Abstract
It is generally believed that proopiomelanocortin (POMC) is expressed exclusively by neurons in the adult rodent brain. Unbeknownst to most researchers, however, Pomc in situ hybridization studies in the rat show specific labeling in the ventral wall of the hypothalamic third ventricle, which is formed by specialized ependymal cells, called tanycytes. Here we characterized this non-neuronal POMC expression in detail using in situ hybridization and immunohistochemical techniques, and report two unique characteristics. First, POMC mRNA and precursor protein expression in non-neuronal cells varies to a great degree as to the extent and abundance of expression. In brains with low-level expression, POMC mRNA and protein was largely confined to a population of tanycytes within the infundibular stalk /caudal median eminence, named here as gamma tanycytes, and a subset of closely located β and α2 tanycytes. In brains with high-level expression, POMC mRNA and protein was observed in the vast majority of α2, β and γ tanycytes. This variability was observed in both adult males and females; of 41 rats between 8 to 15 weeks of age, 17 had low-, 9 intermediate-, and 15 high-level POMC expression in tanycytes. Second, unlike other known POMC-expressing cells, tanycytes rarely contained detectable levels of adrenocorticotropin or α-melanocyte-stimulating hormone. The results indicate either a dynamic spatio-temporal pattern where low and high POMC syntheses in tanycytes occur periodically in each brain, or marked interindividual differences that may persist throughout adulthood. Future studies are required to examine these possibilities and elucidate the physiologic importance of POMC in tanycytes.
Keywords: POMC, median eminence, infundibulum, beta-endorphin, RRID:AB_2307442, RRID:AB_2314007, RRID:AB_91683, RRID:AB_2572293, RRID:AB_221448, RRID:AB_2216104
Introduction
The proopiomelanocortin (Pomc) gene gives rise to peptides that are fundamental regulators of the hormonal system, energy balance and metabolism, either as a pituitary hormone such as adrenocorticotropic hormone (ACTH), or as peptide neurotransmitters in the brain, such as α-melanocyte-stimulating hormone (α-MSH) and β-endorphin (Cawley et al., 2016). Based on studies in the two most widely used laboratory rodent species, mice and rats, it is generally accepted that Pomc is expressed exclusively by neurons in the adult brain (Mercer et al., 2013). Specifically, Pomc expression was described in only two neuronal populations, which are located in the hypothalamic arcuate nucleus and the nucleus of the solitary tract (Mercer et al., 2013). It is surprisingly little known, however, that in situ hybridization (ISH) studies in rats indicate the presence of genuine Pomc mRNA in the ventral lining of the hypothalamic third ventricle (Baubet et al., 1994; Larsen and Mau, 1994; Willesen et al., 1999; Lu et al., 2002; Ross et al., 2009; Sergeyev et al., 2011; Ross et al., 2015), corresponding to the location of specialized ependymal cells, called tanycytes (Rodriguez et al., 2005). This hybridization signal is visible in photographs of many Pomc hybridization studies (Brady et al., 1990; Baubet et al., 1994; Larsen and Mau, 1994; Baker and Herkenham, 1995; Magoul and Tramu, 1997; Ahima et al., 1999; Baskin et al., 1999; Willesen et al., 1999; Bouret et al., 2001; Lu et al., 2002; Reyes and Sawchenko, 2002; Choi et al., 2006; Ross et al., 2009; Resch et al., 2011; Sergeyev et al., 2011; Ross et al., 2015), but specifically mentioned only in a handful of papers (Baubet et al., 1994; Larsen and Mau, 1994; Willesen et al., 1999; Lu et al., 2002; Ross et al., 2009; Sergeyev et al., 2011; Ross et al., 2015). In addition, ISH studies have shown Pomc hybridization signal in numerous cells in the median eminence (Magoul and Tramu, 1997; Lu et al., 2002; Reyes and Sawchenko, 2002; Choi et al., 2006) and pituitary stalk (Baubet et al., 1994; Lu et al., 2002), but many more than what could be attributed to the occasional Pomc neurons normally observed in these locations.
Pomc mRNA expression by non-neuronal cells of the rat hypothalamus has remained unknown to most researchers of the field, and to our best knowledge, has been specifically addressed in only three reports (Ross et al., 2009; Sergeyev et al., 2011; Ross et al., 2015). Ross et al. (2009), who extensively proved the specificity of non-neuronal Pomc hybridization signal, observed that Pomc mRNA in the ependymal layer is regulated by photoperiod in Fischer 344 rats, with higher levels under long photoperiod vs short photoperiod (Ross et al., 2009; Ross et al., 2015). In addition, Sergeyev et al. (2011) reported that Pomc mRNA decreases in β tanycytes in response to administration of the inflammatory agent, bacterial lipopolysaccharide (LPS).
In the present study we characterized non-neuronal POMC expression in the rat hypothalamus and pituitary stalk in detail, and report two unique characteristics. The first is that the extent of non-neuronal POMC expression varies to a great degree in adult rats housed under standard conditions; the second, that tanycytes express the POMC precursor, but rarely contain detectable levels of ACTH or α-MSH.
Materials and Methods
Animals
Male and female Sprague-Dawley rats (Taconic Farms, Germantown, NY) and male Wistar rats (TOXI-COOP KKT, Hungary) were housed under standard conditions (12h light/dark cycle, temperature between 20 to 24 °C, rodent chow, and water ad libitum). Two or three rats were housed in a cage. In total, 58 Sprague-Dawley rats were used for ISH studies, 15 Sprague-Dawley rats for immunofluorescence studies, 3 Wistar rats for the electron microscopic studies and 5 Wistar rats for the tanycyte RNA-Seq analysis. Body weights/ages in each study are presented below or in Table 1. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Tufts Medical Center and the Animal Welfare Committee at the Institute of Experimental Medicine of the Hungarian Academy of Sciences.
Table 1.
Number of rats with low-, intermediate- and high-level POMC mRNA or protein expression in tanycytes in the different experiments. In each experiment, rats were euthanized within 2h of the mid-day period.
| Number of rats euthanized together | Sex | Weight (g) | Age | POMC mRNA or protein levels | ||
|---|---|---|---|---|---|---|
| Low | Intermediate | High | ||||
| Adult rats for Pomc ISH | ||||||
| 4 | M | 240-260 | ∼8 weeks | 3 | 1 | 0 |
| 6 | M | 257-284 | ∼8-9 weeks | 4 | 0 | 2 |
| 6 | F | 224-245 | ∼9-10 weeks | 1 | 2 | 3 |
| 4 | M | 286-293 | ∼9-10 weeks | 0 | 0 | 4 |
| 6 | M | 413-436 | 15 weeks | 2 | 2 | 2 |
| Adult rats for POMC immunofluorescence | ||||||
| 4 | M | 290-320 | ∼10 weeks | 1 | 1 | 2 |
| 4 | M | 290-320 | ∼10 weeks | 2 | 1 | 1 |
| 7 | F | 238-258 | 11 weeks | 5 | 1 | 1 |
| Total: 41 | 17 | 9 | 15 | |||
| Adolescent rats for Pomc ISH | ||||||
| 4 | M | 80-93 | 31 days | 4 | 0 | 0 |
| 4 | F | 67-81 | 31 days | 4 | 0 | 0 |
Tissue preparation for ISH on serial sections
Sprague-Dawley rats were deeply anesthetized with ketamine-xylazine (ketamine: 75 mg/kg; xylazine: 10 mg/kg body weight) and then decapitated. The brains were removed and snap-frozen on powdered dry ice. Then, 18 μm thick coronal sections were cut through the entire extent of the hypothalamic arcuate nucleus using a Leica CM3050 S cryostat (Leica Microsystems, Nussloch GmbH, Germany), thaw-mounted on Superfrost Plus glass slides (Fisher Scientific Co., Pittsburgh, PA), and air-dried. Sections were collected in 1-in-12 series on a total of 24 (2×12) slides per brain. With 6 hypothalamic sections on each slide, a series containing every 12th section fit on 2 slides, one containing the rostral, and another the caudal part of the tanycyte region. The sections were stored at -80°C until processed for ISH.
Radioactive ISH
Radioactive ISH was performed on serial sections of 26 adult and 8 adolescent Sprague-Dawley rats (see Table 1 for sex/age/body weight). The Pomc riboprobe was synthesized in the presence of [35S]-uridine 5′-(alpha-thio) triphosphate (PerkinElmer), and purified with Mini Quick Spin RNA columns (Roche Applied Sciences, Basel, Switzerland). The riboprobe template was mouse cDNA corresponding to bases 532-1007 of mouse Pomc mRNA, GenBank Acc. No. NM_008895.3 (plasmid provided by Dr. Malcolm J. Low). This sequence is 93% homologous with the rat Pomc sequence (504-935 of NM_139326.2). ISH was performed on every 12th coronal section per brain, as previously described for fresh frozen sections (Wittmann et al., 2013), using 50,000 cpm/μl radiolabeled probe concentration. Following stringency washes, sections were dehydrated in ascending series of ethanol, air-dried, and dipped into Kodak NTB autoradiography emulsion (Carestream Health Inc., Rochester, NY). The emulsion coated-slides were placed in light-tight boxes containing desiccant, and stored at 5°C for 8d, when the autoradiograms were developed using Kodak D19 developer (Eastman Kodak Company, Rochester, NY). Slides were immersed into 0.0005% Cresyl Violet acetate (Sigma-Aldrich) for 2 min for fluorescent counterstaining, then dehydrated in ascending series of ethanol followed by xylenes (Sigma-Aldrich), and coverslipped with DPX mountant (Sigma-Aldrich).
Fluorescent ISH combined with immunofluorescence
Fluorescent ISH was performed on serial sections of 16 adult Sprague-Dawley rats. The Pomc riboprobe was labeled with digoxigenin-11-UTP (Roche) by in vitro transcription. ISH was performed on every 12th coronal section, as described previously for fresh frozen sections (Wittmann et al., 2013). Following the hybridization procedure, sections were treated with 0.5% Triton X-100/0.5% H2O2 in PBS (pH 7.4) for 15 min, rinsed in PBS, immersed in maleate buffer (0.1 M maleic acid, 0.15 M NaCl, pH 7.5; 10 min), and in 1% blocking reagent for nucleic acid hybridization (Roche). The sections were incubated overnight in Fab fragments of peroxidase-conjugated sheep anti-digoxigenin antibody (Roche, Cat# 11207733910, RRID:AB_514500; 1:100 in 1% blocking reagent) using CoverWell incubation chambers (Grace Bio-Labs Inc., Bend, OR). After rinses in PBS, the hybridization signal was amplified using the TSA Biotin Tyramide system (Perkin Elmer) for 30 min, followed by Alexa Fluor 488-conjugated Streptavidin (Life Technologies, Grand Island, NY) for 2h, diluted at 1:500 in 1% blocking reagent. Then sections were then incubated in the mixture of a mouse antibody against the neuronal proteins HuC/HuD (Molecular Probes, Cat# A21271, RRID:AB_221448) and chicken anti-vimentin (Millipore, Cat# AB5733, RRID:AB_2216104) (Table 2). The primary antibodies were detected with Alexa Fluor 647-conjugated donkey anti-mouse (Jackson ImmunoResearch Labs, Cat# 715-605-151, RRID:AB_2340863) and Cy3-conjugated donkey anti-chicken IgG (Jackson ImmunoResearch Labs, Cat# 703-165-155, RRID:AB_2340363). Sections were coverslipped with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA).
