Abstract
Background
Uncontrolled hemorrhage is a leading cause of mortality following trauma accounting for up to 40% of deaths. Massive transfusion protocols (MTPs) offer a proven benefit in resuscitation of these patients. Recently, the superiority of thrombelastography (TEG)-guided resuscitation over strategies guided by conventional clotting assays (CCA) has been established. We seek to determine optimal thresholds for r-TEG driven resuscitation.
Methods
R-TEG data were reviewed for 190 patients presenting to our Level 1 Trauma Center from 2010 to 2015. Criteria for inclusion were highest level trauma activation in patients ≥ 18 years of age with hypotension presumed due to acute blood loss. Exclusion criteria included: isolated gun-shot wound to the head, pregnancy and chronic liver disease. Receiver operating characteristic (ROC) analysis was performed to test the predictive performance of r-TEG for massive transfusion requirement defined by need for 1) >10 units of RBCs total or death in the first six hours or 2) >4 units of RBCs in any hour within the first 6 hours. Cut-point analysis was then performed to determine optimal thresholds for TEG-based resuscitation.
Results
ROC analysis of r-TEG yielded areas under the curve (AUC) greater than 70% for all outputs with respect to both transfusion thresholds considered, with exception of activated clotting time (ACT) and lysis at 30 minutes (LY30) for > 4U RBC in any hour in the first 6 hours. Optimal cut-point analysis of the resultant ROC curves was performed and for each value, the most sensitive cut-point was identified, respectively ACT ≥ 128 sec, angle (α) ≤ 65, maximum amplitude (MA) ≤ 55 mm and LY30 ≥ 5%.
Conclusions
Through ROC analysis of prospective TEG data, we have identified optimal thresholds to guide hemostatic resuscitation. These thresholds should be validated in a prospective multicenter trial.
Level of Evidence
Prognostic, level III
Keywords: Injury, Shock, Coagulopathy, Resuscitation, Transfusion
Background
Uncontrolled hemorrhage is the leading cause of preventable trauma-related death accounting for up to 40% of deaths in severely injured hospitalized patients1. The underlying disturbances of the normal clotting system, broadly defined as trauma-induced coagulopathy (TIC), account for the majority of these hemorrhagic deaths2.
Survival curves in this population indicate that half of deaths from exsanguination occur in the first 2 hours from injury and furthermore, that hemorrhage accounts for the vast majority of deaths in the first 24 hours3. Specific injury patterns and other pre-injury factors are often not evident at the time of initial presentation. Therefore, reliable objective means of early recognition and in turn, purposeful intervention are the keys to successfully managing these life-threatening coagulopathies.
Massive transfusion protocols (MTPs) offer a long-proven benefit in resuscitation of patients in hemorrhagic shock. With this concept established, much time and effort has been directed to identify the ideal ratio of products in resuscitation strategy and traditional intervention has been dictated by conventional coagulation assays (CCA), i.e., international normalized ration (INR), partial thromboplastin time (PTT), fibrinogen and platelet count.
A 2013 prospective study by Johansson et al. attributed lower 28-day mortality (12%) and proportion of those deaths resulting from exsanguination (14%) to TEG-guided resuscitation4. Our group has recently confirmed these results in a randomized control trial, showing that a goal-directed, TEG-guided MTP improves survival as compared to MTP guided by CCA based on its individualized, point-of-care, precision hemostatic approach. Furthermore, these results were achieved with less plasma and platelet transfusions during the early phases of resuscitation5. A crucial next step in optimizing this strategy is to establish a system of thresholds based on TEG outputs to guide intervention.
Previous recommendations have been based on healthy individual criteria or associations with transfusion requirements (Johansson4, Holcomb6, Tapia7, and Schochl8,9). In this study, we propose optimal thresholds for r-TEG driven resuscitation based on prospective data collected in severely injured patients at high risk for trauma-induced coagulopathy (TIC).
