Abstract
Objective
To examine the association between first-trimester angiotensin-converting enzyme (ACE) inhibitor exposure and the risk for overall major congenital, cardiac, and central nervous system (CNS) malformations.
Methods
We used a cohort of completed pregnancies linked to liveborn infants derived from Medicaid claims from 2000 to 2010. We examined the risk of malformations associated with first-trimester exposure to an ACE inhibitor. Propensity score based methods were used to control for potential confounders including maternal demographics, medical conditions, exposure to other medications, and measures of health care utilization.
Results
The cohort included 1,333,624 pregnancies, of which 4,107 (0.31%) were exposed to ACE inhibitors during the first trimester. The prevalence of overall malformations in the ACE inhibitor–exposed was 5.9% versus 3.3% in the unexposed (unadjusted relative risk (RR), 1.82; 95% confidence interval (CI) 1.61 to 2.06), of cardiac malformations was 3.4% versus 1.2% (RR 2.95; 95% CI 2.50 to 3.47), and of CNS malformations was 0.27% versus 0.18% (RR 1.46; 95% CI 0.81 to 2.64). After restricting the cohort to pregnancies complicated by chronic hypertension (both exposed and unexposed) and accounting for other confounding factors, there was no significant increase in the risk for any of the outcomes assessed. Relative risks associated with first-trimester ACE inhibitor exposure were 0.89 (95% CI 0.75 to 1.06) for overall malformations, 0.95 (95% CI 0.75 to 1.21) for cardiac malformations, and 0.54 (95% CI 0.26 to 1.11) for CNS malformations.
Conclusions
After accounting for confounders, among women with hypertension, exposure to ACE inhibitors during the first trimester was not associated with an increased risk of major congenital malformations.
Introduction
Angiotensin-converting enzyme (ACE) inhibitors are commonly used antihypertensive medications, particularly in patients with diabetes or renal dysfunction. A recent analysis of the National Health and Nutrition Examination Survey suggested that approximately 40% of women of reproductive age using antihypertensive medications take ACE inhibitors.1 Because of this, it is also a relatively common 1st trimester exposure, accounting for 10 to 20% of all antihypertensive exposures during this part of pregnancy.2,3
While ACE inhibitors are clearly contraindicated in the 2nd and 3rd trimester due to a well recognized fetopathy4–6, the risks of 1st trimester exposure are more poorly defined. A strong association between 1st trimester ACE inhibitors exposure and major cardiovascular and neurological malformations was described in an analysis of Tennessee Medicaid data,7 but other studies suggest that this association may be confounded by the indication of hypertension and associated comorbidities like diabetes.8–11 Data on the teratogenic potential of ACE inhibitors are therefore conflicting, leading to controversy and confusion among physicians and patients regarding the risks of using these drugs in women of reproductive age. The 2013 report from the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy recommends not using ACE inhibitors in women of reproductive age “unless there is a compelling reason, such as the presence of proteinuric renal disease.”12 Resolution of this controversy with large and carefully controlled studies is needed, as evidence of teratogenicity not only informs counseling of patients who are exposed in early pregnancy but also is a major determinate of whether these medications are appropriate to use in women who may inadvertently become pregnant.
We therefore sought to examine the association between first-trimester ACE inhibitor exposure and the risk of major congenital malformations, with careful attention to confounding conditions, using a large, nationwide cohort of pregnancies linked to infants in Medicaid beneficiaries.
Materials and Methods
Study data were drawn from the Medicaid Analytic eXtract (MAX). Medicaid is a joint state-federal health insurance program for people who have a low income. It provided coverage for approximately 40% of births in the United States annually during the study period.13 The MAX is a database that contains the healthcare utilization claims for Medicaid beneficiaries including all diagnoses and procedures associated with inpatient or outpatient healthcare encounters. It also contains data on beneficiaries’ enrollment information including demographic characteristics. Finally, it includes claims for all dispensed outpatient prescription medications.
The Partners Human Research Committee approved the use of this database for research. Using MAX claims from 46 states and the District of Columbia from 2000 to 2010, our group created a pregnancy cohort for pharmacoepidemiologic studies, as described by Palmsten et al.14 To accomplish this, we first identified women aged 12 to 55 who delivered liveborn infants and then linked these women with their offspring using a Medicaid identifier that is shared by families. The last menstrual period (LMP) was estimated for pregnancies in the cohort using a validated algorithm based on the date of delivery and information on the length of gestation in the maternal and infant records.15 The analysis was restricted to pregnancies in which women were eligible for Medicaid from 3 months prior to the LMP through one month postpartum. Pregnancies in which women had restricted benefits, private insurance, or certain capitated managed care programs were excluded as the claims for such patients may be incomplete in MAX. We required that infants be eligible for Medicaid for at least 3 months, unless they died in which case a shorter eligibility period was permitted. We excluded pregnancies exposed to known teratogens during the first trimester including warfarin, antineoplastic agents, lithium, isotretinoin, misoprostol, and thalidomide or in which the infant had a chromosomal abnormality. The MAX pregnancy cohort has been used extensively for studies of drug safety during pregnancy.16–19
For the primary analysis, ACE inhibitor exposure was defined based on a claim for a dispensed outpatient medication from the LMP to day 90 of pregnancy, corresponding to the end of the first trimester. The ACE inhibitors considered in the analysis included benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, and trandolapril (Appendix 1, available online at http://links.lww.com/xxx). ACE inhibitor monotherapy, as well as combinations of ACE inhibitors, and other antihypertensive medications were included. The reference group consisted of women not dispensed an ACE inhibitor during the first trimester. Women exposed to antihypertensives other than ACE inhibitors during the first trimester were excluded from the reference group, as some antihypertensives, for example beta blockers,20 may be associated with an increased risk for malformations. Women who were dispensed ACE inhibitors in the 3 months prior to pregnancy but not during the first trimester were also excluded to avoid exposure misclassification in the reference group.
