Abstract
OBJECTIVE
To identify the factors underlying the recent increase in maternal mortality ratios (maternal deaths per 100,000 live births) in the United States.
METHODS
We carried out a retrospective study with data on maternal deaths and live births in the United States from 1993 to 2014 obtained from the birth and death files of the Centers for Disease Control and Prevention. Underlying causes of death were examined between 1999 and 2014 using International Classification of Diseases, Tenth Revision (ICD-10) codes. Poisson regression was used to estimate maternal mortality rate ratios (RR) and 95% confidence intervals (CI) after adjusting for the introduction of a separate pregnancy question and the standard pregnancy checkbox on death certificates, and adoption of ICD-10.
RESULTS
Maternal mortality ratios increased from 7.55 in 1993, to 9.88 in 1999 and to 21.5 per 100,000 live births in 2014 (RR 2014 vs 1993 2.84, 95% CI 2.49 to 3.24; RR 2014 vs 1999 2.17, 95% CI 1.93 to 2.45). The increase in maternal deaths from 1999 to 2014 was mainly due to increases in maternal deaths associated with two new ICD-10 codes (O26.8 i.e., primarily renal disease and O99 i.e., other maternal diseases classifiable elsewhere); exclusion of such deaths abolished the increase in mortality (RR 1.09, 95% CI 0.94 to 1.27). Regression adjustment for improvements in surveillance also abolished the temporal increase in maternal mortality ratios (adjusted maternal mortality ratios 7.55 in 1993, 8.00 per 100,000 live births in 2013; adjusted RR 2013 vs 1993 1.06, 95% CI 0.90 to 1.25).
CONCLUSION
Recent increases in maternal mortality ratios in the United States are likely an artifact of improvements in surveillance and highlight past underestimation of maternal death. Complete ascertainment of maternal death in populations remains a challenge even in countries with good systems for civil registration and vital statistics.
Introduction
Recent publications1,2 on global trends in maternal mortality have reported substantial increases in maternal deaths in the United States, with maternal mortality ratios (maternal deaths per 100,000 live births) increasing from 12 per 100,000 live births in 1990 to 28 per 100,000 live births in 2013.2 The maternal mortality ratio in the United States in 2013 was higher than that in Azerbaijan, Iran, Kazakhstan, Libya, Saudi Arabia and Uruguay (among others).2 Although a more recent publication3 reported a lower maternal mortality ratio for the United States in 2015, this estimate was still substantially higher than that reported 25 years ago, and also higher than that reported in 46 other countries. Such reports have led to considerable dismay in the United States and calls for prompt clinical action to reduce maternal deaths.4–9
It is difficult to reconcile the maternal mortality ratios in the United States with the lower estimates of these rates in less industrialized countries. Several explanations have been offered to explain the observed temporal increase in maternal mortality including an increase in chronic diseases among reproductive age women (especially obesity) and increasing rates of cesarean delivery.4–12 However, an alternative narrative, which views the rising rates of maternal mortality in United States as an artifact of improved surveillance, implicates several different changes in maternal death surveillance including a) the use of a separate question regarding pregnancy on death certificates by some states, introduced in different years in the 1990s, b) the inclusion of a standard pregnancy check box on the 2003 version of the death certificate, implemented by the states in different years from 2003 onwards c) the introduction of International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes for underlying causes of death in 1999, and d) increasing use of record linkage by states to better identify maternal deaths.11
We carried out a study to address the uncertainty regarding the cause of the temporal increase in maternal mortality ratios in the United States.
Materials and Methods
We carried out a retrospective cohort study with information on maternal deaths and live births in the United States from 1993 to 2014 obtained from the files of the National Center for Health Statistics and the Wide-ranging Online Data for Epidemiologic Research (WONDER) files of the Centers for Disease Control and Prevention. The mortality data in these files included underlying cause-of-death information from death certificates which were coded using International Classification of Diseases version 9 (ICD-9) for the years up to 1998 and using ICD version 10 (ICD-10) from 1999 onwards.
The World Health Organization defines maternal deaths as those that occur during pregnancy or within 42 days of termination of a pregnancy and which are causally linked to pregnancy or its management.13 Although it is estimated that over 99% of deaths are registered in the United States,14 identification of maternal deaths can pose a challenge as recent or current pregnancy may remain unrecognized. For instance, the underlying cause of death for a woman who suffers acute renal failure due to pregnancy complications and dies 5 weeks after delivery may be listed as acute renal failure (and not identified as a maternal death).
The Pregnancy Mortality Surveillance System in the United States identifies pregnancy-related deaths as deaths causally linked to pregnancy and which occur within one year after pregnancy termination.12,15 The determination regarding causality is made using information from death certificates, live or stillbirth certificates and other reports, with causal attribution based on the temporal connection between pregnancy and death, and knowledge of the pathophysiology of pregnancy complications. However, such judgment can be challenging as the causal linkage must sometimes be made in the absence of adequate information on clinical factors (e.g., obesity), prenatal care status (missing in 48% of maternal deaths), live birth order (missing in 59%) and pregnancy outcome (missing in 10%).12
We identified maternal deaths using two approaches. In the primary, more restrictive approach, we included all deaths to women that occurred within 42 days of termination of pregnancy with an underlying cause of death that was related to pregnancy, childbirth or the puerperium i.e., using ICD-9 codes 630–679 or all ICD-10 O chapter codes except those for late maternal deaths beyond 42 days of termination of pregnancy, viz., O96 (death from any obstetric cause occurring more than 42 days but less than 1 year after delivery ) and O97 (death from sequelae of direct obstetric causes; Appendix 1 available online at http://links.lww.com/xxx provides a list of relevant ICD-10 codes). Under the second, less restrictive approach, we examined all maternal deaths including late maternal deaths (i.e., ICD-9 codes 630–679 or all ICD-10 O codes).