Table 2. Antibodies used in this study.
| Antibody | Immunogen | Manufacturer, Catalog#, RRID, species, clonality | Concentration |
|---|---|---|---|
| Primary antibodies | |||
| POMC | N-terminal 26 amino acids of porcine POMC: WCLESSQCQDLSTESNLLACIRACKP | Phoenix Pharmaceuticals, H-029-30, RRID:AB_2307442 rabbit polyclonal | 1:20K for immunofluorescence 1:15K for electron microscopy |
| β-endorphin | rat β-endorphin | Phoenix Pharmaceuticals, H-022-33, RRID:AB_2314007 rabbit polyclonal | 1:10K |
| ACTH | rat ACTH | Phoenix Pharmaceuticals, H-001-21, RRID:AB_2572293 rabbit polyclonal | 1:10K |
| α-MSH | α-MSH | Millipore, AB5087, RRID:AB_91683 sheep polyclonal | 1:10K |
| vimentin | recombinant vimentin | Millipore, AB5733, RRID:AB_2216104 chicken polyclonal | 1:4K after ISH; 1:20K for immunofluorescence |
| HuC/HuD | human HuC/D | Life Technologies, A21271, RRID:AB_221448 mouse monoclonal | 2 μg/ml after ISH; 1 μg/ml for immunofluorescence |
| digoxigenin, peroxidase conjugated | digoxigenin | Roche, 11207733910, RRID:AB_514500 sheep | 1:100 |
| Secondary antibodies | |||
| Alexa 647- anti-mouse | mouse IgG | Jackson Immunoresearch, 715-605-151, RRID:AB_2340863 donkey polyclonal | 1:200 |
| Cy3- anti-chicken | chicken IgY | Jackson Immunoresearch, 703-165-155, RRID:AB_2340363 donkey polyclonal | 1:200 |
| Alexa 488- anti-rabbit | rabbit IgG | Jackson Immunoresearch, 711-545-152, RRID:AB_2313584 donkey polyclonal | 1:400 |
| Alexa 647-anti-chicken | chicken IgY | Jackson Immunoresearch, 703-605-155, RRID:AB_2340379 donkey polyclonal | 1:200 |
| Cy3- anti-mouse | mouse IgG | Jackson Immunoresearch, 715-165-151, RRID:AB_2315777 donkey polyclonal | 1:200 |
| Alexa 488- anti-sheep | sheep IgG | Jackson Immunoresearch, 713-545-147, RRID:AB_2340745 donkey polyclonal | 1:200 |
| Cy3- anti-rabbit | rabbit IgG | Jackson Immunoresearch, 711-165-152, RRID:AB_2307443 donkey polyclonal | 1:200 |
| Biotin-anti-rabbit | rabbit IgG | Jackson Immunoresearch, 711-065-152, RRID:AB_2340593 donkey polyclonal | 1:500 |
Time-course LPS experiment
Radioactive Pomc ISH was performed on sections from a time-course LPS experiment described previously (Wittmann et al., 2015b). Briefly, adult male Sprague-Dawley rats weighing 220-260g (between 7-8 weeks old) were injected ip with either LPS from Escherichia coli (serotype O55:B5; Sigma-Aldrich Co; dissolved in saline) at a dose of 2.5 mg/kg body weight, or with an equal volume of saline as controls. Rats were anesthetized with ketamine/xylazine then decapitated at 2, 4, 9, 24 or 48h following the injections. Fresh-frozen 16 μm thick coronal sections from the mid-level of the arcuate nucleus (between Bregma levels -2.9 and -3.1 mm) were collected and stored as described above. Sections from 4 rats in each group were processed for Pomc ISH; saline-injected control rats were euthanized at 9h (n=2) or 24h (n=2) post-injection.
Immunofluorescence
Adult Sprague-Dawley rats (n=15; see Table 1 for sex/age/body weight) were deeply anesthetized with ketamine-xylazine, and then perfused transcardially with 20 ml 0.01 M PBS (pH 7.4) followed by 120 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed and postfixed by immersion in the same fixative for 2 hours. The brains were cryoprotected in 20% sucrose in PBS overnight, and then snap-frozen on dry ice. One-in-six series of 20 μm coronal sections were cut on a cryostat through the rostrocaudal extent of the arcuate nucleus, collected in cryoprotective solution (30% ethylene-glycol; 25% glycerol; 0.05 M PB), and stored at -20°C until used. Sections were treated with the mixture of 0.5% Triton X-100/0.5% H2O2 for 15 min, rinsed in PBS (3×10 min), and placed in antibody diluent (2% normal horse serum, 0.2 % Photo-flo, 0.2% sodium-azide in PBS) for 20 min to decrease non-specific binding of antibodies. The following three immunofluorescent preparations were made from each brain, each on an individual, full series of 1-in-6 coronal sections: triple immunofluorescence for the N-terminal portion of POMC, vimentin and HuC/D; dual immunofluorescence for β-endorphin and α-MSH; and single immunofluorescence for ACTH. The antibodies and the used concentrations are listed in Table 2. For triple immunofluorescence, sections were first incubated in rabbit anti-POMC (Phoenix Pharmaceuticals, Cat# H-029-30, RRID:AB_2307442) for 2d followed by Alexa 488-conjugated donkey anti-rabbit (Jackson ImmunoResearch Labs, Cat# 711-545-152, RRID:AB_2313584) for 2h. Then, sections were incubated in the cocktail of chicken anti-vimentin and mouse anti-HuC/D antibodies overnight and then in Cy3-conjugated donkey anti-mouse (Jackson ImmunoResearch Labs, Cat# 715-165-151, RRID:AB_2315777) and Alexa 647-conjugated donkey anti-chicken IgG (Jackson ImmunoResearch Labs, Cat# 703-605-155, RRID:AB_2340379) for 2h. For dual immunofluorescence, sections were incubated in the cocktail of rabbit anti-β-endorphin (Phoenix Pharmaceuticals, Cat# H-022-33, RRID:AB_2314007) and sheep anti-α-MSH antisera (Millipore, Cat# AB5087, RRID:AB_91683; gift of Jeffrey B. Tatro) overnight followed by Cy3-conjugated donkey anti-rabbit (Jackson ImmunoResearch Labs, Cat# 711-165-152, RRID:AB_2307443) and Alexa 488-conjugated donkey anti-sheep IgG (Jackson ImmunoResearch Labs, Cat# 713-545-147, RRID:AB_2340745) for 2h. For single ACTH immunofluorescence, sections were incubated in rabbit anti-ACTH serum (Phoenix Pharmaceuticals, Cat# H-001-21, RRID:AB_2572293) for 2d and then in Cy3-conjugated donkey anti-rabbit for 2h. Sections were mounted and coverslipped with DAPI-containing Vectashield (Vector).
Imaging
Darkfield and fluorescent images were captured with a Zeiss Axioplan 2 microscope (Carl Zeiss Ltd., Göttingen, Germany) equipped with a RT SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Confocal images were captured with a Leica SP2 confocal microscope. Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA) was used to create and label composite images and to modify brightness and contrast. Brightness and contrast were modified only in fluorescent images to increase the visibility of low-level signals. Darkfield emulsion autoradiography images of ISH were not modified in any way.
Immuno-electron microscopy for POMC
Three, adult, male Wistar rats, weighing 250–275 g, were deeply anesthetized with ketamine/xylazine, and perfused transcardially with 10 ml PBS, followed by 150 ml fixative containing 4% acrolein and 2% paraformaldehyde in 0.1M PB, pH 7.4. The brains were rapidly removed and postfixed overnight in 4% paraformaldehyde in 0.1M PB. Serial 25μm thick coronal sections containing the median eminence were cut on a Leica VT 1000S vibratome. The sections were treated with 0.5% H2O2 in PBS for 15 min, cryoprotected in 15% sucrose in PBS for 15 min at room temperature and in 30% sucrose in PBS overnight at 4°C and then, quickly frozen over liquid nitrogen and thawed three times to improve antibody penetration into the tissue. The sections were incubated in 2% normal horse serum (NHS) in PBS for 10 min before placed in rabbit anti-POMC serum (1:15K) diluted in 2% NHS in PBS for 4 days at 4°C, followed by overnight incubation in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Labs, Cat# 711-065-152, RRID:AB_2340593) and then in avidin-biotin-peroxidase complex (ABC Elite, Vector; 1:1000) for 2h. The POMC-immunoreactivity was detected with 0.05% DAB/0.15%Ni-ammonium-sulfate/0.005% H2O2 in 0.05 M Tris buffer, pH 7.6. Nickel particles were intensified with Gallyas-silver intensification for 2 min. Sections were osmicated, and then treated with 2% uranyl-acetate in 70% ethanol for 30 min. Following dehydration in an ascending series of ethanol and acetonitrile for 2 min, the sections were flat embedded in Durcupan ACM epoxy resin (Fluka) on liquid release agent (Electron Microscopy Sciences)-coated slides, and polymerized at 56°C for 2 days. After polymerization, 60–70 nm thick ultra-sections were cut with Leica Ultracut UCT ultra microtome. The ultrathin sections were mounted onto Formvar-coated single slot grids, contrasted with 2% lead citrate and examined with a Jeol-100 C transmission electron microscope.
Antibody characterization
As described in the Results section, POMC and β-endorphin immunostainings yielded the same cellular patterns, neurons and variable labeling of tanycytes, as the Pomc ISH studies. To further confirm the specificity of these immunostainings, we performed preabsorption control experiments on adjacent sections from brains with high or intermediate POMC levels in tanycytes. Both the neuronal and tanycyte labeling was completely abolished by preincubation of the POMC antiserum with 40 μg/ml of the immunizing peptide (Table 2) (Cat# 029-30, Phoenix Pharmaceuticals), and by preincubation of the β-endorphin antiserum with 25 μg/ml rat β-endorphin (Phoenix) (Fig. 1A, B). As described in the Results section, ACTH and α-MSH immunofluorescence resulted in only occasional and light labeling of tanycytes. Preincubation of the ACTH antiserum with 25 μg/ml rat ACTH (Phoenix) abolished all labeling (Fig. 1C); preincubation of the α-MSH antiserum with 25 μg/ml α-MSH abolished all signal except a neurofilament-like labeling in the internal zone of the median eminence and pituitary stalk (Fig. 1D). This cross-reaction is rather characteristic of α-MSH antibodies and was reported previously (Drager et al., 1983). We confirmed the specificity of the chicken vimentin antibody by dual immunofluorescence with a mouse monoclonal vimentin antibody (Millipore, Cat# MAB3400, RRID:AB_94843), which resulted in a complete colocalization of the two signals. These two vimentin antibodies recognize different epitopes, as only the chicken, but not the mouse antibody recognizes mouse vimentin. The monoclonal mouse HuC/D antibody has been well characterized and yielded the well-known pattern of neuronal cell bodies (Lee et al., 2012; Wittmann et al., 2015a).
