Abstract
Developmental and physiological changes in children contribute to variation in drug disposition with age. Additionally, critically ill children suffer from various life-threatening conditions that can lead to pathophysiological alterations that further affect pharmacokinetics (PK). Some factors that can alter PK in this patient population include variability in tissue distribution caused by protein binding changes and fluid shifts, altered drug elimination due to organ dysfunction, and use of medical interventions that can affect drug disposition (e.g., extracorporeal membrane oxygenation and continuous renal replacement therapy). Performing clinical studies in critically ill children is challenging because there is large inter-subject variability in the severity and time course of organ dysfunction; some critical illnesses are rare, which can affect subject enrollment; and critically ill children usually have multiple organ failure, necessitating careful selection of a study design. As a result, drug dosing in critically ill children is often based on extrapolations from adults or non-critically ill children. Dedicated clinical studies in critically ill children are urgently needed to identify optimal dosing of drugs in this population. This review will summarize the effect of critical illness on pediatric PK, the challenges associated with performing studies in this vulnerable subpopulation, and the clinical PK studies performed to date for commonly used drugs.
Keywords: clinical pharmacology, critically ill, pediatrics, pharmacokinetic alterations, organ dysfunction
INTRODUCTION
Critically ill patients suffer from life-threatening medical conditions that can be associated with multiple organ dysfunction and a plethora of pathophysiological alterations within the body. Pharmacokinetics (PK) can be altered in this patient population due to variable tissue distribution resulting from fluid shifts, and pH and protein binding changes; drug elimination may also be affected in these children due to renal and hepatic dysfunction. Moreover, life-saving medical interventions (e.g., extracorporeal membrane oxygenation [ECMO]) that are used in these children can contribute to variable drug disposition. In addition to alterations in PK mediated by pathophysiological factors, critically ill children are also undergoing expected physiological and developmental changes with age.
In the absence of critical illness, normal developmental and physiological changes include shifts in body composition and maturation in drug elimination pathways [1–3]. Briefly, body size composition changes significantly from neonates to adulthood. For example, as a percentage of total body weight, total body water is higher and body fat is lower after birth relative to adults [2,4]. For hydrophilic drugs this increased percentage of total body water can result in a greater volume of distribution (VD) (per kilogram of body weight) in newborns, whereas the opposite would be true for highly lipophilic drugs [4]. Maturational changes in drug elimination processes occur mainly due to the ontogeny differences in cytochrome P450 (CYP) enzymes and/or drug transporters. For example, based on in vivo data, it was estimated that CYP3A4 activity does not reach adult values until about 1.3 years of age [5–7]. Renal function also undergoes maturational changes; for example, the glomerular filtration rate reaches 90% of the adult value at 1 year postnatal age [8]. An understanding of how these developmental factors, along with pathophysiological alterations due to critical illness, influence PK changes and exposure-response relationships is important for defining optimal dosing of drugs used in this vulnerable patient population.
In this review, an overview of the factors that can mediate PK changes in critically ill children are discussed, and specific challenges associated with performing clinical research in this patient population are noted. Last, we performed an exhaustive literature search using the U.S. National Library of Medicine’s PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and summarized the relevant clinical pharmacology studies performed to date in critically ill children.
EFFECT OF CRITICAL ILLNESS ON PHARMACOKINETICS
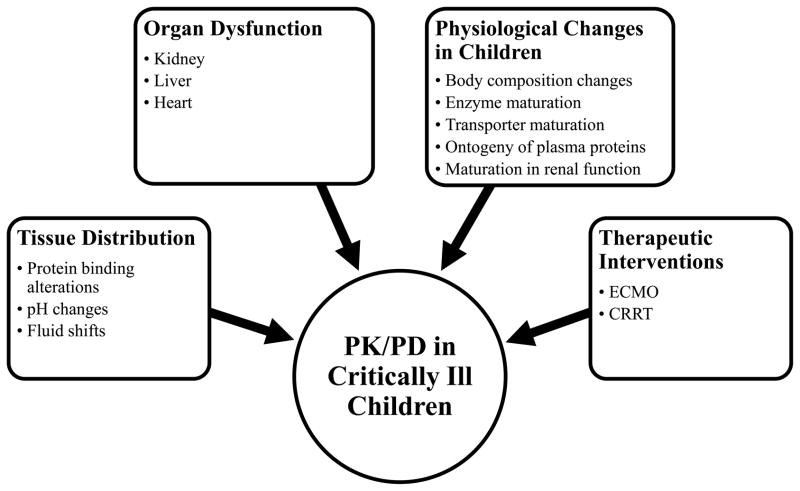
Critical illness alters the disposition of drugs in children through a variety of pathophysiologic mechanisms (Figure 1). First, tissue distribution may be altered due to changes in protein concentrations, blood pH, fluid shifts, and abnormal capillary permeability [9,10]. Drug elimination may be affected by disease related changes in organ function (e.g., heart, kidneys, and liver). Last, use of life saving medical interventions applied in critically ill children, such as ECMO or continuous renal replacement therapy (CRRT) may profoundly alter the physiologic balance, further affecting drug disposition.
Figure 1.
Factors that may cause pharmacokinetic/pharmacodynamic changes in critically ill children.
Alterations in Protein Binding
Changes in protein binding can alter tissue distribution by affecting the fraction of drug that is unbound and able to diffuse into tissues [11]. With critical illness both the synthesis and binding affinity of albumin and α-1 acid glycoprotein (AAG), the predominant drug binding proteins, may change as a result of liver or kidney dysfunction [12–14]. The following are examples of medications with a high degree of protein binding (% protein bound) used in critically ill children: ticarcillin (65%), daptomycin (93–98%), teicoplanin (98%), vancomycin (10–50%), oseltamivir (42%), lorazepam (83%), phenytoin (90%), midazolam (95–98%), and propofol (97–98%) [15–25]. These drugs may undergo protein binding changes in the presence of critical illness. For example, children with acute traumatic brain injury were shown to have significantly altered phenytoin protein binding [26,27].
pH Changes
Acute and chronic respiratory and metabolic acidosis or alkalosis are common pH derangements seen in critically ill children [28,29]. Changes in pH can occur as a result of respiratory dysfunction with carbon dioxide retention, altered renal function with electrolyte imbalance, hypoperfusion with secondary tissue acidosis, or primary metabolic diseases [30]. The pH of individual body compartments, such as the stomach or urine, can also be altered in critically ill children secondary to pharmacological treatment or fluid replacement. The acid dissociation constant (pKa) of a drug dictates the ionization state and distribution at different pH levels. Changes in pH in a specific body compartment may affect drug disposition. For example, methadone, a basic compound (pKa=9.2) administered to critically ill children for opioid abstinence syndrome is mainly unionized in acidic urine and its elimination can be enhanced by acid diuresis that may be observed with critical illness [31].
Fluid Shifts
Critical illness frequently results in marked fluid shifts as a result of altered capillary permeability or perturbations of the oncotic and hydrostatic forces (Starling forces) [32–34]. Disease states such as inflammation, infection, sepsis, liver cirrhosis, renal failure, and congestive heart failure can lead to increased capillary permeability due to direct injury or in response to the inflammatory cascade. Increased intravascular hydrostatic pressure resulting from fluid overload, and decreased tissue oncotic pressure secondary to hypoproteinemia, are also commonly seen in critically ill children. In isolation or in combination, these mechanisms result in a net shift of fluid from the intravascular to the interstitial and extravascular spaces [10,28,35]. Manifestations of these fluid shifts include ascites, pleural effusion, and edema, which can profoundly alter drug distribution. The VD of hydrophilic antibiotics (e.g., aminoglycosides, beta-lactams) may be increased in critically ill patients due to these fluid shifts. The increased VD can reduce the maximum concentration of antibiotics and thereby affect the pharmacodynamic (PD) outcome. For example, the VD of amikacin and gentamicin have been reported to be increased in critically ill children receiving fluid replacement therapy due to increased capillary hydrostatic pressure and fluid shits [36,37].
Renal Dysfunction
Acute kidney injury (AKI) is a prevalent comorbidity in critically ill children characterized by an abrupt decline in renal function. As many as 5.7% of children admitted to the pediatric intensive care unit (PICU) exhibit at least some degree of AKI at the time of admission, and 10% of children develop AKI during their PICU stay [38]. In addition to new onset AKI, children with chronic kidney disease (CKD) or end stage renal disease (ESRD) are frequently admitted to the PICU in critical condition as a result of renal disease exacerbation or comorbidities [39–42]. Alterations in renal function affect many factors that influence the PK of drugs, including impaired renal clearance, enzyme activity, pH changes, and alterations in total body water. For example, AKI is characterized by fluid retention and metabolic acidosis [43–46]. These changes may affect the fraction of drug ionized and alter the tissue distribution and VD of drugs. Together, these mechanisms lead to an altered VD and renal clearance of drugs. For some disease states such as sepsis, traumatic brain injury, and burns, augmented renal clearance (ARC) can also occur. While the exact pathophysiologic mechanisms resulting in ARC remain unknown, increased renal blood flow due to underlying illness or as a result of pharmacologic interventions are likely responsible. ARC has been identified in as many as 65% of adults admitted to a tertiary level ICU, and has been shown to lead to increased renal clearance of some antibiotics in critically ill children [47,48]. The PK of drugs administered to critically ill children (e.g., antibiotics, opioids, anticonvulsants) have been reported to be altered due to renal disease and thus appropriate dose adjustments are warranted based on a patient’s clinical presentation and the extent to which renal function is altered [49–51].
Hepatic Dysfunction
Several hepatic diseases may occur in critically ill children, including acute liver failure, viral or metabolic hepatitis, and cirrhosis [52]. While milder forms of hepatic dysfunction are generally well tolerated, acute liver failure is rare but a potentially fatal medical emergency. Acute liver failure leads to profound physiologic alterations that affect drug disposition, including severe capillary leak, coagulopathy, renal dysfunction, and hypoglycemia. Milder forms of hepatic dysfunction can also alter drug PK due to changes in hepatic blood flow, hepatic enzyme activity, hepatic drug transport, protein binding, and alterations in total body water [3,43,53,54]. Changes in the activity of hepatic enzymes can alter the PK of drugs primarily metabolized in the liver [54,55]. In addition to altered metabolism, liver dysfunction may also result in decreased synthesis of albumin and AAG, leading to reduced plasma concentrations and altered unbound fractions of drugs [56,57]. Increased unbound fractions of drugs such as alfentanil, phenytoin, and morphine have been reported in adults with hepatic dysfunction [58,59]. Finally, impaired protein synthesis and hepatic blood flow resulting from liver disease can cause fluid shifts and ascites, which can affect the VD of hydrophilic drugs such as aminoglycoside antibiotics [3,60,61]. All of the above changes may be particularly relevant for drugs undergoing extensive hepatic first pass metabolism (e.g., antibiotics, opioids, anticonvulsants) [49–51]. Finally, hepatic function may also be affected by iatrogenic alterations in hepatic blood flow, including reduced flows in states of increased intrathoracic pressure secondary to mechanical ventilation, or decreased hepatic perfusion secondary to vasoconstriction by vasopressors (vasopressin, phenylephrine, norepinephrine) [62–66].
Altered Hepatic Metabolism in Other Disease Conditions
Alterations in hepatic metabolism may occur in critically ill children even in the absence of hepatic failure. Inflammatory mediators released during sepsis have been shown to reduce cytochrome P450 mediated metabolism of antipyrine in critically ill children [67]. Similar inflammation may also occur in the setting of traumatic brain injury, altering the hepatic metabolism of phenytoin [26,27]. In addition to alerting drug disposition, inflammatory mediators such as nitric oxide may also directly reduce drug efficacy [68]. Lastly, the hepatic metabolism of high extraction ratio drugs such as propofol may be particularly vulnerable to changes in hepatic blood flow resulting from cardiovascular and other diseases [69].
Cardiovascular Diseases
Both congenital heart defects and acquired heart disease can result in cardiac dysfunction and congestive heart failure (CHF) requiring PICU admission [70,71]. CHF affects drug distribution by altering cardiac output and impairing blood flow to drug clearing organs, such as the liver and kidneys. Both hepatic and renal perfusion pressure may be reduced due to increased central venous pressure resulting from cardiac dysfunction, which further affects organ function. This may result in impaired drug clearance (CL). CHF is also associated with fluid overload, edema, and increased AAG concentrations, factors that can contribute to altered drug distribution [72–74]. Lastly, abdominal venous congestion may impair enteral absorption of drugs, which has been demonstrated for the loop diuretic furosemide [75].