The primary study outcomes were (i) overall major congenital malformations, (ii) cardiac malformations, and (iii) central nervous system (CNS) malformations. Cardiac and CNS malformations have been specifically associated with ACE inhibitors.7 Malformations were defined based on the presence of diagnostic codes from the International Classification of Diseases, 9th revision (Appendix 2, available online at http://links.lww.com/xxx) recorded on two or more days in the infant inpatient or outpatient records or on one or more days if the infant died or underwent a corrective surgical procedure. Because conditions present in the infant are sometimes recorded in the mother’s claims in MAX data, we also identified infant malformations in the maternal record using the same approach, taking care to exclude congenital malformations that were present in the mother. This approach has been shown to identify major congenital malformations (in a validation study of cardiac malformations) with a high positive predictive value.21
Four groups of potential confounders were selected for the analysis either because they represent known risk factors for congenital malformations or because they may represent proxies for such risk factors. These included maternal demographic characteristics, maternal medical or obstetrical conditions, maternal medication exposures, and measures of healthcare utilization. Demographic characteristics assessed included maternal age, race and ethnicity, Medicaid eligibility type, and year of delivery. Maternal medical or obstetrical conditions were assessed during the three months prior to pregnancy until the end of the first trimester and included chronic hypertension, diabetes, dyslipidemia, congestive heart failure, ischemic heart disease, renal disease, overweight or obesity, illicit drug or alcohol abuse, tobacco use, and multiple gestations. The codes used to define chronic hypertension are shown in Appendix 3, available online at http://links.lww.com/xxx. The Obstetric Comorbidity Index, which is designed to summarize the burden of comorbid illness in pregnant women, was calculated for each woman in the cohort.22,23 Maternal medication exposure to other potentially teratogenic medications during the first trimester was also identified, including corticosteroids, danazol, fluconazole, methimazole, propylthiouracil, and synthetic progestins. Additionally, we ascertained exposure to insulin and non-insulin diabetes medications during the three months prior to pregnancy until the end of the first trimester as a marker for diabetes severity. Finally, we defined a number of measures of healthcare utilization during the three months prior to the LMP, which may be markers of general comorbidity or access to healthcare services. These included whether the woman was hospitalized (including the number of days in the hospital), the number of emergency department and outpatient visits, the number of distinct diagnoses reported in the claims, and the number of non-ACE-inhibitor prescription medications used.
We defined the absolute risk of overall, cardiac, and CNS malformations in ACE inhibitor–exposed and ACE inhibitor–unexposed pregnancies in the full cohort and calculated unadjusted relative risks (RR) and 95% confidence intervals (CIs) for each of these outcomes. Then, because the indication of hypertension was expected to be an important confounder in this analysis, we restricted the cohort to women with a diagnosis of chronic hypertension recorded from three months prior to the LMP until the end of the first trimester.
In the chronic hypertension-restricted cohort, we compared baseline characteristics in those who were exposed and unexposed to ACE inhibitors during the first trimester. We then determined the absolute risks of the malformations of interest and calculated RR and 95% CI. In the next step of the analysis, since diabetes is highly prevalent among ACE inhibitor exposed pregnancies and is a strong risk factor for congenital malformations,24 we estimated the association between ACE inhibitors and the malformations of interest adjusting for this covariate.
Finally, to fully account for all measured confounders of the association between ACE inhibitors and malformations, we used a propensity score based approach. The propensity score (PS) was defined using a logistic regression model that estimated the probability of being dispensed an ACE inhibitor during the first trimester based on all of the covariates specified above (including diabetes), without further selection, in the hypertension restricted cohort. After trimming of observations from non-overlapping regions of the propensity score, we created 50 strata based on the distribution of the PS in the exposed pregnancies. The unexposed pregnancies were weighted in the outcome models based on the distribution of the exposed across the PS strata. Adjusted RR and 95% CI were then estimated using generalized linear models. The distribution of covariates in the trimmed population, stratified by ACE inhibitor exposure, is shown for the exposed and the unexposed (with the latter weighted to the distribution of the exposed across the PS strata).