Figure 1.
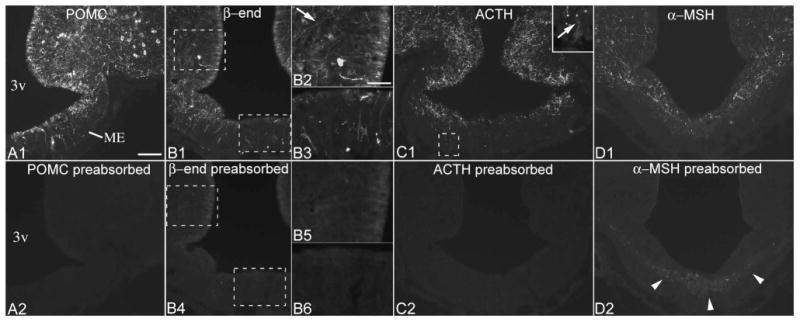
Preabsorption controls for POMC, β-endorphin, ACTH and α-MSH antisera. (A1, A2) Adjacent sections from a brain with high POMC levels in tanycytes demonstrates that preincubation of the POMC antiserum with the immunizing peptide abolishes all POMC immunolabeling in tanycytes and neurons. (B1-B6) Preabsorption control for the β-endorphin antiserum in adjacent sections from a brain with intermediate POMC levels in tanycytes. Specific labeling is present in α2 tanycyte cell bodies and processes (arrow, B2), and in β2 and γ tanycytes (B3). In the section reacted with the preabsorbed antiserum (B4-6), some background labeling remains in the location of α2 tanycyte cell bodies, but this signal is indistinct. (C1, C2) Preabsorption control for the ACTH antiserum; inset shows an ACTH-positive γ tanycyte. (D1, D2) After preincubation of the α-MSH antiserum with excess α-MSH, a neurofilament-like nonspecific labeling remains in the internal median eminence (arrowheads). 3v, third ventricle; ME, median eminence. Scale bar: 100μm; 50μm on B2 (for B2, B3, B5, B6, inset of C1).
RNA-Seq analysis from rat tanycytes
Male, 13 weeks old Wistar rats (n=5) were deeply anesthetized with ketamine-xylazine and transcardially perfused with 70 ml ice cold 10% RNAlater (Sigma) dissolved in 0.1M PBS. The brains were rapidly removed and frozen in isopentane at -40°C. The frozen brains were wrapped in tin foil and stored at -80°C until sectioning. 12 μm thick coronal sections were cut through the rostrocaudal length of the median eminence. The sections were collected on PEN membrane covered slides (Zeiss), rapidly dried on a 42°C hotplate and stored at -80°C. For isolation of tanycytes and the arcuate nucleus, the sections were quickly thawed, fixed in 70% ethanol, stained with cresyl violet, dehydrated in graded solution of ethanol and in xylenes and finally dried in vacuum. The cell bodies of tanycytes or the arcuate nucleus were isolated from the dried sections using PALM MicroBeam Laser microdissection system (Zeiss). The isolated tissues were collected in silicon cap tubes (Zeiss). RNA was isolated using the Arcturus PicoPure RNA isolation kit (Arcturus). The amount and quality of RNA was determined by Agilent 2100 Bioanalyser using RNA 6000 Pico chips. Approximately 0.5-1 ng RNA was isolated from the tanycyte samples of each rat and 5-10 ng RNA from each arcuate nucleus sample. The RIN value of the RNA was between 6.0 and 8.0. The RNA samples were amplified using Ovation RNA amplification system V2 and library was generated for Illumina sequencing. The Illumina next generation sequencing and the bioinformatics analyses were performed by Eurofins. CPM values for each gene were compared between the tanycyte and arcuate nucleus samples with Students't test.
Results
Pomc mRNA expression in non-neuronal cells is highly variable in adult rats
To study Pomc mRNA-expressing cells in the rat hypothalamus and pituitary stalk, we performed both radioactive and fluorescent ISH on serial coronal sections covering the entire rostro-caudal extent of the tanycyte region/arcuate nucleus. Unexpectedly, we found a surprisingly large variability between brains as to the extent and abundance of Pomc mRNA expression in tanycytes and similar non-neuronal cells of the median eminence and pituitary stalk. Therefore, we categorized brains as being of low-, intermediate- or high-level non-neuronal Pomc mRNA (Fig. 2, 3). The high variability in non-neuronal signal was obvious in both the radioactive and fluorescent hybridization preparations, but best demonstrated by radioactive hybridization, particularly when higher radioactivity counts and longer exposure times (8d) were used to increase the sensitivity of detection. In brains with low-level non-neuronal Pomc mRNA, Pomc mRNA was largely confined to a population of cells in the pituitary stalk and a caudal subset of β and the most ventral α tanycytes, located between Bregma levels -3.1 and -3.8 mm (Fig. 3A, 3B, 4A). Hybridization signal was also present in several non-neuronal cells in the caudal median eminence (Fig. 3A, 3B, 4A). Rostral to Bregma level -3.1 mm, only few β tanycytes and non-neuronal median eminence cells contained hybridization signal (Fig. 2A, 2B, 4A). In these brains, the hybridization signal in tanycytes was generally much lower than in Pomc neurons. In brains with high-level non-neuronal Pomc mRNA, the hybridization signal extended rostro-caudally to virtually the entire β tanycyte population (between Bregma -1.7 to -4.0 mm), dorsally to a large portion of α2 tanycytes, and to more non-neuronal cells in the median eminence and pituitary stalk (Fig. 2D, 2E, 3D, 3E, 4B). Pomc mRNA was never observed, however, in the most dorsal tanycyte population, the α1 subtype. In these “high-level” brains, the intensity of tanycyte hybridization signal approximated that of Pomc neurons. In brains with “intermediate-level” non-neuronal Pomc mRNA, the general pattern was similar to “high-level” brains, but the intensity of tanycyte hybridization signal remained well below that of Pomc neurons, particularly in the rostral half of the tanycyte region (Fig. 2C, 3C).
Figure 2.
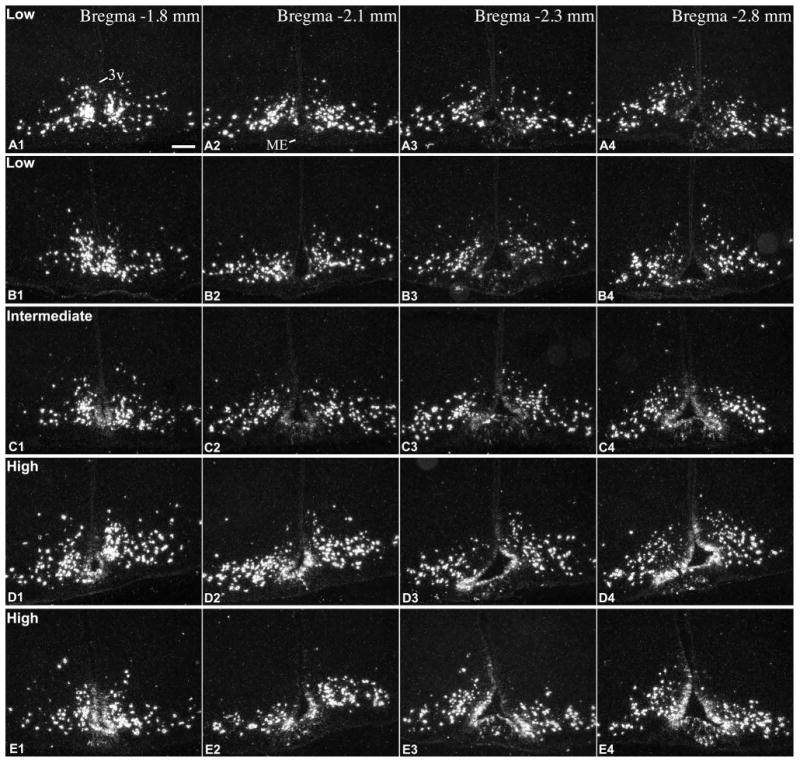
Radioactive ISH demonstrates Pomc mRNA in the rostral part of the tanycyte region in 5 adult rats with different expression levels in tanycytes of the third ventricle (3v) and non-neuronal cells of the median eminence (ME). (A, B) low, (C) intermediate, (D, E) high Pomc mRNA expression in tanycytes. A, B and D are male, C and E are female rats, between 8-10 weeks of age. Images from the caudal part of the tanycyte region from the same brains are presented in Fig. 3. Scale bar: 200μm.
Figure 3.
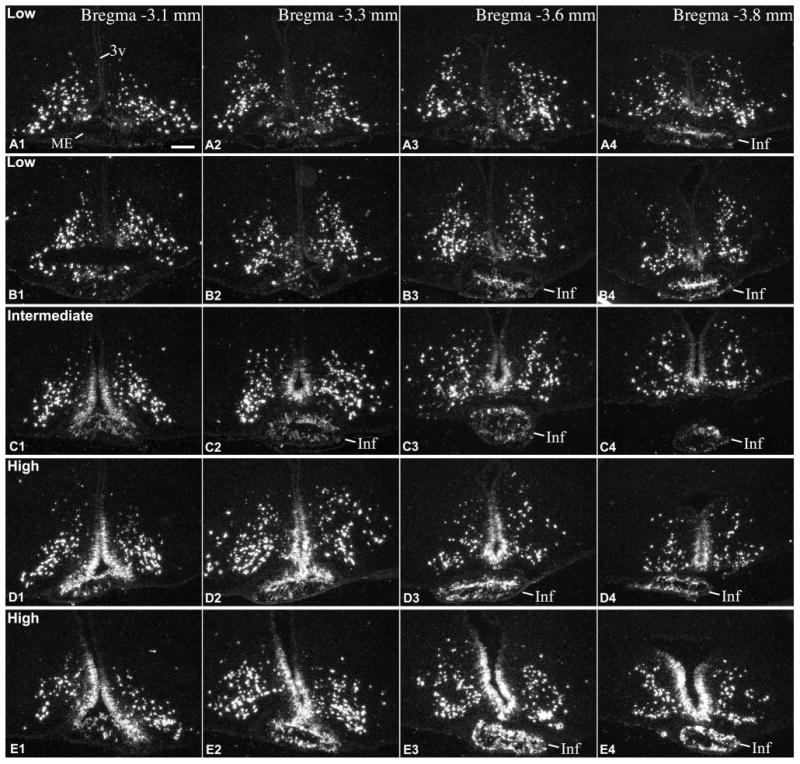
Continuation of Fig.2., radioactive ISH demonstrates Pomc mRNA in the caudal part of the tanycyte region in 5 adult rats with different expression levels in tanycytes of the third ventricle (3v) and non-neuronal cells of the median eminence (ME) and infundibular (pituitary) stalk (Inf). (A, B) low, (C) intermediate, (D, E) high Pomc mRNA expression in tanycytes. A, B and D are male, C and E are female rats, between 8-10 weeks of age. The infundibular stalk appears at different rostro-caudal levels due to differences in the plane of sectioning. Scale bar: 200μm.
Figure 4.