Methods
Our Denver Trauma Activation Protocol (TAP) Database includes all trauma activation adult (>18 years of age) patients who sustained blunt or penetrating injuries, and had a citrated rapid TEG performed by one of our on-site professional research assistants (PRA) with blood collected in the field or at ED presentation, admitted from September 26, 2010 to June 30, 2015 to our Level 1 Trauma Center. Clinicians were blinded to these research data, however, in most cases with evidence of bleeding, TEGs were also ordered by the care team and processed in the hospital clinical lab to guide resuscitation practice. The current study reports the results of the TEGs performed in our research laboratory, which are obtained for all patients enrolled in the database with an IRB approved waiver of consent. The Denver Health Medical Center (DHMC) is an American College of Surgeons verified, state-designated Level 1 Trauma Center. Criteria for inclusion were highest level trauma activation in patients ≥ 18 years of age with hypotension (defined as systolic blood pressure, SBP, ≤70mmHg or SBP≤90mmHg plus heart rate, HR, >108bpm) presumed due to acute blood loss. Exclusion criteria were: unsalvageable injuries (defined by patients in asystole at emergency department arrival), isolated gunshot wounds (GSW) to the head, pregnancy, documented chronic liver disease or known coagulation disorder. The studies contributing to this database were approved by the Colorado Multiple Institution Review Board and performed under a waiver of consent.
TEG (TEG-5000 Analyzer; Haemonetics Corp, Stoughton, MA) was performed on whole blood collected in vacuum tubes with citrate added to prevent clotting prior to analysis. This assay incorporates tissue factor to the whole blood sample immediately before test initiation to expedite results, also known as Rapid-TEG (r-TEG). R-TEG yields the following variables: activated clotting time (ACT; the time to beginning of clot formation, seconds), angle (α; rate of clot strength increase, degrees), maximum amplitude (MA; maximal clot strength achieved, millimeters), and percent clot lysis 30 minutes after MA is achieved (LY30, %). Studies have correlated activated clotting time (ACT) with coagulation factor activity and thrombin generation, angle with fibrinogen concentration and function, maximum amplitude (MA) with platelet–fibrinogen interactions, and percent clot lysis 30 minutes from MA (LY30) with fibrinolysis10.
The transfusion of products other than RBCs during this period was guided by r-TEG criteria proposed by the TEG manufacturers and widely accepted by blood banks as follows: ACT > 110 treated with plasma, angle < 66° treated with cryoprecipitate, MA < 54 mm treated with platelets and elevated LY30 > 3% treated with tranexamic acid (TXA)11, 12, 13. The primary outcome was massive transfusion, defined as one of the following: 1) >4 units of RBCs in any 60-minute period in the first six hours from injury based on the threshold set forth by the PROMMTT trial14 2) > 10 units of RBCs or death in first 6 hours from injury based on findings previously published by our group 15. Additional objective outcomes included, mortality in first 24 hours, ICU-free days <14 and ventilator-free days <21.
Covariates: Shock was defined as admission SBP ≤90mmHg and profound shock as SBP ≤70mmHg. Admission lactate > 5 mg/dL and admission base deficit > 8 mEq/L were used as determinants of critically impaired tissue perfusion. Traumatic brain injury (TBI) was defined as Glasgow Coma Score (GCS) < 8 and head Abbreviated Injury Scale > 2.
Statistical Analysis
Receiver operating characteristic (ROC) curve analyses were performed to test the predictive performance of citrated r-TEG values ACT, angle, MA and LY30 with respect to the stated outcomes. For each of these parameters, we selected the thresholds with the strongest differentiation of the outcome (i.e. massive transfusion) via optimal ROC curve cut-point analysis to identify ideal thresholds for TEG-guided resuscitation. Three methods of cut-point analysis were employed: 1) maximum Youden’s Index (J), 2) shortest distance to (0,1) and 3) sensitivity, specificity equality. We then calculated the mean of these three values to determine the final threshold for each r-TEG output.
Youden’s Index (J) is calculated as: J = Sensitivity + Specificity −1, with a value of 1 representing a perfect test with no false positives or false negatives. The maximum Youden’s Index is the point on the ROC curve where resultant J value is closest to 116.
