Summary
Programmed death and shedding of epithelial cells is a powerful defense mechanism to reduce bacterial burden during infection but this activity cannot be indiscriminate because of the critical barrier function of the epithelium. We report that during cystitis, shedding of infected bladder epithelial cells (BECs) was preceded by the recruitment of mast cells (MCs) directly underneath the superficial epithelium where they docked and extruded their granules. MCs were responding to interleukin-1β (IL-1β) secreted by BECs following inflammasome and caspase-1 signaling. Upon uptake of granule-associated chymase (mouse MC protease 4 (mMCPT4)), BECs underwent caspase-1 associated cytolysis and exfoliation. Thus, infected epithelial cells require a specific cue for cytolysis from recruited sentinel inflammatory cells before shedding.
In Brief (eTOC blurb)
Choi et al. demonstrate that mast cells mediate bladder epithelial cell (BECs) exfoliation, a powerful defense mechanism during infection. Following infection, BECs secrete IL-1β in an inflammasome-dependent manner, which recruits mast cells to the superficial epithelium. Activated mast cells release chymase-containing granules which upon uptake by BECs trigger caspase-1 mediated cytolysis.
Introduction
A common inflammatory response to microbial infection at mucosal surfaces is exfoliation of pathogen-laden epithelial cells. When epithelial cells become overburdened with pathogenic bacteria, they initiate self-destruction processes resulting in reduced microbial burden (Jones et al., 1997). However, this process cannot occur indiscriminately as these epithelial cells also serve an important barrier function protecting the underlying tissue from potentially toxic substances in the lumen of the mucosal tract. Therefore, it is reasonable to expect a regulatory mechanism to coordinate this critical activity.
One of the best examples of epithelial cell shedding following microbial infection occurs in the urinary tract. Urinary tract infections (UTIs) are the second most common bacterial infections in humans (Foxman, 2010) and its treatment and management especially when recurrent or chronic is increasingly a challenge. UTIs are primarily caused by uropathogenic E. coli (UPEC) that gain access to the bladder and rapidly invade superficial bladder epithelial cells (BECs) to avoid being flushed out when urine is voided. The bladder epithelium is a stratified epithelial structure with a luminal layer comprised of fully differentiated and usually binucleated cells (Hicks, 1975). Underneath the superficial cells lie intermediate cells, which have a limited capacity to form tight junctions, followed by basal cells, which are largely responsible for the rapid cell proliferation that occurs following the exfoliation of overlying cells (Mysorekar et al., 2009). When superficial BECs become heavily infected by uropathogens, they spontaneously lose their viability and shed en masse into the urine, a reaction reported to be bacteria-triggered apoptosis (Mulvey et al., 1998). Pyroptosis is a distinct and a more recently discovered form of cell death in response to infection which so far has mostly been observed in macrophages and other hematopoietic cells following bacterial infection (Bergsbaken et al., 2009). A large cytosolic protein complex structure called the inflammasome mediates pyroptosis by evoking the cleavage and activation of caspase-1, a mediator of cytolysis. The inflammasome mediates several other important innate immune responses to microbial challenge including the maturation and secretion of proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 (Bergsbaken et al., 2009). The nature of the cellular immune response mediated by the inflammasome is often dictated by the type of complexes it forms in the subcellular milieu following stimulation (Broz et al., 2010; Chen et al., 2014).
Here, we investigated the possibility that UPEC induced exfoliation of BECs involved inflammasome dependent cytolysis because of our observation that exfoliated BECs from infected mice had undergone lytic cell death. Although inflammasome was implicated, its role in BEC lysis and exfoliation was indirect and lytic cell death required the collaboration of recently recruited MCs. These cells are the source of a critical cytolytic signal that determine which and when infected epithelial cells are shed following infection and this requirement appears be a host mechanism to prevent inappropriate and potentially harmful exfoliation of epithelial cells.
Results
Dynamics of infection-induced shedding and loss in BEC viability
To explore BEC exfoliation, we characterized its dynamics in a murine UTI model. Following catheter mediated instillation of UPEC strain CI5, a frequently utilized and recently sequenced pyelonephritis isolate (Mehershahi et al., 2015; Song et al., 2007), into the bladders of wild-type (WT) C57BL/6 mice, the bulk of BEC exfoliated between 6 and 12 h post-inoculation. Microscopic examination of mouse bladder cross sections revealed that superficial BECs were largely intact at 6 h, but their widespread loss was visually evident by 12 h post-infection (Figure 1A). This finding was supported by quantitation of residual superficial BECs on whole mounts of bladder tissue examined at 6 and 12 h post-infection or after urethral administration of saline control. By 12 h post-infection, up to 75% of superficial BECs had been lost compared to saline controls (Figure 1B). Because loss of superficial BECs has been linked to a reduction in bacterial burden, we quantitated the number of bacteria associated with the epithelium at different time points following bladder infection using standard colony counts of homogenized bladders. A significant drop in bacterial numbers (approximately 90 %) following bladder infection occurred between 6 and 12 h post-infection (Figure 1C), supporting the notion that BEC loss is a powerful mechanism to rapidly reduce infection in the bladder (Mulvey et al., 1998). Microscopy of shed BECs in urines revealed a wide range of bacterial association (heavy and moderate) (Figure 1D, left) to sparsely infected cells (Figure 1D, right).
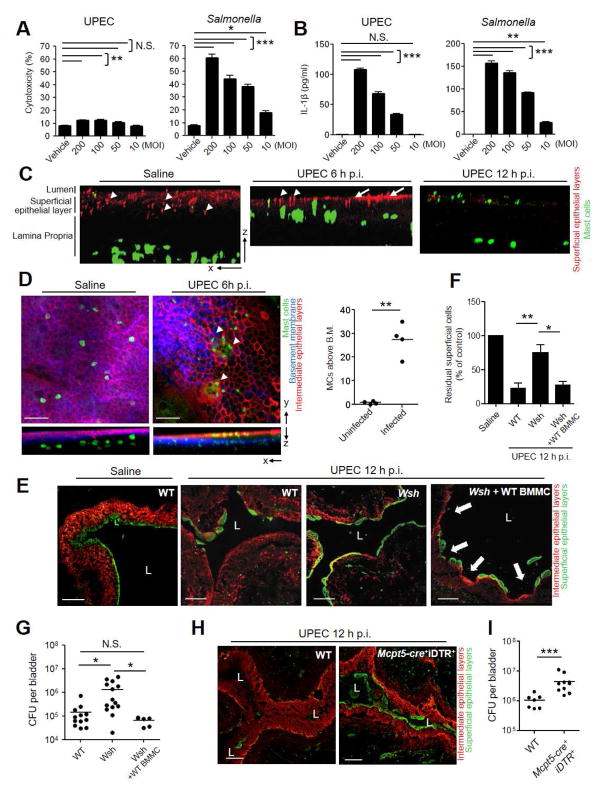
Figure 1. Shedding of mice superficial BECs during bladder infections reduces bacterial load.
(A, B, C) Significant superficial BECs shedding occurs after 6 h UPEC infection. Bladders of saline or UPEC CI5 infected C57BL/6 mice were examined at various time points post-infection (p.i.). (A) Frozen sections of bladders were stained with α-E-cadherin antibody (intermediate epithelium, red) and wheat germ agglutinin (WGA) (superficial epithelium, green). Representative image from the bladders of 3–5 mice per group. “L” indicates lumen. (B) Significantly fewer superficial BECs were observed at 12 h UPEC p.i. compared to saline controls. Bladder whole mounts were stained with α-ZO-1 antibody (tight junction) and WGA. Average number of superficial BECs from ten randomly chosen bladder fields were examined. n = 3–5 mice per group. (C) Mice infected for 12 and 24 h had significantly fewer bacteria per bladder compared to 6 h infected mice. No significant difference in CFUs were observed following 3, 6 and 9 h of infection. (D) Sedimented BECs from urines of UPEC infected mice were stained with crystal violet to show UPEC infected (left) or sparsely infected (right) BEC. (E) Relative proportion of live and dead or dying BECs shed in the urine of UPEC-infected (left) or dispase II-treated (right) mice. Dead or dying cells as indicated by positive ethidium homodimer-2 staining were quantified from randomly selected fields. n = 4–5 mice per group. Data represent 3 independent experiments. Scale bar: (A) 100 μm, (D) 50 μm. *p<0.01, **p<0.05, N.S., not significant., See also Figure S1.
