Abstract
Objective
To analyze the treatment effect of calcium + Vitamin D supplementation, hormone therapy, both, and neither on cardiovascular disease risk factors.
Methods
We conducted a prospective, randomized, double-blind, placebo controlled trial among Women's Health Initiative participants. The predefined primary outcome was low-density lipoprotein cholesterol (LDL-C).
Results
Between September 1993 to October 1998, a total of 68,132 women aged 50-79 were recruited and randomized to the WHI-Dietary Modification (WHI-DM) (n=48,835) and WHI-Hormone Therapy (WHI-HT) trials (n=27,347). Subsequently, 36,282 women from WHI-HT (16,089) and WHI-DM (n=25,210) trials were randomized in the WHI-calcium + Vitamin D (WHI-CaD) trial to 1,000 mg of elemental calcium carbonate plus 400 IU of vitamin D3 daily or placebo. Our study group included 1,521 women who participated in both the HT and CaD trials and were in the 6% subsample of trial participants with blood sample collections at baseline and years 1, 3, and 6. The average treatment effect with 95% confidence interval, for LDL-C, compared to placebo, was −1.6 ,(95th CI–5.5, 2.2) mg/dL for calcium + Vitamin D-alone, −9.0 (95th CI, −13.0, −5.1) mg/dL for hormone therapy alone, and −13.8 (95th CI,−17.8, −9.8) mg/dL for the combination. There was no evidence of a synergistic effect of calcium + Vitamin D + hormone therapy on LDL-C (p-value for interaction (p-int) = 0.26) except in those with low total intakes of vitamin D, for whom there was a significant synergistic effect on LDL (p-int = 0.03).
Conclusion
Reductions in LDL-C were greater among women randomized to both calcium + Vitamin D and hormone therapy than for those randomized to either intervention alone or to placebo. The treatment effect observed in the calcium + Vitamin D + hormone therapy combination group may be additive rather than synergistic. For clinicians and patients deciding to begin calcium + Vitamin D supplementation, current use of hormone therapy should not influence that decision.
INTRODUCTION
Whether hormone therapy and vitamin D (or calcium + vitamin D) has a synergistic relationship on the cardiovascular system in women has gained traction in clinical trials and basic science research1-3. A recent study suggested that menopausal monkeys taking daily oral estrogen and who had greater percent plasma 25OHD3 increases over the course of the study had the least severe cardiovascular disease and greater coronary artery remodeling, compared to those not taking estrogen with lower plasma 25OHD3 concentrations3. Daily use of 1.25 mg conjugated equine estrogen has been shown to increase the biologically active form of vitamin D (1,25(OH)2D) and vitamin D carrier protein in menopausal women4. In ovariectomized rats, estrogen up-regulates the expression of the vitamin D receptors in the small intestine5. An analysis from the Women's Health Initiative (WHI) reported a statistically significant reduction (a synergistic effect) in the risk of hip fracture attributed to hormone therapy among participants randomized to calcium plus vitamin D, compared to WHI-HT trial participants that were randomized to hormone therapy and placebo1.
Because women in the WHI were receiving both vitamin D + calcium, this trial offers an ideal opportunity to study whether there is a synergistic effect on cardiovascular disease risk factors in menopausal women with calcium + vitamin D as well as hormone therapy. The WHI Calcium + vitamin D/ trials were double-blinded, randomized, placebo-controlled studies analyzing multiple health outcomes in menopausal women. In the calcium + vitamin D trial, low-density lipoprotein cholesterol (LDL-C) was significantly reduced for women randomized to calcium + vitamin D6, and for the WHI-HT trials, both estrogen plus progestin and estrogen alone also significantly reduced LDL-C7,8. Moreover, both hormone therapy preparations had statistically significant favorable effects on high-density lipoprotein cholesterol (HDL-C), glucose and waist circumference, but significantly unfavorable effects on triglycerides and systolic blood pressure7,8.
We measured changes in our primary outcome (LDL-C) as well as our secondary outcomes (multiple cardiovascular disease risk factors) in 4 groups of women randomly assigned to different therapeutic combinations: Calcium + vitamin D alone, hormone therapy alone, both hormone therapy and calcium + vitamin D, and neither hormone therapy nor calcium + vitamin D. The study hypothesis is that a statistically significant interaction exists between hormone therapy and calcium + Vitamin D in terms of the effect on primary study endpoints, LDL-C, as well as secondary outcomes including other cardiovascular risk factors. Conceptually, a significant interaction means that we observed a larger benefit among women randomized to both Calcium + Vitamin D and hormone therapy, than the benefit observed among women randomized to only hormone therapy, plus the benefit observed among women randomized to only Calcium + Vitamin D. In other words, a significant interaction corresponds to a synergistic rather than an additive effect relative to the placebo group.