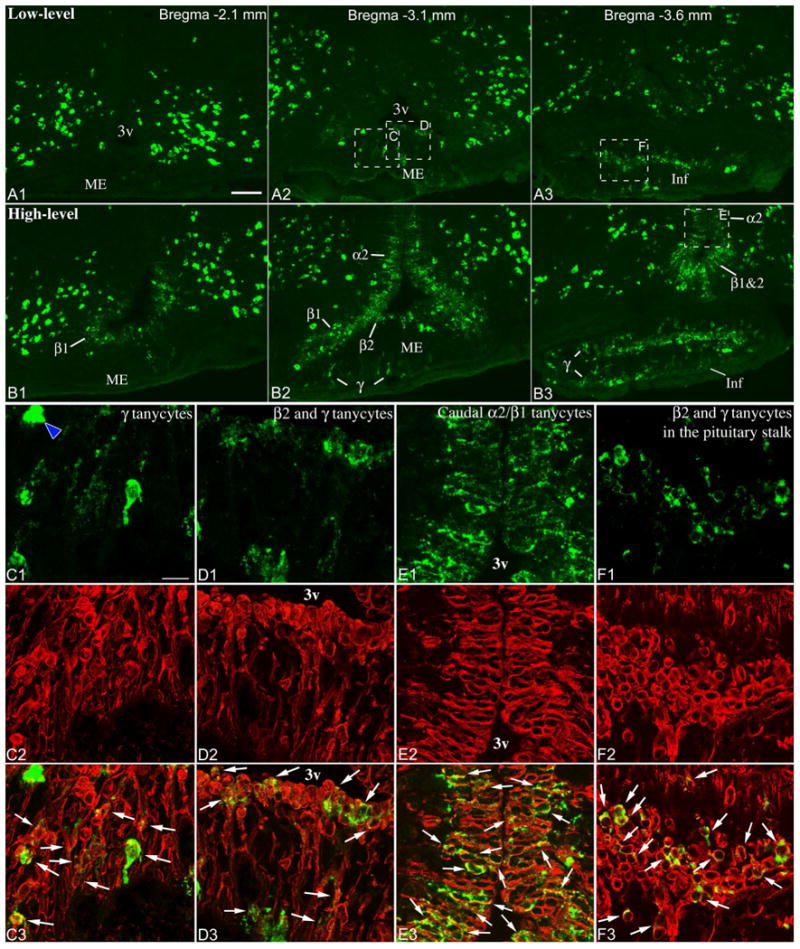
Low- (A1-3) and high-level (B1-3) Pomc mRNA expression in tanycytes, demonstrated by fluorescent ISH. A1-3 is the same brain as in Fig. 2A, 3A; B1-3 is the same brain as in Fig. 2D, 3D. 3v, third ventricle; α2, α2 tanycytes; β1, β1 tanycytes; β2, β2 tanycytes; γ, γ tanycytes; Inf, infundibular stalk; ME, median eminence. Scale bar: 100μm. (C-F) Confocal images of boxed areas from A2, A3 and B3 show combined Pomc ISH (green) and vimentin immunofluorescence (red). Virtually all non-neuronal Pomc mRNA-expressing cells contain the tanycyte marker vimentin (arrows). Blue arrowhead on C1 points to a Pomc neuron in the median eminence. HuC/D immunofluorescence (not shown) allowed unambiguous identification of Pomc neurons. C, D and E are projections of multiple confocal planes; F shows a single confocal plane. Scale bar: 25μm.
This variability was observed in both adult males and females, with similar age and body weight (Table 1), which were euthanized together within 2h of the mid-day period. Among the total 26 rat brains we analyzed with ISH, 10 brains had low, 5 intermediate and 11 high Pomc mRNA levels in tanycytes (Table 1). These 26 rats were euthanized in 5 groups, on different dates. In each group, rats were housed in the same animal room for at least 5 days before euthanasia; rats kept in the same cage could be either in the same or different categories, such as low- vs high-level. Importantly, variability was observed in both young adult rats, between ages 8-10 weeks, and in fully adult, 15 week-old rats (Table 1).
Non-neuronal Pomc mRNA-expressing cells are vimentin-positive tanycytes
To ascertain the identity of non-neuronal cells that express Pomc mRNA and to unambiguously distinguish them from Pomc neurons, we combined fluorescent ISH with immunofluorescence for the tanycyte/ependymal marker, vimentin, and the neuronal marker, HuC/D. In general, non-neuronal Pomc mRNA-expressing cells could be easily distinguished from neurons based solely on different morphology and appearance of the hybridization signal (Fig. 4A, B). In some cases, however, smaller Pomc neurons with less intense signal could be unambiguously identified only by their HuC/D content (data not shown). While few Pomc neurons were regularly found in the median eminence and pituitary stalk, occasionally even within the β-tanycyte layer, we did not observe Pomc mRNA-expressing cells that contained both vimentin and HuC/D (data not shown).
Pomc mRNA-expressing cells in the ventricular wall, corresponding to the location of β1, β2 and α2 tanycytes, always contained vimentin (Fig. 4D, E; for green/magenta image see Supplemental Fig 1.). The vast majority of non-neuronal Pomc mRNA-expressing cells that are located below the β tanycyte layer throughout the external zone of the median eminence also contained vimentin (Fig. 4C, D). These cells were apparently tanycyte-type cells often with an elongated shape perpendicular to the ventricular floor, or with a small, round-shaped cell body with multiple processes (Fig. 4C, D). Pomc mRNA-expressing cells in the pituitary stalk had round or elongated shape with apparent processes and virtually always contained vimentin (Fig. 4A, B, F). Many of these cells were non-ependymal tanycyte-type cells, while others in the rostral part of the stalk were β tanycytes bordering the third ventricular recess.
Since tanycytes are commonly defined as ependymo-glial cells, we searched the literature for previous descriptions to more accurately refer to non-ependymal tanycyte-type cells. We found that early morphological studies described these cells by the name astrocytic tanycytes (Zaborszky and Schiebler, 1978; Bitsch and Schiebler, 1979); however, it was subsequently recognized that they do not represent a transitional form between astrocytes and tanycytes (Rutzel and Schiebler, 1980). More recent studies referred to these cells simply as tanycytes with cell bodies located within the median eminence or infundibular stalk (Hokfelt et al., 1988; Meister et al., 1988; Kawakami, 2000). To easily identify and distinguish these cells from the other tanycyte subtypes, we decided to integrate them into the tanycte nomenclature (Akmayev et al., 1973; Akmayev and Fidelina, 1976) and refer to them as gamma (γ) tanycytes.
LPS-induced inflammation does not alter variable Pomc mRNA expression in tanycytes
The responsiveness of tanycyte gene expression to inflammatory stimuli is well established (Wittmann et al., 2014), and (Sergeyev et al., 2011) reported that Pomc mRNA in tanycytes is downregulated 4h after LPS injection. Thus, we were curious whether LPS administration is able to regulate tanycyte Pomc mRNA levels and abolish its variability. In a time-course experiment, we injected a septic dose of LPS (10-times higher than used in the study by Sergeyev et al.) to young adult rats (∼7-8 weeks old) and assessed Pomc mRNA expression in sections from the mid-level of the tanycyte area. We did not observe any effect of LPS on tanycyte Pomc mRNA. In fact, variability in Pomc mRNA levels was present in all experimental groups: 6 rats belonged to the low (2 control, 2 4h-LPS, 1 24h-LPS, and 1 48h-LPS), 8 to the intermediate (1 control, 2 2h-LPS, 2 9h-LPS, 1 24h-LPS, 2 48h-LPS), and 10 to the high-level category (1 control, 2 2h-LPS, 2 4h-LPS, 2 9h-LPS, 2 24h-LPS, 1 48h-LPS). Particularly remarkable was the variability observed in the 48h-LPS group, despite a substantial weight loss of 20.8 ± 5.8 g compared to their baseline weight (233 ±4.8g; n=4).
Pomc mRNA in tanycytes in adolescent rats
To examine whether variability in non-neuronal Pomc mRNA is present before adulthood, ISH was performed in adolescent, 31 day old male (n=4; BW 80-93g) and female (n=4; BW 67-81g) rats. Hybridization pattern in all of the 8 young rats was highly similar to that observed in “low-level” adult rats. Moderate signal was present in the pituitary stalk, as well as in a portion of β1, β2, α2 and γ tanycytes in mid and caudal levels of the third ventricle and median eminence (Fig. 5). Signal in the rostral half of the tanycyte region was occasional and very light (Fig. 5). Neuronal Pomc hybridization signal was also less intense than in adults, which is in agreement with previous data (Kawagoe et al., 2008).
Figure 5.
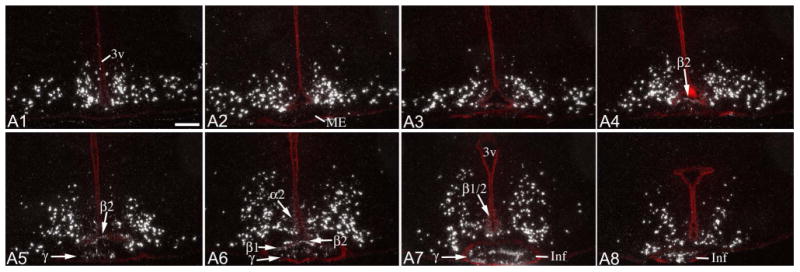
Radioactive ISH demonstrates Pomc mRNA distribution in a 31 day-old, adolescent male rat. Sections are arranged in rostro-caudal order (A1: most rostral; A8: most caudal), with ∼200μm distance between consecutive sections. Fluorescent cresyl-violet staining (red) is overlaid to help identify the location of the third ventricle (3v), median eminence (ME) and infundibular stalk (Inf). Arrows point to non-neuronal signal in α2, β1, β2 and γ tanycytes (γ). Scale bar: 200μm.
Variable POMC protein expression in tanycytes in adult rats
Immunofluorescence using an antibody against the N-terminal portion of POMC yielded the same pattern of cellular expression and variability as Pomc ISH experiments (Fig. 6, 7). In “low-level” brains, POMC was present in tanycytes of the pituitary stalk, and a subset of β and γ tanycytes between Bregma levels -3.1 and -3.8 mm (Fig. 6). Rostral to this level, POMC was present in some β tanycyte processes and γ tanycytes in the median eminence, the number of which varied among “low-level” brains (Fig. 6). In “high-level” brains, POMC was present in the vast majority of β- and α2-tanycytes, and a large number of γ tanycytes, essentially mirroring Pomc mRNA distribution in “high-level” brains (Fig. 7). Of 8 adult male brains, 3 had low, 2 intermediate and 3 high POMC levels in tanycytes; of 7 female brains, 5 had low, 1 intermediate and 1 high POMC levels (Table 1).
Figure 6.