A number of pre-specified sensitivity analyses were performed to test the robustness of our findings. First, we performed a high dimensional PS (hd-PS) analysis, which has been demonstrated to improve confounding control in some circumstances.25 The hd-PS algorithm screens all inpatient and outpatient diagnoses and procedures, in addition to claims for dispensed medications, and prioritizes 200 covariates that may be proxies for unmeasured confounders based on the strength of the association with exposure. These variables are then included in a propensity score along with all investigator specified covariates from the main analysis. Second, to assess any potential impact of exposure misclassification, we redefined exposure based on two dispensings of an ACE inhibitor during the first trimester on the assumption that if a woman is regularly refilling the medication it is likely being taken as prescribed. Third, we restricted the outcomes to claims from the infant record alone to control for the possibility of maternal malformations incorrectly being attributed to the infant. Finally, we re-defined hypertension using all available claims at any time prior to pregnancy through the end of the first trimester (and not just diagnoses recorded in the 3 months prior to the LMP through the end of the first trimester) to increase the sensitivity with which hypertension was captured both in the exposed and unexposed.
The MAX pregnancy cohort only includes information on pregnancies that result in live births. It does not capture pregnancies that result in stillbirth or spontaneous or therapeutic abortions. If the frequency of livebirth is the same in those exposed and non-exposed to ACE inhibitors (within the levels of the covariates included in the propensity score) then RR estimates will be unbiased. However, if this is not the case and live births occur less frequently in the exposed then estimates from the main analysis may be downwardly biased. To quantify the potential impact of missing live births, we used methods previously described in detail.16,17 Briefly, we assumed that the frequency of non-livebirth (including stillbirth and spontaneous or therapeutic abortions) in non-malformed fetuses is 20%. Then, informed by literature-based estimates of the frequency of termination for malformations, we modeled a range of livebirth probabilities in the non-exposed malformed, ranging from 75% to 55% for overall malformations and cardiac malformations and 55% to 35% for CNS malformations (as termination frequencies for CNS malformations are higher than for other anomalies26). We then examined the impact of a 10 to 20% higher frequency of non-livebirth among the ACE inhibitor exposed on the relative risks estimated in the main adjusted analysis.
Results
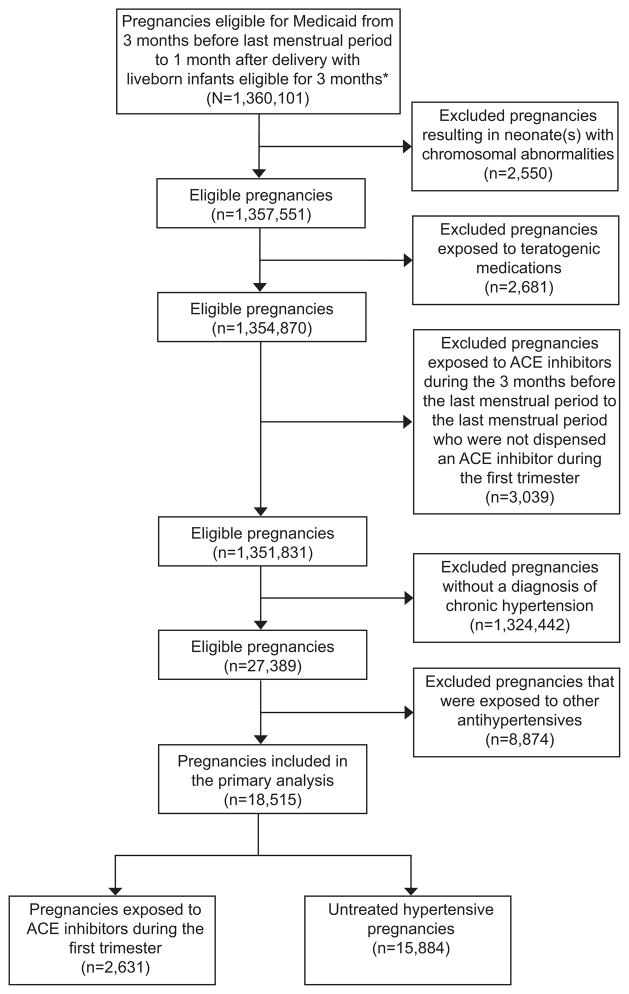
The full cohort included 1,333,624 pregnancies, of which 4,107 (0.31%) were exposed to ACE inhibitors during the first trimester (Figure 1). The hypertension restricted cohort included 18,515 pregnancies of which 2,631 (14.2%) were dispensed an ACE inhibitor during the first trimester. Among these, the most commonly dispensed ACE inhibitors included lisinopril (n=1437; 54.6%), benazepril (n=504; 19.2%), and enalapril (n=282; 10.7%) (see Appendix 1, available online at http://links.lww.com/xxx).
Figure 1.
Patient flowchart. *A short eligibility period is allowed in case of death. ACE, angiotensin-converting-enzyme.
There were important baseline differences in the chronic hypertension-restricted cohort between patients exposed to ACE inhibitors and those who were not (Table 1). ACE inhibitor exposed women were generally older (standardized difference (SD) for maternal age ≥40 years, 0.26) and more likely to be African-American (SD 0.10). They had much higher prevalences of diabetes diagnosis and treatment; 32% of those exposed to ACE inhibitors carried a diagnosis of diabetes compared to about 10% in hypertensive women who were unexposed (SD 0.55). They also had a higher prevalence of renal disease (SD 0.17), ischemic heart disease (SD 0.10), congestive heart failure (SD 0.19), and dyslipdemia (SD 0.23). The Obstetric Comorbidity scores were higher (SD 0.51). Finally, all measures of healthcare utilization assessed were higher among the ACE inhibitor exposed. After balancing these characteristics using the propensity score, the prevalence of all covariates was very similar in the exposed and unexposed, with absolute standardized differences of ≤0.03 across all variables included.