Shortest distance to (0,1) is a similar concept that aims to identify the optimal cut point by isolating the point on the ROC curve closest to the upper-left-hand corner. Depending on shape of the curve, this value will either prioritize sensitivity or specificity indiscriminately17.
Sensitivity, specificity equality identifies the optimal cut point where these 2 values are nearest to equilibrium. In a perfectly symmetric curve, this point would also equal the shortest distance to (0,1)18.
Results
Overall, 190 patients met inclusion criteria (Table 1), of whom 81% were men, and 59% sustained blunt trauma. Age ranged from 18 to 93 years (median 34, IQR 26–47). Median injury severity score (ISS) was 22 (IQR 10–34) and median new injury severity score (NISS) was 27 (IQR 14–43). Median time from injury to ED arrival was 25 (IQR 20.3–32) minutes. Overall, 28.4% of these patients were in shock and 13.2% were in profound shock while 12.6% sustained TBI. With respect to intensive care unit (ICU) course, 22.1% had less than 14 ICU-free days and 26.8% had less than 21 ventilator-free days. The mortality rate was 13.2% (Table 1).
Table 1. Clinical Data –
table provides demographic data about the cohort in total and then stratified by those patients who required massive transfusion and those who did not. Cited in the ‘Results’ section on page 5 of the manuscript.
| All Patients (n=190) | No Massive Transfusion (n=153) | Massive Transfusion (MTP) (n=37) | ||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Shock (SBP ≤ 90mmHg) | 54 | 28.4% | 30 | 32.1% | 24 | 63.2% |
| Profound Shock (SBP ≤ 70mmHg) | 25 | 13.2% | 11 | 7.1% | 14 | 36.8% |
| Lactate > 5 mg/dL | 35 | 35.7% | 21 | 26.9% | 14 | 63.6% |
| Base Deficit > 8mEq/L | 53 | 43.1% | 32 | 32.3% | 21 | 80.8% |
| Traumatic Brain Injury | 24 | 12.6% | 11 | 7.1% | 13 | 34.2% |
| ICU-free Days < 14 | 42 | 22.1% | 13 | 8.5% | 29 | 78.4% |
| Ventilator-free Days <21 | 51 | 26.8% | 20 | 13.1% | 31 | 83.8% |
| Mortality | 25 | 13.2% | 6 | 3.9% | 19 | 51.4% |
| Massive Transfusion Rate | 37 | 19.5% | ||||
| Median | IQR | Median | IQR | Median | IQR | |
| Age – years (n=190) | 34 | 26–47 | 34 | 26–47 | 37 | 27–48 |
| Time to ED -- minutes (n=190) | 25 | 20.3–32 | 25 | 21–31.5 | 24 | 20–39 |
| Injury Severity Score (ISS) (n=133) | 22 | 10–34 | 14 | 9–29 | 38 | 29–43 |
| New Injury Severity Score (NISS) (n=133) | 27 | 14–43 | 17 | 9–29 | 50 | 38–60.8 |
| Glasgow Coma Score (GCS) (n=190) | 14.5 | 5.25–15 | 15 | 11.5–15 | 3 | 3–8 |
| Temperature– C° (n=151) | 36.45 | 36.3–36.8 | 36.55 | 36.3–36.8 | 36.3 | 35.7–36.9 |
| Admission Calcium (n=168) units | 8 | 7.6–8.5 | 8.1 | 7.7–8.5 | 7.7 | 7.2–8.4 |
| Admission Hemoglobin (g/dL) (n=185) | 13.9 | 12.5–15.3 | 14.1 | 13–15.4 | 12.3 | 9.9–14.0 |
| Admission Platelets (1,000/ml) (n=184) | 254.5 | 204–310 | 263 | 213–317 | 176.5 | 116.5–276 |
| Admission International Normalized Ratio(INR) (n=180) | 1.1 | 1.04–1.3 | 1.1 | 1–1.2 | 1.55 | 1.3–2.08 |
| Admission Partial Thromboplastin Time (PTT) (n=180) units | 27.75 | 24.7–33.1 | 26.85 | 23.8–29.8 | 42.9 | 32.6–74.0 |
The r-TEG outputs are depicted in Table 1 and ROC analysis assessing the predictive value of the r-TEG variables for massive transfusion in Table 2. For transfusion threshold > 4 U RBC in any hour in the first six hours post-injury, the areas under the ROC curve (AUC) were 0.69 for ACT, 0.84 for angle, 0.83 for MA and 0.69 for LY30. ACT and LY30 were the only AUCs in this analysis slightly below the 0.70 threshold, suggesting only fair predictive capacity. For massive transfusion defined as need for > 10 U or death within 6 hours post-injury, the AUCs were 0.72 for ACT, 0.80 for angle, 0.81 for MA and 0.72 for LY30 (Table 3).