Extracorporeal Membrane Oxygenation (ECMO)
ECMO is a life support technique used in critically ill patients with serious respiratory and cardiac conditions [76]. ECMO is an adaptation of cardiopulmonary bypass where blood is drained from the patient’s venous system, pumped into an oxygenator via tubing in the ECMO circuit, and returned back to the arterial or venous system [77–79]. Critically ill children may require ECMO under various conditions, including pneumonia, sepsis, congenital heart disease, and pulmonary abnormalities, and may thus be exposed to numerous drugs [80]. Several studies have shown that ECMO use in critically ill children alters the disposition of drug classes discussed in this review (antibiotics, analgesics, anticonvulsants, and sedatives) [81–91]. For example, VD may be increased in critically ill children because of the added volume of the ECMO circuit, as demonstrated for several antibiotics (e.g., vancomycin and gentamicin) [81–84,91]. Additionally, in vitro studies have shown that lipophilic drugs (e.g., midazolam and fentanyl) are adsorbed to the tubing in the ECMO circuit, which may lead to increased VD in critically ill children [85–87]. ECMO may also lead to increased variability of VD and CL. Lastly, renal and hepatic dysfunction are common complications of ECMO support in children, and may impact the CL of drugs used in this patient population (e.g., vancomycin and gentamicin) [64,77–80]. Under the most severe circumstances, patients may be concomitantly supported with ECMO and CRRT, further altering drug disposition [92].
Continuous Renal Replacement Therapy (CRRT)
Renal replacement therapy is indicated for patients with severe AKI experiencing volume overload, electrolyte imbalances, or accumulation of toxins or toxic metabolites. CRRT is a form of renal replacement therapy intended for continuous, around-the-clock use. Because of its continuous use, CRRT is typically better tolerated by critically ill children than other forms of renal replacement therapy [93,94]. Multiple factors can affect drug CL in critically ill children receiving CRRT, including drug and patient characteristics, and the type of CRRT modality employed [95]. The small pore size of the dialysis or hemofiltration membranes limits dialysis or filtration to the unbound fraction of drugs. As a result, drugs with low protein binding are more readily removed by CRRT. Drug clearance by CRRT may also be increased in the setting of hypoproteinemia associated with critical illness. Drugs with a smaller VD, which are predominantly concentrated in the plasma, will also be cleared more readily by CRRT than drugs with extensive tissue distribution [96]. In addition to drug properties, changes in blood or dialysate flow rate can affect transmembrane pressure and drug CL [97,98]. Additionally, some dialysis membranes can adsorb drugs in hemofiltration [97]. Previous studies have shown that the PK of meropenem and ticarcillin were altered in critically ill children supported with CRRT [99,100].
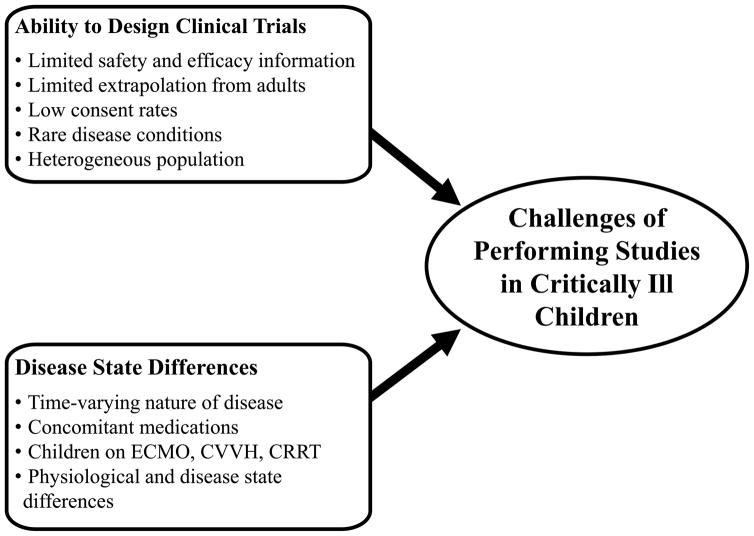
CHALLENGES ASSOCIATED WITH PERFORMING STUDIES IN CRITICALLY ILL CHILDREN
In spite of the extensive use of drugs in critically ill children, PK studies to inform appropriate dosing are limited. This is largely due to the challenges associated with conducting clinical studies in the pediatric population and specifically in critically ill children (Figure 2) [101,102]. In general, challenges associated with performing clinical trials in children include low consent rates, limited blood volume, and small number of subjects with a given disease. These challenges are typically magnified in critically ill children.
Figure 2.
Challenges associated with performing studies in critically ill children.
Performing clinical trials in critically ill children has additional challenges. First, critically ill children usually have multiple organ failure, necessitating careful selection of a study design. Unfortunately, there is often limited safety, efficacy and target exposure information of drugs used in critically ill children, which further complicates the design of clinical studies. Also, the effect of critical illness on PK can vary from adults, making extrapolation of available information difficult. Second, many critical illnesses are relatively rare, which further complicates subject enrollment. Third, the time varying nature of many critical illnesses requires that subjects be followed for a longer period of time and appropriate measure of disease function be collected. Last, critically ill children are administered a wide array of concomitant medications and are undergoing medical interventions that can alter the PK of drugs studied and confound the drug effects of interest.
PHARMACOKINETIC STUDIES IN CRITICALLY ILL CHILDREN
Clinical pharmacology studies in critically ill children have been performed for drugs widely used in this patient population. In many cases there were significant PK alterations in critically ill children relative to non-critically ill children or adults. The PK alterations of anti-infectives and non-anti-infectives are summarized in Tables I and II, respectively. Alterations in weight-normalized CL and VD compared to those in non-critically ill children obtained from the literature (or in adults when PK parameters were not available for non-critically ill children) are presented. In some cases the PK is highly variable in critically ill children and therefore no trends could be detected. The following section summarizes the PK alterations of anti-infectives and non-anti-infectives drugs studied in critically ill children.
Table I.
Pharmacokinetics changes of anti-infectives in critically ill children compared to non-critically ill children or adults.
| PK changes relative to
non-critically ill children or adults |
|||||
|---|---|---|---|---|---|
| Drugs | Sample size | Age (range) | Clearance | Volume of Distribution | References |
| Aminoglycosides | |||||
| Amikacin | 70–200a | 1 week – 17 years | ↑ | ↑ | [104,105] |
| Gentamicin | 33–644a | 0–20.59 months | ↔ | ↑ | [106–108,112– 114] |
| Netilmicin | 200 | 26.4–41 weeks GA | ↔ | ↑ | [116–119] |
| Tobramycin | 1 | 4 months | ↑ | [138] | |
| Beta-Lactam Antibiotics | |||||
| Cefotaxime | 37 | 0.67–199 days | ↔ | ↑ | [121–124] |
| Imipenem | 19 | 0.02–19 years | ↔ | ↔ | [125–127] |
| Meropenem | 287 | 0–18 years | ↔ | ↑ | [99] |
| 1 | 8 months | ↑ | [130] | ||
| Piperacillin | 13 | 9 months– 6 years | ↔ | ↔ | [130,131] |
| Ticarcillin | 3 | 6–16 years | ↔ | ↔ | [100,132] |
| Others | |||||
| Daptomycin | 1 | 13 years old | ↑ | ↔ | [123] |
| Teicoplanin | 21 | 7 days–12 years | ↔ | ↔ | [143,144] |
| Vancomycin | 20–22a | 1 months – 16 years | ↔ | ↑ | [136,137,139] |
| 12 | 37–42 weeks GA | ↓ | ↑ | [84,140,141] | |
| 1 | 4 months | ↑ | [138] | ||
| Antifungals | |||||
| Fluconazole | 8 | 6–59 days | ↔ | ↔ | [147,148] |
| 40 | 1 day –17 years | ↔ | ↑ | [149] | |
| Voriconazole | 1 | 17 years | ↑ | ↓ | [151] |
| Antivirals | |||||
| Acyclovir | 16 | 27–40 weeks GA and 1–56 days PNA | ↔ | ↔ | [152,153] |
Age is expressed as postnatal age unless otherwise stated and GA is the gestational age expressed in weeks
Abbreviations: NA, not available; ↑, increased compared to weight-normalized parameters in non-critically ill children or adults; ↓, decreased compared to weight-normalized parameters in non-critically children or adults; ↔, no change compared to weight-normalized parameters in non-critically children or adults
range across multiple studies
Table II.
Pharmacokinetics changes of non-anti-infectives in critically ill children compared to non-critically ill children or adults.
| PK changes relative to
non-critically ill children or adults |
|||||
|---|---|---|---|---|---|
| Drugs | Sample size | Age (range) | Clearance | Volume of Distribution | References |
| Analgesics | |||||
| Fentanyl | 7 | 27–39 weeks GA | ↔* | ↑ | [158] |
| Anticonvulsants | |||||
| Lorazepam | 10 | 37–41 weeks GA | ↓ | ↓ | [168,169] |
| Gastric acid suppressants | |||||
| Ranitidine | 9–27a | 1 day–17.1 yearsa | ↔* | ↔* | [170–174] |
| Omeprazole | 13–40a | 1month–19 yearsa | ↔* | ↔* | [175–177] |
| Sedatives | |||||
| Midazolam | 39–83a | 1 month–17 yearsa | ↓ | ↔ | [179,183] |
| Propofol | 28 | 0.1–182 months | ↔ | ↔ | [184] |
| 21 | 1 week–12 years | ↓ | ↑ | [188] | |
| Vasopressors | |||||
| Dobutamine | 11–47a | 0.1–17 years | ↔* | ↑ | [191–193,197,198] |
| Dopamine | 14, 11a | 27–42 weeksa GA | ↑ | ↔ | [200,201] |
| Epinephrine | 6–39a | 0.1–16 yearsa | ↓ | ↔ | [203,204] |
| Norepinephrine | 38 | 0–182 months | ↔ | ↔ | [205] |
Abbreviations: NA, not available; ↑, increased compared to weight-normalized parameters in non-critically ill children or adults; ↓, decreased compared to weight-normalized parameters in non-critically children or adults; ↔, no change compared to weight-normalized parameters in non-critically children or adults
range across multiple studies
Highly variable parameter within the population
Age is expressed as postnatal age unless otherwise stated and GA is the gestational age expressed in weeks
Aminoglycosides
Aminoglycoside antibiotics are prescribed to infants and children to treat serious Gram-negative infections including sepsis, meningitis, complicated intra-abdominal or urinary tract infections, and pulmonary exacerbations of cystic fibrosis [103]. Aminoglycosides are highly polar cations that are primarily excreted unchanged in the urine by glomerular filtration. They have infrequently been associated with nephrotoxicity and ototoxicity, and most PK studies in critically ill children have focused on optimizing dosing to minimize toxicity risk while maintaining efficacy.
Amikacin
Amikacin is particularly effective against gentamicin-resistant organisms (Enterobacteriaceae, Pseudomonas aeruginosa, and Serratia marcescens) and has been studied in critically ill children ranging from 1 week to 17 years of age. The mean amikacin CL ranged from 0.063 to 0.085 L/hr/kg and the mean central VD ranged from 0.18 to 0.24 L/kg [104,105]. Critically ill children had larger VD estimates and prolonged elimination half-lives compared to non-critically ill adults, possibly due to localized or generalized edema [104]. Among pediatric burn patients, the CL of amikacin was increased compared to healthy adult volunteers; however, amikacin concentrations were comparable to other critically ill children [105].
Gentamicin
Gentamicin is a widely used aminoglycoside and it has been studied extensively in critically ill neonates [106–110] and children up to 15 years of age [111,112]. The mean gentamicin CL ranged from 0.028 to 0.07 L/hr/kg, the mean half-life ranged from 3.85 to 9.23 hours, and the mean or median central VD ranged from 0.39 to 0.98 L/kg [106–108,112]. In comparison, non-critically ill children had a mean CL of 0.042 L/hr/kg, mean half-life ranging from 2–8 hours, and a mean VD ranging from 0.3–0.43 L/kg [113,114]. Similar to amikacin, critically ill children have an extended half-life due at least in part to the larger VD. Volume expansion during critical illness could be a result of capillary leak associated with sepsis and septic shock causing peripheral edema, excess fluid administered to prevent hypotension, and fluid retention during sepsis and renal failure. However, one study reported that gestational age, but not fluid intake, correlated with CL or VD in critically ill neonates with sepsis, while fluid retention was negatively correlated with Cmax [106].