Approximately 85% of shed cells were either dead or dying by lysis, based on their complete or partial acquisition of the membrane-impermeable dye ethidium homodimer-2 (Figure 1E, left, S1, top). The remaining BECs did not remain viable for long, even when placed in growth medium (data not shown). In contrast, when we purposefully induced BEC exfoliation by instilling dispase II, a mild proteolytic agent, into mouse bladders, close to 77% of shed BECs were viable (Figure 1E, right, S1, bottom). Thus, in contrast to BECs shed via non-infectious means, cells exfoliated during bacterial infection had already undergone lytic cell death or destined to die shortly thereafter.
Heavy bacterial burden is not sufficient for BEC exfoliation
We sought to verify the above observations in an in vitro assay system which allows assessment of both death and shedding in the human 5637 BEC line (5637 BECs). Infection with UPEC CI5 strain only induced minimal lytic cell death of 5637 BECs even at MOI as high as 200 (Figure 2A, left). In contrast, when we employed a Salmonella Typhimurium SL1344 strain, dose dependent and significant cell death occurred (Figure 2A, right). Salmonella induced cell lysis is a classical example of pyroptosis, a response involving inflammasome activation (Bergsbaken et al., 2009). When we compared secretion of IL-1β a marker of inflammasome activation from UPEC and Salmonella infected 5637 BECs, both pathogens evoked high and comparable amounts of IL-1β secretion (Figure 2B), indicating that inflammasome was also activated in UPEC infected 5637 BECs. Nevertheless, the failure of the UPEC strain to trigger death of 5637 BECs in vitro, in spite of the fact that in vivo it caused extensive exfoliation suggests that the pathogen or its byproducts were not sufficient to trigger bladder exfoliation and that additional host factors are involved. That bacterial factors are secondary to host factors in triggering BEC exfoliation also comes from the finding that when we substituted the pyelonephritis UPEC CI5 strain for UPEC NU14, a well characterized cystitis strain (Mulvey et al., 1998), in the infection of mouse bladders, we found comparable BEC exfoliation and residual bacterial burden (data not shown).
Figure 2. Shedding of UPEC infected BEC is dependent on MCs.
(A, B) UPEC infections to triggers secretion of IL-1β but fails to evoke lytic cell death of 5637 BECs in vitro. BECs were exposed to UPEC or Salmonella at different MOIs. After 12 h, culture supernatants were analyzed (A) for LDH release and (B) for IL-1β secretion. (C, D) MC migration into the bladder epithelium following infection. Uninfected and infected mouse bladders were harvested at 6 h and (C) 12 h p.i., whole mount stained with α-ZO-1 antibody (urothelium, red) and avidin (MCs, green). In-depth Z stack imaging of whole mounted bladders were displayed as indicated axis. Representative images of n = 3 mice per treatment. (D) Whole mounts were stained for intermediate epithelia (red), basal membrane (blue), and MCs (green). Images were processed as described in figure 2C. (Right) 4–5 random fields per bladder were Z stack-imaged, and the number of MCs that crossed basement membrane (B.M.) per field enumerated. (E) Shedding of BECs in infected MC sufficient but not MC-deficient mice. WT, MC-deficient (Wsh), and MC-repleted Wsh mice (Wsh+ BMMC) were infected with UPEC for 12 h. Frozen sections of bladders were stained for superficial (green) and intermediate (red) epithelia. (F) Whole mount quantification of superficial BECs reveal Wsh mice retain significantly more cells than WT and Wsh+BMMC mice. n = 3–5 mice per group. (G) Wsh mice have significantly higher bladder bacterial counts than WT and Wsh+BMMC mice. (H, I) Failure to exfoliate and increased bacterial burden in bladders of conditional MC-deficient mice. WT or Mcpt5-Cre+iDTR+ mice were inoculated with UPEC after MC depletion with DT. Collapsed bladders (12 h p.i.) from each group were stained and viewed (E). (I) Bladder bacterial counts presented as CFUs. Data represent 3–4 independent experiments. “L” indicates lumen. Scale bar: 100 μm. *p<0.05, **p<0.01, ***p<0.001, N.S.: Not Significant., See also Figure S2.
MCs are necessary for BEC shedding in vivo
To elucidate host factors contributing to BEC exfoliation in vivo, we examined cell types present in close proximity to the superficial epithelium in control and infected mouse bladders at different time points post infection using whole mount microscopy. From analyses of in-depth Z stacks, in addition to the usual neutrophil response to infection (data not shown), a remarkable influx of MCs directly underneath the epithelium of the bladder was observed as early as 6 h post-infection (Figure 2C, middle, right). MCs, which are normally found in the lamina propria and detrusor muscle region of bladders (Figure 2C, left), were recruited directly underneath the urothelium (Figure 2C, middle). By 12 h post-infection, a population of MCs were once again present in the lamina propria region (Figure 2C, right). Additionally, ZO-1 staining of superficial uroepithelium after 6 h in infected bladders was markedly different (Figure 2C, middle, arrows) compared with uninfected controls (Figure 2C, left, arrowheads) which is indicative of perturbation in the tight junctions between superficial epithelial cells. At 12 h ZO-1 staining is largely absent probably due to the loss of BECs. Microscopic studies of the infected bladder epithelium further revealed several MCs, having migrated across the laminin staining basement membrane, and were now aggregated underneath superficial epithelial cells (Figure 2D, middle, arrowheads) prior to their shedding (Figure 2D, left). We found MCs had crossed the basement membrane and entered the transitional epithelium in UPEC infected but not in uninfected WT mice (Figure 2D, right) suggesting a possible role for recruited MCs in BECs shedding during infection.