Table 1.
Baseline characteristics of chronic hypertensive women stratified by ACE inhibitor use. The Medicaid Analytic Extract, 2000 to 2010
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| ACE inhibitor exposed | Non-exposed | ACE inhibitor exposed* | Non-exposed* | Standardized difference | |
| Total | 2,631 | 15,884 | 2,626 | 15,449 | |
| Age, years | |||||
| ≤19 | 86(3.3) | 1755(11.1) | 86(3.3) | 472(3.0) | 0.02 |
| 20 to 24 | 290(11) | 4958(31.2) | 290(11) | 1615(10.2) | 0.03 |
| 25 to 29 | 667(25.4) | 4554(28.7) | 667(25.4) | 4072(25.7) | −0.01 |
| 30 to 34 | 789(30) | 2762(17.4) | 787(30) | 4937(31.2) | −0.03 |
| 35 to 39 | 569(21.6) | 1421(9.0) | 566(21.6) | 3407(21.5) | 0 |
| ≥40 | 230(8.7) | 434(2.7) | 230(8.8) | 1342(8.5) | 0.01 |
| Race/ethnicity | |||||
| Caucasian | 827(31.4) | 5462(34.4) | 825(31.4) | 5128(32.4) | −0.02 |
| African-American | 1331(50.6) | 7211(45.4) | 1328(50.6) | 7926(50) | 0.01 |
| Hispanic | 274(10.4) | 1726(10.9) | 274(10.4) | 1648(10.4) | 0 |
| Asian | 68(2.6) | 352(2.2) | 68(2.6) | 410(2.6) | 0 |
| Native American | 25(1) | 245(1.5) | 25(1) | 145(0.9) | 0 |
| Other | 55(2.1) | 566(3.6) | 55(2.1) | 326(2.1) | 0 |
| Unknown | 51(1.9) | 322(2) | 51(1.9) | 263(1.7) | 0.02 |
| Comorbidities | |||||
| Diabetes | 829(31.5) | 1565(9.9) | 824(31.4) | 4859(30.7) | 0.02 |
| Renal Disease | 134(5.1) | 309(2) | 134(5.1) | 813(5.1) | 0 |
| Dyslipidemia | 321(12.2) | 911(5.7) | 320(12.2) | 1915(12.1) | 0 |
| Congestive heart failure | 94(3.6) | 129(0.8) | 89(3.4) | 475(3) | 0.02 |
| Ischemic heart disease | 57(2.2) | 153(1) | 55(2.1) | 321(2) | 0 |
| Tobacco use | 122(4.6) | 879(5.5) | 122(4.7) | 751(4.7) | 0 |
| Drug abuse or dependence | 43(1.6) | 326(2.1) | 43(1.6) | 259(1.6) | 0 |
| Alcohol abuse or dependence | 24(0.9) | 143(0.9) | 24(0.9) | 160(1) | −0.01 |
| Multifetal gestation | 186(7.1) | 877(5.5) | 186(7.1) | 1095(6.9) | 0.01 |
| Obstetric Comorbidity Score | |||||
| ≤1 | 346(13.2) | 5211(32.8) | 346(13.2) | 2039(12.9) | 0.01 |
| 2 | 504(19.2) | 3702(23.3) | 504(19.2) | 3050(19.3) | 0 |
| ≥3 | 1781(67.7) | 6971(43.9) | 1776(67.6) | 10757(67.9) | −0.01 |
| Medication exposures | |||||
| Non-insulin diabetes medications | 592 (22.5) | 517 (3.3) | 587(22.4) | 3359(21.2) | 0.03 |
| Insulin | 511(19.4) | 609(3.8) | 506(19.3) | 3045(19.2) | 0 |
| Measures of healthcare utilization | |||||
| Number of generic medications (other than ACE inhibitor) ≥5 | 1177(44.7) | 4170(26.3) | 1172(44.6) | 7087(44.7) | 0 |
| Number of outpatient visits ≥6 | 879(33.4) | 5481(34.5) | 878(33.4) | 5252(33.1) | 0.01 |
Numbers are different from unadjusted analysis due to trimming of observations in the non-overlapping portions of the propensity score distribution. Additional covariates included in the propensity score included baseline measures of healthcare utilization including hospitalization, emergency room visits, Medicaid eligibility criteria, number of distinct diagnoses, overweight/obesity, and exposure to potentially teratogenic medications. ACE=Angiotensin converting enzyme
In the full cohort, the prevalence of overall malformations in the ACE inhibitor exposed was 5.9% versus 3.3% in the unexposed (unadjusted relative risk (RR), 1.82; 95% confidence interval (CI) 1.61 to 2.06), of cardiac malformations was 3.4% versus 1.2% (RR 2.95; 95% CI 2.50 to 3.47), and of CNS malformations was 0.27% versus 0.18% (RR 1.46; 95% CI 0.81 to 2.64) (Table 2). After restricting the cohort to those with a diagnosis of chronic hypertension, the association between exposure to ACE inhibitors and CNS malformations was non significant (RR 1.07, 95% CI 0.51 to 2.27) and was markedly attenuated for overall malformations (RR 1.35,RR, 95% CI 1.13 to 1.61) and cardiac malformations (RR 1.79, 95% CI 1.39 to 2.30). Adjustment for diabetes resulted in no significant associations: for overall malformations the adjusted RR was 0.97, 95% CI 0.79 to 1.19, for cardiac malformations 1.08, 95% CI 0.81 to 1.44, and for CNS malformations 0.68, 95% CI 0.30 to 1.54. In the propensity score analysis which adjusted for all covariates, the estimates did not suggest an increase in the risk of malformations associated with first trimester ACE inhibitor exposed: for overall malformations the fully adjusted RR was 0.89, 95% CI 0.75 to 1.06, for cardiac malformations 0.95, 95% CI 0.75 to 1.21, and for CNS malformations 0.54, 95% CI 0.26 to 1.11.
