, Cha HJ. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1-mediated downregulation of intrinsic stress signaling pathways. Stem Cells. 2009;27:1963–1975. doi: 10.1002/stem.121. [DOI] [PubMed] [Google Scholar]
- 20.Bahmani B, Roudkenar MH, Halabian R, Jahanian-Najafabadi A, Amiri F, Jalili MA. Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress Chaperones. 2014;19:685–693. doi: 10.1007/s12192-014-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwaka TP. Use of genetically modified stem cells in experimental gene therapies. In: Templeton NS, editor. Gene and Cell Therapy: Therapeutic Mechanisms and Strategies, third edition. Boca Raton, FL: Taylor & Francis/CRC Press; 2009. pp. 731–736. [Google Scholar]
- 22.Akita T, Murohara T, Ikeda H, Sasaki K, Shimada T, Egami K, Imaizumi T. Hypoxic preconditioning augments efficacy of human endothelial progenitor cells for therapeutic neovascularization. Lab Invest. 2003;83:65–73. doi: 10.1097/01.lab.0000050761.67879.e4. [DOI] [PubMed] [Google Scholar]
- 23.Volkmer E, Kallukalam BC, Maertz J, Otto S, Drosse I, Polzer H, Bocker W, Stengele M, Docheva D, Mutschler W, Schieker M. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng Part A. 2010;16:153–164. doi: 10.1089/ten.TEA.2009.0021. [DOI] [PubMed] [Google Scholar]
- 24.Baksh D, Davies JE, Zandstra PW. Adult human bone marrow-derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp Hematol. 2003;31:723–732. doi: 10.1016/s0301-472x(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 25.Eipers PG, Kale S, Taichman RS, Pipia GG, Swords NA, Mann KG, Long MW. Bone marrow accessory cells regulate human bone precursor cell development. Exp Hematol. 2000;28:815–825. doi: 10.1016/s0301-472x(00)00183-1. [DOI] [PubMed] [Google Scholar]
- 26.Baksh D, Davies JE, Zandstra PW. Soluble factor cross-talk between human bone marrow-derived hematopoietic and mesenchymal cells enhances in vitro CFU-F and CFU-O growth and reveals heterogeneity in the mesenchymal progenitor cell compartment. Blood. 2005;106:3012–3019. doi: 10.1182/blood-2005-01-0433. [DOI] [PubMed] [Google Scholar]
- 27.Papadimitropoulos A, Piccinini E, Brachat S, Braccini A, Wendt D, Barbero A, Jacobi C, Martin I. Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion. PLoS One. 2014;9:e102359. doi: 10.1371/journal.pone.0102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975;271:261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301:C362–C372. doi: 10.1152/ajpcell.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 31.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 32.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 33.Moussavi-Harami F, Duwayri Y, Martin JA, Moussavi-Harami F, Buckwalter JA. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: consequences for tissue engineering. Iowa Orthop J. 2004;24:15–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, Wan Safwani WK. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem Biophys Res Commun. 2014;448:218–224. doi: 10.1016/j.bbrc.2014.04.096. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- 36.Krinner A, Zscharnack M, Bader A, Drasdo D, Galle J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Prolif. 2009;42:471–484. doi: 10.1111/j.1365-2184.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zscharnack M, Poesel C, Galle J, Bader A. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs. 2009;190:81–93. doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]
- 38.Boyette LB, Creasey OA, Guzik L, Lozito T, Tuan RS. Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Transl Med. 2014;3:241–254. doi: 10.5966/sctm.2013-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Rendon E, Hale SJ, Ryan D, Baban D, Forde SP, Roubelakis M, Sweeney D, Moukayed M, Harris AL, Davies K, Watt SM. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- 40.Kalpakci KN, Brown WE, Hu JC, Athanasiou KA. Cartilage tissue engineering using dermis isolated adult stem cells: the use of hypoxia during expansion versus chondrogenic differentiation. PLoS One. 2014;9:e98570. doi: 10.1371/journal.pone.0098570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller J, Benz K, Ahlers M, Gaissmaier C, Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplant. 2011;20(10):1589–1602. doi: 10.3727/096368910X564094. [DOI] [PubMed] [Google Scholar]
- 42.Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3(2):9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Pei M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:703–712. doi: 10.1089/ten.TEA.2010.0339. [DOI] [PubMed] [Google Scholar]
- 44.Pilgaard L, Lund P, Duroux M, Lockstone H, Taylor J, Emmersen J, Fink T, Ragoussis J, Zachar V. Transcriptional signature of human adipose tissue-derived stem cells (hASCs) preconditioned for chondrogenesis in hypoxic conditions. Exp Cell Res. 2009;315:1937–1952. doi: 10.1016/j.yexcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Khan WS, Adesida AB, Hardingham TE. Hypoxic conditions increase hypoxia-inducible transcription factor 2alpha and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res Ther. 2007;9:R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 47.Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 48.Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010;18:1345–1354. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111:13954–13959. doi: 10.1073/pnas.1410977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 52.