MCs are highly granulated immune regulatory cells that mobilize immunity against microbial attack and toxic venom (Malaviya et al., 1996; Metz et al., 2006). Following bacterial infection, bladder MCs degranulate, releasing granules that contain pre-formed mediators including histamine, tumor necrosis factor (TNF), and serine proteases (Wernersson and Pejler, 2014). Evidence for a specific role for MC TNF in the recruitment of neutrophils and in the early clearance of infecting bacteria from the bladder and other mucosal sites comes from comparative studies of UTIs in WT and MC-deficient (W/Wv) mice (Malaviya et al., 2004). MC have previously been implicated in modulating epithelial permeability in the infected gut (McDermott et al., 2003) and during inflammatory diseases such as inflammatory bowel disease (Santos et al., 2001) and neurogenic cystitis (Chen et al., 2006; Chen et al., 2007). Given this role and the MC’s role in bacterial clearance in the bladder, we investigated whether they contributed to BEC exfoliation by comparing the configuration of the superficial epithelium in WT and MC deficient mice following infection. Historically, the MC deficient mouse models employed have been c-Kit dependent such as KitW-sh/KitW-sh (Wsh) mice. Since Kit deficiency also causes some complex alterations in the immune system new mouse models of MC-deficiency based on the Cre-loxP system may be preferred (Dudeck et al., 2011; Feyerabend et al., 2011). We undertook our studies first employing Kit-dependent models and then confirmed these findings using Kit-independent MC deficient models. We examined frozen infected bladder sections from WT and MC-deficient Wsh mice at 12 h post-infection, a time point where a marked loss of superficial epithelial cells occurs. Unlike WT mice, which had largely lost their superficial layer (Figure 2E, left), the superficial bladder epithelia of MC-deficient Wsh mice were still intact (Figure 2E, middle). Quantification of superficial BECs in whole mount infected bladders indicated that while WT mice experienced a significant loss of superficial cells, Wsh mice failed to loose the superficial epithelium (Figure 2F). However, the phenotype was restored when Wsh mice had their MCs repleted (Wsh + WT BMMC) by adoptive transfer of bone marrow-derived MCs (BMMCs) (Figures 2E, right and Figure 2F), revealing a specific role for MCs in epithelial cell exfoliation. Predictably, Wsh mice retained significantly higher (approximately 10-fold higher average) numbers of bacteria in the bladder compared to WT and repleted mice at 12 h post- infection (Figure 2G). The repletion of mouse bladders was conducted as we have described previously (Chan et al., 2013). To further confirm the role of MCs, we repeated our in vivo experiments with Mcpt5-cre+iDTR+ mice, in which we conditionally depleted MCs in a kit-independent manner (Dudeck et al., 2011). With repeated i.v. administration of Diphtheria Toxin (DT) into Mcpt5-cre+iDTR+ mice, we eliminated MCs from all organs tested including the bladder (Figure S2A, S2B). Consistent with the Wsh mouse model, the absence of MCs in these mice significantly inhibited BEC exfoliation (Figure 2H, S2C, S2D), and experienced higher bacterial burden (Figure 2I) than WT mice. There was no significant difference in the BEC exfoliation and residual bacterial burden between control DT treated WT and Mcpt5-cre+ mice following infection (Figure S2C–S2E).
Since MCs evoke a vigorous neutrophil response during bladder infection (Malaviya et al., 2004), we investigated if neutrophils contributed to BEC exfoliation. We depleted the neutrophil population in WT mice by i.p. infusion of a neutrophil depleting antibody (clone:1A8). For controls, an equal amount of an isotype antibody was administered (clone: 2A3). After establishing over 95% depletion of Ly6G+CD11b+ cells in the blood of the test mice (Figure S4F, S4G), we infected the bladders of both groups of mice with UPEC. We found marked but comparable BEC loss in both groups of mice (Figure S4H, S4I), indicating that neutrophils had limited role in breakdown of bladder integument.
BEC derived IL-1β is responsible for MCs recruitment
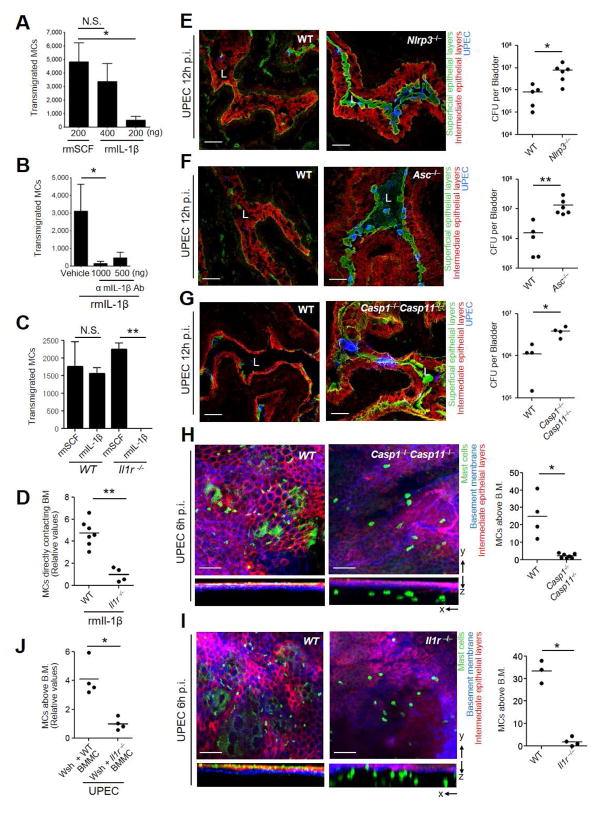
Since BECs exposed to UPEC released ample amounts of IL-1β, a known mediator of migration in certain cell types (Oliveira et al., 2008; Rider et al., 2011; White et al., 2008), we investigated if IL-1β promoted recruitment of MCs. We undertook a standard transwell migration assay where we placed BMMCs in the upper chamber of a transwell chamber and loaded recombinant mouse IL-1β (rmIL-1β) into the basolateral compartment of the chamber to create a migratory gradient. We assayed for migration of MCs to the lower chamber. Recombinant mouse stem cell factor (rmSCF), a well-known MC chemo-attractant, was tested in a parallel assay (Okayama and Kawakami, 2006). We observed dose-dependent MCs migration in response to rmIL-1β (Figure 3A) whose magnitude was comparable to MC responses to rmSCF (Figure 3A). The specificity of the MC migratory response for IL-1β was deduced by its sensitivity to α-mIL-1β antibody (Figure 3B) and by the fact that BMMC derived from an Il1r−/− mice failed to respond to IL-1β (Figure 3C). Next, we administered 200 ng of rmIL-1β into bladders of WT and Il1r−/− mice. 4 h later, we observed a 5-fold increase in MC population accumulating in basement membrane of the bladder of WT mice compared to Il1r−/− mice where most of the MCs still remained deep in the lamina propria (Figure 3D, S3). Thus, IL-1β is a potent recruiter of MCs.
Figure 3. Inflammasome mediated IL-1β recruits MCs to the site of BEC infection.
(A, B, C) IL-1β is a potent chemoattractant for MCs in vitro. (A, C) WT or Il1r−/− BMMCs were cultured on the apical side of trans-wells and rmSCF or rmIL-1β was placed on the basolateral side of the wells and migration of MCs into the basolateral side was assessed after 4 h of incubation. (B) Same as above except that rmIL-1β was pre-incubated with increasing doses of α-IL-1β antibody or vehicle (D) In vivo MC recruitment by IL-1β. WT or Il1r−/− mice were intravesicularly administered with rmIL-1β (200 ng). 4h post-treatment, whole mounted bladders were assessed for recruited MCs in direct contact with the basement membrane of WT and Il1r−/− mice. Data is presented as relative values. (E–G) Inflammasome components: Nlrp3, Asc and Casp1Casp11 are required for bladder exfoliation. 12 h p.i. with UPEC, infected bladders from (E) Nlrp3−/−, (F) Asc−/− or (G) Casp1−/−Casp11−/− mice were processed and examined. (E–G) are frozen sections of collapsed bladders stained for superficial (green), and intermediate (red) epithelia and UPEC (blue). Right panel are bladder bacterial counts presented as CFUs. (H, I, J) Defects in inflammasome reduces MC migration into infected bladder epithelium. Whole mount bladders from (H) WT and Casp1−/−Casp11−/− mice (I) WT and Il1r−/− mice, were stained for intermediate epithelia (red), basal membrane (blue), MCs (green). In-depth Z stacks were displayed as X–Y and X–Z axis. (H, I) Right panel depict MC counts above the basement membrane. (J) MC counts above the basement membrane 6 h p.i. in Wsh + WT BMMCs and Wsh + Il1r−/− BMMCs (presented as relative counts). (D, H, I, J) Quantification of MCs that had crossed the B.M. 4–5 random fields per bladder were Z stack-imaged, and the number of MCs that crossed B.M. or reached B.M. per field was enumerated. Representative images are shown. Scale bar: 100 μm. “L” indicates lumen. *p<0.05, **P<0.01, N.S.: Not Significant., See also Figure S3.