Temporal changes in maternal mortality were estimated by comparing maternal mortality ratios (per 100,000 live births) in 2014 vs 1993. Changes were assessed by age, race and place of death. Analysis of temporal trends by the underlying cause of death was restricted to the years between 1999 and 2014 when the cause of death was coded using ICD-10. Temporal changes were quantified using ratios of maternal mortality ratios (referred to maternal mortality rate ratios; RR), 95% confidence intervals (CI) and P values for a linear trend in proportions. We also examined temporal changes in overall and cause-specific maternal mortality ratios in California, as a declining temporal trend in maternal deaths has been reported in this state.16
Maternal mortality rates were also quantified using the number of women aged 15–44 years as the denominator. Temporal trends in such maternal death rates were contrasted with the rate of death from all causes among women aged 15–44 years. Cause-specific deaths among women 15–44 years were also examined for deaths from circulatory system causes, renal causes and diabetes mellitus and contrasted with rates of maternal death from these same causes.
Maternal deaths were modeled using ecological Poisson regression whereby such deaths in a population in a given year were modeled as a function of population characteristics in that year. Thus state-year (and not an individual woman) was the unit of analysis i.e., each state and the District of Columbia were represented by 22 observations for the years between 1993 and 2014 (n=1,122). The dependent variable for Poisson regression was the number of maternal deaths offset by the number of live births in each state-year. Two analyses were carried out to assess temporal changes in maternal deaths as defined by the more and less restrictive approaches to defining maternal death i.e., with late maternal deaths identified by ICD-10 codes, O96 and O97, excluded and included in the definition of maternal death. Year was modeled as an independent variable using indicator variables. Indicator variables were also used to model improvements in surveillance for maternal death, namely, the separate question regarding pregnancy on the death certificate used by some states,17 ICD-10 coding and the standard pregnancy checkbox introduced on the 2003 version of the death certificate. The standard pregnancy checkbox was adopted by a steadily increasing number of states at different points in the years between 2003 and 2014 (Figure 1A). Crude temporal trends by year were contrasted with trends adjusted for improvements in surveillance for maternal death. Goodness of fit of Poisson regression models was assessed using deviance statistics and Pearson’s Chi-square and variance estimates were corrected for overdispersion through appropriate scaling. Results of the Poisson regression analysis were confirmed using a model with a random intercept. Ethics approval was not sought for the study as it was based on publically available data.
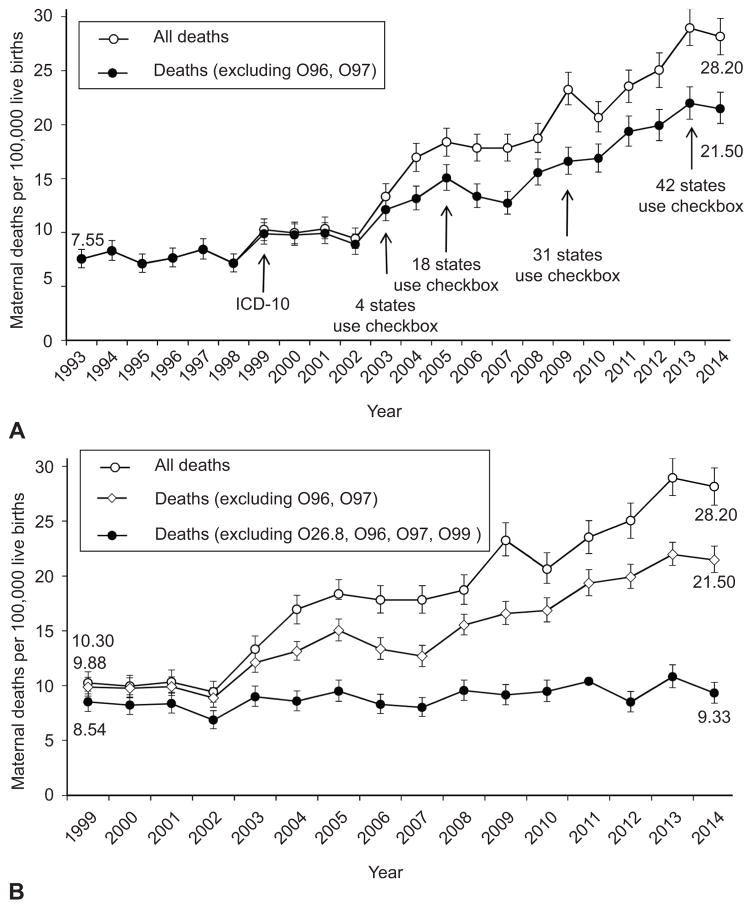
Figure 1.
Maternal mortality ratios and 95% confidence intervals (CI) in the United States, 1993–2014, including and excluding late maternal deaths (identified by International Classification of Diseases, 10th Revision [ICD-10] codes O96 and O97) (A), and maternal mortality ratios and 95% CIs in the United States, 1993–2014, including and excluding late maternal deaths (O96 and O97) and excluding four new ICD-10 codes (O26.8, O96, O97, and O99) (B). The year when the ICD-10 coding system and the standard pregnancy checkbox on death certificates were introduced are shown in A.