Furthermore, a study performed in newborn infants ≥ 37 weeks gestational age receiving high-frequency mechanical ventilation and conventional mechanical ventilation found that infants receiving high-frequency mechanical ventilation had a slower mean (±standard deviation) (± SD) elimination rate constant [0.081 (± 0.02) versus 0.10 hr−1 (± 0.02)], a prolonged mean half-life [9.23 (±2.91) versus 7.07 hours (± 1.14)], more rapid clearances [(0.07 (± 7.04) versus 0.05 (± 0.01) L/hr/kg], and a larger mean VD [(0.98 (± 0.46) versus 0.49 (±0.09) L/kg)] compared to infants receiving conventional mechanical ventilation [107]. The authors hypothesized that patients receiving high frequency mechanical ventilation might have an increased half-life due to decreased cardiac output and renal blood flow, resulting in impaired renal elimination of gentamicin [107]. These PK changes might also be influenced by the higher severity of illness in infants receiving high-frequency mechanical ventilation, although the study did try to control for severity of illness by only including infants that received a full 7–10 course of therapy [107]. The serum creatinine was slightly higher in the high-frequency mechanical ventilation group compared to the conventional mechanical ventilation group but it did not reach statistical significance [107].
Netilmicin
Netilmicin is considered to have the lowest potential for nephrotoxicity and ototoxicity of all aminoglycosides [115]. Critically ill neonates given netilmicin along with fluid therapy or parenteral nutrition during the first week of life had a mean VD of 0.46–0.53 L/kg, half-life of 6.8–12.8 hours, and CL of 0.03–0.05 L/hr/kg [116]. There was no statistically significant difference between the PK parameters of netilmicin among neonates receiving either fluid therapy or parenteral nutrition [116]. Non-critically ill newborns had a slightly smaller mean VD (0.41–0.52 L/kg), a shorter mean half-life (4.6–8.5 hours) and similar mean CL (0.04–0.06 L/hr/kg) [117–119]. The slightly higher VD in critically ill neonates was possibly due to an expansion of the extracellular compartment from the hypertonic osmotic load administered during parenteral nutrition [116]. Another study performed in critically ill children (1 day to 15.5 years of age) reported that the majority (65%) of children with elevated trough levels had acute onset of renal insufficiency [120]. This can be expected since netilmicin is excreted by the kidneys.
Beta-lactam antibiotics
Cefotaxime
Cefotaxime is an antibiotic used in neonates on ECMO because it is active against many of the pathogens involved in neonatal and ECMO-related infections. In 37 neonates receiving cefotaxime and on ECMO with a median (range) post-natal age of 3.3 days (0.67–199), the median (range) CL, VD, and half-life were 0.36 L/h (0.19–0.75), 1.82 L (0.73–3.02), and 3.5 hours (1.6–6.8), respectively [121]. Although cefotaxime CL was similar to non-ECMO treated neonates (0.36 L/h versus 0.2–0.55 L/h), the VD was larger (1.82 L versus 0.68 to 1.14 L) relative to non-ECMO treated [121–124]. The authors suggested that hemodilution or capillary leakage of protein-bound drug into the extravascular compartment might have increased the VD in ECMO treated neonates, especially in the early phase of ECMO.
Imipenem
Imipenem-cilastatin was the first carbapenem approved for use in children, including neonates, and dosing recommendations are supported by adequate and well-controlled studies [125,126]. One study reported that the PK of imipenem in critically ill children (median [range] age of 0.8 years [0.02 to 12.9]) was highly variable with unpredictable plasma concentrations observed in several children [127]. The mean (± SD) steady-state half-life, CL and VD were 1.35 hours (± 0.38), 0.34 L/h/kg (± 0.14), and 0.46 L/kg (± 0.25), respectively [127]. These values were similar to the values reported in other non-critically ill children and healthy adults [125,126]. Nonetheless, some physiological parameters during critical illness altered imipenem PK, including blood pressure, creatinine clearance, and bicarbonate and lactate levels. For instance, elimination rates were reduced in patients with high lactate and low bicarbonate levels, possibly due to poor perfusion to the kidneys, and elimination rates correlated with creatinine clearance [127].
Meropenem
Meropenem is approved to treat bacterial meningitis, intra-abdominal infections, and complicated skin infections in pediatric patients 3 months of age and older [128]. Meropenem has also been studied in children with CRRT and ECMO [99,129]. Peak meropenem concentrations were decreased in children receiving CRRT with fluid overload by an average of 6.1% in patients with 10% fluid overload, 11.5% with 20% fluid overload, and 16% with 30% fluid overload [99]. However, fluid overload did not affect CL or the target attainment of 40% and 75% time above the minimum inhibitory concentration (4 mg/mL) [99]. In addition, a single case report in an 8 month old male infant on ECMO suggests that meropenem CL (4.14, 4.88, and 4.52 mL/kg/min on day 1 (8 h), day 3 (72 h), and day 9 (216 h), respectively) was slightly higher than estimates from healthy volunteers (4 mL/kg/min) [129].
Piperacillin
Piperacillin/tazobactam is broad spectrum antibiotic frequently prescribed as empiric therapy in critically ill children. Despite its frequent use, only one study has evaluated the population PK in critically ill children (median [range] age of 2 years [9 months–6 years]) [130]. The mean (± SD) estimates for piperacillin CL, volume of the central compartment, and elimination half-lives were 0.299 (± 0.128) L/hr/kg, 0.249 (± 0.211) L/kg, and 1.39 (± 0.62) h, respectively [130]. The piperacillin CL and VD in these critically ill children were similar to those reported in non-critically ill children aged 6 months through 12 years of age [130,131]. These non-critically ill children had a CL ranging from 0.282 to 0.354 L/hr/kg and a VD ranging from 0.28–0.30 L/kg [131].
Ticarcillin
Ticarcillin is a beta-lactam used in children for serious infections such as septicemia, peritonitis, and bone and skin infections. Limited PK studies have been published in critically ill children receiving ticarcillin. The PK has been studied in three children (ages 6, 6.5, and 16 years of age) receiving ticarcillin and two of the three children also received CRRT and ECMO. The mean (± SD) VD and CL for ticarcillin was 0.26 (± 0.01) L/kg and 0.038 (± 0.003) L/kg/hr, respectively [100]. The VD and non-renal CL of ticarcillin was comparable to patients with cystic fibrosis and healthy adult patients [132].
Lipopeptides
Daptomycin
Daptomycin is a lipopeptide antibiotic used for the treatment of complicated skin infections, bacteremia, meningitis and endocarditis. There is limited information about the PK of daptomycin in critically ill children. A single case report has reported the PK of daptomycin in a 13 year old male with vancomycin-resistant Enterococcus faecium endocarditis receiving 8 mg/kg of daptomycin [133]. His estimated renal clearance was approximately 120 mL/min/1.73 m2 or higher. This critically ill adolescent had a faster elimination rate, shorter half-life, and an increased CL with similar VD compared to healthy adults. The steady state half-life, CL, VD and area under the curve (AUC) from 0–24 hrs in this critically ill child were 4.58 hrs, 13.47 mL/h/kg, 0.089 L/kg and 593.92 μg*hr/mL [133]. In comparison, the average half-life, CL, VD and AUC from 0–24 hrs in healthy adults receiving 8 mg/kg were 9.0 hours, 7.2 mL/h/kg, 0.092 L/kg, and 1130 μg*hr/mL, respectively. The increase in CL for this critically ill adolescent compared to healthy adults may be due to age related differences in renal function or ARC during critical illness [134].
Glycopeptides
Vancomycin
Vancomycin is a glycopeptide antibiotic used for the treatment of serious Gram-positive infections such as methicillin-resistant staphylococci bacteremia, skin and soft tissue infections, and staphylococcal endocarditis. Vancomycin PK has been well studied in critically ill children as well as in infants receiving ECMO and CRRT [84,135–138]. In critically ill children the mean VD ranged from 0.44 to 0.81 L/kg, the mean (± SD) CL was 1.95 (± 1.1) mL/kg/min, and the mean half-life ranged from 3.1 to 5.3 hours [136,137]. These values are relatively similar to those reported in healthy adults: mean systemic CL of 1.84 mL/min/kg and a mean central compartment VD of 0.584 L/kg [139]. However, the VD may be altered in critically ill children. Fluid resuscitation is often administered to critically ill patients due to the systemic inflammatory response and this can be expected to increase extravascular volume. One study reported that children with a positive fluid balance had higher vancomycin VD compared to children with a negative fluid balance (0.6 L/kg versus 0.3 L/kg) [136]. In another study, critically ill infants had large initial VD from aggressive fluid resuscitation resulting in high variability in maximum concentrations and elimination half-lives [137].
Vancomycin PK was also reported to be altered in infants receiving ECMO: mean (± SD) steady state VD of 1.1 (± 0.5) L/kg, CL of 0.78 (± 0.19) mL/min/kg, and half-life of 16.9 (± 9.5) h [84]. Infants undergoing ECMO had a VD that was increased by 50%, a lower CL, and a prolonged half-life up to 100% compared to published reports in non-ECMO treated infants [84,140,141]. In addition, the hemofiltration CL in a 4-month infant on continuous veno-venous hemofiltration (CVVH) receiving vancomycin and tobramycin was increased (CL 0.27 to 0.80 mL/min for vancomycin and CL 0.32 to 0.91 mL/min for tobramycin), which required dosage modification for both vancomycin and tobramycin [138]. Thus, the PK values for vancomycin, especially the VD, are highly variable in critically ill children and more vigilant vancomycin drug monitoring might be necessary in this population.
Teicoplanin
Teicoplanin is a glycopeptide antibiotic with a similar antibacterial spectrum to vancomycin, but with a more favorable side effect profile and it is available as an intramuscular and intravenous formulation [142]. Although teicoplanin might be preferable in children due to its longer half-life and ease of administration, only one study has studied the PK in critically ill children. In this study of 21 children ranging in ages from 7 days to 12 years, the mean central volume was 0.38 L/kg, the terminal half-life was 17.4 hours, and total CL was 45 mL/h/kg [143] These PK parameters were comparable to non-critically ill (ages 2–11 years) children: VD of 0.31–0.68 L/kg, half-life of 6.5–18.1 hours, and CL of 29–51 mL/h/kg [144]. However, only 11% of trough levels were greater than 10 mg/L, which is considered a desirable trough level in children. The authors concluded that some critically ill children might have lower levels of teicoplanin because of an increased VD, possibly due to altered plasma protein concentrations and volume expansion treatment, diuretics, or from the excess volume administered from multiple drug infusion [143].
Antifungals (Azole Antifungals)
Triazoles are commonly prescribed antifungals with activity against many Candida species. The triazole antifungals that are FDA approved and commonly used in children include fluconazole, voriconazole, and posaconazole. The triazole antifungals undergo hepatic metabolism and have the potential to inhibit CYP450 enzymes. One exception is that fluconazole exhibits minimal metabolism and is primarily eliminated in the urine. Posaconazole has not been used as frequently in children because the oral formulation requires a high-fat meal for optimal absorption and the safety and efficacy of the intravenous formulation has not been established in children [145,146].
Fluconazole
Fluconazole is used in children to treat oropharyngeal, systemic, and invasive candidiasis, cryptococcal meningitis, and other fungal infections. Fluconazole PK parameters were highly variable in 8 critically ill infants (median gestation age at birth of 37 weeks and postnatal age of 17 days) with median (interquartile range) CL values of 16 (13–21) mL/h/kg, VD of 1051 (858–1461) mL/kg, and a half-life of 56 (26–80) h [147]. These PK parameters were comparable to non-critically infants (median gestation age at birth of 26 weeks and postnatal age of 16 days) with a CL and VD of about 22 mL/h/kg and 1004 mL/kg for a 32 week gestation infant, respectively [148]. Another study in 40 critically ill children with median (range) age of 22 days (1 day to 17 years) reported that the median (range) population VD was 45% higher in children receiving ECMO compared to critically ill children not on ECMO [1.35 L/kg (0.81–1.81) versus 0.93 L/kg (0.55, 1.37)] while CL estimates were similar [0.018 L/kg/h (0.011–0.043) versus 0.018 L/kg/h (0.008–0.042)] [149]. The higher VD in children receiving ECMO is likely due to the large volume of exogenous blood required to prime the circuit [149]. Additionally, one study reported that fluconazole trough levels were significantly lower in children with cancer [150].