Table 2. Rapid Citrated TEG data.
provides median and interquartile range of the four TEG outputs analyzed across the cohort. Cited in the ‘Results’ section on page 5 of the manuscript.
| Output | Median | Interquartile Range |
|---|---|---|
| Activated Clotting Time (ACT) units | 121 | 113–136 |
| Angle (α) units | 70 | 64.4–74.1 |
| Maximum Amplitude (MA) units | 61.5 | 54.5–65.5 |
| Lysis at 30 Minutes (LY30) % | 1.9 | 1.0–3.6 |
Table 3. Area under the Receiver Operating Characteristics Curves (AUC) with 95% confidence intervals and Optimal Cut-points.
In 2 distinct but not labeled sections a) provides area under the curve (AUC) from ROC analysis of each TEG output with respect to each definition of massive transfusion considered and b) provides the optimal cut point from each of three methods of cut point analysis used with respect to each of the four TEG outputs analyzed. Cited in the ‘Results’ section on page 3 of the manuscript.
| MTP defined: | ACT | LY30 | Angle | MA | |
|---|---|---|---|---|---|
| > 10U RBC or death in 6 hours (n=30) | AUC (95% CI) | 0.72 (0.61–0.82) | 0.72 (0.59–0.84) | 0.80 (0.70–0.90) | 0.81 (0.72–0.90) |
| > 4U RBC/hour in the first 6 hours (n=30) | AUC (95% CI) | 0.68 (0.58–0.80) | 0.69 (0.56–0.81) | 0.84 (0.76–0.91) | 0.83 (0.75–0.91) |
| Death within 24 hours postinjury | AUC (95% CI) | 0.77 (0.65–0.89) | 0.70 (0.51–0.88) | 0.73 (0.58–0.89) | 0.71 (0.56–0.86) |
| < 14 ICU free days | AUC (95% CI) | 0.67 (0.58–0.76) | 0.60 (0.48–0.72) | 0.74 (0.66–0.83) | 0.77 (0.69–0.85) |
| < 21 ventilator free days | AUC (95% CI) | 0.64 (0.54–0.74) | 0.54 (0.42–0.66) | 0.73 (0.64–0.82) | 0.75 (0.67–0.84) |
| Optimal cut-points | Method | ACT (sec) | LY30 (%) | Angle (°) | MA (mm) |
| > 10U RBC or DEATH in 6 Hours | Youden index (J) | 128 | 7.7 | 62.3 | 54 |
| Distance to (0,1) | 128 | 3.4 | 62.3 | 55.5 | |
| Sen=Spec | 128 | 2.8 | 66.9 | 57.5 | |
| > 4U RBC/hour in the first 6 hours | Youden index (J) | 139 | 7.7 | 62.3 | 54 |
| Distance to (0,1) | 128 | 2.6 | 65.0 | 55.5 | |
| Sen=Spec | 128 | 2.7 | 66.5 | 57.5 | |
| Death within 24 hours post-injury | Youden index (J) | 128 | 9.5 | 65.3 | 47.5 |
| Distance to (0,1) | 136 | 3.8 | 65.3 | 55.5 | |
| Sen=Spec | 136 | 2.7 | 66.5 | 60 | |
| < 14 ICU free days | Youden index (J) | 128 | 3.8 | 71 | 55.5 |
| Distance to (0,1) | 128 | 2.8 | 66.9 | 55.5 | |
| Sen=Spec | 128 | 2.2 | 68.6 | 59.5 | |
| < 21 ventilator free days | Youden index (J) | 144 | 7.7 | 65 | 55.5 |
| Distance to (0,1) | 128 | 3.8 | 65.3 | 55.5 | |
| Sen=Spec | 128 | 2.1 | 68.8 | 60 |
RBC: red blood cells, Sen=sensitivity, Spec: specificity
Cut point analysis on each of these curves yielded a range of optimum thresholds for TEG parameters for the massive transfusion outcome. As shown in Table 3, most of the cutoffs for the TEG parameters were consistent across the optimality criteria (Youden Index, Sen=spec, distance (0,1)), with the exception of LY30, for which we noted a difference between the Youden Index and the other two optimality criteria. The TEG cutoffs for massive transfusion were also remarkably consistent with the cutoffs determined for the three objective outcomes (death, ICU and ventilator free days).