Results
There were 4,000,240 live births in the United States in 1993, and 302 maternal deaths (excluding late maternal deaths), yielding a maternal mortality ratio of 7.55 per 100,000 live births. The maternal mortality ratio increased steadily to 21.5 per 100,000 live births in 2014 (RR 2.84, 95% CI 2.49–3.24; Figure 1A). Age-specific maternal mortality ratios among women <25 years, 25–34 years and 35–44 years increased two-fold between 1993 and 2014, while maternal deaths among women ≥45 years increased more substantially (Table 1). The temporal increase in maternal mortality ratios among Whites between 1993 and 2014 was significantly higher than the increase among African Americans. Inpatient hospital deaths decreased, while maternal deaths at home and in hospice increased significantly (Table 1).
Table 1.
Maternal deaths and maternal mortality ratios per 100,000 live births (MMR) by age, race and place of death, United States, 1993 and 2014.
| Determinant | 1993
|
2014
|
Rate ratio 2014 vs 1993 | 95% confidence interval | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Deaths | Live births | MMR/Proportion† | Deaths | Live births | MMR/Proportion† | ||||
| Maternal deaths not including late maternal deaths* | |||||||||
| Age <25 years | 84 | 1,551,774 | 5.41 | 125 | 1,134,414 | 11.0 | 2.04 | 1.54–2.68 | <0.001 |
| 25–34 | 136 | 2,030,013 | 6.70 | 295 | 2,226,450 | 13.2 | 1.98 | 1.61–2.42 | <0.001 |
| 35–44 | 82 | 416,124 | 19.7 | 264 | 618,769 | 42.7 | 2.17 | 1.69–2.77 | <0.001 |
| ≥45 | 0 | 2,329 | 0.00 | 171 | 8,443 | 2025.3 | - | - | 0.001 |
| Race: White | 152 | 3,149,833 | 4.83 | 520 | 3,019,863 | 17.2 | 3.57 | 2.98–4.28 | <0.001 |
| African American | 135 | 658,875 | 20.5 | 291 | 640,562 | 45.4 | 2.21 | 1.81–2.72 | <0.001 |
| Other | 15 | 191,532 | 7.83 | 45 | 327,651 | 13.7 | 1.75 | 0.98–3.15 | 0.06 |
| Place of death: Inpatient | 202 | - | 66.9 | 530 | - | 61.9 | 0.93 | 0.84–1.02 | 0.15 |
| Outpatient/ER | 55 | - | 18.2 | 140 | - | 16.4 | 0.90 | 0.68–1.19 | 0.48 |
| Home | 30 | - | 9.93 | 128 | - | 15.0 | 1.51 | 1.03–2.19 | 0.03 |
| Hospice | 0 | - | 0.00 | 24 | - | 2.8 | - | - | 0.05 |
| Other | 14 | - | 4.64 | 34 | - | 4.0 | 0.86 | 0.47–1.57 | 0.24 |
|
| |||||||||
| Total | 302 | 4,000,240 | 7.55 | 856 | 3,988,076 | 21.5 | 2.84 | 2.49–3.24 | <0.001 |
Late maternal deaths identified by ICD-10 codes O96 and O97 not included among maternal deaths.
Age- and race-specific maternal mortality ratios expressed per 100,000 live births; rates by place of death expressed as a proportion of all maternal deaths.
Maternal mortality ratios (excluding late maternal deaths) increased from 9.88 in 1999 to 21.5 per 100,000 live births in 2014 (RR 2.17, 95% CI 1.93–2.45; Table 2). However, maternal deaths due to complications of labor and delivery (ICD-10 codes O60–O75) declined significantly over the same period (RR 0.43, 95% CI 0.27–0.68; Appendix 2, http://links.lww.com/xxx). There was no significant change in maternal deaths due to abortive outcomes (O00–O07), edema, proteinuria and hypertensive disorders (O10–O16), maternal care related to the fetus and amniotic cavity (O30–O48), and complications predominantly related to the puerperium (O85–O92). However, deaths due to other maternal disorders predominantly related to pregnancy (O20–O29) and deaths due to other obstetric problems not elsewhere classified (O95, O98 and O99) increased substantially between 1999 and 2014 (RR 10.0, 95% CI 6.85–14.7 and 5.88, 95% CI 4.38–7.89, respectively; Appendix 2, http://links.lww.com/xxx).
Table 2.
Overall and cause-specific maternal mortality ratios per 100,000 live births (MMRs), United States 1999 to 2014.