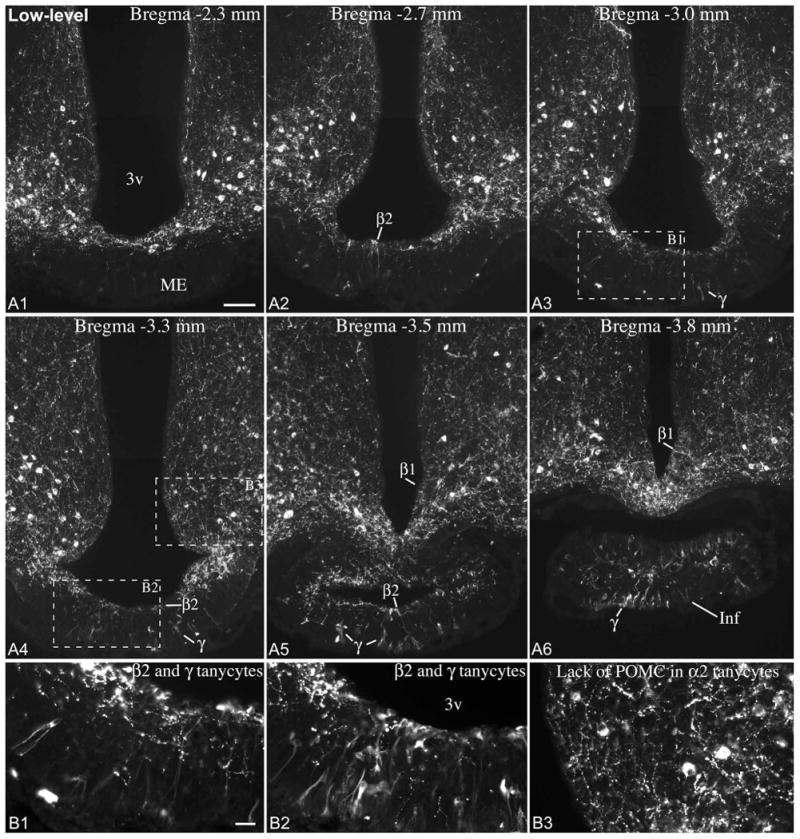
(A) POMC immunofluorescence from an adult male rat with low POMC levels in tanycytes. POMC is expressed in a small subset of β2 and β1 tanycytes in the wall of the third ventricle (3v), as well as in γ tanycytes in the median eminence (ME) and infundibular stalk (Inf). Scale bar: 100μm. (B1, B2) Higher magnification of boxed areas in A3 and A4 shows POMC-expressing β2 tanycytes and γ tanycytes in the median eminence. (B3) Higher magnification of boxed area from A4 shows the lack of POMC in α2 tanycytes. Scale bar: 25μm.
Figure 7.
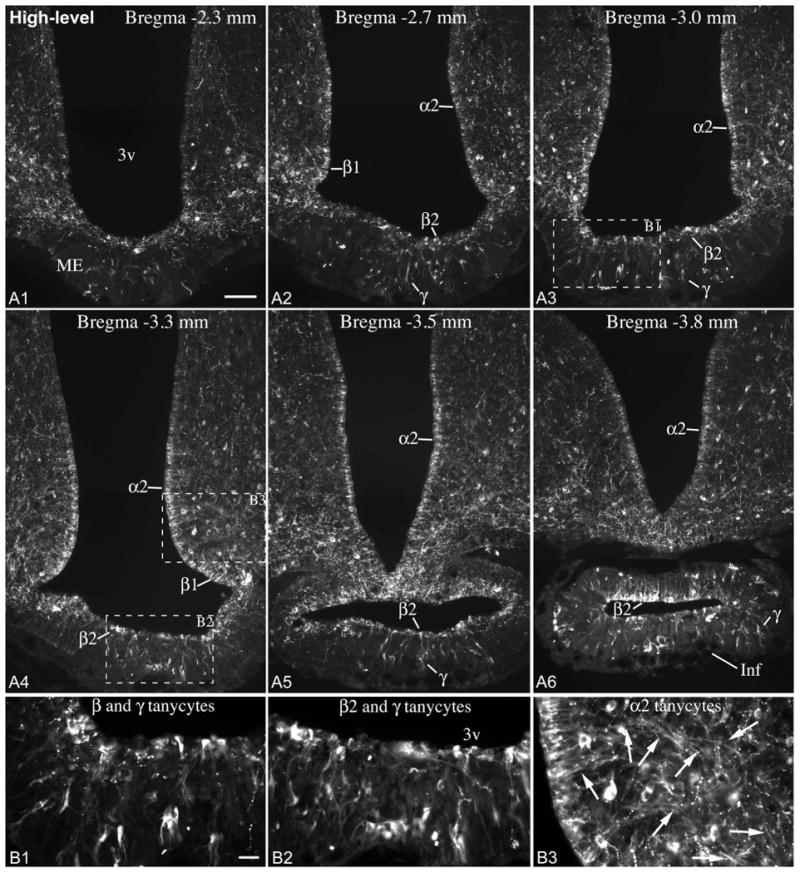
(A) POMC immunofluorescence from an adult male rat with high POMC levels in tanycytes. The majority of α2, β1 and β2 tanycytes lining the third ventricle (3v), and a large number of γ tanycytes in the median eminence (ME) and infundibular stalk (Inf) express POMC. Scale bar: 100μm. (B1-B3) Higher magnification of boxed areas in A3 and A4 show POMC-expressing β, γ and α2 tanycytes. Arrows on B3 point to the POMC-positive processes of α2 tanycytes. Scale bar: 25μm.
The varying shapes and morphological characteristics of POMC-positive γ tanycytes (Fig. 6, 7), clearly delineated by the POMC immunofluorescent signal, were essentially identical to the original descriptions of these cells (Zaborszky and Schiebler, 1978; Bitsch and Schiebler, 1979). Triple immunofluorescence studies confirmed our combined ISH/immunofluorescence findings that POMC-positive γ tanycytes were virtually always vimentin positive and comprised separate population from POMC neurons that were vimentin-negative but HuC/D-positive (Fig. 8F-I). By location, POMC neurons of the median eminence were found exclusively in the internal zone, close to and occasionally within the β-tanycyte-layer (Fig. 8F-I). POMC-positive γ tanycytes, however, extended from the subependymal through the external zone (Fig. 6, 7, 8F-I).
Figure 8.
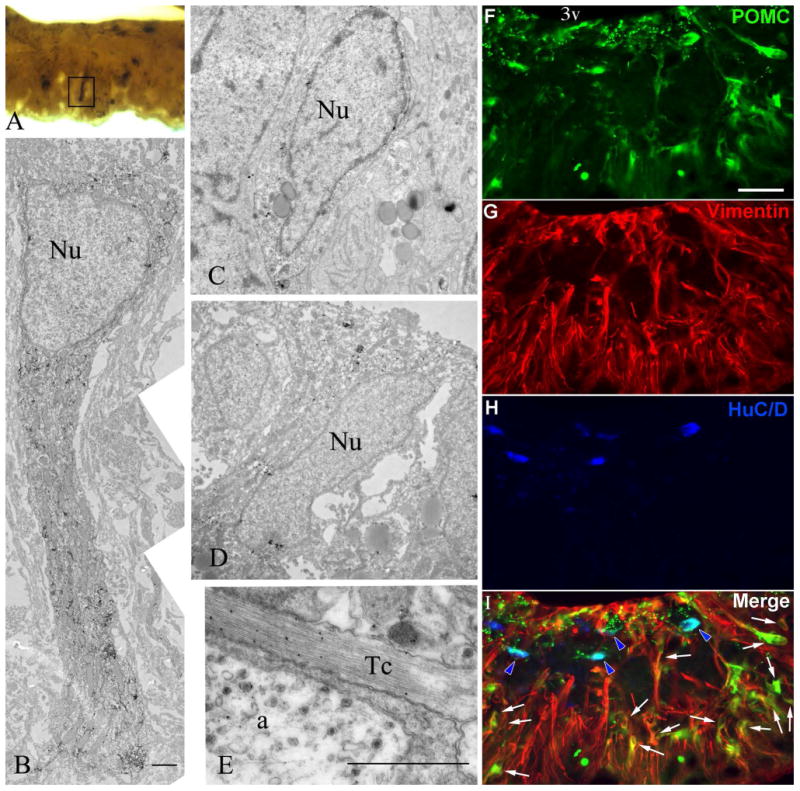
(A-E) Immuno-electron microscopic detection of POMC in tanycytes. The ultrastructure of a POMC-immunoreactive γ tanycyte in the external zone of the median eminence (boxed area of A) is shown in (B). POMC immunoreaction is labeled by silver grains. Note the high concentration of mitochondria in the highly immunolabeled short process of the γ tanycyte. (C) and (D) illustrate cell bodies of POMC-immunoreactive β- tanycytes lining the floor of the third ventricle. (E) Silver grains denoting POMC-immunoreactivity are associated with microfilaments in a tanycyte process. Nu= nucleus; a= axon varicosity; Tc= tanycyte process. Scale bar = 2 μm. (F-I) Triple immunofluorescence from the rostral median eminence of a brain with high-level POMC in tanycytes. POMC (green) is present in both vimentin-positive (red) γ tanycytes (arrows) and HuC/D-positive (blue) neurons (blue arrowheads), but these two cell types form separate populations. 3v, third ventricle. Scale bar: 50μm.
By electron microscopic examination of the median eminence, we detected POMC immunoreactivity in cell bodies and processes of β tanycytes as well as in γ tanycytes (Fig. 8A-E). Gamma tanycytes in the median eminence had ultrastructural characteristics similar to β tanycytes, including numerous elongated mitochondria in their processes, and several large lipid drops (Brawer, 1972; Akmayev and Fidelina, 1976; Zaborszky and Schiebler, 1978; Rodriguez et al., 1979) (Fig. 7A-E). Their processes often terminated on capillaries.
Immunoreactivity of POMC-derived peptides in tanycytes
β-endorphin immunofluorescence resulted in highly similar patterns and variability as the POMC staining, but labeled substantially fewer tanycytes in the same brains (Fig. 9). Namely, in “low-level” brains, non-neuronal β-endorphin staining was confined to some γ tanycytes in the pituitary stalk and median eminence, as well as few β-tanycyte cell bodies and processes (Fig. 9A, B). In “high-level” brains, β-endorphin staining labeled many more β and γ tanycytes, as well as α2 tanycytes and their processes (Fig. 9C, D). Immunofluorescence using anti-ACTH serum labeled only occasional γ tanycytes in the median eminence and the pituitary stalk (Fig. 10A, B). Their number was independent on whether the brain had low or high POMC level in tanycytes; in some brains there were 1-2 cells in each section, while in others there were no clear ACTH-positive γ tanycytes. The signal in γ tanycytes was always much lighter than in neurons or axons (Fig. 10A, B). In addition, in brains with high POMC levels in tanycytes we noted a very light ACTH immunoreactivity primarily in α2 tanycyte cell bodies (Fig. 10C, D). α-MSH immunoreactivity that was clearly above background level was very rare in tanycytes. Very light signal was observed occasionally in γ tanycytes in the median eminence and pituitary stalk, and in a few tanycyte-processes in brains with high-level POMC in tanycytes (Fig. 10E, F).
Figure 9.