Voriconazole
Voriconazole has extended coverage compared to fluconazole and has activity against yeasts and molds including Candida krusei, Candida glabrata, and Aspergillus. Voriconazole is used to treat invasive and pulmonary aspergillosis [146]. A single case report studied the PK parameters of voriconazole in a 17 year old adolescent male before and during ECMO. Before ECMO, the half-life was 24.7 hours, VD was 1.58 L/kg, and CL was 47.91 mL/min [151]. During ECMO, the half-life decreased to 21 hours, the VD decreased slightly to 1.38 L/kg, and the CL increased slightly to 49.33 mL/min [151]. Peak and trough levels were similar on the first day of ECMO compared to levels prior to ECMO, despite the fact that the dose increased from 280 to 400 mg twice daily to account for the loss due to binding [151]. However, two days after starting ECMO, the peak and trough levels increased and the patient experienced liver toxicity. The authors speculate that voriconazole initially was sequestered in the circuit but that plasma concentrations began to increase after binding sites in the circuit were saturated [151].
Antivirals
Acyclovir
Acyclovir is an antiviral drug commonly used to treat neonatal herpes simplex infections. Only one study has investigated the PK of acyclovir in critically ill neonates with varying degrees of renal and hepatic dysfunction [152]. In this study, neonates with a median (range) gestational age of 38 weeks (27–40) had wide variability in PK parameters with CL ranging from 0.03–0.27 L/hr/kg, half-life ranging from 3.8 to 44.5 hours, and mean steady state VD ranging from 0.42 to 6.51 L/kg [152]. These PK parameters were similar to children up to 3 months of age with normal renal function: mean CL (0.268 L/kg/h), mean steady state VD (1.08 L/kg), and mean half-life (3.80 hours) and to adults with varying degrees of renal function [152,153]. As expected based on acyclovir’s renal elimination, half-life was correlated with serum creatinine concentration: 5.0 hours for serum creatinine < 1 mg/dL and 15.6 hours for serum creatinine > 1 mg/dL [152]. The authors concluded that neonates with hepatic or renal dysfunction accumulate acyclovir when dosed without adjustment for organ dysfunction [152].
Oseltamivir
Although influenza is usually self-limiting in healthy adults, it can result in hospitalizations and mortality in infants and young children. Oseltamivir is an influenza virus neuraminidase inhibitor used for the treatment and prophylaxis of influenza infection [154]. Oseltamivir is given as the pro-drug, oseltamivir phosphate, which is then converted to the active form, oseltamivir carboxylate by hepatic esterases. In critically ill infants and children (ages 0–12 years) receiving intravenous oseltamivir, oseltamivir carboxylate AUC from 0 to the last sample time point (3.67 to 12 hr) ranged from 1,700 to 11,500 ng*hr/mL compared to an AUC from 0 to 24 hr of 2410 and 1850 ng*hr/mL in non-critically ill children with ages 3–5 and 1–2 years, respectively [155,156]. In addition, the oseltamivir carboxylate AUC from 0 to 12 hours ranged from 987.1 to 10,642 ng*hr/mL in three children receiving the oseltamivir suspension through the nasogastric or duodenal tube during ECMO administration [157]. Two of these children had higher oseltamivir carboxylate plasma concentrations compared to children and adults not on ECMO, while the third child had suboptimal plasma concentrations due to gastric bleeding and decreased gastric motility [157]. The authors concluded that ECMO support does not appear to significantly alter oseltamivir PK although further studies are needed [157]. Although oseltamivir had elevated exposure in some critically ill children, it was well-tolerated in this study.
Analgesics
Fentanyl
Fentanyl is an analgesic drug administered to critically ill children experiencing severe pain or agitation. Fentanyl PK data in critically ill children is limited. One study reported an increased VD and prolonged elimination half-life after continuous administration in 7 critically ill children (mean gestational age 32 ±4 weeks compared to that reported in adults and older children [158]. The authors concluded that this was likely due to the age-related developmental changes in younger children (e.g., changing fat and muscle masses, total body water and extent of protein binding) [158]. It was suggested that fentanyl dosage must be individually titrated to achieve optimal PD effect in critically ill children [158].
Anticonvulsants
Levetiracetam
Levetiracetam is used for the management of status epilepticus in critically ill children. It is <10% protein bound, is not extensively metabolized via the cytochrome P450 pathway, and is largely eliminated unchanged by the kidneys [159]. Because of these characteristics, levetiracetam has a lower susceptibility to drug interactions compared to other antiepileptic drugs [160]. Prior studies have evaluated the PK of levetiracetam in critically ill children with epilepsy [160–164]. Levetiracetam PK in adults and children has been reported to be different [161,165]. In children with epilepsy (2–46 months of age), a shorter mean half-life (5.3±1.3 hours) and a more rapid oral levetiracetam CL (1.46±0.42 mL/min/kg) relative to healthy adults (6–8 hours and 0.96 mL/min/kg, respectively) were reported [161,165]. The authors suggest that these PK changes may be due to the age-related physiological differences in children with epilepsy versus adults [2,166].
Lorazepam
Lorazepam is an anticonvulsant used for the treatment of epileptic seizures. Two studies have evaluated the PK of lorazepam in critically ill pediatric patients [167,168]. Both of these studies have reported that the PK of lorazepam is different in critically ill children as compared to adult patients. A PK study in 10 critically ill neonates (median [range] gestational age of 40 weeks [37–41]) with seizures had a mean (± SD) decreased VD (0.76 L/kg [± 0.37]) and CL (0.23 mL/min/kg [± 0.11]) compared to healthy adults (1.3 L/kg and 1.21 mL/min/kg, respectively) [168,169]. Consequently, the elimination half-life in critically ill neonates was much higher (40.2 hours) relative to adults (12.9 hours) [168,169]. The authors suggest that these differences in VD may be due to a lower percentage of adipose tissue (as a fraction of total body weight) in neonates or secondary to differences in protein binding compared to the adult population [168]. Similarly, the reduced CL in the critically ill neonatal population may be due to reduced glucuronidation of lorazepam compared to adults [168].
Gastric acid suppressants
Ranitidine
Ranitidine is commonly administered to critically ill children with upper gastrointestinal bleeding and stress-related mucosal damage. One study reported mean values for CL of 836 mL/hr/kg, VD of 1.61 L/kg, and half-life of 2.1 hours in 9 critically ill children [170]. Another study in 13 term neonates undergoing ECMO reported a reduced mean (±SD) CL (252 mL/hr/kg [±154]), comparable VD (1.8 L/kg [± 0.55]) and increased half-life (6.6 hours [± 2.75]) compared to infants not being on ECMO [171]. The authors suggested that these changes may be due to a reduced glomerular filtration rate in some of the neonates undergoing ECMO [171]. Another study reported that ranitidine CL in critically ill children increased during the study period possibly due to concomitant administration of CYP inducers resulting in a reduced efficacy when recommended doses were administered [172]. All studies report that the ranitidine PK is highly variable in both critically ill adults and children and thus it is not possible to reliably compare the alterations [170–174].
Omeprazole
Omeprazole is a proton pump inhibitor commonly used for different gastrointestinal conditions in critically ill children. Although widely used, limited studies have reported the PK of omeprazole in critically ill children [175–177]. Most studies reported that omeprazole PK was variable in critically ill children and the overall PK parameters were similar to those reported in adults [175–177]. Further studies are required to study omeprazole PK in critically ill children with different age groups and disease conditions.
Sedatives
Midazolam
Midazolam is commonly used in critically ill pediatric patients because it has a rapid elimination compared to other benzodiazepines [178]. Previous studies have shown that midazolam CL increases with age and weight in pediatric patients [179,180]. One study investigated the PK of midazolam in critically ill children divided in different age groups: group 1, infants < 12 months (n = 16); group 2, children 1–2 years (n = 12); and group 3, children age 3 years and older (n = 10). Midazolam CL in children 1–2 years of age was lower compared to children 3 years of age and older (CL 2.3 mL/min/kg versus 13 mL/min/kg) [179,181]. However, a recent study in children 1 month to 17 years of age showed that while age and weight did not have an influence on CYP3A mediated clearance, critical illness was an important determinant of reduced midazolam CL in children [182]. The authors concluded that this may be due to reduced CYP3A4/5 activity secondary to inflammation that occurs with critical illness [182]. Another study performed in 83 critically ill children demonstrated that inflammation and organ failure can significantly reduce midazolam CL likely due to an alteration of cytochrome P450 3A-mediated metabolism. In this study, C-reactive protein levels and the number of failing organs (cardiovascular, renal, respiratory, hematologic, or hepatic), in addition to body weight, were used as continuous covariates on midazolam CL. Based on modeling and simulation results, the authors concluded that critically ill patients receiving CYP3A substrates may be at a higher risk of toxicity due to increased drug levels [183].
Propofol
Propofol is a frequently used sedative in children and adults, and several pediatric studies have investigated the effect of critical illness on its disposition [184–188]. In a PK study of propofol administered to 28 critically ill, mechanically ventilated children (age 0.1–182 months), the disposition was reported to be comparable to previous studies in adults [184]. However, another study reported that propofol CL was reduced and steady-state VD was increased in children that had undergone cardiac surgery [188]. The authors concluded that the increased VD was likely due to reduced plasma protein binding associated with critical illness [188].
Vasopressors and inotropes
Dobutamine
Dobutamine is used off-label for the treatment of hemodynamic insufficiencies and cardiac failure in critically ill children [189,190]. Dobutamine PK in critically ill children has been studied in several clinical trials [191–195]. Wide variability in dobutamine PK has been observed in pediatric patients as in the adult population [191–196]. Across four studies in critically ill children, the mean (range) CL was 94.2 mL/min/kg (40–178) [191–193,197,198]; with wide ranges of dobutamine CL observed in all studies and one study reported a CL range of 12.5–1319 mL/min/kg [195]. The mean (range) VD in 27 PICU patients (aged 1 month to 16 years) was 1.13 L/kg (0.07–5.64), which is slightly higher than that reported in adults with severe heart failure (0.202 L/kg) [195,198,199]. Given that dobutamine is a hydrophilic drug, this difference may be due to the fluid shifts resulting from critical illness. Despite the variability in PK across various studies, the observed PD of dobutamine in critically ill children has been less variable and increases in heart rate and blood pressure have been reported. Thus, additional studies are needed to better understand the sources of variability in dobutamine PK in critically ill children.
Dopamine
Dopamine is administered to critically ill children for the treatment of cardiac failure and shock. Dopamine PK in critically ill children has been studied previously in children with a gestational age (range) of 27–42 weeks [200,201]. The mean (range) CL and VD of dopamine were reported as 115 mL/min/kg (62–168) and 1.8 L/kg (0.6–4) [200]. Limited information is available on dopamine disposition in critically ill adults and one study reported a lower dopamine CL with a mean (± SD) of 70 mL/min/kg (±36) [202].
Epinephrine and norepinephrine
Epinephrine and norepinephrine are catecholamines that are exogenously administered to critically ill children during cardiopulmonary resuscitation, anaphylaxis, or to prevent low cardiac output following cardiopulmonary bypass surgery. There is a significant variability in the PK and PD of exogenously administered epinephrine and norepinephrine. Several studies have investigated the PK/PD of epinephrine [203,204] and norepinephrine [205] in critically ill children. In critically ill children ranging from 0.5 to 16 years of age, epinephrine was reported to have a CL ranging between 15.6–79.2 mL/min/kg and the PK was characterized by a one-compartment model with linear elimination. Epinephrine CL in critically ill children was lower than reported in one adult study (mean [± SD] CL 144.8 mL/min/kg) [203,204,206]. The authors suggested that this may be due to changes in combination of different factors (e.g., plasma concentrations of platelets, electrolytes, calcium, caffeine) that alter the PK of catecholamines in critically ill children [204]. Similarly the CL of norepinephrine was 6.6 L/h/kg and the PK was well-characterized by a one-compartment model with linear elimination [205]. However, there was no difference in the PK of norepinephrine in critically ill children relative to adults [205].
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, drug disposition in critically ill children in some cases is unaltered, whereas for other widely used drugs the PK is altered and highly variable in this patient population. These PK alterations may be due, at least in part, to physiological and developmental factors that can dictate differences between children and adults. Additionally, critical illness and disease state changes can significantly affect the PK of drugs. Collectively these PK changes can lead to altered PD outcomes in critically ill children. For example, the PK of ranitidine was variable in critically ill children leading to reduced target attainment of gastric pH control despite treatment with the recommended doses [172]. Similarly, the altered PK of vancomycin in critically ill children may have resulted in reduced antimicrobial efficacy as evidenced by only half of critically ill children reaching target vancomycin AUC24/MIC ratio of >400 in a single center study of 22 children [136]. Thus, in both cases, the authors highlight the requirement for appropriate dose adjustments to attain optimal efficacy in critically ill children [136,172].