| Year | Number of maternal deaths | MMR per 100,000 live births | Pre- eclampsia (O11, O14) | Eclampsia (O15) | Diabetes mellitus (O24) | Liver disorders (O26.6) | Other sp. pregnancy conditions (O26.8) | APH and PPH (O44–46, O72) | Other maternal diseases (O99) | Circulatory system diseases (O99.4) | MMR excluding (O26.8, O99) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 391 | 9.88 | 1.11 | 0.83 | 0.10 | 0.18 | 0.23 | 0.51 | 1.11 | 0.40 | 8.54 |
| 2000 | 396 | 9.76 | 1.03 | 0.42 | 0.12 | 0.17 | 0.25 | 0.37 | 1.28 | 0.44 | 8.23 |
| 2001 | 399 | 9.91 | 0.99 | 0.55 | 0.05 | 0.02 | 0.40 | 0.52 | 1.14 | 0.79 | 8.37 |
| 2002 | 357 | 8.88 | 0.75 | 0.37 | 0.07 | 0.12 | 0.97 | 0.35 | 1.04 | 0.55 | 6.86 |
| 2003 | 495 | 12.1 | 0.76 | 0.59 | 0.17 | 0.15 | 0.90 | 0.44 | 2.20 | 0.81 | 9.00 |
| 2004 | 540 | 13.1 | 0.49 | 0.61 | 0.22 | 0.22 | 1.51 | 0.58 | 3.04 | 0.85 | 8.58 |
| 2005 | 623 | 15.1 | 0.60 | 0.39 | 0.29 | 0.27 | 1.88 | 0.48 | 3.67 | 1.38 | 9.50 |
| 2006 | 569 | 13.3 | 0.66 | 0.40 | 0.19 | 0.33 | 2.20 | 0.28 | 2.84 | 0.98 | 8.30 |
| 2007 | 548 | 12.7 | 0.53 | 0.53 | 0.16 | 0.39 | 1.74 | 0.32 | 2.94 | 1.25 | 8.02 |
| 2008 | 660 | 15.5 | 0.78 | 0.33 | 0.33 | 0.52 | 2.21 | 0.42 | 3.77 | 1.34 | 9.56 |
| 2009 | 685 | 16.6 | 0.53 | 0.44 | 0.31 | 0.56 | 2.98 | 0.36 | 4.45 | 1.40 | 9.15 |
| 2010 | 674 | 16.9 | 0.63 | 0.35 | 0.45 | 0.55 | 2.90 | 0.55 | 4.48 | 1.50 | 9.48 |
| 2011 | 765 | 19.3 | 0.81 | 0.51 | 0.43 | 0.73 | 3.67 | 0.38 | 5.29 | 1.47 | 10.4 |
| 2012 | 787 | 19.9 | 0.51 | 0.30 | 0.40 | 0.51 | 5.01 | 0.35 | 6.40 | 1.95 | 8.50 |
| 2013 | 864 | 22.0 | 0.46 | 0.43 | 0.66 | 0.81 | 5.06 | 0.51 | 6.10 | 1.73 | 10.8 |
| 2014 | 856 | 21.5 | 0.33 | 0.28 | 0.85 | 0.83 | 5.27 | 0.35 | 6.87 | 1.98 | 9.33 |
|
| |||||||||||
| Rate ratio | - | 2.17 | 0.29 | 0.33 | 8.44 | 4.68 | 23.2 | 0.64 | 6.18 | 4.90 | 1.09 |
| 95% CI | - | 1.93, 2.45 | 0.16, 0.54 | 0.17, 0.65 | 2.99, 23.8 | 2.07, 10.6 | 11.9, 45.1 | 0.39, 1.08 | 4.50, 8.50 | 2.86, 8.36 | 0.94, 1.27 |
| Rate Difference | - | 11.6 | −0.78 | −0.55 | 0.75 | 0.65 | 5.04 | −0.16 | 5.76 | 1.58 | 0.79 |
| 95% CI | - | 9.19, 14.3 | −0.93, −0.51 | −0.69, −0.29 | 0.20, 2.28 | 0.19, 1.73 | 2.51, 10.1 | −0.31, 0.04 | 3.89, 8.33 | 0.74, 2.94 | −0.51, 2.30 |
| P value* | - | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.09 | <0.001 | <0.001 | 0.24 |
Overall maternal mortality ratios do not include late maternal deaths.
Rate ratios and rate differences express change in maternal mortality ratios between 1993 and 2014.
CI denotes confidence interval, APH antepartum hemorrhage and PPH refers to postpartum hemorrhage. Other sp. pregnancy conditions refers to other specified pregnancy related conditions including pregnancy related renal disease, exhaustion, fatigue and peripheral neuritis (O26.8).
P-value based on a Chi-square test for linear trend in proportions.
Detailed cause-specific maternal mortality ratios showed that maternal deaths from preeclampsia and eclampsia decreased 3-fold (Table 2). Death rates from antepartum and postpartum hemorrhage decreased by 36%, although this decline was not statistically significant. Maternal deaths from diabetes mellitus (O24; RR 8.44, 95% CI 2.99–23.8), liver disorders (O26.6; RR 4.68, 95% CI 2.07–10.6), other specified pregnancy-related conditions (O26.8 i.e., primarily renal disease; RR 23.2, 95% CI 11.9–45.1), other maternal diseases classifiable elsewhere but complicating pregnancy, childbirth and the puerperium (O99; RR 6.18, 95% CI 4.50–8.50) and diseases of the circulatory system (O99.4; RR 4.90, 95% CI 2.86–8.36) increased several-fold (Table 2).
The rate difference (RD) in overall maternal mortality ratios (excluding late maternal deaths) in 2014 vs 1999 was 11.6 per 100,000 live births (Table 2). This change was primarily due to increases in other specified pregnancy-related conditions (O26.8; RD 5.04 per 100,000 live births, Table 2) and other maternal diseases classifiable elsewhere but complicating pregnancy, childbirth and the puerperium (O99; RD 5.76). The exclusion of these two conditions eliminated the temporal increase in maternal mortality ratios between 1999 and 2014 (RD 0.79 per 100,000 live births, 95% CI -0.51 to 2.30; Table 2 and Figure 1B).