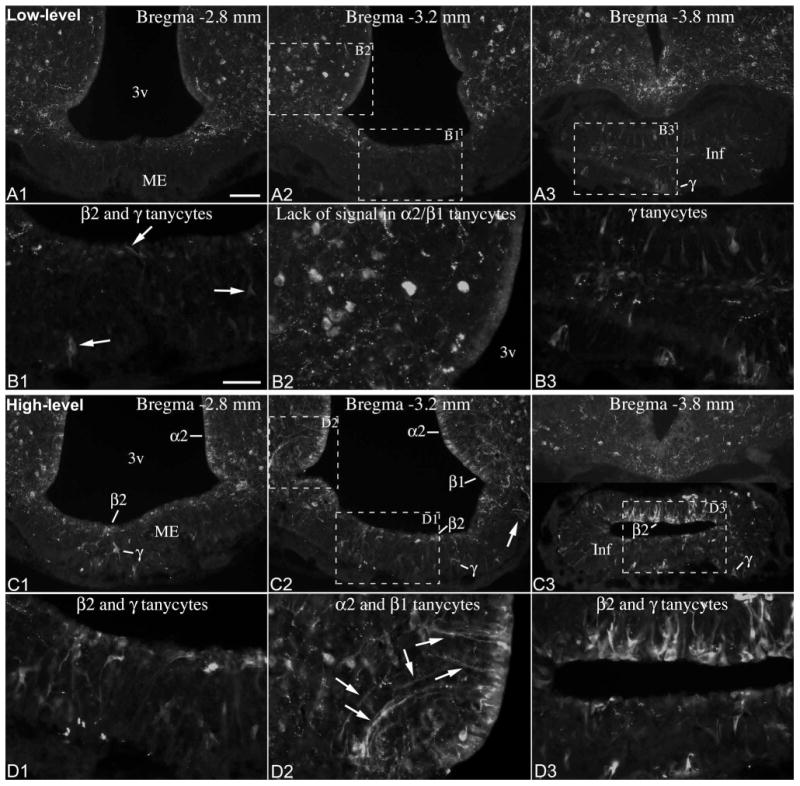
(A1-3) β-endorphin-immunofluorescence in a brain with low-level POMC in tanycytes (same brain as in Fig. 6.). (B1-3) Higher magnification of boxed areas in A1-A3 shows a β-endorphin-immunoreactive β2 tanycyte and γ tanycytes in the median eminence (arrows in B1); the lack of signal in α2 and β1 tanycytes (B2); and numerous immunopositive γ tanycytes in the pituitary stalk (B3). (C1-3). β-endorphin-immunofluorescence in a brain with high-level POMC in tanycytes (same brain as in Fig. 7.). Arrow on C2 indicates intensely labeled tanycyte endfeet in the lateral median eminence. (D1-D3) Higher magnification of boxed areas in C1-C3 shows β-endorphin-immunoreactive β2 tanycytes and γ tanycytes in the median eminence (D1); α2 and β1 tanycyte cell bodies and processes (arrows); and intensely labeled β2 and γ tanycytes of the pituitary stalk (D3). 3v, third ventricle; Inf, infundibular stalk; ME, median eminence. Scale bar: 100μm on A (for A and C); 50μm on B (for B, D).
Figure 10.
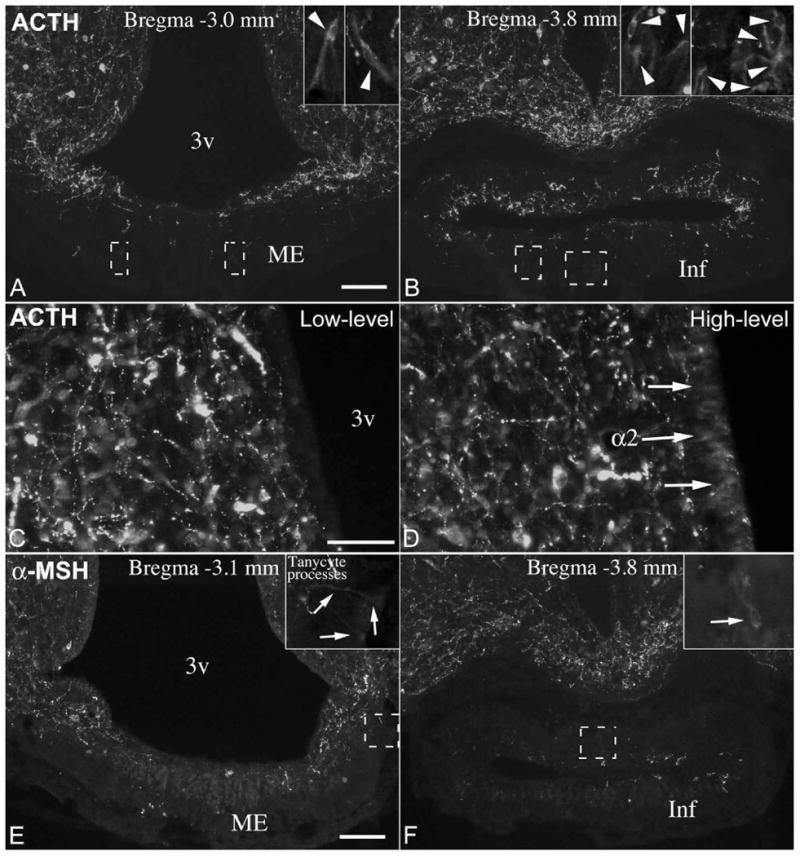
(A, B) ACTH immunofluorescence in an adult male rat brain with few ACTH-immunoreactive γ tanycytes in the median eminence (ME) and pituitary stalk (Inf). Insets show ACTH-positive γ tanycytes in boxed areas. (C, D) The cell body layer of α2 tanycytes lacks ACTH immunofluorescence in a brain with low-level POMC in tanycytes (C), but a low-level signal (arrows) can be detected in a brain with high-level POMC in tanycytes (D). (E, F) α-MSH immunofluorescence in tanycyte processes and in an γ tanycyte in the pituitary stalk. Insets show boxed areas in higher magnification. Field in E is identical with field shown in Fig. 9. C2. 3v, third ventricle. Scale bar: 100μm on A and E (for A, B, E, F); 50μm on C (for C, D).
Expression of POMC-processing enzymes in tanycytes
The scarce ACTH and α-MSH immunoreactivity in tanycytes suggested that there may be little processing of the POMC precursor in these cells. This would be in agreement with previous ISH studies that show no positive signal in tanycytes for the prohormone-convertase 1 and 2 (PC1, PC2) (Cullinan et al., 1991; Schafer et al., 1993) that cleave the POMC precursor to generate ACTH, and further to α-MSH, respectively (Cawley et al., 2016). To further examine whether genes involved in POMC-processing are expressed in tanycytes, we used RNA-Seq analysis on the transcriptome of rat tanycytes (α1, α2, β1, β2) that were isolated by laser capture microdissection. Expression levels were compared to samples obtained by the same method from the adjacent arcuate nucleus. Expression values for PC1, PC2, carboxypeptidase E, peptidylglycine alpha-amidating monooxygenase, and secretogranin V (or 7B2) mRNAs were significantly lower in tanycyte samples than in the arcuate nucleus (Table 3), in agreement with ISH studies that show predominantly neuronal expression patterns for these genes in the hypothalamus (Cullinan et al., 1991; Schafer et al., 1993; Marcinkiewicz et al., 1994). PC2 mRNA had the lowest value in tanycytes, 7.4 ± 0.4, which was below the cutoff value of 10.0 considered for positive expression. The second lowest expression, PC1 mRNA with 16.2 ± 0.8, was only slightly above the cutoff value. Pomc mRNA levels were similar in the tanycyte sample and the arcuate nucleus sample (347.2 ± 36.2 in tanycytes vs 397.2 ± 37.0 in the arcuate nucleus).
Table 3.
Next-Generation sequencing expression values for Pomc and genes involved in POMC-processing in tanycytes and neighboring arcuate nucleus collected by laser capture microdissection. Tissues were collected from 5 male Wistar rats, statistical comparison was made by Student's t-test.
| Tanycytes | Arcuate nucleus | ||
|---|---|---|---|
| Description | Mean ± SE (normalized CPM) | Mean ± SE (normalized CPM) | P value |
| Pomc, proopiomelanocortin | 347.2 ± 36.2 | 397.2 ± 37.0 | 0.4921 |
| Cpe, carboxypeptidase E | 529.8 ± 33.5 | 1331.6 ± 44.2 | <0.0001 |
| Pcsk1, proprotein convertase subtilisin/kexin type 1 | 16.2 ± 0.8 | 51.7 ± 1.5 | <0.0001 |
| Pcsk2, proprotein convertase subtilisin/kexin type 2 | 7.4 ± 0.4 | 50.2 ± 4.9 | 0.0002 |
| Pam, peptidylglycine alpha-amidating monooxygenase | 204.8 ± 9.5 | 306.1 ± 6.3 | 0.0002 |
| Scg5, secretogranin V (7B2 protein) | 36.3 ± 9.1 | 184.0 ± 46.0 | <0.0001 |
Discussion
It has been well known that POMC, rather uniquely among hormones/peptide transmitters, is expressed by two, very different cell types in the hypothalamic-pituitary complex: hormone-producing cells of the pituitary (corticotrophs and melanotrophs) and neurons of the arcuate nucleus. The findings of this study demonstrate that in rats there is yet a third, POMC-expressing cell-type in the hypothalamus-pituitary complex, which is the tanycyte. In fact, POMC-expressing tanycytes in the pituitary stalk effectively bridge the anatomical gap between pituitary corticotrophs/melanotrophs and arcuate POMC neurons, thus POMC expression from the pituitary to the hypothalamus is anatomically continuous but distributed in three different cell types.
Tanycyte Pomc ISH signal in previous studies
In preparation for this study we reviewed virtually all available literature that includes Pomc ISH in the rat hypothalamus. The list and categorization of these more than 200 papers is provided in the Appendix. We found 57 papers with photographs of emulsion autoradiographic or non-isotopic ISH where the images included at least a portion of the third ventricle wall and thus, the presence of signal in tanycytes could be assessed. Of these, 15 papers had images with very clear Pomc hybridization signal in tanycytes (Brady et al., 1990; Baubet et al., 1994; Larsen and Mau, 1994; Baker and Herkenham, 1995; Magoul and Tramu, 1997; Ahima et al., 1999; Baskin et al., 1999; Willesen et al., 1999; Bouret et al., 2001; Lu et al., 2002; Reyes and Sawchenko, 2002; Choi et al., 2006; Ross et al., 2009; Resch et al., 2011; Sergeyev et al., 2011); the relevant figures and data from these papers are summarized in Table 4. These papers collectively confirm that Pomc mRNA is expressed in tanycytes of different rat strains, both males and females, and under standard housing conditions (Table 4). In 42 papers, Pomc hybridization signal could not be unambiguously identified in tanycytes (see Appendix). This included cases where tanycytes were clearly negative or the signal was too light to be considered positive; where background levels were too high, the image resolution too low, or too little portion of the ventricular wall was included in the image. It is important to note, however, that in the majority of these papers, the radioactive or alkaline phosphatase Pomc hybridization signal was light or moderate at best in Pomc neurons. Therefore, tanycytes that generally express less Pomc mRNA than neurons may appear negative due to the low sensitivity of the detection. Nevertheless, tanycytes appeared to be negative or unclear even in some images with well-developed hybridization signal in neurons [for example, Fig 2. in (Reyes and Sawchenko, 2002) and Fig 5. in (Cullinan et al., 1991)]. We believe that the varying labeling of tanycytes seen in published papers at least in part reflects the natural variability of Pomc mRNA levels, which probably contributed to the fact that only few researchers have recognized Pomc mRNA expression by tanycytes.