Bladder exfoliation and MC recruitment is NLRP3 inflammasome dependent
We investigated the role of components in the inflammasome activation cascade employing genetically modified mice. We compared bladder bacterial load following UPEC infection between WT C57BL/6 and mice deficient in various (Nlrp3−/−, Asc−/− or Casp1−/−Casp11−/−) inflammasome components. The bacterial load in each of the genetically modified mice was significantly higher than corresponding WT mice (Figures 3E–G). Consistent with this finding, bladder cross-sections revealed the persistence of a large swathe of bacteria and an intact superficial epithelium in each of the infected genetically modified mice (Figures 3E–G).
We also closely examined the precise MC location in the bladder mucosa of these mutant mice. In the bladders that failed to exfoliate, MCs remained in the lamina propria, having failed to cross the basement membrane into the transitional epithelial layer. Shown in Figure 3H is the relative location of MCs in WT and Casp1−/−Casp11−/− mice. We compared the number of MCs that had crossed the basement membrane and entered the transitional epithelium between uninfected and UPEC infected WT mice. While UPEC infection induces MC to migrate in WT mice, we found that MC did not migrate in Casp1−/−Casp11−/− mice, despite UPEC infection (Figure 3H). To confirm the role of IL-1β in MC migration we examined the location of MCs in Il1r−/− mice after infection and found, as expected little or no MC migration into the superficial epithelium following infection (Figure 3I). To more definitively establish that MC recruitment during UPEC infection of the bladder was specifically mediated by IL-1β, we compared the recruitment of MCs into the bladder epithelial regions of Wsh mice repleted with WT BMMC or with BMMC from Il1r−/− mice. Up to 4 fold increase in the MC population in Wsh mice repleted with WT BMMC compared to Wsh mice repleted with Il1r−/− BMMC (Figure 3J). Taken together, our data suggest that BEC derived IL-1β is the critical determinant responsible for MC recruitment during UPEC infection. Cumulatively, there was limited exfoliation in spite of a heavy bacterial load in BECs of mice that failed to secrete IL-1β due to defects in inflammasome signaling. Importantly, these mice also failed to recruit MCs out of the lamina propria.
Lytic cell death of UPEC-infected BEC is triggered upon endocytosis of MC granules
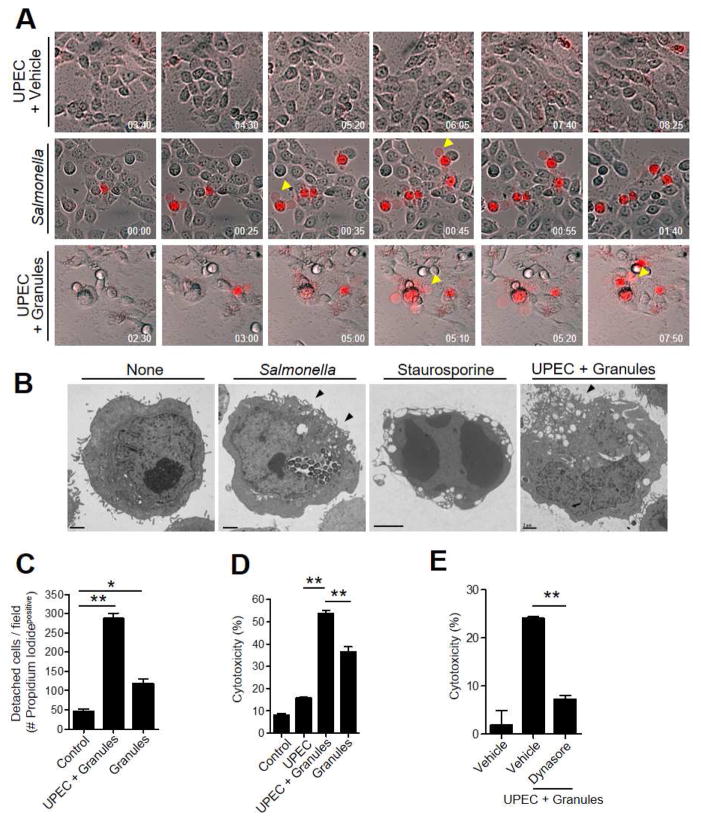
Our in vivo studies have suggested that a signal of a heavy bacterial burden on BECs was insufficient for exfoliation of BECs. There appeared an additional need to recruit MCs to the site of infection. Since MCs are characterized by release, upon stimulation, of numerous highly stable granules (Wernersson and Pejler, 2014), we wondered if loss of viability and shedding of BECs could be linked to uptake of MC granules. We studied in vitro time-lapse images of UPEC-infected 5637 BECs in a medium containing a membrane impermeable fluorescent dye, propidium iodide, following exposure to MC granules from a rat basophilic leukemia (RBL-2H3) MC line (Kunder et al., 2009). These 300 nm particles exhibited the characteristic round morphology of MC granules (Figure S4A). A few hours after exposure to MC granules, we observed UPEC-infected 5637 BECs undergoing lytic cell death, visualized by the entry of propidium iodide into cells (Figure 4A, bottom, arrowheads, Movie 3). This manner of cell death involving cell membrane eruptions is distinct from apoptosis but resembled cytolysis (Bergsbaken et al., 2009). Oxidative stress-induced necrosis was ruled out here also because the Nexrox-2 inhibitor had no significant effect in reducing MC granule-mediated cytotoxicity in infected 5637 BECs (Figure S4B). As Salmonella Typhimurium mediated-cell death is the prototype for pyroptosis (Bergsbaken et al., 2009), we compared our findings with Salmonella-infected 5637 BECs and observed a morphologically identical form of cell death (Figure 4A, middle, arrowheads, Movie 2). Very little cell death in UPEC-infected 5637 BECs was observed in the absence of granules during the same incubation period (Figure 4A, top, Movie 1). Transmission electron microscopy (TEM) of the cellular morphology of 5637 BECs treated with staurosporine, an inducer of apoptosis, and UPEC-infected 5637 BECs treated with MC granules was performed. Whereas staurosporine-treated BECs lost most of their cytoplasmic contents and underwent nuclear fragmentation, MC granule-treated infected 5637 BECs retained their cytoplasmic contents without any nuclear fragmentation (Figure 4B). The morphological changes observed in MC granule-treated infected 5637 BECs were limited to plasma membrane perturbation, which was also seen in Salmonella infected BECs (Figure 4B, arrowheads).
Figure 4. UPEC-infected BEC undergo lytic cell death following endocytosis of MC granules.
(A, B) Cell lysis and intracellular cytotoxicity induced by UPEC+granules or Salmonella. Infected 5637 BECs were exposed to vehicle, MC granules or Salmonella. (A) 1 h post-exposure, media were replaced with propidium iodide and time lapse imaging performed. Arrowheads depict sites of release of cellular contents. (B) 5637 BECs were exposed to vehicle, the apoptosis inducer staurosporin (1 μM), UPEC+granules or Salmonella for 16 h. Thereafter, cell cross sections were observed by TEM. Arrow heads depict localized membrane perturbations. (C, D, E) Endocytosis of MC granules induce BEC detachment and death of 5637 BECs in vitro. (C) Quantification of propidium iodide positive BECs following exposure to vehicle, UPEC+granules or granules. (D) LDH release from BECs following exposure to vehicle, UPEC, UPEC+granules or granules (E) LDH release from BECs following pretreatment with vehicle or dynasore (30 min 100 μM), followed by UPEC+granules. Data represent 3 independent experiments. Scale bar: 2 μm. *p<0.05, **P<0.001, See also Movies S1, S2, S3 and Figure S4.