Table 2.
Risk for Congenital Malformations after Exposure to ACE inhibitors During the First Trimester of Pregnancy. The Medicaid Analytic Extract, 2000 to 2010
| Full cohort | Hypertension restricted cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk, N (%) |
Relative risk (95% confidenc e intervals) |
Risk, N (%) |
Relative risk (95% confidence intervals) |
|||||
| Congenital malformation s |
Exposed (n=4,10 7) |
Unexposed (n=1,329,51 7) |
Unadjuste d |
Exposed (n=2,63 1) |
Unexpose d (n=15,884 ) |
Unadjuste d |
Diabete s adjuste d |
Propensit y score adjusted |
| Overall | 244 (5.94) | 43,323 (3.26) | 1.82 (1.61–2.06) | 142 (5.40) | 634 (3.99) | 1.35 (1.13–1.61) | 0.97 (0.79–1.19) | 0.89 (0.75–1.06) |
| Cardiovascular | 139 (3.38) | 15,272 (1.15) | 2.95 (2.50–3.47) | 77 (2.93) | 260 (1.64) | 1.79 (1.39–2.30) | 1.08 (0.81–1.44) | 0.95 (0.75–1.21) |
| Central nervous system | 11 (0.27) | 2,433 (0.18) | 1.46 (0.81–2.64) | * | 45 (0.28) | 1.07 (0.51–2.27) | 0.68 (0.30–1.54) | 0.54 (0.26–1.11) |
Cell size <11 which cannot be disclosed in accordance with the data use agreement. ACE=Angiotensin converting enzyme
Relative risk estimates across the sensitivity analyses performed were consistent with those of the main analysis for overall malformations and cardiac malformations and none of the point estimates from these analyses suggested an increase in risk associated with ACE inhibitor exposure (Table 3). However, due to the paucity of events, the risk estimates were less stable and the confidence intervals were relatively wide for CNS malformations.
Table 3.
Sensitivity Analyses Examining the Risks for Congenital Malformations after First Trimester Exposure to ACE inhibitors. The Medicaid Analytic Extract, 2000 to 2010
| Total | Outcome events | Propensity score adjusted | |||
|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | ||
| High dimensional propensity score adjustment | |||||
| Overall | 2443 | 14363 | 131 | 587 | 0.88 (0.74–1.05) |
| Cardiovascular | 2443 | 14363 | 68 | 238 | 0.80 (0.62–1.02) |
| Central nervous system | 2443 | 14363 | * | 41 | 1.03 (0.49–2.17) |
| Exposure based on 2 dispensings in 1st trimester | |||||
| Overall | 805 | 14879 | 50 | 604 | 0.95 (0.72–1.25) |
| Cardiovascular | 805 | 14879 | 23 | 247 | 0.86 (0.57–1.29) |
| Central nervous system | 805 | 14879 | * | 41 | 1.41 (0.62–3.22) |
| Indefinite look back to define hypertension | |||||
| Overall | 3423 | 95028 | 191 | 3656 | 0.91 (0.79–1.05) |
| Cardiovascular | 3423 | 95028 | 107 | 1419 | 0.96 (0.79–1.16) |
| Central nervous system | 3423 | 95028 | * | 214 | 0.61 (0.31–1.17) |
| Outcome defined based on codes in the infant claims alone | |||||
| Overall | 2626 | 15449 | 122 | 549 | 0.87 (0.72–1.05) |
| Cardiovascular | 2626 | 15449 | 62 | 214 | 0.89 (0.68–1.15) |
| Central nervous system | 2626 | 15449 | * | 40 | 0.49 (0.23–1.06) |
Cell size <11 which cannot be disclosed in accordance with the data use agreement. ACE=Angiotensin converting enzyme. Numbers are different from unadjusted analysis due to trimming of observations in the non-overlapping portions of the propensity score distribution.