Maternal mortality ratios including late maternal deaths increased from 7.55 in 1993 to 28.2 per 100,000 live births in 2014 (RR 3.73, 95% CI 3.28–4.24, Figure 1A and Appendix 3 available online at http://links.lww.com/xxx]). Late maternal deaths i.e., obstetric deaths >42 days and <1 year after delivery (O96) and deaths from sequelae of obstetric causes (O97) increased from 0.38 in 1999 to 6.69 per 100,000 live births in 2014 (RR 17.7, 95% CI 10.5–29.7, Appendix 2, http://links.lww.com/xxx). Exclusion of the codes O26.8 (other specified pregnancy-related conditions), O99 (other maternal diseases classifiable elsewhere but complicating pregnancy, childbirth and the puerperium) and late maternal deaths (O96 and O97) abolished the temporal increase in these maternal mortality ratios (Figure 1B).
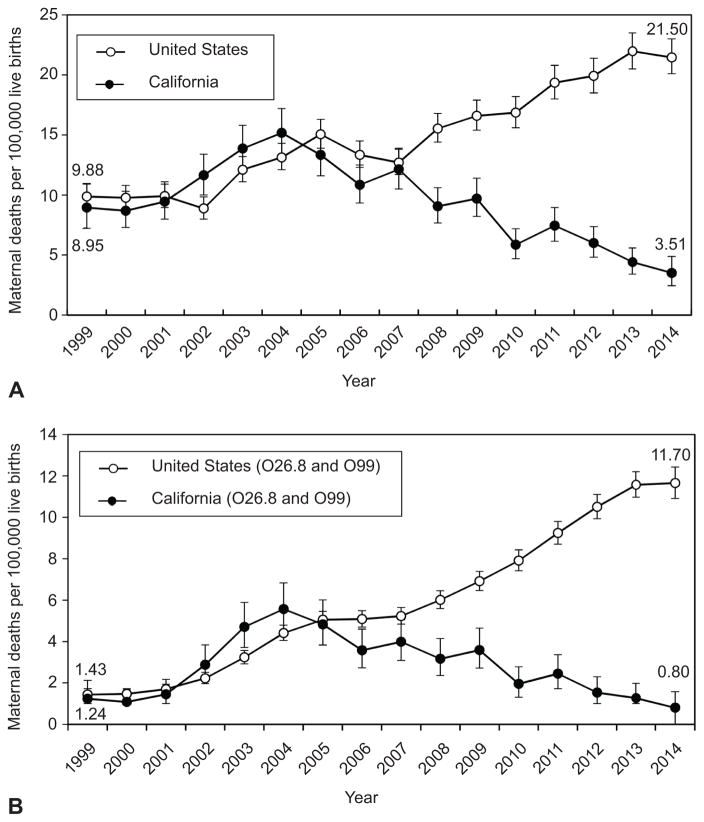
Maternal mortality ratios in California did not follow the steadily increasing pattern of maternal deaths in the United States; rates increased from 1999 to 2004 before declining until 2014 (Figure 2A). Nevertheless, the maternal mortality pattern in California was heavily influenced by changes in the frequency of maternal deaths identified by the four new ICD-10 codes. Rates of maternal death from other specified pregnancy-related conditions (O26.8) and other maternal diseases classifiable elsewhere (O99) increased from 1.08 in 1999–2001 to 5.57 per 100,000 live births in 2003–05, before declining to 1.27 per 100,000 live births in 2012–14 (Figure 2B). Rates of obstetric death >42 days and <1 year after delivery and death from sequelae of obstetric causes (O96 and O97) followed a different pattern; rates increased from <1 per 100,000 live births in 1999, peaked in 2009 at 18.8 before declining to 15.3 per 100,000 live births in 2014.
Figure 2.
Maternal mortality ratios in the United States and California, 1999–2014 (A) and maternal mortality ratios in the United States and California restricted to maternal deaths due to other specified pregnancy-related conditions (O26.8) and other maternal diseases classifiable elsewhere but complicating pregnancy, childbirth, and the puerperium (O99) (B). All rates shown, except the maternal mortality ratios in the United States, are 3-year moving averages.
All cause-mortality among women aged 15–44 years decreased steadily from 864.9 deaths per million in 1999 to 780.6 deaths per million women aged 15–44 years in 2014 (Appendix 4 [Figure 1A], http://links.lww.com/xxx). This decline was in contrast to rates of maternal death among women aged 15–44 years, which increased from 6.28 per million in 1999 to 10.8 per million among women aged 15–44 years in 2014. Among women 15–44 years, rates of death from circulatory system causes, renal causes and diabetes mellitus decreased between 1999 and 2014, while maternal death rates from these same causes increased (Appendix 4 [Figure 1B to 1D], http://links.lww.com/xxx).
The results of ecological Poisson regression analyses are presented in Table 3. The temporal increase in crude rates of maternal death (excluding late maternal deaths) was abolished by adjustment for improvements in surveillance. The adjusted model showed that the separate question on death certificates, ICD-10 coding and the standard pregnancy checkbox were associated with higher rates of maternal death. Compared with the maternal mortality ratio in 1993, adjusted maternal mortality ratios were stable between 1994 and 1998, significantly lower from 1999 to 2004 and 2006 to 2008 and not significantly different from the baseline rate in more recent years (Appendix 5, Figure 2A, http://links.lww.com/xxx). Poisson regression of maternal deaths including late maternal deaths yielded similar results (Appendix 5 [Figure 2B] and Appendix 6, http://links.lww.com/xxx). Random intercept Poisson regression models also yielded the same findings.
Table 3.