PK/PD changes in children may vary during a treatment period and dose adjustments (upward or downward titration) may be needed over time. Although efforts have been made to study PK alterations in critically ill children, the small sample sizes and extreme variability observed affected the overall conclusions that could be drawn from some studies. Additional clinical studies are needed to better understand the impact of PK/PD changes for drugs with a narrow therapeutic index, large molecule drugs, and for those that are predominantly eliminated through the hepatic and renal pathways. Disease mediated changes during critical illness highlight the need for developing robust disease progression models in this population. Future clinical trials should study the role of biomarkers when evaluating the influence of critical illness over time. In some cases, treatment of critically ill patients can be adjusted based on corresponding levels of biomarkers. For example, ventilator settings can be adjusted based on oxygen saturation or partial oxygen pressure (pO2) measurements over time [207,208]. Also, a high C-reactive protein (≥ 75 mg/L) measured within 24 hours before ICU discharge was found to be associated with an increased risk of adverse outcome after ICU discharge [209]. Cardiac biomarkers such as lactate, troponin, or B-type natriuretic peptide have all shown some utility in the assessment of children with critical cardiac disease, mostly when their overall temporal trend, rather than a single measurement, are analyzed [210]. In a retrospective review of infants and children after heart surgery, persistent elevation of plasma lactate levels >2 mmol/L for 24–48 hours was a superior predictor of mortality compared to initial or peak plasma lactate levels [211]. In the pediatric septic shock population, cardiac troponin elevation at the time of admission has been associated with myocardial depression and disease severity [212]. The use of biomarkers to guide pharmacotherapy in critically ill children holds great promise, but population specific validation and PK/PD studies are needed to identify clinically relevant biomarkers that can help guide appropriate treatment adjustments over time [213].
Further efforts should be made to elucidate the effect of therapeutic interventions such as ECMO and CRRT on PK alterations. Additionally, critically ill children with impaired organ function may be at a higher risk of DDI when concomitant medications are administered. Last, critical illness may alter drug efficacy and safety and future studies should be designed to better understand the effect of critical illness on clinical and PD outcomes. Because changes in PK/PD can lead to potentially sub-therapeutic or toxic levels of drugs in critically ill children, clinical studies in this vulnerable population are important for rational dose selection.
ABBREVIATIONS
- AAG
α-1 acid glycoprotein
- AKI
Acute Kidney Injury
- ARC
Augmented Renal Clearance
- AUC
Area under the concentration versus time curve
- CHF
Congestive Heart Failure
- CKD
Chronic Kidney Disease
- CL
Clearance
- CRRT
Continuous Renal Replacement Therapy
- CVVH
Continuous Veno-Venous Hemofiltration
- CYP
Cytochrome P450 enzymes
- ECMO
Extracorporeal Membrane Oxygenation
- ESRD
End Stage Renal Disease
- PD
Pharmacodynamics
- PICU
Pediatric Intensive Care Unit
- PK
Pharmacokinetics
- VD
Volume of Distribution
Footnotes
CONFLICT OF INTEREST
D.G. receives support for research from the National Institute for Child Health and Human Development (K23HD083465), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry (Cempra, Inc. and Jacobus Pharmaceutical Company, Inc.) for drug development in adults and children. C.P.H. receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117). The remaining authors have no funding to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which had no role in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication.
References
- 1.Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 2.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 3.Zuppa AF, Barrett JS. Pharmacokinetics and pharmacodynamics in the critically ill child. Pediatr Clin North Am. 2008;55:735–55. xii. doi: 10.1016/j.pcl.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Tayman C, Rayyan M, Allegaert K. Neonatal pharmacology: extensive interindividual variability despite limited size. J Pediatr Pharmacol Ther. 2011;16:170–84. doi: 10.5863/1551-6776-16.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem F, Johnson TN, Abduljalil K, Tucker GT, Rostami-Hodjegan A. A re-evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin Pharmacokinet. 2014;53:625–36. doi: 10.1007/s40262-014-0140-7. [DOI] [PubMed] [Google Scholar]
- 6.Ince I, Knibbe CAJ, Danhof M, de Wildt SN. Developmental changes in the expression and function of cytochrome P450 3A isoforms: evidence from in vitro and in vivo investigations. Clin Pharmacokinet. 2013;52:333–45. doi: 10.1007/s40262-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009 doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- 8.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol Springer-Verlag. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 9.Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255–71. vi. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez D, Conrado DJ, Theuretzbacher U, Derendorf H. The effect of critical illness on drug distribution. Curr Pharm Biotechnol. 2011;12:2030–6. doi: 10.2174/138920111798808211. [DOI] [PubMed] [Google Scholar]
- 11.Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010;9:929–39. doi: 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- 12.Haller C. Hypoalbuminemia in renal failure: pathogenesis and therapeutic considerations. Kidney Blood Press Res. 2005;28:307–10. doi: 10.1159/000090185. [DOI] [PubMed] [Google Scholar]
- 13.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–71. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 14.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 15.Lodise TP, Lomaestro B, Rodvold KA, Danziger LH, Drusano GL. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob Agents Chemother. 2004;48:4718–24. doi: 10.1128/AAC.48.12.4718-4724.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallner JJ. Binding of Drugs by Albumin Plasma Protein. J Pharm Sci. 1977;66:447–65. doi: 10.1002/jps.2600660402. [DOI] [PubMed] [Google Scholar]
- 17.Libke RD, Clarke JT, Ralph ED, Luthy RP, Kirby WM. Ticarcillin vs carbenicillin: clinical pharmacokinetics. Clin Pharmacol Ther. 1975;17:441–6. doi: 10.1002/cpt1975174441. [DOI] [PubMed] [Google Scholar]
- 18.Lee BL, Sachdeva M, Chambers HF. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991;35:2505–8. doi: 10.1128/aac.35.12.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akt P, Lortholary O, Fauvelle F, Tod M, Genereau T, Louchahi M, et al. Pharmacokinetics of teicoplanin during plasma exchange. Clin Microbiol Infect. 1999;5:213–8. doi: 10.1111/j.1469-0691.1999.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 20.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 21.Dutkowski R, Thakrar B, Froehlich E, Suter P, Oo C, Ward P. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 2003;26:787–801. doi: 10.2165/00002018-200326110-00004. [DOI] [PubMed] [Google Scholar]
- 22.Peterson GM, McLean S, Aldous S, Von Witt RJ, Millingen KS. Plasma protein binding of phenytoin in 100 epileptic patients. Br J Clin Pharmacol. 1982;14:298–300. doi: 10.1111/j.1365-2125.1982.tb01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swart EL, de Jongh J, Zuideveld KP, Danhof M, Thijs LG, Strack van Schijndel RJM. Population pharmacokinetics of lorazepam and midazolam and their metabolites in intensive care patients on continuous venovenous hemofiltration. Am J Kidney Dis. 2005;45:360–71. doi: 10.1053/j.ajkd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Halliday NJ, Dundee JW, Collier PS, Loughran PG, Harper KW. Influence of plasma proteins on the onset of hypnotic action of intravenous midazolam. Anaesthesia. 1985;40:763–6. doi: 10.1111/j.1365-2044.1985.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 25.Servin F, Desmonts JM, Haberer JP, Cockshott ID, Plummer GF, Farinotti R. Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology. 1988;69:887–91. doi: 10.1097/00000542-198812000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Stowe CD, Lee KR, Storgion SA, Phelps SJ. Altered phenytoin pharmacokinetics in children with severe, acute traumatic brain injury. J Clin Pharmacol. 2000;40:1452–61. [PubMed] [Google Scholar]
- 27.O’Mara NB, Jones PR, Anglin DL, Cox S, Nahata MC. Pharmacokinetics of phenytoin in children with acute neurotrauma. Crit Care Med. 1995;23:1418–24. doi: 10.1097/00003246-199508000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–51. doi: 10.1097/CCM.0b013e3181961bff. quiz 859. [DOI] [PubMed] [Google Scholar]
- 29.Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65:757–73. doi: 10.1007/s00228-009-0678-8. [DOI] [PubMed] [Google Scholar]
- 30.Keyes JL. Blood-gas analysis and the assessment of acid-base status. Heart Lung. 1976;5:247–55. [PubMed] [Google Scholar]
- 31.Johnson PN, Boyles KA, Miller JL. Selection of the initial methadone regimen for the management of iatrogenic opioid abstinence syndrome in critically ill children. Pharmacotherapy. 2012;32:148–57. doi: 10.1002/PHAR.1001. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AE. Capillary fluid filtration. Starling forces and lymph flow. Circ Res. 1981;49:557–75. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- 33.Ellis D. Pathophysiology, Evaluation, and Management of Edema in Childhood Nephrotic Syndrome. Front Pediatr. 2015;3:111. doi: 10.3389/fped.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little RC, Ginsburg JM. The physiologic basis for clinical edema. Arch Intern Med. 1984;144:1661–4. doi: 10.1001/archinte.144.8.1661. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Ishihara H, Hashiba E, Matsui A, Matsuki A. Detection of histamine-induced capillary protein leakage and hypovolaemia by determination of indocyanine green and glucose dilution method in dogs. Intensive Care Med. 1999;25:304–10. doi: 10.1007/s001340050840. [DOI] [PubMed] [Google Scholar]
- 36.Dasta JF, Armstrong DK. Variability in aminoglycoside pharmacokinetics in critically ill surgical patients. Crit Care Med. 1988;16:327–30. doi: 10.1097/00003246-198804000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Beckhouse MJ, Whyte IM, Byth PL, Napier JC, Smith AJ. Altered aminoglycoside pharmacokinetics in the critically ill. Anaesth Intensive Care. 1988;16:418–22. doi: 10.1177/0310057X8801600406. [DOI] [PubMed] [Google Scholar]
- 38.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–9. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 39.Chang J-W, Jeng M-J, Yang L-Y, Chen T-J, Chiang S-C, Soong W-J, et al. The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int. 2015;87:632–9. doi: 10.1038/ki.2014.299. [DOI] [PubMed] [Google Scholar]
- 40.Naik S, Sharma J, Yengkom R, Kalrao V, Mulay A. Acute kidney injury in critically ill children: Risk factors and outcomes. Indian J Crit Care Med. 2014;18:129–33. doi: 10.4103/0972-5229.128701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin SM, Balestracci A, Aprea V, Bolasell C, Wainsztein R, Debaisi G, et al. Acute kidney injury in critically ill children: incidence and risk factors for mortality. Arch argentinos Pediatr. 2013;111:411–6. doi: 10.5546/aap.2013.eng.412. [DOI] [PubMed] [Google Scholar]
- 42.Bailey D, Phan V, Litalien C, Ducruet T, Mérouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 43.Krishnan V, Murray P. Pharmacologic issues in the critically ill. Clin Chest Med. 2003;24:671–88. doi: 10.1016/s0272-5231(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 44.Mitch WE, Wilcox CS. Disorders of body fluids, sodium and potassium in chronic renal failure. Am J Med. 1982;72:536–50. doi: 10.1016/0002-9343(82)90523-x. [DOI] [PubMed] [Google Scholar]
- 45.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocktaeschel J, Morimatsu H, Uchino S, Goldsmith D, Poustie S, Story D, et al. Acid-base status of critically ill patients with acute renal failure: analysis based on Stewart-Figge methodology. Crit Care. 2003;7:R60. doi: 10.1186/cc2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 2014;42:520–7. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 48.De Cock PAJG, Standing JF, Barker CIS, de Jaeger A, Dhont E, Carlier M, et al. Augmented renal clearance implies a need for increased amoxicillin-clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother. 2015;59:7027–35. doi: 10.1128/AAC.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25:525–52. doi: 10.1177/0269216311406313. [DOI] [PubMed] [Google Scholar]
- 50.Eyler RF, Mueller BA. Antibiotic pharmacokinetic and pharmacodynamic considerations in patients with kidney disease. Adv Chronic Kidney Dis. 2010;17:392–403. doi: 10.1053/j.ackd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Asconapé JJ. Use of antiepileptic drugs in hepatic and renal disease. Handb Clin Neurol. 2014;119:417–32. doi: 10.1016/B978-0-7020-4086-3.00027-8. [DOI] [PubMed] [Google Scholar]
- 52.Trey C. The critically ill child: acute hepatic failure. Pediatrics. 1970;45:93–8. [PubMed] [Google Scholar]
- 53.Piñeiro-Carrero VM, Piñeiro EO. Liver. Pediatrics. 2004;113:1097–106. [PubMed] [Google Scholar]
- 54.Rodighiero V. Effects of liver disease on pharmacokinetics. An update Clin Pharmacokinet. 1999;37:399–431. doi: 10.2165/00003088-199937050-00004. [DOI] [PubMed] [Google Scholar]
- 55.Thakkar N, Gonzalez D, Cohen-Wolkowiez M, Massaro MM, Bernhardt J, Zane NR, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744–7. doi: 10.1038/jp.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barry M, Keeling PW, Weir D, Feely J. Severity of cirrhosis and the relationship of alpha 1-acid glycoprotein concentration to plasma protein binding of lidocaine. Clin Pharmacol Ther. 1990;47:366–70. doi: 10.1038/clpt.1990.41. [DOI] [PubMed] [Google Scholar]