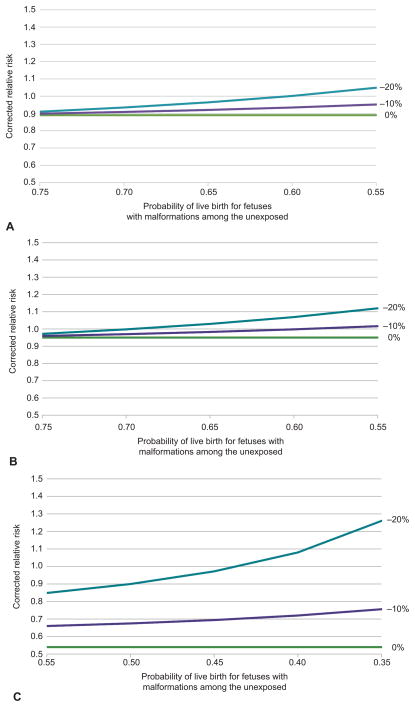
The analyses of the impact of missing non-live births are shown in Figures 2a to 2c. For overall malformations, the most extreme scenario considered was a probability of livebirth of 55% (20% terminated or spontaneously aborted or stillborn for reasons other than malformations and 25% for malformations) and a 20% absolute decrease in the probability of livebirth in the ACE inhibitor exposed compared to the non-exposed (for both malformed and non-malformed fetuses). Under these conditions, the estimate of RR would shift from 0.89 (as estimated in the PS adjusted analysis) to 1.05. Using the same assumptions, the RR for cardiac malformations shifts from 0.95 to 1.12 in the most extreme scenario. For CNS malformations, a higher frequency of termination for malformations was modeled. The most extreme scenario considered in this setting modeled a 35% probability of livebirth in the unexposed with malformations (20% terminated or spontaneously aborted or stillborn for reasons other than malformations and 45% for malformations) and a 20% absolute decrease in the probability of livebirth in the ACE inhibitor exposed. In this scenario, the corrected RR estimate shifts substantially upward, from 0.54 to 1.26.
Figure 2.
For the analysis of overall (A) and cardiac malformations (B), we assume 20% of nonmalformed pregnancies in the unexposed end in non–live birth (spontaneous or therapeutic abortion or stillbirth) and between 25% and 45% of the pregnancies complicated by malformation end in non–live birth. The 3 curves show the impact of non–live birth frequencies in the angiotensin-converting-enzyme (ACE) inhibitor exposed that are 0%, 10%, or 20% higher. For the analysis of central nervous system malformations (C), we assume 20% of non-malformed pregnancies in the unexposed end in non–live birth, but that 45% to 65% of malformed pregnancies in the exposed end in non–live birth. The 3 curves again show the impact of non–live birth frequencies in the ACE inhibitor exposed that are 0%, 10%, or 20% higher.
Discussion
In this study based on a cohort of over 1.3 million pregnancies, after accounting for relevant confounders, we did not observe an increase in the risk of overall malformations, cardiac malformations, or CNS malformations associated with first-trimester ACE inhibitor exposure. While it is important for clinicians to discontinue ACE inhibitors prior to the second trimester to avoid the fetopathy associated with late pregnancy exposure, our data suggest that exposure early in pregnancy during the period of organogenesis does not confer an increase in the risk of malformations.
Clinically this finding is important as it suggests that this class of medication is appropriate for use in women of reproductive age who may become pregnant, provided they are able to present for prenatal care prior to the end of the first trimester. ACE inhibitors are considered a first line medication in the treatment of hypertension, particularly in patients with diabetes27 and chronic renal disease28. However, current ACOG recommendations suggest avoiding this antihypertensive in women of reproductive age in the absence of a compelling indication12 because of the concern of inadvertent exposure during early pregnancy. Our results suggest a critical reevaluation of this recommendation.
Our findings differ from a previous study using Tennessee Medicaid Claims, which reported ACE inhibitor exposure was associated with a 2.7-fold increase in malformations overall, a 3.7-fold increase in cardiac malformations, and a 4.4-fold increase in CNS malformations.7 However, that analysis did not adjust for the indication of hypertension. Though not traditionally considered a risk factor for malformations and while the mechanism underlying the association is not well understood, several recent studies suggest that hypertension may be an independent risk factor for malformations.8,29,30. The Tennessee Medicaid analysis may not have adequately adjusted for the presence of diabetes. Further, the analysis only included 209 ACE inhibitor exposed pregnancies resulting in very wide confidence intervals. It is notable that our unadjusted estimates were more consistent with the findings from the Tennessee data, with a significant observed increase in the risk associated with exposure. This observation indicates that the overall risk for malformation is higher in women eligible to receive ACE inhibitors compared with the general population, but the increased risk appears to be attributable to the underlying conditions of hypertension and diabetes in this population and not attributable to ACE inhibitor use. Indeed, after fully accounting for all comorbid conditions that could increase the risk for malformations, the relative risk estimates for ACE inhibitors were near or below the null value. This lack of risk is consistent with a more recent study which examined 755 ACE inhibitor exposed pregnancies from Kaiser Permanente Northern California.8 In that analysis, when the control group was specified as pregnancies to women with untreated hypertension, there was no increase in risk of overall, cardiac, or CNS malformations attributable to ACE inhibitor exposure.