Table 4.
Details of Pomc in situ hybridization studies (15 published papers) showing hybridization signal in tanycytes of the rat hypothalamus.
| Reference | Fig # | Visible Non-neuronal Signal | Strain | Sex | Light Cycle | Detection Method | Probe |
|---|---|---|---|---|---|---|---|
|
Brady 1990 Neuroendocrinology 52(5): 441-7. |
1. | α2, β, tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 48mer oligo |
|
Baubet 1994 Brain Res Mol Brain Res 26(1-2): 163-8. |
1. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 30mer oligo |
|
Larsen 1994 Peptides 15(5): 783-90. |
4. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 48mer oligo |
|
Baker 1995 J Comp Neurol 358(4): 518-30. |
2. | α2, β, γ tanycytes | Sprague-Dawley | M | N/A | Radioactive | 48mer oligo |
|
Magoul 1997 Neurosci Lett 223(2): 93-6. |
2. | α2, β, γ tanycytes | Wistar | M | N/A | Radioactive | 51mer oligo |
|
Ahima 1999 Endocrinology 140(11): 4923-31. |
6. | α2, β tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 925 b riboprobe |
|
Baskin 1999 Diabetes 48(4): 828-33. |
1. | α2, β tanycytes | Wistar | M | 12L:12D | Fluorescence | riboprobe |
|
Willesen 1999 Neuroendocrinology 70(5): 306-16. |
3. | α2, β, γ tanycytes | Wistar | F | 12L:12D | Alkaline phosphatase | 465 b riboprobe |
|
Bouret 2001 Endocrinology 142(9): 4055-65. |
3. | α2, β, γ tanycytes | Wistar | F | 14L:10D | Alkaline phosphatase | 409 b riboprobe |
|
Lu 2002 Endocrinology 143(10): 3905-15. |
1. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 936 b riboprobe |
|
Reyes 2002 J Neurosci 22(12): 5091-9. |
3. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | 500 b riboprobe |
|
Choi 2006 Brain Res 1087(1): 83-6. |
2. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | riboprobe |
|
Ross 2009 J Neuroendocrinol 21(7): 610-9. |
6. | α2, β tanycytes | Fischer 344 (& SD) | M | 16L:8D; 8L:16D | Radioactive | 344 b riboprobe |
|
Resch 2011 Am J Physiol Regul Integr Comp Physiol 301(6): R1625-34. |
5. | α2, β, γ tanycytes | Sprague-Dawley | M | 12L:12D | Radioactive | riboprobe |
|
Sergeyev 2011 Bull Exp Biol Med 150(4): 443-5. |
1. | β, γ tanycytes | Outbred albino | M | N/A | Radioactive | 54mer oligo |
Diversity of POMC expression by tanycyte subtypes
The present study reveals an unexpected diversity among tanycytes with respect to their capacity to express POMC. First, POMC expression was observed in every tanycyte subtype, except the α1 in which we never observed POMC mRNA or protein. Second, consistent POMC expression in tanycytes was restricted to a core region that included the pituitary stalk and the nearby mid-caudal region of the median eminence and third ventricle. Here we always observed a substantial population of POMC-expressing tanycytes, mostly from the β and γ subtypes. Outside this region, POMC expression varied among adult brains from only a few β and γ tanycytes to the vast majority of α2, β and γ tanycytes. Therefore, POMC expression in most α2 β and γ tanycytes is conditional and driven by a yet undetermined factor.
The results shed new light on a tanycyte subtype that has received little attention since its first description. Gamma tanycytes, described originally as astrocytic tanycytes, represent the most numerous non-ependymal cell type in the rat median eminence (Zaborszky and Schiebler, 1978). As defined by (Rutzel and Schiebler, 1980), these cells belong to the tanycyte series developmentally, but do not come into contact with the ventricular surface and morphologically somewhat differ from ventricular tanycytes. Their gene expression profile, however, is apparently very similar to α and/or β tanycytes, including vimentin, dopamine- and cyclic adenosine-3′:5′-monophosphate- regulated phosphoprotein (DARPP-32) (Hokfelt et al., 1988; Meister et al., 1988), type 2 deiodinase (Tu et al., 1997), organic anion-transporting polypeptide 1c1 (Wittmann et al., 2015a), kainite-preferring glutamate receptor subunit GluR7 (Eyigor and Jennes, 1998), and POMC, among others.
Variable POMC levels in tanycytes
To the best of our knowledge, the natural variability of tanycyte POMC mRNA and protein levels among adult rat brains is a unique phenomenon among most genes expressed in the hypothalamus. We found that low- and high-level POMC expression in tanycytes occur with about equal frequency between ages 8 and 15 weeks, ∼40% each, whereas the incidence of intermediate-level expression was lower, ∼20%. Interestingly, Pomc mRNA levels were uniformly low in 8 adolescent rats, suggesting that higher POMC levels in tanycytes may appear only after reaching adulthood. Theoretically, two possibilities could explain the variation in adult rats. One possibility is that the level of POMC expression in tanycytes remains constant in a given adult brain, but can markedly vary from one animal to another. A more intriguing possibility, however, is that POMC expression in tanycytes is dynamic, with high to low expression levels occurring periodically in each adult brain. This explanation would imply that Pomc gene expression in tanycytes is activity dependent, and may be induced by one or more specific signals that act periodically upon tanycytes. In this regard, it is important to consider that POMC expression levels in the tanycyte population follow a gradient pattern. This is especially apparent in intermediate-level brains, where the high mRNA levels present in the caudal core region decrease gradually in the rostral direction. Based on this pattern, we can speculate that upon some initiation signal, POMC expression may “spread” from core region tanycytes to adjacent tanycytes, and ultimately to most of the tanycyte population. The transmitting signal between tanycytes may be POMC itself, or it may spread via gap junctions and other cell-cell contacts, which are known to heavily interconnect tanycytes (Brawer, 1972; Hatton and Ellisman, 1982; Orellana et al., 2012). Future studies will be necessary to examine these possibilities. However, we believe that the present results favor the hypothesis of a spatio-temporally dynamic POMC expression pattern over a constant expression pattern with marked interindividual differences.
POMC processing in tanycytes
Another interesting feature of tanycyte POMC expression is the very low levels of ACTH and α-MSH-immunoreactivity that indicates little processing of POMC to these peptides. This is probably explained by the very low level of PC1 in tanycytes, according to our RNA-Seq analysis and previous ISH studies (Cullinan et al., 1991; Schafer et al., 1993). The lack of a substantial amount of ACTH in tanycytes may be of importance, as its secretion into the portal blood could potentially disrupt the normal feedback mechanism of cortisol on the hypothalamic-pituitary-adrenocortical axis. Interestingly, β-endorphin immunoreactivity was substantial in tanycytes, but we did not detect PC2, which is the primary enzyme that cleaves β-endorphin from POMC (Cawley et al., 2016). Thus, it is possible that β-endorphin immunoreactivity in tanycytes mostly if not entirely represents the POMC precursor and/or the intermediate β lipotropin, both of which can be recognized by β-endorphin antibodies (Miller et al., 2003; Laurent et al., 2004). Alternatively, there may be a PC2-independent mechanism to produce β-endorphin in tanycytes, the existence of which was suggested by residual β-endorphin production in the hypothalamus of PC2 knockout mice (Allen et al., 2001). Future studies are required to determine the full extent of proteolytic POMC processing in tanycytes.
Potential functional implications
The physiological significance of POMC in rat tanycytes remains to be determined and at present remains highly speculative. Depending on the potential target cells of tanycyte-derived POMC, we suggest three potential roles for POMC in tanycytes. One is regulating the release of hypohysiotropic hormones via acting on hypophysiotropic axons in the median eminence. Hypophysiotropic axons in the rat median eminence contain large amounts of μ opioid receptors (Abbadie et al., 2000) that bind β-endorphin, and tanycyte-derived β-endorphin would be the best-positioned ligand to access these receptors. In addition, radioligand binding studies demonstrated that hypophysiotropic axons in the median eminence bind ACTH with high affinity (van Houten et al., 1981; Van Houten et al., 1985). This is probably due to the presence of melanocortin 4 receptors that hypophysiotropic neurons express (Tatro, 1990; Kishi et al., 2003), although the presence of these receptor proteins on their axons remains to be determined. Therefore, it is possible that a low amount of ACTH and α-MSH released from tanycytes may also modulate the activity of hypophysiotropic axons. A second possible function is that tanycyte-derived POMC, acting in a paracrine/autocrine manner, may be involved in the proliferative/neurogenic functions of tanycytes themselves. Recent studies in mice demonstrated that tanycytes have stem cell properties and are capable of producing new neurons and glial cells during adulthood (Lee et al., 2012; Haan et al., 2013; Robins et al., 2013). Studies in rats suggest a similar neural progenitor function for tanycytes in adulthood (Xu et al., 2005; Perez-Martin et al., 2010). Interestingly, N-terminal POMC peptides, such as pro-gamma-MSH, have mitogenic effects on the rat adrenal cortex (Bicknell, 2016), raising the possibility that N-terminal POMC peptides released from tanycytes may promote hypothalamic neurogenesis by increasing the proliferation of tanycytes. Lastly, N-terminal POMC peptides secreted by tanycytes into the portal capillaries of the median eminence may regulate pituitary functions, such as proliferation of adult pituitary stem cells or pituitary hormone release.
Conclusions
In conclusion, the present study reveals a novel, unique type of POMC expression in tanycytes in the adult rat hypothalamus and pituitary stalk. Future studies will be necessary to investigate the cause of variability, the exact nature of POMC-processing, the functional significance, and the potential existence of tanycyte POMC expression in other species.
Supplementary Material
Footnotes
This work was supported by NIH grant R21AG050663, a grant from the Dr. Gerald J. and Dorothy R. Friedman New York Foundation for Medical Research, The Hilda and Preston Davis Foundation for Eating Disorders Research, the Hungarian National Brain Research Program, Hungarian Scientific Research Fund OTKA K109415, and Seventh EU Research Framework Programme (Health-F2-2010-259772).
Conflict of interest statement: The authors have no conflicts of interests to disclose.