Discussion
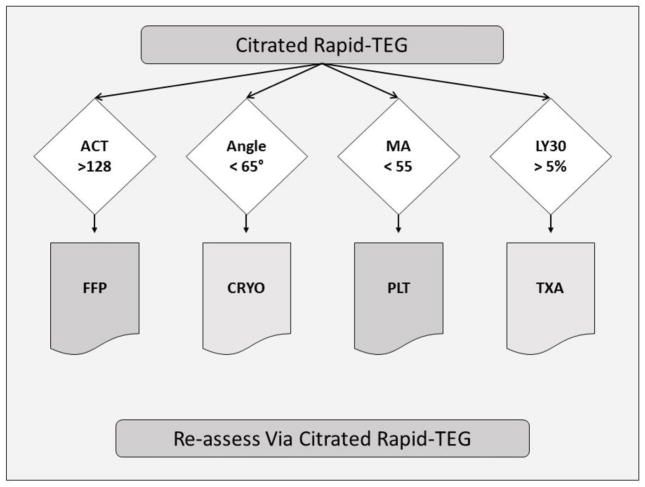
In this study we determined the degree of discrimination offered by r-TEG output values ACT, angle, MA and LY30 with respect to massive transfusion need. Based on these data, the optimal thresholds for TEG-guided resuscitation are as follows: ACT ≥ 128 seconds, angle ≤ 65°, MA ≤ 55mm and LY30 ≥ 5% (Figure 1). We considered two definitions of massive transfusion based on the literature. The > 10 U RBC in the first six hours threshold originated from work of our group first published in 2008 that challenged the historical definition of > 10 U RBC in first 24 hours. This modification was based on the fact that 80% of RBC transfusions were completed in the first six hours and that transfusion need in this 6-hour window was among the most significant determinants of mortality. The > 4 U RBC given in any hour in the first six threshold was suggested by the multi-center, prospective PROMMT trial in the recent publication by Moren et. al. demonstrating a significant mortality difference in patients who receive > 4U per any hour within the first 6 hours14.
Figure 1. TEG-guided Resuscitation Thresholds.
a schematic representation of the appropriate use of the thresholds in a TEG-guided MTP. Cited in the ‘Discussion’ section on page 6 of the manuscript.
In our study, we found that AUCs were consistent for each r-TEG value considered across the range of transfusion requirements as were the thresholds determined from optimal cut point analysis of the ROC curves. Of the r-TEG outputs considered, angle and MA consistently yielded the strongest ROC signals for all definitions of massive transfusion considered. AUC was ≥ 80% for both of these outputs with respect to both transfusion requirements representing good to excellent discrimination. It is logical that these values would provide crucial insight to transfusion need as they serve as surrogates for clot strength. It is also noteworthy that angle provides a comparably strong signal to that yielded by MA for eventual transfusion need since the angle value is available significantly earlier in the real-time output of the r-TEG tracing allowing for earlier clinical intervention.