Next, we quantified the cytotoxic effect of MC granules upon BECs. Here, we chose to examine cell detachment as a measure of cell death. Since cell detachment in the infected bladder was closely linked to loss of BEC viability, we investigated whether cell detachment occurred in adherent in vitro cultured 5637 BECs and if it paralleled loss in cell viability. We cultured the 5637 BECs to confluence on cell culture plates and infected cells with UPEC. We applied isolated MC granules onto the 5637 BECs, and after 16 h we collected shed cells and examined them for viability using propidium iodide dye, which fluorescently stains dead cells. Compared to untreated 5637 BECs controls or untreated infected 5637 BECs, MC granules induced a significant loss of viability in infected 5637 BECs (Figure 4C, S4C). Isolated MC granules also caused significant loss of viability even in uninfected 5637 BECs, albeit not to the same level as infected 5637 BECs (Figure 4C, S4C). When we stained residual cells on plates with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) dye, which fluorescently stains live cells, large dark patches indicating cell exfoliation were observed (Figure S4D). The number of detached cells amongst adherent in vitro cultured 5637 BECs closely paralleled their loss in viability, revealing that cell detachment was an effective parameter to monitor cell viability. Another quantitative measure of cytotoxicity, was assessment of the culture medium for lactate dehydrogenase (LDH) release. Significant cell death was induced by MC granules, which is consistent with the propidium iodide dye staining assays (Figure 4D). Next we examined if internalization of MC granules was necessary for cytolysis by comparing cytolysis in vehicle treated 5637 BECs and 5637 BECs pretreated with dynasore, a dynamin inhibitor that blocks cellular phagocytic activity. Dynasore treated 5637 BECs were protected from MC granule-induced cell death (Figure 4E) and detachment (Figure S4E). Therefore, internalization by BECs was necessary for MC granules to manifest its cytotoxic actions.
Endocytosed granules induce disruption of lysosomal vesicles
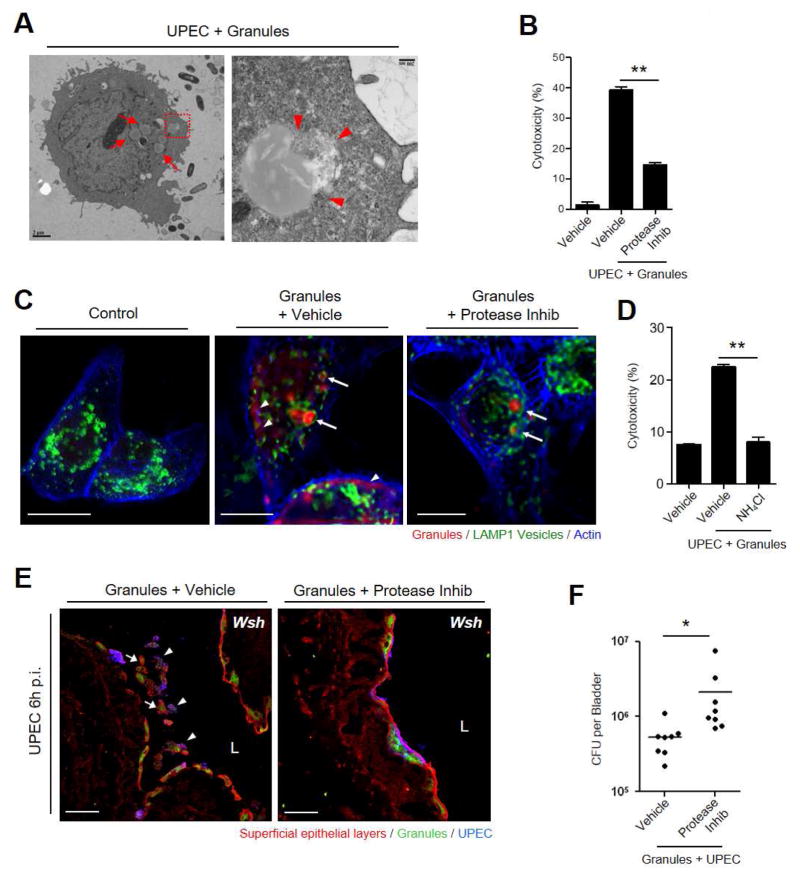
To elucidate how MCs elicited cell lysis, we investigated the fate of MC granules inside 5637 BECs following uptake through high resolution TEM which revealed the presence of multiple intracellular MC granules per cell (Figure 5A, left, arrows). In several cases, the intracellular granules appeared to be breaking out of their endocytic compartment (Figure 5A, right, arrowheads) implying intrinsic proteolytic abilities. MC granules contain various kinds of inflammatory mediators, prominently proteases (Wernersson and Pejler, 2014). We tested whether cytotoxicity in BEC was linked to these proteases. Using LDH assay, we confirmed the contribution of proteolytic enzymes borne in MC granules to cytotoxicity by showing that pretreating MC granules with a protease inhibitor cocktail (protease inhib: 10 μM aprotinin, 100 μM leupeptin, 100 μg/ml Soybean Trypsin Inhibitor, and 50 μM chymostatin) prevented lysis of 5637 BECs following uptake of treated MC granules (Figure 5B, S5A). Using fluorescently-conjugated avidin (Nakamura et al., 2013) as a probe for MC granules, we observed that following exposure to 5637 BECs, MC granules were harbored within LAMP1+ lysosomal compartments (Figure 5C, middle, arrows). Diffuse avidin staining (arrowheads) was also detected in the cytosol of these cells (Figure 5C, middle), suggesting disruption of lysosomes and seepage of granule components into the cytosol. To see if MC proteolytic enzymes were responsible for the breakdown of lysosomal membranes, we once again examined the effects of pretreating MC granules with the protease inhibitor cocktail prior to exposure to infected BECs. In contrast to untreated granules, protease inhibitor cocktail-treated MC granules remained strictly within LAMP1+ compartments with limited seepage into the cytosol (Figure 5C, right). Through pretreatment of infected 5637 BECs with ammonium chloride, we also ascertained the importance of the acidification of granule-containing compartments in cell death (Figure 5D). Similarly, neutralization of LAMP1+ compartment with ammonium chloride (NH4Cl) also prevented MC granules mediated BEC shedding (Figure S5B). Cumulatively, MC granule-induced cytotoxicity appears to be initiated by their endocytosis and subsequent seepage of granule contents from lysosomes of infected BECs.
Figure 5. Protease from endocytosed granules induce cell death and exfoliation.
(A) Breakdown of endocytosed MC granules and leakage of contents into BEC cytosol. BECs with intracellular bacteria and MC granules (arrows). Magnified image of granule intruding into cytosol (arrowheads). (B) Protease inhibitors block granule-induced cytoxicity in infected BECs. Granules were pre-incubated (30 min) with or without protease inhibitor cocktail and added to pre-infected 5637 BECs. Culture supernatants were analyzed for LDH release. (C) Endocytozed granules are encased in LAMP1+ lysosomes (arrows). Cells were stained for granules (avidin, red), actin (phalloidin, blue), and lysosome (α-LAMP1 antibody, green). Granule contents (stained red) appear diffusely in cytosol (arrowheads) (middle), indicating disruption of lysosome. Disruption of lysosome and leakage of granule contents is blocked in presence of protease inhibitor (right). (D) Inhibitors of endosome acidification block granule-induced cytotoxicity in infected 5637 BECs. Granules were added to pre-infected cells with or without pre-treatment (2 h) with NH4Cl, followed by LDH release assay. (E) Instillation of MC granules, but not protease inhibitor cocktail-treated granules, into UPEC-infected bladders of Wsh mice triggers exfoliation of superficial cells. Detached superficial cells (WGA, red) containing granules (avidin, green) could be seen in the lumen (L) (left), with (arrowheads) or without (arrows) UPEC. Bladders were harvested after 6 h p.i. (F) Granule treatment but not granule+protease inhib reduced bacterial load in the bladders of Wsh mice. Scale bar: (A) 2 μm (left), 200 nm (right), (C) 10 μm, (E) 50 μm. Data represent 3 independent experiments. *p<0.05, **P<0.001, See also Figure S5.