It is notable that the point estimate for the relative risk of CNS malformations shifted to 0.54 after full adjustment for potential confounders. There are several potential explanations for why the point estimate might appear to be protective, even in the presence of the lack of effect of ACE inhibitor exposure on the risk of the outcome. The first is the marked instability of the relative risk estimate, generated by the fact that there are fewer than 11 cases in the ACE exposed and only 41 in the reference group. Indeed, the wide confidence interval associated with the estimate (which includes the null) and the shifts in the point estimate across the multiple sensitivity analyses demonstrate this instability. Second, an observed protective estimate could be due to higher termination frequencies for malformations in the ACE inhibitor exposed than the non-exposed, as shown in our sensitivity analyses examining the impact of missing live births. Finally, if treatment with ACE inhibitors is a marker for better management of chronic conditions associated with risk of malformations, like diabetes, then there may be residual confounding that bias the risk estimates downward. This said, our results are clearly not consistent with a substantial increase in the risk for CNS malformations associated with ACE inhibitor exposure.
Our study has a number of important strengths. Our data are drawn from the claims of Medicaid beneficiaries nationwide. The cohort created using these data represents one of the largest and most comprehensive pregnancy cohorts developed for the study of drug safety during pregnancy. The number of ACE inhibitor exposed pregnancies included in the study was approximately 3-times larger than any prior study examining the teratogenicity of this class of medications, allowing for relatively precise estimates of risk to be made. Because the MAX pregnancy cohort contains complete healthcare utilization information on all included women from 3 months prior to pregnancy through delivery, there is capture of rich information regarding potential confounders including medical conditions and medication exposures. These confounders were adjusted for using advanced epidemiological methods including propensity score stratification and high-dimensional propensity score analyses. We confirmed the robustness of the findings with respect to overall malformations and cardiac malformations in multiple sensitivity analyses, although for CNS malformations the estimates were unstable owing to a very low number of outcomes.
Our study is also subject to certain limitations inherent in its design. Our exposure definition is based on a dispensed medication during the first trimester. While it is a reasonable assumption that filled prescriptions are taken, this cannot be empirically confirmed. To overcome this potential limitation, we performed a sensitivity analysis in which we required two dispensings of an ACE inhibitor during the first trimester, as it is likely that if a woman refills her medication, it is being taken regularly. This analysis, which is expected to define exposure with greater specificity, yielded risk estimates that were comparable to those of the main analysis. The MAX data also lack certain potential confounders including measures of the severity of comorbidities like renal disease or diabetes and patient characteristics like BMI. We addressed this potential limitation through the inclusion of multiple proxy variables for these conditions in the propensity score, and the use of a high-dimensional propensity score (which included 200 empirically defined variables in addition to pre-specified confounders). Given the null associations reported, it is unlikely that residual confounding is a significant issue for our analyses. It is also important to note that due to small numbers of events, the risk estimates for CNS malformations are unstable. The adjusted RR emerging for the assessment of the impact of only considering livebirths are thus also imprecisely estimated. Therefore, the conclusions regarding the impact of ACE inhibitors on CNS malformations must be made cautiously.
An additional potential limitation is that we define the presence of malformations based on administrative coding of the condition. However, previous work from our group has validated this approach with a chart review, finding a high positive predictive value for certain major congenital malformations defined using algorithms that rely on codes on multiple dates or corrective surgery.21 Finally, our cohort includes only live births (as did the other two large previous cohort studies examining this issue).7,8 To address this concern, we conducted a series of sensitivity analyses exploring the impact of differential livebirth frequencies in the exposed and unexposed and the potential impact on the risk estimates from the main analysis. In the analysis of overall malformations and cardiac malformations, even under the most extreme assumptions, the corrected relative risk estimates were less than 1.2. Finally, while our study does not suggest an association with overall malformations, cardiac malformations or CNS malformations in aggregate, we cannot exclude an association with specific defects.
In conclusion, our results suggest that ACE inhibitor exposure during the first trimester is not associated with an increase in the risk for congenital malformations after accounting for the underlying indication of hypertension and confounding factors such as the presence of diabetes. Our findings suggest that ACE inhibitors can be safely used in women of reproductive age, although it remains imperative to transition women off of these medications early in pregnancy to avoid the known adverse fetal effects associated with late pregnancy exposure.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH (Bethesda, Maryland, United States) under Award Number K08HD075831 (BTB), the National Heart Lung and Blood Institute at the National Institutes of Health under grant number K24HL096141 (EWS), and the National Institute of Mental Health under grant number K01MH099141(KH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SOURCE
Division of Pharmacoepidemiology & Pharmacoeconomics, Department of Medicine, Brigham & Women’s Hospital and Harvard Medical School
Presented at the 31st International Conference on Pharmacoepidemiology and Therapeutic Risk Management, August 22–26, 2015, Boston, Massachusetts.
Financial Disclosure
Sonia Hernandez-Diaz has consulted for Boehringer-Ingelheim and UCB for unrelated projects. The Pharmacoepidemiology Program at the Harvard School of Public Health is supported by Pfizer, Takeda, Bayer, and Asisa. Krista F Huybrechts, Brian T Bateman, and Sonia Hernandez-Diaz are investigators on grants to the Brigham and Women’s Hospital from Lilly and Pfizer and Brian T Bateman on grants from Baxalta, unrelated to the topic of this article. Brian T Bateman consults for Optum for unrelated projects. The other authors did not disclose any potential conflict of interest.