ACT and LY30 yielded weaker, but still significant ROC signals of AUC 0.72 for both with respect to the need for > 10U RBC or death in the first six hours representing good discrimination, but fell just below this threshold for ACT and LY30 with respect to the need for > 4U RBC in the first hour (0.68 and 0.69 respectively). ROC analysis proved a suboptimal method for establishing cut points for these two TEG outputs. This is likely explained by the more complex and non-linear relationship these outputs have with the outcomes considered. For example, our group has previously demonstrated the quadratic relationship between LY30 and early mortality19.
Potential thresholds were considered using the three most commonly used methods of cut point analysis including the maximum Youden’s index, shortest distance to (0,1) and sensitivity, specificity equality. The range of optimal cut points yielded by these methods (ACT of 128–139, angle of 62.3–66.9, MA of 54–57.5 and LY30 of 2.6–7.7) was consistent with our group’s prior clinical experience.
The next objective was to move from a recommended range to distinct thresholds. We determined that our strongest recommendations could be made from the strongest ROC curves regardless of which definition of massive transfusion generated the curve. Thus, we elected to use the ROC curve yielded by >10U RBC or death in the first 6 hours to derive our recommendations for ACT and LY30 based on the stronger AUCs (0.72 vs. 0.68 and 0.72 vs. 0.69 respectively). And conversely, we employed the ROC curves produced by the > 4U RBC in any hour in the first six to determine the optimal threshold for angle and MA again based on the relative strength of these curves (0.84 vs. 0.80 and 0.83 vs. 0.81 respectively).
In comparison, Holcomb et al.’s 2012 study of consecutive trauma admissions, which concluded that r-TEG could replace CCA, used both correlation and multivariate regression analysis to validate predetermined cutoffs for TEG values based on associations with transfusion requirements 6. For instance, they assessed an ACT > 128 seconds, the same cut point arrived at through our analysis because of its historical association with INR > 1.5. In their cohort, an ACT > 128 sec was associated with an odds ratio of 1.7 for prediction of early blood requirement and an odds ratio of 1.95 for prediction of massive transfusion. Holcomb and colleagues also employed the same threshold recommendation for MA (< 55) but differed with respect to angle (< 56 vs. ≤ 65 in our analysis) and LY30 (> 3% vs. ≥ 5% in our analysis). Other key differences between these studies include: 1) We used massive transfusion as our primary outcome rather than need for early transfusion. 2) By selecting for patients with hypotension presumed due to acute blood loss, we assessed a cohort with more severe injuries as evidenced by increased injury severity score (ISS) (median (IQR) 22 (10–34) vs. 17 (9–26), increased base deficit > 8 mEq/L (43% > 8 vs. 25% > 5) and increased massive transfusion rate (19.5% vs 5%).
In conclusion, these thresholds, to our knowledge, represent the first based on an analysis of severely injured patients at high risk for TIC and provide an important standard in the evolution of TEG-guided resuscitation. Our experience can serve as a building block for a multicenter trial, which should aim to refine these recommendations for specific patient subgroups and to account for the diversity of interventions employed by different trauma centers. Furthermore, refinement of the TEG-guided resuscitation strategy should include optimizing the respective clinical interventions for each given r-TEG output.
Table 4. Mean Optimal Cut Point – value (AUC).
Provides the mean value generated from 3 methods of cut point analysis with respect to each of 4 TEG outputs and for both massive transfusion definitions. Include following ‘Results’ section of the manuscript.
| Mean cutpoint | ACT (sec) | LY30 (%) | Angle (°) | MA (mm) |
|---|---|---|---|---|
| > 10U RBC or DEATH in 6 Hours | 128 (0.72) | 5 (0.72) | 64 (0.80) | 55 (0.81) |
| > 4U RBC/hour within 6 hours postinjury | 139 (0.69) | 4 (0.69) | 65 (0.84) | 55 (0.83) |
| Death within 24 hours postinjury | 133 (0.68) | 5 (0.72) | 66 (.071) | 54 (0.64) |
| < 14 ICU-free days | 128 (0.61) | 3 (0.64) | 69 (0.68) | 57 (0.69) |
| < 21 Ventilator-free days | 133 (0.61) | 5 (0.63) | 66 (0.66) | 57 (0.69) |
Table 5. Values (%) of TEG Output Thresholds with respect to MTP.