- 57.Pacifici GM, Viani A, Taddeucci-Brunelli G, Rizzo G, Carrai M, Schulz HU. Effects of development, aging, and renal and hepatic insufficiency as well as hemodialysis on the plasma concentrations of albumin and alpha 1-acid glycoprotein: implications for binding of drugs. Ther Drug Monit. 1986;8:259–63. doi: 10.1097/00007691-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Bower S, Sear JW, Roy RC, Carter RF. Effects of different hepatic pathologies on disposition of alfentanil in anaesthetized patients. Br J Anaesth. 1992;68:462–5. doi: 10.1093/bja/68.5.462. [DOI] [PubMed] [Google Scholar]
- 59.Olsen GD, Bennett WM, Porter GA. Morphine and phenytoin binding to plasma proteins in renal and hepatic failure. Clin Pharmacol Ther. 1975;17:677–84. doi: 10.1002/cpt1975176677. [DOI] [PubMed] [Google Scholar]
- 60.Lanao JM, Dominguez-Gil A, Macias JG, Diez JL, Nieto MJ. The influence of ascites on the pharmacokinetics of amikacin. Int J Clin Pharmacol Ther Toxicol. 1980;18:57–61. [PubMed] [Google Scholar]
- 61.Sampliner R, Perrier D, Powell R, Finley P. Influence of ascites on tobramycin pharmacokinetics. J Clin Pharmacol. 1984;24:43–6. doi: 10.1002/j.1552-4604.1984.tb01812.x. [DOI] [PubMed] [Google Scholar]
- 62.Perkins MW, Dasta JF, DeHaven B. Physiologic implications of mechanical ventilation on pharmacokinetics. DICP. 1989;23:316–23. doi: 10.1177/106002808902300408. [DOI] [PubMed] [Google Scholar]
- 63.Meier-Hellmann A, Bredle DL, Specht M, Spies C, Hannemann L, Reinhart K. The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock. Intensive Care Med. 1997;23:31–7. doi: 10.1007/s001340050287. [DOI] [PubMed] [Google Scholar]
- 64.Obritsch MD, Bestul DJ, Jung R, Fish DN, MacLaren R. The role of vasopressin in vasodilatory septic shock. Pharmacotherapy. 2004;24:1050–63. doi: 10.1592/phco.24.11.1050.36144. [DOI] [PubMed] [Google Scholar]
- 65.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12:28–32. [PubMed] [Google Scholar]
- 66.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet. 2002;41:959–98. doi: 10.2165/00003088-200241120-00003. [DOI] [PubMed] [Google Scholar]
- 67.Carcillo JA, Doughty L, Kofos D, Frye RF, Kaplan SS, Sasser H, et al. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003;29:980–4. doi: 10.1007/s00134-003-1758-3. [DOI] [PubMed] [Google Scholar]
- 68.Wong HR, Carcillo JA, Burckart G, Shah N, Janosky JE. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit Care Med. 1995;23:835–42. doi: 10.1097/00003246-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Peeters MYM, Aarts LPHJ, Boom FA, Bras LJ, Tibboel D, Danhof M, et al. Pilot study on the influence of liver blood flow and cardiac output on the clearance of propofol in critically ill patients. Eur J Clin Pharmacol. 2008;64:329–34. doi: 10.1007/s00228-007-0399-9. [DOI] [PubMed] [Google Scholar]
- 70.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 71.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 72.Edwards JE, Race GA, Scheifley CH. Albuminuria in congestive heart failure. Circulation. 1956;13:329–33. doi: 10.1161/01.cir.13.3.329. [DOI] [PubMed] [Google Scholar]
- 73.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 74.Lima JJ, Binkley PF, Johnson J, Leier CV. Dose- and time-dependent binding and kinetics of pindolol in patients with congestive heart failure. J Clin Pharmacol. 1986;26:253–7. doi: 10.1002/j.1552-4604.1986.tb03519.x. [DOI] [PubMed] [Google Scholar]
- 75.Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congest Heart Fail. 9:287–92. doi: 10.1111/j.1527-5299.2003.02399.x. [DOI] [PubMed] [Google Scholar]
- 76.Dalton HJ, Macrae DJ Pediatric Acute Lung Injury Consensus Conference Group. Extracorporeal support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S111–7. doi: 10.1097/PCC.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 77.Watt K, Li JS, Benjamin DK, Cohen-Wolkowiez M. Pediatric cardiovascular drug dosing in critically ill children and extracorporeal membrane oxygenation. J Cardiovasc Pharmacol. 2011;58:126–32. doi: 10.1097/FJC.0b013e318213aac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care BioMed Central. 2000;4:156–68. doi: 10.1186/cc689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Himebauch AS, Kilbaugh TJ, Zuppa AF. Pharmacotherapy during pediatric extracorporeal membrane oxygenation: a review. Expert Opin Drug Metab Toxicol. 2016:1–10. doi: 10.1080/17425255.2016.1201066. [DOI] [PubMed] [Google Scholar]
- 81.Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989;33:817–9. doi: 10.1128/aac.33.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990;9:562–6. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 83.Hoie EB, Swigart SA, Leuschen MP, Willett LD, Bolam DL, Goodrich PD, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–5. [PubMed] [Google Scholar]
- 84.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40:1139–42. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosen DA, Rosen KR, Silvasi DL. In vitro variability in fentanyl absorption by different membrane oxygenators. J Cardiothorac Anesth. 1990;4:332–5. doi: 10.1016/0888-6296(90)90041-d. [DOI] [PubMed] [Google Scholar]
- 86.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–6. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 87.Mulla H, Lawson G, von Anrep C, Burke MD, Upton DU, Firmin RK, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–6. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 88.Chauhan S, Subin S. Extracorporeal membrane oxygenation, an anesthesiologist’s perspective: physiology and principles. Part 1. Ann Card Anaesth. 2011;14:218–29. doi: 10.4103/0971-9784.84030. [DOI] [PubMed] [Google Scholar]
- 89.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–17. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 90.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–70. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 91.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 18:1082–6. [PubMed] [Google Scholar]
- 92.Shekar K, Fraser JF, Taccone FS, Welch S, Wallis SC, Mullany DV, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care. 2014;18:565. doi: 10.1186/s13054-014-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldstein SL. Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial. 24:187–91. doi: 10.1111/j.1525-139X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 94.Bridges BC, Askenazi DJ, Smith J, Goldstein SL. Pediatric renal replacement therapy in the intensive care unit. Blood Purif. 2012;34:138–48. doi: 10.1159/000342129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Awdishu L, Bouchard J. How to optimize drug delivery in renal replacement therapy. Semin Dial. 2011;24:176–82. doi: 10.1111/j.1525-139X.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 96.Wong W-T, Choi G, Gomersall CD, Lipman J. To increase or decrease dosage of antimicrobials in septic patients during continuous renal replacement therapy: the eternal doubt. Curr Opin Pharmacol. 2015;24:68–78. doi: 10.1016/j.coph.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Böhler J, Donauer J, Keller F. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl. 1999:S24–8. [PubMed] [Google Scholar]
- 98.Schetz M, Ferdinande P, Van den Berghe G, Verwaest C, Lauwers P. Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med Springer-Verlag. 1995;21:612–20. doi: 10.1007/BF01700172. [DOI] [PubMed] [Google Scholar]
- 99.Nehus EJ, Mouksassi S, Vinks AA, Goldstein S. Meropenem in children receiving continuous renal replacement therapy: clinical trial simulations using realistic covariates. J Clin Pharmacol. 2014;54:1421–8. doi: 10.1002/jcph.360. [DOI] [PubMed] [Google Scholar]
- 100.Lindsay CA, Bawdon R, Quigley R. Clearance of ticarcillin-clavulanic acid by continuous venovenous hemofiltration in three critically ill children, two with and one without concomitant extracorporeal membrane oxygenation. Pharmacotherapy. 1996;16:458–62. [PubMed] [Google Scholar]
- 101.Field MJ, Behrman RE. The Necessity and Challenges of Clinical Research Involving Children. National Academies Press; US: 2004. Children I of M (US) C on CRI. [PubMed] [Google Scholar]
- 102.Bavdekar SB. Pediatric clinical trials. Perspect Clin Res. 2013;4:89–99. doi: 10.4103/2229-3485.106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McCracken GH. Aminoglycoside toxicity in infants and children. Am J Med. 1986;80:172–8. doi: 10.1016/0002-9343(86)90497-3. [DOI] [PubMed] [Google Scholar]
- 104.Marik PE, Havlik I, Monteagudo FS, Lipman J. The pharmacokinetic of amikacin in critically ill adult and paediatric patients: comparison of once- versus twice-daily dosing regimens. J Antimicrob Chemother. 1991;27(Suppl C):81–9. doi: 10.1093/jac/27.suppl_c.81. [DOI] [PubMed] [Google Scholar]
- 105.Sherwin CMT, Wead S, Stockmann C, Healy D, Spigarelli MG, Neely A, et al. Amikacin population pharmacokinetics among paediatric burn patients. Burns. 2014;40:311–8. doi: 10.1016/j.burns.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Martínková J, Pokorná P, Záhora J, Chládek J, Vobruba V, Selke-Krulichová I, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clin Ther. 2010;32:2400–14. doi: 10.1016/j.clinthera.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 107.Bhatt-Mehta V, Donn SM. Gentamicin pharmacokinetics in term newborn infants receiving high-frequency oscillatory ventilation or conventional mechanical ventilation: a case-controlled study. J Perinatol. 2003;23:559–62. doi: 10.1038/sj.jp.7210985. [DOI] [PubMed] [Google Scholar]
- 108.Hoff DS, Wilcox RA, Tollefson LM, Lipnik PG, Commers AR, Liu M. Pharmacokinetic outcomes of a simplified, weight-based, extended-interval gentamicin dosing protocol in critically ill neonates. Pharmacotherapy. 2009;29:1297–305. doi: 10.1592/phco.29.11.1297. [DOI] [PubMed] [Google Scholar]
- 109.Moffett BS, Rossano JW. Use of 4-mg/kg/24-hour empiric aminoglycoside dosing in preoperative neonates with congenital heart disease. Ann Pharmacother. 2012;46:1193–7. doi: 10.1345/aph.1Q792. [DOI] [PubMed] [Google Scholar]
- 110.Semchuk W, Shevchuk YM, Sankaran K, Wallace SM. Prospective, randomized, controlled evaluation of a gentamicin loading dose in neonates. Biol Neonate. 1995;67:13–20. doi: 10.1159/000244137. [DOI] [PubMed] [Google Scholar]
- 111.Lopez SA, Mulla H, Durward A, Tibby SM. Extended-interval gentamicin: population pharmacokinetics in pediatric critical illness. Pediatr Crit Care Med. 2010;11:267–74. doi: 10.1097/PCC.0b013e3181b80693. [DOI] [PubMed] [Google Scholar]
- 112.Zakova M, Pong S, Trope A, Atenafu EG, Papaioannou V, Bitnun SA, et al. Dose derivation of once-daily dosing guidelines for gentamicin in critically ill pediatric patients. Ther Drug Monit. 2014;36:288–94. doi: 10.1097/FTD.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 113.Bass KD, Larkin SE, Paap C, Haase GM. Pharmacokinetics of once-daily gentamicin dosing in pediatric patients. J Pediatr Surg. 1998;33:1104–7. doi: 10.1016/s0022-3468(98)90540-1. [DOI] [PubMed] [Google Scholar]
- 114.Botha JH, du Preez MJ, Adhikari M. Population pharmacokinetics of gentamicin in South African newborns. Eur J Clin Pharmacol. 2003;59:755–9. doi: 10.1007/s00228-003-0663-6. [DOI] [PubMed] [Google Scholar]
- 115.Mathews A, Bailie GR. Clinical pharmacokinetics, toxicity and cost effectiveness analysis of aminoglycosides and aminoglycoside dosing services. J Clin Pharm Ther. 1987;12:273–91. doi: 10.1111/j.1365-2710.1987.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 116.Bacopoulou F, Skouroliakou M, Markantonis SL. Netilmicin in the neonate: pharmacokinetic analysis and influence of parenteral nutrition. Pharm world {&} Sci PWS. 2009;31:365–8. doi: 10.1007/s11096-009-9278-z. [DOI] [PubMed] [Google Scholar]
- 117.Bergan T, Michalsen H. Pharmacokinetic assessment of netilmicin in newborns and older children. Infection. 1982;10:153–8. doi: 10.1007/BF01640766. [DOI] [PubMed] [Google Scholar]
- 118.Gosden PE, Bedford KA, Dixon JJ, Speidel BD, Leaf AA, Macgowan AP. Pharmacokinetics of once-a-day netilmicin (4 mg/kg) in neonates. J Chemother. 2001;13:270–6. doi: 10.1179/joc.2001.13.3.270. [DOI] [PubMed] [Google Scholar]
- 119.Ettlinger JJ, Bedford KA, Lovering AM, Reeves DS, Speidel BD, MacGowan AP. Pharmacokinetics of once-a-day netilmicin (6 mg/kg) in neonates. J Antimicrob Chemother. 1996;38:499–505. doi: 10.1093/jac/38.3.499. [DOI] [PubMed] [Google Scholar]
- 120.Wagner BP, Pfenninger J. Once daily dosing of netilmicin in neonatal and pediatric intensive care. Intensive Care Med. 1994;20:365–7. doi: 10.1007/BF01720910. [DOI] [PubMed] [Google Scholar]
- 121.Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2010;54:1734–41. doi: 10.1128/AAC.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bertels RA, Semmekrot BA, Gerrits GP, Mouton JW. Serum concentrations of cefotaxime and its metabolite desacetyl-cefotaxime in infants and children during continuous infusion. Infection. 2008;36:415–20. doi: 10.1007/s15010-008-7274-1. [DOI] [PubMed] [Google Scholar]
- 123.Kafetzis DA, Brater DC, Kapiki AN, Papas CV, Dellagrammaticas H, Papadatos CJ. Treatment of severe neonatal infections with cefotaxime. Efficacy and pharmacokinetics. J Pediatr. 1982;100:483–9. doi: 10.1016/s0022-3476(82)80466-6. [DOI] [PubMed] [Google Scholar]
- 124.McCracken GH, Threlkeld NE, Thomas ML. Pharmacokinetics of cefotaxime in newborn infants. Antimicrob Agents Chemother. 1982;21:683–4. doi: 10.1128/aac.21.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bégué PC, Baron S, Challier P, Fontaine JL, Lasfargues G. Pharmacokinetic and clinical evaluation of imipenem/cilastatin in children and neonates. Scand J Infect Dis Suppl. 1987;52:40–5. [PubMed] [Google Scholar]
- 126.Claesson G, Eriksson M, Rogers JD. Pharmacokinetics of imipenem/cilastatin sodium in children with peritonitis. Pharmacol Toxicol. 1992;71:103–6. doi: 10.1111/j.1600-0773.1992.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 127.Giannoni E, Moreillon P, Cotting J, Moessinger A, Bille J, Décosterd L, et al. Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob Agents Chemother. 2006;50:2563–8. doi: 10.1128/AAC.01149-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.AstraZeneca. [Date Accessed: 2016-04-07];MERREM ® I.V. FOR INTRAVENOUS USE ONLY. 2006 :3–25. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050706s022lbl.pdf.