MATERIALS AND METHODS
The WHI clinical trials were designed to evaluate the risks and benefits of dietary modification (DM), hormone therapy, and supplementation with calcium + Vitamin D. The protocol and consent forms were approved by the institutional review boards for all participating institutions (see Acknowledgements in Appendix 1 online at http://links.lww.com/xxx).
Like previously published secondary analyses9, the WHI-HT trials data was combined to improve statistical power, and further justified because both WHI arms had the same qualitative effects on the measured cardiovascular disease risk factors. These results can apply to a population similar to those enrolled in the WHI HT trials; 40% without a uterus taking estrogen therapy or placebo, and 60% with an intact uterus taking estrogen and progestogen therapy or placebo. The WHI is the largest cohort (N=16,089) randomized to both hormone therapies (active or placebo) and calcium + Vitamin D (active or placebo)1, from whom blood data was collected on 1,521 participants. Since we are using preexisting data, power calculations were not performed10,11.
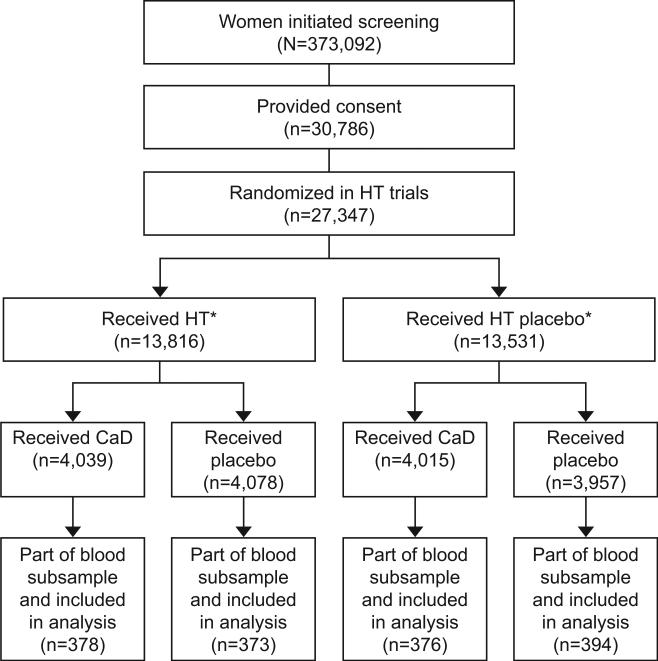
A total of 68,132 women aged 50-79 were recruited between September 1993 to October 1998 and were randomly assigned into the WHI-DM trial, WHI-HT trials, or both. A total of 27,347 women in the two parallel WHI-HT trials were randomized to 0.625 mg of conjugated equine estrogen alone or placebo among women that had a hysterectomy, or 0.625 mg of conjugated equine estrogen plus 2.5 mg of medroxyprogesterone acetate) or placebo taken daily among women that had not had a hysterectomy. A total of 48,835 women in the WHI-DM trial were randomized to a dietary modification intervention (dietary modification to lower total fat intake; n = 19,541) or Comparison (usual diet; n = 29,294) group. At the first or second annual visit, 36,282 eligible women from WHI-HT (16,089) and WHI-DM (n=25,210) trials were randomized further to calcium + Vitamin D (1,000 mg of elemental calcium [carbonate] plus 400 IU of vitamin D3 daily supplementation [n=18,176]) or placebo (n=18,106)), with 14% (n=5017) of participants in both the DM and Hormone Therapy trials. The eligibility criteria to be enrolled in the calcium + Vitamin D trial included many safety parameters (e.g., no previous hypercalcemia or renal calculi) and no competing risk indicators (e.g., no medical condition associated with survival of less than three years). Eligibility for the WHI-HT trial included post-menopausal (as defined previously12) who were between 50 and 79 years at initial screening. Analysis included women that participated in both the calcium + Vitamin D trial and WHI-HT trial (either Estrogen and progestogen or Estrogen alone) and were also part of the 6% blood subsample, Figure 1 (n=1,521). Because the calcium + Vitamin D trial was initiated after 1 year of the WHI-HT, year 1 of the WHI-HT was considered as baseline for the calcium + Vitamin D trial. Lipids along with other cardiovascular risk factors (blood pressure, weight, waist circumference, waist-hip-ratio, glucose, insulin) were measured at baseline and years 1, 3, and 6 after randomization into the hormone therapy trials. The cardiovascular disease risk factors, which were measured after the estrogen plus progestin trial and the conjugated equine estrogen-alone trial stopped on July 7th 2002 and February 29th 2004, respectively, were censored. Details of biomarker analysis and laboratory methods have been published previously13.