Next, we investigated whether the effect of MC proteases in vitro are applicable in vivo. Predictably, exogenous intravesicular application of MC granules would spontaneously induce significant exfoliation of BECs in the bladders of MC deficient mice (Wsh) that are naturally defective in their capacity to exfoliate BECs. To test this notion, we transurethrally administered MC granules into the bladders of UPEC-infected Wsh mice (Figures 5E, 5F). 6 h post-infection, we harvested the bladders to determine the status of the superficial epithelium. We observed marked detachment of infected (arrowheads) as well as uninfected (arrows) superficial BECs (Figure 5E, left). Exfoliated epithelia were positively stained for MC granules (arrows), however, when these MC granules were pre-incubated with protease inhibitor cocktail prior to administration, we observed comparable uptake of granules but limited shedding of superficial BECs (Figure 5E, right). Correspondingly, infected mice administered MC granules harbored significantly fewer bacteria in their bladders, compared to infected mice given granules pretreated with protease inhibitor cocktail (Figure 5F). These in vivo studies point to a key role played by MC proteases in promoting BEC shedding following infection in mice. These granules appeared intracellular indicating that MC granules might be provoking exfoliation from within.
Cytolysis and exfoliation of BEC evoked by recombinant MC chymase
To our knowledge, neither MCs nor their proteases have previously been implicated in cell death, we sought to identify the specific MC protease involved. As chymase is a serine protease that is highly expressed in MC granules (Wernersson and Pejler, 2014), we investigated the actions of mMCPT4, the functional homologue of human chymase (CMA1), on BEC shedding during UPEC infection in Mcpt4−/− and WT mice (Tchougounova et al., 2003). Since mMCPT4 is only expressed in MCs, any physiological consequence of its absence can be linked to MCs. Markedly less exfoliation of BECs and increased bladder bacterial burden was observed in Mcpt4−/− mice compared to WT counterparts (Figures 6A, 6B), directly implicating mMCPT4 in the death of BECs. To confirm that the origin of mMCPT4 in the bladder is the MC, we systemically repleted Wsh mice with BMMCs derived from WT or Mcpt4−/− mice. When both groups of mice were infected, only mice repleted with WT BMMC exhibited significant exfoliation of BECs and reduced residual bacterial load (Figure S6). It is noteworthy that granzyme, a granule-stored serine protease produced by professional cytotoxic cells, was previously shown to evoke cellular apoptotic death by directly cleaving and activating procaspases-3 in target cells (Goping et al., 2003) a reaction apparently independent of any inflammasome complex. Therefore, we wondered if MC chymase was capable of directly cleaving and activating caspase-1, a mediator of cytolysis and if this reaction was sufficient to cause death of BECs. We generated a fusion protein of recombinant mMCPT4-TAT fused to a membrane permeable oligopeptide (RKKRRQRRR), which enabled rapid penetration of the fusion protein into the BEC cytosol upon exposure (Gump and Dowdy, 2007). 5637 BECs underwent dose dependent lytic cell death (Figure 6C). To mimic the in vivo actions of isolated MC granules, we instilled the fusion protein into the bladders of UPEC infected Wsh mice and observed spontaneous loss of the superficial epithelium, resulting in significant loss in bacterial load (Figures 6D, 6E). Thus, in both in vitro and in vivo assays, recombinant rodent chymase evoked death of BECs. We examined if mMCPT4-TAT induced cell death was attributable to cleavage and activation of procaspase-1. When isolated procaspase-1 was exogenously exposed to mMCPT4-TAT, dose dependent cleavage of procaspase-1 occurred (Figure 6F), which paralleled an increase in caspase-1 activity (Figure S6C). Thus, mMCPT4-TAT mediated cytolysis of BECs correlated with direct cleavage of the zymogen, caspase-1.
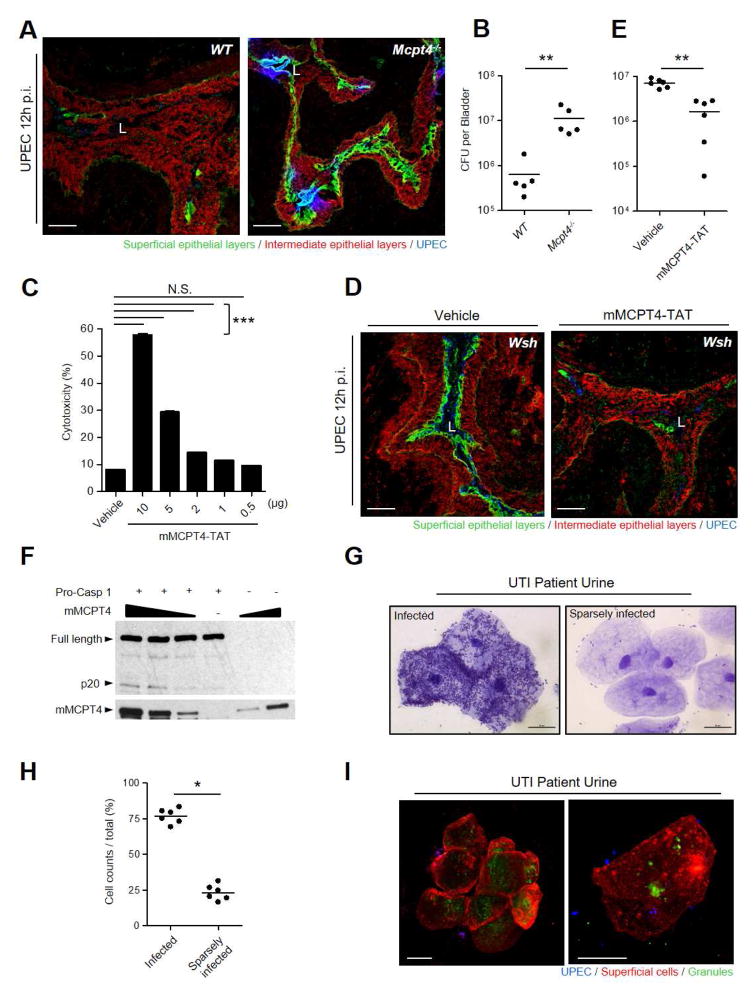
Figure 6. mMCPT4 induces cytolysis and exfoliation of BEC.
(A, B) Bladders of Mcpt4−/− mice exfoliated less and had higher CFU than WT 12 h p.i. (A, D) Frozen sections of collapsed bladders stained for superficial epithelia (green), intermediate epithelia (red), and UPEC (blue). (C) rmMCPT4-TAT induce lytic cell death of 5637 BECs in vitro. Various doses of rmMCPT4-TAT were added to 5637 BECs, followed by LDH assay 12 h post-treatment. (D, E) mMCPT4-TAT induced exfoliation of infected bladder epithelium in MC deficient (Wsh) mice is associated with reduced bacterial burden. Bladders of Wsh mice were infected with UPEC for 1 h, followed by intravesicular instillation of rmMCPT4-TAT or vehicle. Bladders were examined 12 h later. (E) Bacterial burden in bladders. (F) Direct cleavage of procaspase 1 mediated by rmMCPT4-TAT. Increasing concentrations of rmMCPT4-TAT were incubated with recombinant procaspase 1 for 1h. Full-length and cleaved procaspase as well as rmMCPT4-TAT-His6 were detected by α-caspase 1 antibody (p20 active or full length unit) and α-His6 antibody, respectively. (G–I) Examination of BECs from urine samples (5 randomly selected patients with acute UTIs). (G) Crystal violet staining of sedimented superficial BECs showing highly UPEC infected (left) and sparsely infected (right) BECs. (H) Relative numbers of bacteria-infected and sparsely infected BECs in urine (n=565 cells). (I) MC granule remnants visualized inside urothelial cells shed in urine of UTI patients. A cluster of cells (left) and two isolated cells at a higher magnification (right). Sedimented BECs were stained for superficial BECs (WGA, red), UPEC (α-E. coli antibody, blue), and MC granules (avidin, green). Scale bar: (A, D) 100 μm, (G, I) 20 μm. “L” indicates lumen. Data represent 2–3 independent experiments. *p<0.05, **P<0.01, ***P<0.001, See also Figure S6.