Maternal mortality ratios per 100,000 live births (MMRs) and crude and adjusted maternal mortality rate ratios, United States, 1993 to 2014.
| Year | Maternal mortality ratio | Unadjusted
|
Adjusted*
|
||||
|---|---|---|---|---|---|---|---|
| Rate Ratio | 95%confidence interval | P value | Rate ratio | 95% confidence interval | P value | ||
| Separate question | - | 0.71 | 0.65–0.78 | <0.001 | 1.09 | 1.00–1.19 | 0.05 |
| ICD-10 coding | - | 1.92 | 1.74–2.12 | <0.001 | 1.75 | 1.37–2.23 | <0.001 |
| Checkbox | - | 2.12 | 1.99–2.27 | <0.001 | 1.73 | 1.56–1.92 | <0.001 |
| 1993 | 7.55 | 1.00 | Reference | Reference | 1.00 | Reference | Reference |
| 1994 | 8.28 | 1.10 | 0.83–1.46 | 0.50 | 1.10 | 0.84–1.44 | 0.49 |
| 1995 | 7.10 | 0.94 | 0.70–1.27 | 0.69 | 0.94 | 0.71–1.25 | 0.69 |
| 1996 | 7.63 | 1.01 | 0.76–1.35 | 0.92 | 1.01 | 0.77–1.33 | 0.93 |
| 1997 | 8.43 | 1.12 | 0.84–1.48 | 0.42 | 1.12 | 0.85–1.46 | 0.42 |
| 1998 | 7.13 | 0.95 | 0.71–1.27 | 0.71 | 0.94 | 0.72–1.25 | 0.69 |
| 1999 | 9.88 | 1.31 | 1.00–1.72 | 0.02 | 0.75 | 0.60–0.94 | 0.01 |
| 2000 | 9.76 | 1.30 | 0.99–1.70 | 0.05 | 0.74 | 0.59–0.93 | 0.008 |
| 2001 | 9.91 | 1.32 | 1.01–1.72 | 0.02 | 0.75 | 0.60–0.94 | 0.01 |
| 2002 | 8.88 | 1.18 | 0.90–1.55 | 0.10 | 0.67 | 0.53–0.85 | 0.001 |
| 2003 | 12.1 | 1.61 | 1.24–2.08 | <0.001 | 0.79 | 0.65–0.97 | 0.02 |
| 2004 | 13.1 | 1.75 | 1.35–2.25 | <0.001 | 0.83 | 0.68–1.00 | 0.05 |
| 2005 | 15.1 | 2.00 | 1.56–2.56 | <0.001 | 0.90 | 0.75–1.07 | 0.23 |
| 2006 | 13.3 | 1.77 | 1.38–2.28 | <0.001 | 0.75 | 0.63–0.90 | 0.002 |
| 2007 | 12.7 | 1.69 | 1.31–2.17 | <0.001 | 0.70 | 0.58–0.84 | <0.001 |
| 2008 | 15.5 | 2.06 | 1.62–2.64 | <0.001 | 0.81 | 0.68–0.97 | 0.02 |
| 2009 | 16.6 | 2.20 | 1.73–2.81 | <0.001 | 0.87 | 0.73–1.03 | 0.10 |
| 2010 | 16.9 | 2.24 | 1.75–2.86 | <0.001 | 0.86 | 0.72–1.02 | 0.08 |
| 2011 | 19.3 | 2.57 | 2.02–3.27 | <0.001 | 0.98 | 0.82–1.15 | 0.77 |
| 2012 | 19.9 | 2.65 | 2.08–3.36 | <0.001 | 0.97 | 0.82–1.14 | 0.70 |
| 2013 | 22.0 | 2.92 | 2.31–3.70 | <0.001 | 1.06 | 0.90–1.25 | 0.48 |
| 2014 | 21.5 | 2.82 | 2.25–3.61 | <0.001 | -† | -† | -† |
Maternal mortality ratios do not include late maternal deaths.
Adjusted for a separate pregnancy question on death certificates, International Statistical Classification of Diseases and Related Health Problems (ICD-10) coding, and the standard pregnancy checkbox on death certificates.
Indicator variable for the year 2014 aliased as it was a linear combination of the other variables (collinearity). The regression equation is: Log P(maternal death) = −9.5306+(0.0946*v1994)+( −0.0575*v1995) +(0.0116*v1996)+(0.1105*v1997)+( −0.0566*v1998)+( −0.2895*v1999)+( −0.3015*v2000) +(−0.2875*v2001)+( −0.3976*v2002)+( −0.2340*v2003)+( −0.1911*v2004)+( −0.1108*v2005) +(−0.2859*v2006)+( −0.3548*v2007)+( −0.2083*v2008)+( −0.1430*v2009)+( −0.1527*v2010) +(−0.0250*v2011)+( −0.0329*v2012)+(0.0580*v2013)+(0.0873*Separate question)+(0.5577*ICD10) +(0.5480*Standard checkbox).
Supplementary analyses showed that 91.1% of late maternal deaths (O96, O97) did not have any other cause of death in the multiple causes of death fields. Similarly, 86.9% of deaths due to other maternal diseases classifiable elsewhere (O99) did not mention any other cause of death. Most deaths (79.7%) due to other specified pregnancy-related conditions (O26.8) listed other maternal diseases classifiable elsewhere (O99) under multiple causes of death.