Figure 1.
Flow diagram of the Women's Health Initiative (WHI) trials of menopausal hormone therapy (HT) and calcium plus vitamin D (CaD). There were 27,347 women randomized in the menopausal HT trials, and 16,089 participants were also randomized in the CaD trial. Of these, 1,521 were part of the blood subsample and included in this analysis. *The WHI clinical trial used a partial fractional design, so not all HT trial participants were later randomized to CaD; details have been previously published.1 Among the 16,089 participants in the WHI-CaD trail, 5,017 were also a part of the WHI-DM cohort.
Repeated measures models with an unstructured variance-covariance matrix were used to model longitudinal means. Per the randomized partial-factorial design, means were assumed to be equal at baseline for all four hormone therapy + randomization groups, and equal at year 1 from hormone therapy randomization for calcium + Vitamin D randomization groups; the latter is the calcium + Vitamin D ‘baseline’ measure. Constraining the ‘baseline’ means to be equal prior to randomization is the most efficient use of the data14,15. To allow for parsimonious 1-degree-of-freedom estimates of treatment effects (treatment group minus placebo) and interactions (calcium + Vitamin D + Hormone Therapy), the post- randomization means at years 3 & 6 are averaged.
For the subgroup analysis, we analyzed whether particular subgroups may modify the calcium + Vitamin D + hormone therapy interaction on our main outcome variable, low-density lipoprotein cholesterol (LDL-C) (i.e., whether a synergistic effect of calcium + Vitamin D + hormone therapy might occur for particular subgroups). Statistical significance was based on a test of interaction. We looked at a total of 13 prespecified baseline subgroups, see Table 1. No adjustment for multiple testing was made; at most, one interaction was expected to be significant by chance alone. The cut points for total vitamin D intake and total calcium were also chosen a-priori. The lower cut point was suggested previously1 where the effect of hormone therapy appeared to be null for values of total vitamin D below 200 IU and calcium below 800 mg.
Table 1.
Pre-specified baseline subgroups analyzed for test of interaction in subgroup analysis
| Subgroups | Characteristics |
|---|---|
| Age (Years) | 50-59 |
| 60-69 | |
| 70-79 | |
| Race/Ethnicity | White |
| Black | |
| Hispanic | |
| Other | |
| Body Mass Index (BMI, kg/m2) | Normal, <25 |
| Overweight, 25-<30 | |
| Obese >=30 | |
| Current Smoker | No |
| Yes | |
| Alcohol use | Nondrinker |
| <=7 drinks/week | |
| >7 drinks/week | |
| Physical activity (Tertiles, METs/week) | <2.5 |
| 2.5-10.5 | |
| >=10.5 | |
| Bilateral oophorectomy | No |
| Yes | |
| Vasomotor symptoms | None |
| Mild | |
| Moderate | |
| Severe | |
| History of hyperlipidemia (self-report or medications) | No |
| Yes | |
| Hypertension (self-report of treated hypertension or high blood pressure) | No |
| Yes | |
| Treated for diabetes | No |
| Yes | |
| Total vitamin D (dietary and supplements, IU/day) | 200 |
| 400 | |
| 600 | |
| Total calcium (dietary, supplements, medications, mg/day) | 800 |
| 1200 | |
| 1600 |
To address the skewed distributions of triglycerides, glucose, insulin, and waist-hip-ratio log-transformation was used, and geometric means are reported. Statistical significance of synergistic effect was based on tests of interaction. A significant interaction corresponds to a synergistic rather than an additive effect relative to the placebo group. To graphically show the presence of an additive or synergistic effect between calcium + Vitamin D and hormone therapy across all cardiovascular disease biomarkers, Z-scores (treatment effect divided by standard error) are shown. All analyses were done with SAS version 9.4 and figures were drawn with R 3.1. All p values are two-sided and p values ≤ 0.05 were regarded as significant.