Finally, in an attempt to link some of the in vivo findings in mouse models to humans, we subjected exfoliated BECs obtained from urines of patients with UTIs to microscopy. A wide variation in the bacterial load on the exfoliated BECs was observed in each patient with as many as 25 % of them having sparse or no adherent bacteria (Figures 6G, 6H), which consistent with the notion that the presence of a heavy bacterial load was not the only signal for exfoliation. We found evidence of MC involvement in the exfoliation of BECs as many shed cells stained positive for either MC granules or granule contents, directly implicating MCs in the exfoliation process (Figure 6I).
Discussion
The extensive exfoliation of bladder cells following bacterial infection was previously assumed to be the consequence of apoptotic cell death triggered when host cells became heavily infected (Mulvey et al., 1998). Predictably, individual cells lining the bladder epithelium upon infection would “silently” and indiscriminately shed depending on their bacterial load while exposing the underlying tissue to obnoxious and toxic contents of urine which, however, is not the case. Here, we reveal that death and shedding of BECs during UTIs is a carefully regulated host activity that specifically involves MCs, which are prominent immune regulatory cells. During infection, these cells cross the basement membrane, traverse the intermediate epithelium of the bladder and then aggregate underneath the superficial epithelium to release their cytolytic granules. This finding echoes earlier suggestions that MCs may migrate out of the lamina propria into the urothelial region and that these cells may contribute to epithelial lesions during neurogenic cystitis (Chen et al., 2006; Chen et al., 2007). The dual requirement of inflammatory cues followed by a prompt from recruited MCs before shedding of BECs can occur could be a host requirement to avoid inappropriate shedding of epithelial cells, for example when innocuous commensal bacteria deposit on the epithelium. This host strategy also ensures that a versatile immune regulatory cell is proximally localized at the break in the epithelial barrier before any underlying tissue is exposed to urine contents. What actually activates these recruited MCs in the infected superficial epithelium is currently not known. We have previously noticed that crosslinking of CD48 molecules on MC membranes by FimH adhesins on UPEC can result in MC degranulation (Malaviya et al., 2004). However, cationic peptides secreted by stressed BECs, such as β–defensins, may also activate MCs through Mas-related gene X2 (Subramanian et al., 2013). While MCs can potentially recruit neutrophils and other immune cells into the exposed site to combat infection through the secretion of TNF and various chemokines (Malaviya et al., 1996), they can also abruptly halt local inflammatory reactions through the release of IL-10 (Chan et al., 2013). Recently, it was shown that in mice models of UTI, about 6 h following UPEC infection, a time point that our current studies indicate is when loss of the epithelial barrier begins, MCs abruptly switch from a proinflammatory program into an anti-inflammatory one (Chan et al., 2013). This activity was shown to prevent development of harmful immune response to urine contents associated with the loss of the epithelium following bacterial infection (Chan et al., 2013). However, this activity could also serve to facilitate the rapid regeneration of superficial epithelium (Chan et al., 2013), which cannot occur unless inflammation is halted. In any case, the current studies reveal that in addition to regulating pro- and anti-inflammatory reactions in the bladder, MCs play a critical role in directly mediating exfoliation of BECs a powerful host defense mechanism to reduce bacterial load. This MC- induced exfoliation of epithelium is distinct from the previously described role of these cells in inducing increased mucosal permeability during certain inflammatory conditions, which mainly involves the cleavage of tight junctions by MC proteases (McDermott et al., 2003). Although our data point to a major role for MCs in mediating exfoliation during bladder infections, we cannot rule out the complementary role of UPEC-associated virulence factors such as FimH (Mulvey et al., 1998) and hemolysin (Nagamatsu et al., 2015) in this activity as these factors are necessary for bacterial colonization of the bladder in spite of the powerful natural defenses that are present.
MC chymase is currently best known for promoting pathological activities at various inflammatory sites (Wernersson and Pejler, 2014) but they have also been implicated in certain beneficial activities such as degradation of toxic venom (Metz et al., 2006) and in degrading local proinflammatory cytokines to temper harmful inflammation (Zhao et al., 2005). However, none of these activities explain why large amounts of these proteases are specifically stored within discrete MC granules or why these proteases still remain associated with granule remnants even after their extracellular release. MC granules are internalized by infected and, to a lesser extent, uninfected BECs, resulting in the detachment and death of these cells. The “readily phagocytozable” MC granules serve as highly effective vehicles for intracellular delivery of concentrated amounts of chymase to achieve cell death. Another major protease found in MC granules is tryptase which becomes functionally active only at acidic pHs (Wernersson and Pejler, 2014). Although not proven, granular tryptase could contribute to membrane breakdown of lysosomes following uptake by BECs and cause seepage of lysosomal contents including granule-associated chymase into the cytosol. MC chymase can potentially evoke cell lysis from the cytosol via multiple mechanisms and we have shown that at least one possible mechanism is direct cleavage and activation of caspase-1. This cytolytic pathway resemble the actions of granzymes which are also granule associated cytolytic proteases released by professional cytotoxic cells such as natural killer cells and T cells to lyse infected cells (Lieberman, 2003). Within target cells, granule-borne granzyme leak out of endosome compartments into the cytosol via pore channels formed by perforin, another granule component (Lieberman, 2003). Once in the cytosol, granzyme cleaves and activates specific caspases as suggested here for MC chymase to cause apoptotic cells death which is not typically associated with inflammation. Thus, unlike cell death induced by professional cytotoxic cells, MC induced death of target cells is likely to further enhance inflammatory response.
Although MCs appear to be the primary mediators of epithelial cell shedding and death in the bladder following infection, we could not immediately rule out the contribution of cytolytic granules produced by other immune cells, especially those recruited by MCs (as these cells are also not present in the bladder of MC deficient mice). Indeed, large numbers of neutrophils are recruited by MCs within hours to sites of infection, and they are rich in proteolytic granules (Malaviya et al., 1996). However, neutrophil depletion studies revealed that these cells failed to contribute to BEC exfoliation. BECs may also not be the only targets of extracellularly released cytolytic granules by MCs during infection. Various phagocytic cells have been known for decades to readily internalize extracellularly released granules at inflammatory sites, but the outcome of this action was poorly understood (Baggiolini et al., 1982). If these internalized granules also cause death in these cells, this could represent a powerful homeostatic mechanism for restricting inflammation caused by immune cells, particularly spent cells.
We have observed that IL-1β is a potent inducer of MC migration which is released by BECs upon infection by UPEC. MCs lacking IL-1β receptor were found incapable of mediating a migratory response to this proinflammtory cytokine. It is noteworthy that the IL-1β response of BECs is not accompanied by cell death even though inflammasome components Nlrp3, Asc and activated caspase-1 are integral to this response. Why cell death was not observed at this time is not known but there are increasing examples of caspase-1 activation resulting in IL-1β secretion but no accompanying cell death (Broz et al., 2010; Chen et al., 2014). Presumably, the specific composition of the inflammasome superstructure that is formed following cell activation dictates whether or not IL-1β secretion is also accompanied by cell death.
Shedding of infected epithelial cells is a powerful host mechanism to rapidly reduce bacterial burden on various mucosal sites following infection. Our studies focusing exclusively on the bladder reveal that following UPEC infection, BEC shedding is mediated by MCs which migrate to the superficial epithelium and extrude cytolytic granules. Although MCs are in very close proximity to the epithelium at other mucosal sites, whether they also regulate epithelial cell shedding at these mucosal sites remain to be investigated.