Discussion
Our study suggests that the reported substantial increase in maternal mortality in the United States between 1993 and 2014 was likely a consequence of improvements in maternal death surveillance and changes in the coding of maternal deaths. Regression adjustment for the separate pregnancy question on death certificates, ICD-10 codes and the standard pregnancy checkbox on death certificates eliminated the increase in maternal mortality rates between 1993 and 2014. Exclusion of maternal deaths associated with the four new ICD-10 codes that identified late maternal deaths (O96, O97), other specified pregnancy-related conditions (O26.8) and other maternal diseases classifiable elsewhere (O99) also abolished the temporal increase in maternal mortality between 1999 and 2014. Temporal patterns of maternal mortality in California, including both the increase from 1999 to 2003–05 and the subsequent decline to 2014, were also substantially influenced by the same new ICD-10 codes. Other findings which suggest that the temporal increase in maternal mortality was due to changes in maternal death surveillance include large increases in the relatively more difficult to identify maternal deaths such as those to women ≥45 years, and those occurring at home or in hospice, and steady declines in all-cause and cause-specific mortality among all women aged 15–44 years.
Maternal deaths due to diseases of the circulatory system, diabetes mellitus, and liver disorders increased significantly. However, the absolute increase in deaths from these conditions was small (RD 2014 vs 1999: 1.58, 0.75 and 0.65 per 100,000 live births, respectively). Reductions in maternal deaths of a similar magnitude occurred among deaths due to complications of labor and delivery (RD 2014 vs 1999 –0.87 per 100,000 live births), preclampsia (RD −0.78), and eclampsia (RD −0.55). Equally encouraging was the nonsignificant decline in deaths due to antepartum and postpartum hemorrhage, despite significant increases in postpartum hemorrhage in the United States.18–20 The abovementioned increases and decreases in specific causes of maternal death contributed little to the large increase in overall maternal mortality ratios between 1999 and 2014 (RD 11.6 per 100,000 live births).
Reports of temporal increases in maternal mortality rates in the United States have led to shock and soul searching by clinicians.4,8 In fact, maternal deaths from conditions historically associated with high case fatality rates including preeclampsia, eclampsia, complications of labor and delivery, antepartum and postpartum hemorrhage and abortion either declined substantially or remained stable between 1999 and 2014. Nevertheless, maternal mortality and especially severe maternal morbidity from such causes remain issues of serious concern and the clinical audit of such cases needs serious consideration.21
The new ICD-10 codes for obstetric deaths >42 days and <1 year (O96) and deaths due to sequelae of obstetric causes (O97) were introduced in order to capture late maternal deaths from causes not identified under the ICD-9 system. Although some of these deaths represent cases where death was delayed beyond the puerperal period because of intensive care and other interventions, a substantial fraction of such deaths likely represent deaths never previously captured by ICD-9 codes. Increases in maternal deaths identified by the new ICD-10 codes for other specific pregnancy-related conditions (O26.8) and other maternal diseases classifiable elsewhere but complicating pregnancy, childbirth and the puerperium (O99) also represent deaths previously coded using non-pregnancy chapter codes in ICD-9. Although some of the increase in such deaths may reflect an increase in chronic disease in women of reproductive age, the decline in all cause and cause-specific mortality rates among women aged 15–44 years, and the decline in maternal deaths from these two causes in California from 2003–05 to 2014 suggests that coding issues are a more likely explanation for the large increase in maternal deaths from these two causes. The contrasting pattern in all maternal deaths and in O26.8 and O99 deaths in the United States versus California is particularly intriguing. The sharp increase in such deaths in California between 1999 and 2004 and the subsequent equally rapid decline appear to be artefacts of coding and reporting rather than a brief epidemic of renal, circulatory and other chronic disease deaths among pregnant women followed by a rapid decline in such chronic disease deaths.
The steady temporal declines in all-cause and cause-specific mortality rates among women aged 15–44 years contrast sharply with the rising rates of maternal death among women aged 15–44 years. Improvements in medical care have led to reductions in deaths among women of reproductive age, while these medical advances and others, such as assisted reproduction, have increased the fecundity of women with chronic disease. The combination of chronic disease and pregnancy may have resulted in an increase in severe maternal morbidity22 and death, although such a phenomenon cannot explain the two- to three-fold increase in maternal mortality ratios between 1993 and 2014. The large 23-fold increase in deaths due to other specified pregnancy-related conditions (O26.8; primarily deaths due to renal causes) between 1999 and 2014 illustrates this point. Hospital-based studies from the United States which exclusively used ICD-9 codes show that between 1999–2001 and 2010–11 obstetric acute renal failure associated with dialysis and obstetric acute renal failure associated with death only increased by 31% and 71%, respectively.23
Some insight into maternal mortality in the United States can be obtained through international comparisons. Vital statistics-based maternal mortality ratios in Canada doubled following the adoption of ICD-10 coding;24 and cause-specific maternal mortality ratios in the United States compare favorably with those in Canada for deaths from hypertension, hemorrhage and circulatory system diseases.25 Maternal mortality rates in the United Kingdom in 2011–13 were also mostly comparable with those in the United States for preeclampsia and eclampsia, hemorrhage, cardiac diseases, direct and indirect maternal deaths and late maternal deaths.26 Of particular note, is the overall rate of maternal death (including direct, indirect and late maternal deaths) which was 23.1 per 100,000 maternities in the United Kingdom and 25.8 per 100,000 live births in the United States in 2011–13 despite vastly different schemes of case ascertainment.