Resources Cited: Roche, Cat# 11207733910, RRID:AB_514500
Molecular Probes, Cat# A21271, RRID:AB_221448
Millipore, Cat# AB5733, RRID:AB_2216104
Jackson ImmunoResearch Labs, Cat# 715-605-151, RRID:AB_2340863
Jackson ImmunoResearch Labs, Cat# 703-165-155, RRID:AB_2340363
Phoenix Pharmaceuticals, Cat# H-029-30, RRID:AB_2307442
Jackson ImmunoResearch Labs, Cat# 711-545-152, RRID:AB_2313584
Jackson ImmunoResearch Labs, Cat# 715-165-151, RRID:AB_2315777
Jackson ImmunoResearch Labs, Cat# 703-605-155, RRID:AB_2340379
Phoenix Pharmaceuticals, Cat# H-022-33, RRID:AB_2314007
Millipore, Cat# AB5087, RRID:AB_91683
Jackson ImmunoResearch Labs, Cat# 711-165-152, RRID:AB_2307443
Jackson ImmunoResearch Labs, Cat# 713-545-147, RRID:AB_2340745
Phoenix Pharmaceuticals, Cat# H-001-21, RRID:AB_2572293
Jackson ImmunoResearch Labs, Cat# 711-065-152, RRID:AB_2340593
Millipore, Cat# MAB3400, RRID:AB_94843
Role of authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GW. ISH and immunofluorescence: GW. Electron microscopy: EF. RNA-Seq experiment: AS, BG, CF. Drafting of the manuscript: GW. Critical revision of the manuscript for important intellectual content: GW, AS, EF, BG, CF, RML. Obtained funding: GW, BG, CF, RML. Study supervision: RML.
Literature cited
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419(2):244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140(11):4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV. Morphological aspects of the hypothalamic-hypophyseal system. VI. The tanycytes: their relation to the sexual differentiation of the hypothalamus. An enzyme-histochemical study. Cell Tissue Res. 1976;173(3):407–416. doi: 10.1007/BF00220328. [DOI] [PubMed] [Google Scholar]
- Akmayev IG, Fidelina OV, Kabolova ZA, Popov AP, Schitkova TA. Morphological aspects of the hypothalamic-hypophyseal system. IV. Medial basal hypothalamus. An experimental morphological study. Z Zellforsch Mikrosk Anat. 1973;137(4):493–512. doi: 10.1007/BF00307226. [DOI] [PubMed] [Google Scholar]
- Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, Lundblad JR, Washburn CL, Pintar JE. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. J Neurosci. 2001;21(16):5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol. 1995;358(4):518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48(4):828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- Baubet V, Fevre-Montange M, Gay N, Debilly G, Bobillier P, Cespuglio R. Effects of an acute immobilization stress upon proopiomelanocortin (POMC) mRNA levels in the mediobasal hypothalamus: a quantitative in situ hybridization study. Brain Res Mol Brain Res. 1994;26(1-2):163–168. doi: 10.1016/0169-328x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Bicknell AB. N-terminal POMC peptides and adrenal growth. J Mol Endocrinol. 2016 doi: 10.1530/JME-15-0269. [DOI] [PubMed] [Google Scholar]
- Bitsch P, Schiebler TH. [Postnatal development of the median eminence in the rat] Z Mikrosk Anat Forsch. 1979;93(1):1–20. [PubMed] [Google Scholar]
- Bouret S, Chuoi-Mariot MT, Prevot V, Croix D, Takumi T, Jegou S, Vaudry H, Beauvillain JC, Mitchell V. Evidence that TGF beta may directly modulate POMC mRNA expression in the female rat arcuate nucleus. Endocrinology. 2001;142(9):4055–4065. doi: 10.1210/endo.142.9.8361. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Brawer JR. The fine structure of the ependymal tanycytes at the level of the arcuate nucleus. J Comp Neurol. 1972;145(1):25–41. doi: 10.1002/cne.901450103. [DOI] [PubMed] [Google Scholar]
- Cawley NX, Li Z, Loh YP. 60 YEARS OF POMC: Biosynthesis, trafficking and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016 doi: 10.1530/JME-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Blake V, Cole S, Fernstrom JD. Effects of chronic fenfluramine administration on hypothalamic neuropeptide mRNA expression. Brain Res. 2006;1087(1):83–86. doi: 10.1016/j.brainres.2006.02.129. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Day NC, Schafer MK, Day R, Seidah NG, Chretien M, Akil H, Watson SJ. Neuroanatomical and functional studies of peptide precursor-processing enzymes. Enzyme. 1991;45(5-6):285–300. doi: 10.1159/000468902. [DOI] [PubMed] [Google Scholar]
- Drager UC, Edwards DL, Kleinschmidt J. Neurofilaments contain alpha-melanocyte-stimulating hormone (alpha-MSH)-like immunoreactivity. Proc Natl Acad Sci U S A. 1983;80(20):6408–6412. doi: 10.1073/pnas.80.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyigor O, Jennes L. Identification of kainate-preferring glutamate receptor subunit GluR7 mRNA and protein in the rat median eminence. Brain Res. 1998;814(1-2):231–235. doi: 10.1016/s0006-8993(98)01056-7. [DOI] [PubMed] [Google Scholar]
- Haan N, Goodman T, Najdi-Samiei A, Stratford CM, Rice R, El Agha E, Bellusci S, Hajihosseini MK. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J Neurosci. 2013;33(14):6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton JD, Ellisman MH. The distribution of orthogonal arrays in the freeze-fractured rat median eminence. J Neurocytol. 1982;11(2):335–349. doi: 10.1007/BF01258250. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Foster G, Schultzberg M, Meister B, Schalling M, Goldstein M, Hemmings HC, Jr, Ouimet C, Greengard P. DARPP-32 as a marker for D-1 dopaminoceptive cells in the rat brain: prenatal development and presence in glial elements (tanycytes) in the basal hypothalamus. Adv Exp Med Biol. 1988;235:65–82. doi: 10.1007/978-1-4899-2723-1_6. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Yamamoto Y, Kubo K, Dobashi K, Asayama K, Ueta Y, Shirahata A. Postnatal development of galanin-like peptide mRNA expression in rat hypothalamus. Regul Pept. 2008;145(1-3):133–140. doi: 10.1016/j.regpep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Kawakami S. Glial and neuronal localization of ionotropic glutamate receptor subunit-immunoreactivities in the median eminence of female rats: GluR2/3 and GluR6/7 colocalize with vimentin, not with glial fibrillary acidic protein (GFAP) Brain Res. 2000;858(1):198–204. doi: 10.1016/s0006-8993(00)01980-6. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Mau SE. Effect of acute stress on the expression of hypothalamic messenger ribonucleic acids encoding the endogenous opioid precursors preproenkephalin A and proopiomelanocortin. Peptides. 1994;15(5):783–790. doi: 10.1016/0196-9781(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology. 2004;145(2):519–528. doi: 10.1210/en.2003-0829. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143(10):3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- Magoul R, Tramu G. Tachykinin-induced changes in beta-endorphin gene expression in the rat arcuate nucleus. Neurosci Lett. 1997;223(2):93–96. doi: 10.1016/s0304-3940(97)13407-3. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Touraine P, Chretien M. Pan-neuronal mRNA expression of the secretory polypeptide 7B2. Neurosci Lett. 1994;177(1-2):91–94. doi: 10.1016/0304-3940(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Meister B, Hokfelt T, Tsuruo Y, Hemmings H, Ouimet C, Greengard P, Goldstein M. DARPP-32, a dopamine- and cyclic AMP-regulated phosphoprotein in tanycytes of the mediobasal hypothalamus: distribution and relation to dopamine and luteinizing hormone-releasing hormone neurons and other glial elements. Neuroscience. 1988;27(2):607–622. doi: 10.1016/0306-4522(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci. 2013;7:19. doi: 10.3389/fnins.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J Neurochem. 2003;86(3):556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Saez JC, Garcia MA. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60(1):53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Cifuentes M, Grondona JM, Lopez-Avalos MD, Gomez-Pinedo U, Garcia-Verdugo JM, Fernandez-Llebrez P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31(9):1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1625–1634. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Sawchenko PE. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J Neurosci. 2002;22(12):5091–5099. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Gonzalez CB, Delannoy L. Cellular organization of the lateral and postinfundibular regions of the median eminence in the rat. Cell Tissue Res. 1979;201(3):377–408. doi: 10.1007/BF00236998. [DOI] [PubMed] [Google Scholar]
- Ross AW, Johnson CE, Bell LM, Reilly L, Duncan JS, Barrett P, Heideman PD, Morgan PJ. Divergent regulation of hypothalamic neuropeptide Y and agouti-related protein by photoperiod in F344 rats with differential food intake and growth. J Neuroendocrinol. 2009;21(7):610–619. doi: 10.1111/j.1365-2826.2009.01878.x. [DOI] [PubMed] [Google Scholar]
- Ross AW, Russell L, Helfer G, Thomson LM, Dalby MJ, Morgan PJ. Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet. PLoS One. 2015;10(3):e0119763. doi: 10.1371/journal.pone.0119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutzel H, Schiebler TH. Prenatal and early postnatal development of the glial cells in the median eminence of the rat. Cell Tissue Res. 1980;211(1):117–137. doi: 10.1007/BF00233728. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Day R, Cullinan WE, Chretien M, Seidah NG, Watson SJ. Gene expression of prohormone and proprotein convertases in the rat CNS: a comparative in situ hybridization analysis. J Neurosci. 1993;13(3):1258–1279. doi: 10.1523/JNEUROSCI.13-03-01258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyev VG, Vegeyeva OA, Akmayev IG. Effect of intraperitoneal administration of bacterial lipopolysaccharide on synthesis of pro-opiomelanocortin mRNA in rat tanycytes. Bull Exp Biol Med. 2011;150(4):443–445. doi: 10.1007/s10517-011-1164-8. [DOI] [PubMed] [Google Scholar]
- Tatro JB. Melanotropin receptors in the brain are differentially distributed and recognize both corticotropin and alpha-melanocyte stimulating hormone. Brain Res. 1990;536(1-2):124–132. doi: 10.1016/0006-8993(90)90016-5. [DOI] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138(8):3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- van Houten M, Khan MN, Khan RJ, Posner BI. Blood-borne adrenocorticotropin binds specifically to the median eminence-arcuate region of the rat hypothalamus. Endocrinology. 1981;108(6):2385–2387. doi: 10.1210/endo-108-6-2385. [DOI] [PubMed] [Google Scholar]
- Van Houten M, Khan MN, Walsh RJ, Baquiran GB, Renaud LP, Bourque C, Sgro S, Gauthier S, Chretien M, Posner BI. NH2-terminal specificity and axonal localization of adrenocorticotropin binding sites in rat median eminence. Proc Natl Acad Sci U S A. 1985;82(4):1271–1275. doi: 10.1073/pnas.82.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70(5):306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Wittmann G, Harney JW, Singru PS, Nouriel SS, Reed Larsen P, Lechan RM. Inflammation-inducible type 2 deiodinase expression in the leptomeninges, choroid plexus, and at brain blood vessels in male rodents. Endocrinology. 2014;155(5):2009–2019. doi: 10.1210/en.2013-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521(14):3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Mohacsik P, Balkhi MY, Gereben B, Lechan RM. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS. 2015a;12:21. doi: 10.1186/s12987-015-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Szabon J, Mohacsik P, Nouriel SS, Gereben B, Fekete C, Lechan RM. Parallel regulation of thyroid hormone transporters OATP1c1 and MCT8 during and after endotoxemia at the blood-brain barrier of male rodents. Endocrinology. 2015b;156(4):1552–1564. doi: 10.1210/en.2014-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192(2):251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Schiebler TH. [Glia of median eminence. Electron microscopic studies of normal, adrenalectomized and castrated rats] Z Mikrosk Anat Forsch. 1978;92(4):781–799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