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 53.Biddlestone J, Bandarra D, Rocha S. The role of hypoxia in inflammatory disease (review) Int J Mol Med. 2015;35:859–869. doi: 10.3892/ijmm.2015.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 55.Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Haase VH. Renal cancer: Oxygen meets metabolism. Exp Cell Res. 2012;318:1057–1067. doi: 10.1016/j.yexcr.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 59.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 60.Fandrey J, Gorr TA, Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lord-Dufour S, Copland IB, Levros LC, Jr, Post M, Das A, Khosla C, Galipeau J, Rassart E, Annabi B. Evidence for transcriptional regulation of the glucose-6-phosphate transporter by HIF-1alpha: Targeting G6PT with mumbaistatin analogs in hypoxic mesenchymal stromal cells. Stem Cells. 2009;27:489–497. doi: 10.1634/stemcells.2008-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 64.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 66.Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dröge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- 68.Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-kappaB and HIF-1, two extremes. Free Radic Biol Med. 2002;33:1231–1242. doi: 10.1016/s0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- 69.Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 72.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 73.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281(41):30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 74.Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, Liu J, Geng Z, Wang Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94:33–39. doi: 10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2alpha is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56:3297–3306. doi: 10.1002/art.22878. [DOI] [PubMed] [Google Scholar]
- 76.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 77.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 78.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 79.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94:400–407. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- 80.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 81.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krinner A, Hoffmann M, Loeffler M, Drasdo D, Galle J. Individual fates of mesenchymal stem cells in vitro. BMC Syst Biol. 2010;4:73. doi: 10.1186/1752-0509-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. J Cell Sci. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001;9(Supplement A):S36–S40. doi: 10.1053/joca.2001.0442. [DOI] [PubMed] [Google Scholar]
- 86.Chiou M, Xu Y, Longaker MT. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644–652. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- 87.Hagmann S, Moradi B, Frank S, Dreher T, Kämmerer PW, Richter W, Gotterbarm T. FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 2013;46:396–407. doi: 10.1111/cpr.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan WS, Tew SR, Adesida AB, Hardingham TE. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther. 2008;10:R74. doi: 10.1186/ar2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, Quarto R. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 90.Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1:771–782. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JH, Lee MC, Seong SC, Park KH, Lee S. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;17:991–1002. doi: 10.1089/ten.TEA.2010.0277. [DOI] [PubMed] [Google Scholar]
- 92.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 93.Pizzute T, Li J, Zhang Y, Davis M, Pei M. FGF ligand dependent proliferation and chondrogenic differentiation of synovium-derived stem cells and concomitant adaptation of Wnt/MAPK signals. Tissue Eng Part A. 2016 doi: 10.1089/ten.tea.2016.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 95.Solchaga LA, Penick K, Goldberg VM, Caplan AI, Welter JF. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009–1019. doi: 10.1089/ten.tea.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckley CT, Kelly DJ. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J Mech Behav Biomed Mater. 2012;11:102–111. doi: 10.1016/j.jmbbm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Sampat SR, O'Connell GD, Fong JV, Alegre-Aguarón E, Ateshian GA, Hung CT. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:2259–2265. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, van Osch GJ. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports. 2015;4:459–472. doi: 10.1016/j.stemcr.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Centola M, Tonnarelli B, Schären S, Glaser N, Barbero A, Martin I. Priming 3D cultures of human mesenchymal stromal cells toward cartilage formation via developmental pathways. Stem Cells Dev. 2013;22:2849–2858. doi: 10.1089/scd.2013.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanada S, Ochi M, Kojima K, Sharman P, Yasunaga Y, Hiyama E. Possibility of selection of chondrogenic progenitor cells by telomere length in FGF-2-expanded mesenchymal stromal cells. Cell Prolif. 2006;39:575–584. doi: 10.1111/j.1365-2184.2006.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 103.Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 104.Liu L, Digirolamo CM, Navarro PA, Blasco MA, Keefe DL. Telomerase deficiency impairs differentiation of mesenchyma stem cells. Exp Cell Res. 2004;194:1–8. doi: 10.1016/j.yexcr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 105.Delorme B, Ringe J, Pontikoglou C, Gaillard J, Langonné A, Sensebé L, Noël D, Jorgensen C, Häupl T, Charbord P. Specific lineage-priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells. 2009;27:1142–1151. doi: 10.1002/stem.34. [DOI] [PubMed] [Google Scholar]