Experimental Procedures
Mouse and bacterial strains
Six- to eight-week-old female mice were utilized for in vivo experiments. The following moue strains were employed: C57BL/6, KitW-sh/W-sh, Casp1−/−Casp11−/−, Asc−/−, Nlrp3−/−, iDTR, Il1r−/− (Jackson Laboratories), Mcpt4−/− (generated as described previously) (Tchougounova et al., 2003) and Mcpt5-cre+ (a gift from Dr. Axel Roers, University of Technology, Dresden). MCs in Mcpt5-cre+iDTR+ mice were conditionally depleted as follows: 8-week-old Mcpt5-cre+iDTR+ mice, C57BL/6 littermates, or Mcpt5-cre+ mice were given 5 i.v. injections of 100 ng of diphtheria toxin/mouse within 1 week. UPEC strain CI5 (Song et al., 2007) or Salmonella Typhymurium strain SL1344 was utilized for murine infection experiments or in vitro experiment. All animal experiments were performed with the approval of the Duke University Animal Care and Use Committee.
Culture of BMMCs and repletion of MC-deficient mice
BMMCs culture from WT C57BL/6, Il1r−/−, or Mcpt4−/− mice or repletion of Wsh mice was performed as previously described (Chan et al., 2013). Briefly, to replete Wsh mice, 107 BMMCs were intravenously infused and allowed to repopulate for 15 weeks, after which mice were utilized for in vivo infection experiments as described in the previous section.
In vitro cell culture
The human 5637 bladder epithelial cell line (ATCC) was cultured in RPMI 1640 medium containing 10% FBS, 1% HEPES, 1% sodium pyruvate, and 1% glucose (all from Gibco).
Mast cell migration assays
A Chemotaxis assay kit (Trevigen) was utilized and detailed procedures are described in the Supplemental section.
Immunofluorescent staining and microscopy
For frozen tissue samples, frozen bladder tissue were sectioned and fixed in ice-cold acetone. After blocking with 1% BSA-PBS (Gibco), samples were stained with primary and secondary antibody. For whole mount bladders visualization, bladders were fixed for 2 h in 4 % PFA and blocked with buffer having 0.3 % Trion, 2.5 % normal goat serum (Gibco) in 1% BSA-PBS. Then, samples were stained with primary and secondary antibodies. For imaging human BEC, 5637 cells were grown on glass cover slip and treated as described. 4 % PFA fixed cells and 0.1 % saponin in 1% BSA-PBS solution permeabilized and blocked the samples. Primary antibody and secondary antibodies and phalloidin-Alexa647 (Invitrogen) stained samples. A Nikon ECLIPSE TE200 and Zeiss 780 upright confocal microscope were used for obtaining confocal images via a channel-series approach. For in vitro live imaging, 5637 BECs were grown on MatTek plate (MatTek Corporation) and were infected with UPEC or applied with granules as described. After propidium iodide (Molecular Probes) was applied to the media of cells, cells were placed under live cell station which is maintained at 37°C and 5% CO2 with humidification. Zeiss Axio Observer microscope captured live moments.
Caspase-1 cleavage
20 μg of mouse recombinant procaspase-1 were incubated with increasing amounts (0, 10, 20 μg) of rmMCPT4 for 1 h at 37°C in 27 mM Tris-HCl buffer containing 150 mM NaCl pH 7.4. Procaspase-1 cleavage was visualized by immunoblot using α-caspase-1 (p20) (Adipogen). mMCPT4 was detected by α-His6 antibody (Roche).
Statistical analyses
Statistical analyses were performed using GraphPad Prism v.6 (GraphPad Software). Bacterial numbers were analyzed with the Mann-Whitney U test or one-way or two-way ANOVA and Tukey’s post-test, and p<0.05 was considered statistically significant. Error bar represent ±SEM.
Additional Experimental Procedures
Detailed experimental procedures pertaining to BMMCs culture, in vivo infections, in vitro assays, MC granule related studies, and microscopy are available in Supplemental experimental procedures.
Supplementary Material
Highlights.
Mast cells trigger bladder epithelial cell (BEC) exfoliation during E. coli infection.
During infection, BECs secrete interleukin-1β which recruits mast cells.
Mast cells release granules which are endocytosed by BECs triggering lytic death.
Mast cell chymase is the critical component in granules causing cell death.
Acknowledgments
We thank A. Roers for the Mcpt5-Cre+iDTR+ mice. We thank L. Hellman for providing the Mcpt4 clone, and S. Dowdy for the pTAT-2.1 vector. We thank J. Shipley-Phillips and M. Dykstra for assistance with electron microscopy. J. Hart is gratefully acknowledged for critical review of this manuscript. We thank R. Valdivia, P. Seed, G. Taylor, and T. Lechler for helpful suggestions and comments. S.N. Abraham is the cofounder and chief scientific officer for Mastezellen Bio Inc. This work was funded by U.S. National Institutes of Health grants: U01-AI082107; R01-AI096305; R56-DK095198.
Footnotes
Author Contributions
H.C. and S.E.B. conducted the majority of the experiments; C.Y.C. assisted with Wsh mouse repletion and in the design of the study; A.J.M., E.M., Y.M., and M.A provided in sightful suggestions and helped editing of the manuscript; H.C., S.E.B. and S.N.A. designed the study and wrote the manuscript; and the other authors read the paper and commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baggiolini M, Horisberger U, Martin U. Phagocytosis of mast cell granules by mononuclear phagocytes, neutrophils and eosinophils during anaphylaxis. International archives of allergy and applied immunology. 1982;67:219–226. doi: 10.1159/000233022. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nature reviews Microbiology. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell host & microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2014;8:570–582. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol. 2006;175:754–759. doi: 10.1016/S0022-5347(05)00171-0. [DOI] [PubMed] [Google Scholar]
- Chen MC, Keshavan P, Gregory GD, Klumpp DJ. RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. Am J Physiol Renal Physiol. 2007;292:F1372–1379. doi: 10.1152/ajprenal.00472.2006. [DOI] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, Peschke K, Vohringer D, Waskow C, Krieg T, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, Radermacher P, Moller P, Benoist C, Mathis D, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Goping IS, Barry M, Liston P, Sawchuk T, Constantinescu G, Michalak KM, Shostak I, Roberts DL, Hunter AM, Korneluk R, Bleackley RC. Granzyme B-induced apoptosis requires both direct caspase activation and relief of caspase inhibition. Immunity. 2003;18:355–365. doi: 10.1016/s1074-7613(03)00032-3. [DOI] [PubMed] [Google Scholar]
- Gump JM, Dowdy SF. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Hicks RM. The mammalian urinary bladder: an accommodating organ. Biological reviews of the Cambridge Philosophical Society. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. The American journal of pathology. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. The Journal of experimental medicine. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nature reviews Immunology. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Abraham SN, Malaviya R. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunology letters. 2004;91:103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehershahi KS, Abraham SN, Chen SL. Complete Genome Sequence of Uropathogenic Escherichia coli Strain CI5. Genome Announc. 2015;3 doi: 10.1128/genomeA.00558-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell host & microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E871–880. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation. 2008;31:36–46. doi: 10.1007/s10753-007-9047-x. [DOI] [PubMed] [Google Scholar]
- Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. Journal of immunology. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell host & microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. beta-Defensins activate human mast cells via Mas-related gene X2. Journal of immunology. 2013;191:345–352. doi: 10.4049/jimmunol.1300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. The Journal of experimental medicine. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nature reviews Immunology. 2014;14:478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- White SR, Fischer BM, Marroquin BA, Stern R. Interleukin-1beta mediates human airway epithelial cell migration via NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1018–1027. doi: 10.1152/ajplung.00065.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. Journal of immunology. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.