References
- 1.Bateman BT, Shaw KM, Kuklina EV, Callaghan WM, Seely EW, Hernandez-Diaz S. Hypertension in Women of Reproductive Age in the United States: NHANES 1999–2008. PloS ONE. 2012;7(4):e36171. doi: 10.1371/journal.pone.0036171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade SE, Raebel MA, Brown J, et al. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiology and drug safety. 2008 Mar;17(3):240–247. doi: 10.1002/pds.1550. [DOI] [PubMed] [Google Scholar]
- 3.Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension. 2012 Oct;60(4):913–920. doi: 10.1161/HYPERTENSIONAHA.112.197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr M, Jr, Cohen MM., Jr ACE inhibitor fetopathy and hypocalvaria: the kidney-skull connection. Teratology. 1991 Nov;44(5):485–495. doi: 10.1002/tera.1420440503. [DOI] [PubMed] [Google Scholar]
- 5.Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991 Jul;78(1):128–135. [PubMed] [Google Scholar]
- 6.Pryde PG, Sedman AB, Nugent CE, Barr M., Jr Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol. 1993 Mar;3(9):1575–1582. doi: 10.1681/ASN.V391575. [DOI] [PubMed] [Google Scholar]
- 7.Cooper W, Hernandez-Diaz S, Arbogast P, et al. Major Congenital Malformations after First-Trimester Exposure to ACE Inhibitors. New England Journal of Medicine. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 8.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931. doi: 10.1136/bmj.d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walfisch A, Al-maawali A, Moretti ME, Nickel C, Koren G. Teratogenicity of angiotensin converting enzyme inhibitors or receptor blockers. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2011 Aug;31(6):465–472. doi: 10.3109/01443615.2011.579197. [DOI] [PubMed] [Google Scholar]
- 10.Polifka JE. Is there an embryopathy associated with first-trimester exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists? A critical review of the evidence. Birth defects research. Part A, Clinical and molecular teratology. 2012 Aug;94(8):576–598. doi: 10.1002/bdra.23027. [DOI] [PubMed] [Google Scholar]
- 11.Koren G. Hypertension: ACE inhibitor use in pregnancy--setting the record straight. Nature reviews Cardiology. 2012 Jan;9(1):7–8. doi: 10.1038/nrcardio.2011.179. [DOI] [PubMed] [Google Scholar]
- 12.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013 Nov;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 13.Garcia G. Maternal and Child Health (MCH) Update: States Increase Eligibility for Children’s Health in 2007. [Accessed March 5, 2012];Electronic citation. Available at: http://www.nga.org/files/live/sites/NGA/files/pdf/0811MCHUPDATE.PDF;jsessionid=7B47A647247DD4E5CB9B709C8F9797AE.
- 14.Palmsten K, Huybrechts KF, Mogun H, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PLoS One. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiology and drug safety. 2013 Jan;22(1):16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. The New England journal of medicine. 2014 Jun 19;370(25):2397–2407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman BT, Hernandez-Diaz S, Fischer MA, et al. Statins and congenital malformations: cohort study. BMJ. 2015;350:h1035. doi: 10.1136/bmj.h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai RJ, Huybrechts KF, Hernandez-Diaz S, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. Jama. 2015 Jun 2;313(21):2142–2151. doi: 10.1001/jama.2015.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yakoob MY, Bateman BT, Ho E, et al. The risk of congenital malformations associated with exposure to beta-blockers early in pregnancy: a meta-analysis. Hypertension. 2013 Aug;62(2):375–381. doi: 10.1161/HYPERTENSIONAHA.111.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernandez-Diaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiology and drug safety. 2014 Jun;23(6):646–655. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol. 2013 Nov;122(5):957–965. doi: 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalfe A, Lix LM, Johnson JA, et al. Validation of an obstetric comorbidity index in an external population. BJOG : an international journal of obstetrics and gynaecology. 2015 Dec;122(13):1748–1755. doi: 10.1111/1471-0528.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. American journal of obstetrics and gynecology. 2008 Sep;199(3):237, e231–239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009 Jul;20(4):512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson E, Ehrenstein V, Norgaard M, et al. Estimating the proportion of all observed birth defects occurring in pregnancies terminated by a second-trimester abortion. Epidemiology. 2014 Nov;25(6):866–871. doi: 10.1097/EDE.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine. 2016 Apr 19;164(8):542–552. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 28.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014 Feb 5;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 29.Bateman BT, Huybrechts KF, Fischer MA, et al. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. American journal of obstetrics and gynecology. 2015 Mar;212(3):337 e331–314. doi: 10.1016/j.ajog.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan A, Lee LJ, Mitchell LE, Agopian AJ. Maternal Hypertension During Pregnancy and the Risk of Congenital Heart Defects in Offspring: A Systematic Review and Meta-analysis. Pediatric cardiology. 2015 Oct;36(7):1442–1451. doi: 10.1007/s00246-015-1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.