- 106.Handorf AM, Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alegre-Aguarón E, Sampat SR, Xiong JC, Colligan RM, Bulinski JC, Cook JL, Ateshian GA, Brown LM, Hung CT. Growth factor priming differentially modulates components of the extracellular matrix proteome in chondrocytes and synovium-derived stem cells. PLoS One. 2014;9:e88053. doi: 10.1371/journal.pone.0088053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varas L, Ohlsson LB, Honeth G, Olsson A, Bengtsson T, Wiberg C, Bockermann R, Järnum S, Richter J, Pennington D, Johnstone B, Lundgren-Akerlund E, Kjellman C. Alpha10 integrin expression is up-regulated on fibroblast growth factor-2-treated mesenchymal stem cells with improved chondrogenic differentiation potential. Stem Cells Dev. 2007;16:965–978. doi: 10.1089/scd.2007.0049. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Pizzute T, Pei M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014;358:633–649. doi: 10.1007/s00441-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 111.Toh WS, Foldager CB, Pei M, Hui JH. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev. 2014;10:686–696. doi: 10.1007/s12015-014-9526-z. [DOI] [PubMed] [Google Scholar]
- 112.Pei M, Li JT, Shoukry M, Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011;22:333–343. doi: 10.22203/ecm.v022a25. discussion 343. [DOI] [PubMed] [Google Scholar]
- 113.He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809–3821. doi: 10.1089/ten.TEA.2009.0188. [DOI] [PubMed] [Google Scholar]
- 114.Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19:1144–1154. doi: 10.1089/ten.TEA.2012.0351. [DOI] [PubMed] [Google Scholar]
- 115.Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17:3067–3076. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pei M, Zhang Y, Li J, Chen D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22:889–900. doi: 10.1089/scd.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014;10:289–298. doi: 10.4161/15476278.2014.970089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35:642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 119.Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35:4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 120.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 121.Zeiger AS, Hinton B, Van Vliet KJ. Why the dish makes a difference: quantitative comparison of polystyrene culture surfaces. Acta Biomater. 2013;9:7354–7361. doi: 10.1016/j.actbio.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 122.Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol. 2012;227:2163–2174. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulze-Tanzil G, Mobasheri A, de Souza P, John T, Shakibaei M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis Cartilage. 2004;12:448–458. doi: 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 124.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 125.Cha MH, Do SH, Park GR, Du P, Han KC, Han DK, Park K. Induction of re-differentiation of passaged rat chondrocytes using a naturally obtained extracellular matrix microenvironment. Tissue Eng Part A. 2013;19:978–988. doi: 10.1089/ten.tea.2012.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He F, Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine (Phila Pa 1976) 2012;37:459–469. doi: 10.1097/BRS.0b013e31821fcc64. [DOI] [PubMed] [Google Scholar]
- 127.Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine (Phila Pa 1976) 2012;37:1538–1547. doi: 10.1097/BRS.0b013e31825150bf. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Pizzute T, Pei M. Anti-inflammatory strategies in cartilage repair. Tissue Eng Part B Rev. 2014;20:655–668. doi: 10.1089/ten.teb.2014.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Pizzute T, Li J, He F, Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - A feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials. 2015;64:88–97. doi: 10.1016/j.biomaterials.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 131.Pizzute T, Zhang Y, He F, Pei M. Ascorbate-dependent impact on cell-derived matrix in modulation of stiffness and rejuvenation of infrapatellar fat derived stem cells toward chondrogenesis. Biomed Mater. 2016;11:045009. doi: 10.1088/1748-6041/11/4/045009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. doi: 10.1016/j.actbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 134.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brinckmann J, Hunzelmann N, Kahle B, Rohwedel J, Kramer J, Gibson MA, Hubmacher D, Reinhardt DP. Enhanced fibrillin-2 expression is a general feature of wound healing and sclerosis: potential alteration of cell attachment and storage of TGF-beta. Lab Invest. 2010;90:739–752. doi: 10.1038/labinvest.2010.49. [DOI] [PubMed] [Google Scholar]