The strengths of our study include a congruence of findings from different analyses including those based on underlying cause of death and ecological regression, and also other supportive findings such as those related to age, race, place of death, all-cause mortality among women 15–44 years, and multiple causes of death. The limitations of our study include a lack of clinical detail regarding maternal deaths in our data sources, although reports that document the rising trend in maternal death in the United States are also based on these same sources. The timing of the introduction of the standard pregnancy checkbox in the regression modeling was approximate as some states introduced this change mid-year whereas our analyses by year assumed use of the checkbox for the full year. We were also unable to model increasing use of record linkage for ascertaining maternal deaths.
In summary, our study shows that increases in maternal mortality ratios in the United States between 1993 and 2014 coincided with improvements in maternal death surveillance including better identification of pregnancy status on death certificates and use of new ICD-10 codes for determining the underlying cause of death. Although there may have been some increase in maternal deaths due to chronic diseases (such as diseases of the circulatory system, diabetes and liver disease), and definite reductions in maternal death due to obstetric causes (such as preeclampsia, eclampsia and complications of labor and delivery), the overall picture is not consistent with any serious deterioration in maternal health or maternal health services in the United States.
Supplementary Material
Acknowledgments
Giulia M. Muraca is the recipient of a Vanier Canada Graduate Scholarship and is also supported by a Canadian Institutes of Health Research (CIHR) grant on severe maternal morbidity (MAH- 15445). KSJ is supported by the British Columbia Children’s Hospital Research Institute and holds a CIHR Chair in maternal, fetal, and infant health services research (APR-126338).
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva: World Health Organization; 2014. Trends in maternal mortality: 1990 to 2013. Available from: http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2013/en/ [Google Scholar]
- 3.WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva: World Health Organization; 2015. Trends in maternal mortality: 1990 to 2015. Available from: http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en/ [Google Scholar]
- 4.Main EK, Menard MK. Maternal mortality: Time for national action. Obstet Gynecol. 2013;122:735–6. doi: 10.1097/AOG.0b013e3182a7dc8c. [DOI] [PubMed] [Google Scholar]
- 5.Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt) 2014;23:3–9. doi: 10.1089/jwh.2013.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neggers YH. Trends in maternal mortality in the United States. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.04.001. pii: S0890-6238(16)30051-X. [DOI] [PubMed] [Google Scholar]
- 7.ACOG Statement on Maternal Mortality. The American College of Obstetricians and Gynecologists. 2015 May 4; Available from: http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Maternal-Mortality.
- 8.Chescheir N. Enough already. Obstet Gynecol. 2015;125:1–4. doi: 10.1097/AOG.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 9.Maternal mortality: Exceptionally deadly. The Economist. Philadelphia: Jul 18, 2015. Available from: http://www.economist.com/news/united-states/21657819-death-childbirth-unusually-common-america-exceptionally-deadly. [Google Scholar]
- 10.Agarwal P. Maternal mortality and morbidity in the United States of America. Bull World Health Organ. 2015;93:135. doi: 10.2471/BLT.14.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maron DF. Has Maternal Mortality Really Doubled in the U.S.? Scientific American. 2015 Jun 8; Available from: http://www.scientificamerican.com/article/has-maternal-mortality-really-doubled-in-the-u-s/
- 12.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 13.International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Vol. 2. World Health Organization; Geneva: 1992. [PubMed] [Google Scholar]
- 14.Mortality data from the National Vital Statistics System. Centers for Disease Control (CDC) MMWR Morb Mortal Wkly Rep. 1989;38:118–23. [PubMed] [Google Scholar]
- 15.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 16.Maternal mortality: California and the United States. California Maternal Quality Care Collaborative. Stanford CA: 2016. (available at https://www.cmqcc.org/focus-areas/maternal-mortality/california-and-us) [Google Scholar]
- 17.Hoyert DL. Maternal mortality and related concepts. National Center for Health Statistics. Vital Health Stat. 2007;3(33) [PubMed] [Google Scholar]
- 18.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353, e1. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209:449, e1–7. doi: 10.1016/j.ajog.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw D, Guise JM, Shah N, Gemzell-Danielsson K, Joseph KS, Levy B, Wong F, et al. Drivers of maternity care in high-income countries: can health systems support woman- centred care? Lancet. 2016 Sep 14; doi: 10.1016/S0140-6736(16)31527-6. pii: S0140-6736(16)31527-6. [DOI] [PubMed]
- 22.Martin AS, Monsour M, Kissin DM, Jamieson DJ, Callaghan WM, Boulet SL. Trends in severe maternal morbidity after assisted reproductive technology in the United States, 2008–2012. Obstet Gynecol. 2016;127:59–66. doi: 10.1097/AOG.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrabadi A, Dahhou M, Joseph KS, Kramer MS. Investigation of a rise in obstetric acute renal failure in the United States, 1999–2011. Obstet Gynecol. 2016;127:899–906. doi: 10.1097/AOG.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 24.Lisonkova S, Bartholomew S, Liu S, Liston RM, Joseph KS for the Maternal Health Study Group of the Canadian Perinatal Surveillance System. Temporal trends in maternal mortality in Canada based on Vital Statistics data. J Obstet Gynaecol Can. 2011;33:1011–19. doi: 10.1016/S1701-2163(16)35050-2. [DOI] [PubMed] [Google Scholar]
- 25.Public Health Agency of Canada. Perinatal Health Indicators for Canada 2013: a report of the Canadian Perinatal Surveillance System. Ottawa: 2013. [Google Scholar]
- 26.Knight M, Tuffnell D, Kenyon S, Shakespeare J, Gray R, Kurinczuk JJ, editors. on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care. Surveillance of maternal deaths in the UK 2011–13 and lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–13. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.