- 129.Cies JJ, Moore WS, Dickerman MJ, Small C, Carella D, Chopra A, et al. Pharmacokinetics of continuous-infusion meropenem in a pediatric patient receiving extracorporeal life support. Pharmacotherapy. 2014;34:e175–9. doi: 10.1002/phar.1476. [DOI] [PubMed] [Google Scholar]
- 130.Cies JJ, Shankar V, Schlichting C, Kuti JL. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J. 2014;33:168–73. doi: 10.1097/INF.0b013e3182a743c7. [DOI] [PubMed] [Google Scholar]
- 131.Reed MD, Goldfarb J, Yamashita TS, Lemon E, Blumer JL. Single-dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother. 1994;38:2817–26. doi: 10.1128/aac.38.12.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Groot R, Hack BD, Weber A, Chaffin D, Ramsey B, Smith AL. Pharmacokinetics of ticarcillin in patients with cystic fibrosis: a controlled prospective study. Clin Pharmacol Ther. 1990;47:73–8. doi: 10.1038/clpt.1990.11. [DOI] [PubMed] [Google Scholar]
- 133.Akins RL, Haase MR, Levy EN. Pharmacokinetics of daptomycin in a critically ill adolescent with vancomycin-resistant enterococcal endocarditis. Pharmacotherapy. 2006;26:694–8. doi: 10.1592/phco.26.5.694. [DOI] [PubMed] [Google Scholar]
- 134.Dvorchik B, Arbeit RD, Chung J, Liu S, Knebel W, Kastrissios H. Population pharmacokinetics of daptomycin. Antimicrob Agents Chemother. 2004;48:2799–807. doi: 10.1128/AAC.48.8.2799-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reiter PD, Doron MW. Vancomycin cerebrospinal fluid concentrations after intravenous administration in premature infants. J Perinatol. 16:331–5. [PubMed] [Google Scholar]
- 136.Giachetto GA, Telechea HM, Speranza N, Oyarzun M, Nanni L, Menchaca A. Vancomycin pharmacokinetic-pharmacodynamic parameters to optimize dosage administration in critically ill children. Pediatr Crit Care Med. 2011;12:e250–4. doi: 10.1097/PCC.0b013e3181fe4047. [DOI] [PubMed] [Google Scholar]
- 137.Gous AG, Dance MD, Lipman J, Luyt DK, Mathivha R, Scribante J. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth Intensive Care. 1995;23:678–82. doi: 10.1177/0310057X9502300603. [DOI] [PubMed] [Google Scholar]
- 138.Armstrong DK, Hidalgo HA, Eldadah M. Vancomycin and tobramycin clearance in an infant during continuous hemofiltration. Ann Pharmacother. 1993;27:224–7. doi: 10.1177/106002809302700219. [DOI] [PubMed] [Google Scholar]
- 139.Golper TA, Noonan HM, Elzinga L, Gilbert D, Brummett R, Anderson JL, et al. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther. 1988;43:565–70. doi: 10.1038/clpt.1988.74. [DOI] [PubMed] [Google Scholar]
- 140.Schaad UB, McCracken GH, Nelson JD. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–26. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 141.Naqvi SH, Keenan WJ, Reichley RM, Fortune KP. Vancomycin pharmacokinetics in small, seriously ill infants. Am J Dis Child. 1986;140:107–10. doi: 10.1001/archpedi.1986.02140160025021. [DOI] [PubMed] [Google Scholar]
- 142.Brogden RN, Peters DH. Teicoplanin. A reappraisal of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:823–54. doi: 10.2165/00003495-199447050-00008. [DOI] [PubMed] [Google Scholar]
- 143.Sánchez A, López-Herce J, Cueto E, Carrillo A, Moral R. Teicoplanin pharmacokinetics in critically ill paediatric patients. J Antimicrob Chemother. 1999;44:407–9. doi: 10.1093/jac/44.3.407. [DOI] [PubMed] [Google Scholar]
- 144.Reed MD, Yamashita TS, Myers CM, Blumer JL. The pharmacokinetics of teicoplanin in infants and children. J Antimicrob Chemother. 1997;39:789–96. doi: 10.1093/jac/39.6.789. [DOI] [PubMed] [Google Scholar]
- 145.Bell EA. Pharmacotherapy with systemic antifungal medications. Infectious Diseases in Children. 2011 [Google Scholar]
- 146.Cohen-Wolkowiez M, Moran C, Benjamin DK, Smith PB. Pediatric antifungal agents. Curr Opin Infect Dis. 2009;22:553–8. doi: 10.1097/QCO.0b013e3283321ccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Piper L, Smith PB, Hornik CP, Cheifetz IM, Barrett JS, Moorthy G, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30:375–8. doi: 10.1097/INF.0b013e318202cbb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK, Sullivan JE, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52:4043–9. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Watt KM, Gonzalez D, Benjamin DK, Brouwer KLR, Wade KC, Capparelli E, et al. Fluconazole population pharmacokinetics and dosing for prevention and treatment of invasive Candidiasis in children supported with extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 2015;59:3935–43. doi: 10.1128/AAC.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van der Elst KCM, Pereboom M, van den Heuvel ER, Kosterink JGW, Schölvinck EH, Alffenaar J-WC. Insufficient fluconazole exposure in pediatric cancer patients and the need for therapeutic drug monitoring in critically ill children. Clin Infect Dis. 2014;59:1527–33. doi: 10.1093/cid/ciu657. [DOI] [PubMed] [Google Scholar]
- 151.Spriet I, Annaert P, Meersseman P, Hermans G, Meersseman W, Verbesselt R, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. 2009;63:767–70. doi: 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 152.Englund JA, Fletcher CV, Balfour HH. Acyclovir therapy in neonates. J Pediatr. 1991;119:129–35. doi: 10.1016/s0022-3476(05)81053-4. [DOI] [PubMed] [Google Scholar]
- 153.GlaxoSmithKline. [Date Accessed: 2016-04-20];Zovirax Product Insert. 2003 http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018828s030,020089s019,019909s020lbl.pdf.
- 154.Genentech. [Date Accessed: 2016-03-11];Tamiflu® (oseltamivir phosphate) Prescribing Information. 2014 http://www.gene.com/download/pdf/tamiflu_prescribing.pdf.