- 136.Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- 137.Olivieri J, Smaldone S, Ramirez F. Fibrillin assemblies: extracellular determinants of tissue formation and fibrosis. Fibrogenesis Tissue Repair. 2010;3:24. doi: 10.1186/1755-1536-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Udalova IA, Ruhmann M, Thomson SJ, Midwood KS. Expression and immune function of tenascin-C. Crit Rev Immunol. 2011;31:115–145. doi: 10.1615/critrevimmunol.v31.i2.30. [DOI] [PubMed] [Google Scholar]
- 139.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 140.Kaur S, Soto-Pantoja DR, Stein EV, Liu C, Elkahloun AG, Pendrak ML, Nicolae A, Singh SP, Nie Z, Levens D, Isenberg JS, Roberts DD. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Sci Rep. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward M, Ajuwon KM. Regulation of pre-adipocyte proliferation and apoptosis by the small leucine-rich proteoglycans, biglycan and decorin. Cell Prolif. 2011;44:343–351. doi: 10.1111/j.1365-2184.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 143.Serra R, Chang C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today. 2003;69:333–351. doi: 10.1002/bdrc.10023. [DOI] [PubMed] [Google Scholar]
- 144.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park JS, Kim HY, Kim HW, Chae GN, Oh HT, Park JY, Shim H, Seo M, Shin EY, Kim EG, Park SC, Kwak SJ. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech Ageing Dev. 2005;126:551–559. doi: 10.1016/j.mad.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 146.Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, Chun JS. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 147.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 148.Liu JX, Hu B, Wang Y, Gui JF, Xiao W. Zebrafish eaf1 and eaf2/u19 mediate effective convergence and extension movements through the maintenance of wnt11 and wnt5 expression. J Biol Chem. 2009;284:16679–16692. doi: 10.1074/jbc.M109.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ryu JH, Chun JS. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem. 2006;281:22039–22047. doi: 10.1074/jbc.M601804200. [DOI] [PubMed] [Google Scholar]
- 150.Estes BT, Diekman BO, Guilak F. Monolayer cell expansion conditions affect the chondrogenic potential of adipose-derived stem cells. Biotechnol Bioeng. 2008;99:986–995. doi: 10.1002/bit.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J, Jones B, Zhang Y, Vinardell T, Pei M. Low-density expansion protects human synovium-derived stem cells from replicative senescence: a preliminary study. Drug Deliv Transl Res. 2012;2:363–374. doi: 10.1007/s13346-012-0094-y. [DOI] [PubMed] [Google Scholar]
- 152.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 153.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 154.Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176–1185. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li X, Tamama K, Xie X, Guan J. Improving Cell Engraftment in Cardiac Stem Cell Therapy. Stem Cells Int. 2016;2016:7168797. doi: 10.1155/2016/7168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Naderi-Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int. 2015;39:23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]
- 157.Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones. 2015;20:237–251. doi: 10.1007/s12192-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khatiwala R, Cai C. Strategies to Enhance the Effectiveness of Adult Stem Cell Therapy for Ischemic Heart Diseases Affecting the Elderly Patients. Stem Cell Rev. 2016;12:214–223. doi: 10.1007/s12015-016-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li J, Ohliger J, Pei M. Significance of epigenetic landscape in cartilage regeneration from the cartilage development and pathology perspective. Stem Cells Dev. 2014;23:1178–1194. doi: 10.1089/scd.2014.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson AB, Barton MC. Hypoxia-induced and stress-specific changes in chromatin structure and function. Mutat Res. 2007;618:149–162. doi: 10.1016/j.mrfmmm.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muscari C, Giordano E, Bonafè F, Govoni M, Pasini A, Guarnieri C. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013;20:63. doi: 10.1186/1423-0127-20-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng T, Yang C, Weber N, Kim HT, Kuo AC. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem Biophys Res Commun. 2012;426:544–550. doi: 10.1016/j.bbrc.2012.08.124. [DOI] [PubMed] [Google Scholar]
- 166.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matsubara T, Tsutsumi S, Pan H, Hiraoka H, Oda R, Nishimura M, Kawaguchi H, Nakamura K, Kato Y. A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun. 2004;313:503–508. doi: 10.1016/j.bbrc.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 168.Cheng HW, Tsui YK, Cheung KM, Chan D, Chan BP. Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue Eng Part C Methods. 2009;15:697–706. doi: 10.1089/ten.TEC.2008.0635. [DOI] [PubMed] [Google Scholar]
- 169.Lindner U, Kramer J, Behrends J, Driller B, Wendler NO, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 170.Pei M, Li J, Zhang Y, Liu G, Wei L, Zhang Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014;356:391–403. doi: 10.1007/s00441-014-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He F, Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med. 2013;7:73–84. doi: 10.1002/term.505. [DOI] [PubMed] [Google Scholar]
- 172.Li J, He F, Pei M. Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res. 2011;345:357–365. doi: 10.1007/s00441-011-1212-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.