Provides Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) values for each for the recommended optimal TEG thresholds. Include following ‘Results’ section of the manuscript.
| TEG Output | Sensitivity | Specificity | PREDICTIVE VALUE OF A POSITIVE | PREDICTIVE VALUE OF A NEGATIVE |
|---|---|---|---|---|
| ACT > 128 sec | 64 | 67 | 66 | 65 |
| LY30 > 5% | 54 | 91 | 86 | 66 |
| Angle < 65 degrees | 70 | 81 | 79 | 73 |
| MA < 55mm | 70 | 82 | 79 | 73 |
Acknowledgments
Source of Funding: This study was supported in part by National Institutes of Health grants T32-GM008315 (National Institute of General Medical Sciences), P50-GM0492221 (National Institute of General Medical Sciences), and UM 1HL120877 (National Heart, Lung, and Blood Institute) and in part by Colorado Clinical and Translational Science Award Grant UL1 TR001082 (National Center for Advancing Translational Science). Additional research support was provided by Haemonetics.
Footnotes
Author Contribution Statement
Peter M Einersen was primary author, responsible for statistical analysis and direct preparation of the manuscript text and figures. Ernest E Moore was the lead principal investigator on this project and provided direct input to the direction of the analysis of the data and writing of the manuscript. Angela Sauaia contributed in the preparation of the manuscript. Michael P Chapman, Hunter B Moore and Eduardo Gonzalez contributed significantly to study design and preparation of the manuscript. Christopher C Silliman and Anirban Banerjee provided oversight to the strategic aims of the paper and preparation of the manuscript.
References
- 1.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. doi: 10.1097/SLA.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson PI, Sorensen AM, Larsen CF, Windelov NA, Stensballe J, Perner A, Rasmussen LS, Ostrowski SR. Low hemorrhage-related mortality in trauma patients in a Level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion. 2013;53(12):3088–99. doi: 10.1111/trf.12214. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256(3):476–86. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 7.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–85. doi: 10.1097/TA.0b013e31827e20e0. discussion 85–6. [DOI] [PubMed] [Google Scholar]
- 8.Schochl H, Voelckel W, Grassetto A, Schlimp CJ. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J Trauma Acute Care Surg. 2013;74(6):1587–98. doi: 10.1097/TA.0b013e31828c3171. [DOI] [PubMed] [Google Scholar]
- 9.Schochl H, Schlimp CJ, Voelckel W. Potential value of pharmacological protocols in trauma. Curr Opin Anaesthesiol. 2013;26(2):221–9. doi: 10.1097/ACO.0b013e32835cca92. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost. 2010;36(7):723–37. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore HB, Moore EE, Chin TL, Gonzalez E, Chapman MP, Walker CB, Sauaia A, Banerjee A. Activated clotting time of thrombelastography (T-ACT) predicts early postinjury blood component transfusion beyond plasma. Surgery. 2014;156(3):564–9. doi: 10.1016/j.surg.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–7. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’Ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–14. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 14.Moren AM, Hamptom D, Diggs B, Kiraly L, Fox EE, Holcomb JB, Rahbar MH, Brasel KJ, Cohen MJ, Bulger EM, et al. Recursive partitioning identifies greater than 4 U of packed red blood cells per hour as an improved massive transfusion definition. J Trauma Acute Care Surg. 2015;79(6):920–4. doi: 10.1097/TA.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 16.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Van Calster B, Pencina MJ. Performance measures for prediction models and markers: evaluation of predictions and classifications. Rev Esp Cardiol. 2011;64(9):788–94. doi: 10.1016/j.recesp.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem. 2008;54(4):729–37. doi: 10.1373/clinchem.2007.096032. [DOI] [PubMed] [Google Scholar]
- 19.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7. doi: 10.1097/TA.0000000000000341. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]