- 155.Muñoz FM, Anderson EJ, Deville JG, Clinch B, Kamal MA. Pharmacokinetics and safety of intravenous oseltamivir in infants and children in open-label studies. Int J Clin Pharmacol Ther. 2015;53:531–40. doi: 10.5414/CP202307. [DOI] [PubMed] [Google Scholar]
- 156.Oo C, Hill G, Dorr A, Liu B, Boellner S, Ward P. Pharmacokinetics of anti-influenza prodrug oseltamivir in children aged 1–5 years. Eur J Clin Pharmacol. 2003;59:411–5. doi: 10.1007/s00228-003-0639-6. [DOI] [PubMed] [Google Scholar]
- 157.Wildschut ED, de Hoog M, Ahsman MJ, Tibboel D, Osterhaus ADME, Fraaij PLA. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One. 2010;5:e10938. doi: 10.1371/journal.pone.0010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Santeiro ML, Christie J, Stromquist C, Torres BA, Markowsky SJ. Pharmacokinetics of continuous infusion fentanyl in newborns. J Perinatol. 1997;17:135–9. [PubMed] [Google Scholar]
- 159.UCB. Keppra injection (levetiracetam) package insert. 2008. [Date Accessed: 2016-03-23]. [Google Scholar]
- 160.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med. 2009;10:505–10. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Glauser TA, Mitchell WG, Weinstock A, Bebin M, Chen D, Coupez R, et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia. 2007;48:1117–22. doi: 10.1111/j.1528-1167.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 162.Toublanc N, Sargentini-Maier ML, Lacroix B, Jacqmin P, Stockis A. Clin Pharmacokinet. Vol. 47. Springer International Publishing; 2008. Retrospective population pharmacokinetic analysis of levetiracetam in children and adolescents with epilepsy: dosing recommendations; pp. 333–41. [DOI] [PubMed] [Google Scholar]
- 163.Pellock JM, Glauser TA, Bebin EM, Fountain NB, Ritter FJ, Coupez RM, et al. Pharmacokinetic Study of Levetiracetam in Children. Epilepsia. 2002;42:1574–9. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 164.Snoeck E, Jacqmin P, Sargentini-Maier ML, Stockis A. Modeling and simulation of intravenous levetiracetam pharmacokinetic profiles in children to evaluate dose adaptation rules. Epilepsy Res. 2007;76:140–7. doi: 10.1016/j.eplepsyres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 165.Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43:707–24. doi: 10.2165/00003088-200443110-00002. [DOI] [PubMed] [Google Scholar]
- 166.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–86. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 167.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, randomized comparison of i. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–98. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 168.McDermott CA, Kowalczyk AL, Schnitzler ER, Mangurten HH, Rodvold KA, Metrick S. Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr. 1992;120:479–83. doi: 10.1016/s0022-3476(05)80925-4. [DOI] [PubMed] [Google Scholar]
- 169.Greenblatt DJ, Shader RI, Franke K, MacLaughlin DS, Harmatz JS, Allen MD, et al. Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans. J Pharm Sci. 1979;68:57–63. doi: 10.1002/jps.2600680119. [DOI] [PubMed] [Google Scholar]
- 170.Wiest DB, O’Neal W, Reigart JR, Brundage RC, Gillette PC, Yost RL. Pharmacokinetics of ranitidine in critically ill infants. Dev Pharmacol Ther. 1989;12:7–12. [PubMed] [Google Scholar]
- 171.Wells TG, Heulitt MJ, Taylor BJ, Fasules JW, Kearns GL. Pharmacokinetics and pharmacodynamics of ranitidine in neonates treated with extracorporeal membrane oxygenation. J Clin Pharmacol. 1998;38:402–7. doi: 10.1002/j.1552-4604.1998.tb04443.x. [DOI] [PubMed] [Google Scholar]
- 172.Lugo RA, Harrison AM, Cash J, Sweeley J, Vernon DD. Pharmacokinetics and pharmacodynamics of ranitidine in critically ill children. Crit Care Med. 2001;29:759–64. doi: 10.1097/00003246-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 173.Fontana M, Massironi E, Rossi A, Vaglia P, Gancia GP, Tagliabue P, et al. Ranitidine pharmacokinetics in newborn infants. Arch Dis Child. 1993;68:602–3. doi: 10.1136/adc.68.5_spec_no.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ilett KF, Nation RL, Tjokrosetio R, Thompson WR, Oh TE, Cameron PD. Pharmacokinetics of ranitidine in critically ill patients. Br J Clin Pharmacol. 1986;21:279–88. doi: 10.1111/j.1365-2125.1986.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Solana MJ, Colom H, López-Herce J, Urbano J, González R, López J, et al. Population pharmacokinetics of omeprazole in critically ill pediatric patients. Ther Drug Monit. 2014;36:519–27. doi: 10.1097/FTD.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 176.Solana MJ, López-Herce J. Pharmacokinetics of intravenous omeprazole in critically ill paediatric patients. Eur J Clin Pharmacol. 2010;66:323–30. doi: 10.1007/s00228-009-0774-9. [DOI] [PubMed] [Google Scholar]
- 177.Jacqz-Aigrain E, Bellaich M, Faure C, Andre J, Rohrlich P, Baudouin V, et al. Pharmacokinetics of intravenous omeprazole in children. Eur J Clin Pharmacol. 1994;47:181–5. doi: 10.1007/BF00194970. [DOI] [PubMed] [Google Scholar]
- 178.Jacqz-Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol. 1992;42:329–32. doi: 10.1007/BF00266357. [DOI] [PubMed] [Google Scholar]
- 179.Hughes J, Gill AM, Mulhearn H, Powell E, Choonara I. Steady-state plasma concentrations of midazolam in critically ill infants and children. Ann Pharmacother. 1996;30:27–30. doi: 10.1177/106002809603000104. [DOI] [PubMed] [Google Scholar]
- 180.Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–72. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- 181.Vet NJ, de Hoog M, Tibboel D, de Wildt SN. The effect of critical illness and inflammation on midazolam therapy in children. Pediatr Crit Care Med. 2012;13:e48–50. doi: 10.1097/PCC.0b013e3181fe406d. [DOI] [PubMed] [Google Scholar]
- 182.Ince I, de Wildt SN, Peeters MYM, Murry DJ, Tibboel D, Danhof M, et al. Critical illness is a major determinant of midazolam clearance in children aged 1 month to 17 years. Ther Drug Monit. 2012 Aug;34(4):381–9. doi: 10.1097/FTD.0b013e31825a4c3a. [DOI] [PubMed] [Google Scholar]
- 183.Vet NJ, Brussee JM, de Hoog M, Mooij MG, Verlaat CWM, Jerchel IS, et al. Inflammation and Organ Failure Severely Affect Midazolam Clearance in Critically Ill Children. Am J Respir Crit Care Med. 2016;194:58–66. doi: 10.1164/rccm.201510-2114OC. [DOI] [PubMed] [Google Scholar]
- 184.Reed MD, Yamashita TS, Marx CM, Myers CM, Blumer JL. A pharmacokinetically based propofol dosing strategy for sedation of the critically ill, mechanically ventilated pediatric patient. Crit Care Med. 1996;24:1473–81. doi: 10.1097/00003246-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 185.Peeters MYM, Allegaert K, Blussé van Oud-Alblas HJ, Cella M, Tibboel D, Danhof M, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0. 5 fixed-exponent allometric model. Clin Pharmacokinet. 2010;49:269–75. doi: 10.2165/11319350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 186.Knibbe CAJ, Zuideveld KP, Aarts LPHJ, Kuks PFM, Danhof M. Allometric relationships between the pharmacokinetics of propofol in rats, children and adults. Br J Clin Pharmacol. 2005;59:705–11. doi: 10.1111/j.1365-2125.2005.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kataria BK, Ved SA, Nicodemus HF, Hoy GR, Lea D, Dubois MY, et al. The pharmacokinetics of propofol in children using three different data analysis approaches. Anesthesiology. 1994;80:104–22. doi: 10.1097/00000542-199401000-00018. [DOI] [PubMed] [Google Scholar]
- 188.Rigby-Jones AE, Nolan JA, Priston MJ, Wright PMC, Sneyd JR, Wolf AR. Pharmacokinetics of propofol infusions in critically ill neonates, infants, and children in an intensive care unit. Anesthesiology. 2002;97:1393–400. doi: 10.1097/00000542-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 189.Driscoll DJ, Gillette PC, Duff DF, Nihill MR, Gutgesell HP, Vargo TA, et al. Hemodynamic effects of dobutamine in children. Am J Cardiol. 1979;43:581–5. doi: 10.1016/0002-9149(79)90016-x. [DOI] [PubMed] [Google Scholar]
- 190.Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane database Syst Rev. 2003:CD001242. doi: 10.1002/14651858.CD001242. [DOI] [PubMed] [Google Scholar]
- 191.Berg RA, Padbury JF. Sulfoconjugation and renal excretion contribute to the interpatient variation of exogenous catecholamine clearance in critically ill children. Crit Care Med. 1997;25:1247–51. doi: 10.1097/00003246-199707000-00030. [DOI] [PubMed] [Google Scholar]
- 192.Berg RA, Donnerstein RL, Padbury JF. Dobutamine infusions in stable, critically ill children: pharmacokinetics and hemodynamic actions. Crit Care Med. 1993;21:678–86. doi: 10.1097/00003246-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 193.Habib DM, Padbury JF, Anas NG, Perkin RM, Minegar C. Dobutamine pharmacokinetics and pharmacodynamics in pediatric intensive care patients. Crit Care Med. 1992;20:601–8. doi: 10.1097/00003246-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 194.Martinez AM, Padbury JF, Thio S. Dobutamine pharmacokinetics and cardiovascular responses in critically ill neonates. Pediatrics. 1992;89:47–51. [PubMed] [Google Scholar]
- 195.Schwartz PH, Eldadah MK, Newth CJ. The pharmacokinetics of dobutamine in pediatric intensive care unit patients. Drug Metab Dispos. 19:614–9. [PubMed] [Google Scholar]
- 196.Klem C, Dasta JF, Reilley TE, Flancbaum LJ. Variability in dobutamine pharmacokinetics in unstable critically ill surgical patients. Crit Care Med. 1994;22:1926–32. [PubMed] [Google Scholar]
- 197.Berg RA, Padbury JF, Donnerstein RL, Klewer SE, Hutter JJ. Dobutamine pharmacokinetics and pharmacodynamics in normal children and adolescents. J Pharmacol Exp Ther. 1993;265:1232–8. [PubMed] [Google Scholar]
- 198.Mahoney L, Shah G, Crook D, Rojas-Anaya H, Rabe H. A Literature Review of the Pharmacokinetics and Pharmacodynamics of Dobutamine in Neonates. Pediatr Cardiol. 2016;37:14–23. doi: 10.1007/s00246-015-1263-9. [DOI] [PubMed] [Google Scholar]
- 199.Kates RE, Leier CV. Dobutamine pharmacokinetics in severe heart failure. Clin Pharmacol Ther. 1978;24:537–41. doi: 10.1002/cpt1978245537. [DOI] [PubMed] [Google Scholar]
- 200.Bhatt-Mehta V, Nahata MC, McClead RE, Menke JA. Dopamine pharmacokinetics in critically ill newborn infants. Eur J Clin Pharmacol. 1991;40:593–7. doi: 10.1007/BF00279976. [DOI] [PubMed] [Google Scholar]
- 201.Padbury JF, Agata Y, Baylen BG, Ludlow JK, Polk DH, Goldblatt E, et al. Dopamine pharmacokinetics in critically ill newborn infants. J Pediatr. 1987;110:293–8. doi: 10.1016/s0022-3476(87)80176-2. [DOI] [PubMed] [Google Scholar]
- 202.Juste RN, Moran L, Hooper J, Soni N. Dopamine clearance in critically ill patients. Intensive Care Med. 1998;24:1217–20. doi: 10.1007/s001340050747. [DOI] [PubMed] [Google Scholar]
- 203.Oualha M, Urien S, Spreux-Varoquaux O, Bordessoule A, D’Agostino I, Pouard P, et al. Pharmacokinetics, hemodynamic and metabolic effects of epinephrine to prevent post-operative low cardiac output syndrome in children. Crit Care. 2014;18:R23. doi: 10.1186/cc13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fisher DG, Schwartz PH, Davis AL. Pharmacokinetics of exogenous epinephrine in critically ill children. Crit Care Med. 1993;21:111–7. doi: 10.1097/00003246-199301000-00021. [DOI] [PubMed] [Google Scholar]
- 205.Oualha M, Tréluyer J-M, Lesage F, de Saint Blanquat L, Dupic L, Hubert P, et al. Population pharmacokinetics and haemodynamic effects of norepinephrine in hypotensive critically ill children. Br J Clin Pharmacol. 2014;78:886–97. doi: 10.1111/bcp.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wilkie FL, Halter JB, Prinz PN, Benedetti C, Eisdorfer C, Atwood B, et al. Age-related changes in venous catecholamines basally and during epinephrine infusion in man. J Gerontol. 1985;40:133–40. doi: 10.1093/geronj/40.2.133. [DOI] [PubMed] [Google Scholar]
- 207.Marshall JC, Marshall JC, Reinhart K, Pierrakos C, Vincent JL, Group BDW, et al. Biomarkers in critical illness: good answers, but what is the question? J Crit Care Elsevier. 2012;27:519–21. doi: 10.1016/j.jcrc.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 208.Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet (London, England) Elsevier. 2012;379:2252–61. doi: 10.1016/S0140-6736(12)60480-2. [DOI] [PubMed] [Google Scholar]
- 209.Gülcher SS, Bruins NA, Kingma WP, Boerma EC. Elevated C-reactive protein levels at ICU discharge as a predictor of ICU outcome: a retrospective cohort study. Ann Intensive Care. 2016;6:5. doi: 10.1186/s13613-016-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Domico M, Allen M. Biomarkers in Pediatric Cardiac Critical Care. Pediatr Crit Care Med. 2016;17:S215–21. doi: 10.1097/PCC.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 211.Kalyanaraman M, DeCampli WM, Campbell AI, Bhalala U, Harmon TG, Sandiford P, et al. Serial blood lactate levels as a predictor of mortality in children after cardiopulmonary bypass surgery. Pediatr Crit Care Med. 2008;9:285–8. doi: 10.1097/PCC.0b013e31816c6f31. [DOI] [PubMed] [Google Scholar]
- 212.Fenton KE, Sable CA, Bell MJ, Patel KM, Berger JT. Increases in serum levels of troponin I are associated with cardiac dysfunction and disease severity in pediatric patients with septic shock. Pediatr Crit Care Med. 2004;5:533–8. doi: 10.1097/01.PCC.0000144711.97646.0C. [DOI] [PubMed] [Google Scholar]
- 213.Bai JPF, Barrett JS, Burckart GJ, Meibohm B, Sachs HC, Yao L. Strategic biomarkers for drug development in treating rare diseases and diseases in neonates and infants. AAPS J Springer. 2013;15:447–54. doi: 10.1208/s12248-013-9452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]