Results
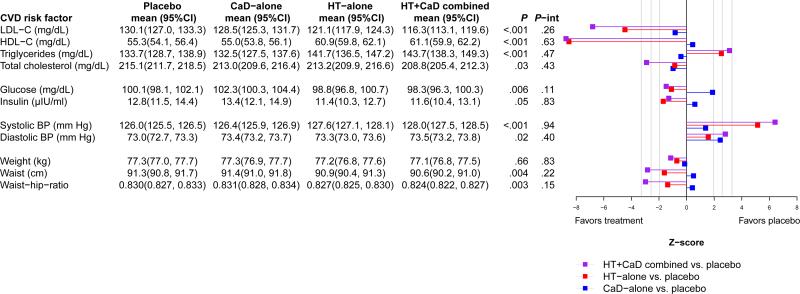
Baseline characteristics were similarly distributed by treatment groups (Table 2). The effects of calcium + Vitamin D + hormone therapy, on all of the cardiovascular disease risk factors except insulin, were larger in magnitude, and in the same direction, as the effects of hormone therapy alone, regardless of the size or direction of the calcium + Vitamin D effect. In other words, the addition of calcium + Vitamin D enhanced the effects of hormone therapy, either in a positive or a negative direction. However, none of the hormone therapy + calcium + Vitamin D interactions were statistically significant (Figure 2), therefore did not provide statistical evidence for the synergistic effects of hormone therapy + calcium + Vitamin D. For example, although hormone therapy + calcium + Vitamin D had a stronger effect on LDL-C compared to either hormone therapy alone or calcium + Vitamin D alone, the observed effects were additive (p-int = 0.26). Estimates for the primary analysis were precise; the 95% CI for mean LDL-C in all four treatment groups was +/− 3mg/dL. The effects on LDL-C (active minus placebo) were −1.6 (−5.5, 2.2) mg/dL for calcium + Vitamin D-alone, −9.0 (−13.0, −5.1) mg/dL for hormone therapy alone, and −13.8 (−17.8, −9.8) mg/dL for calcium + Vitamin D + hormone therapy (Figure 3). Appendix 2, available online at http://links.lww.com/xxx, displays the profile-means for our primary endpoint, LDL-C- by randomization groups during the study. To investigate the influence of temporal trends, we limited post-randomization follow-up to year 3, and observed a similar pattern (p-int = 0.44); the treatment effects on LDL-C for this sensitivity analysis were −1.5 (−5.1, 2.2) mg/dL for calcium + Vitamin D-alone, −13.9. (−17.8, −10.1) mg/dL for hormone therapy alone, and −17.4 (−21.2, −13.6) mg/dL for calcium + Vitamin D + hormone therapy. Lastly, a sensitivity analysis was conducted to account for compliance to study pills. Specifically, LDL-C measurements that occurred after a participant became non-adherent (took < 80% of study pills) were censored. Resulting model estimates produced a similar additive pattern without any evidence for a synergistic effect (p-interaction = 0.66).
Table 2.
Baseline characteristics for the subsample with blood collections of the 2×2 factorial portion of the WHI HT and Calcium + Vitamin D trials (n = 1,521) by randomization group
| Placebo (n=394) | Calcium + Vitamin D-alone (n=376) | Hormone Therapy-alone (n=373) | Hormone Therapy+Calcium + Vitamin D (n=378) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | N | % | P-Value |
| Age at screening, y, mean (SD) | 62.3 | (7.4) | 61.7 | (7.0) | 62.0 | (7.0) | 62.3 | (6.8) | 0.61 |
| Age group at screening (10 yr intervals) | 0.44 | ||||||||
| 50-59 | 162 | 41.1 | 157 | 41.8 | 154 | 41.3 | 141 | 37.3 | |
| 60-69 | 154 | 39.1 | 163 | 43.4 | 153 | 41.0 | 171 | 45.2 | |
| 70-79 | 78 | 19.8 | 56 | 14.9 | 66 | 17.7 | 66 | 17.5 | |
| Race/ethnicity | 0.13 | ||||||||
| White | 186 | 47.2 | 169 | 44.9 | 190 | 50.9 | 199 | 52.6 | |
| Black | 114 | 28.9 | 101 | 26.9 | 89 | 23.9 | 79 | 20.9 | |
| Other | 94 | 23.9 | 106 | 28.2 | 94 | 25.2 | 100 | 26.5 | |
| Hormone use | 0.77 | ||||||||
| Never used | 267 | 67.8 | 245 | 65.2 | 255 | 68.4 | 263 | 69.6 | |
| Past user | 97 | 24.6 | 92 | 24.5 | 84 | 22.5 | 85 | 22.5 | |
| Current user1 | 30 | 7.6 | 39 | 10.4 | 34 | 9.1 | 30 | 7.9 | |
| Baseline vasomotor symptoms, % | 0.50 | ||||||||
| None | 202 | 51.4 | 203 | 54.6 | 205 | 55.4 | 203 | 54.4 | |
| Mild | 127 | 32.3 | 99 | 26.6 | 106 | 28.6 | 99 | 26.5 | |
| Moderate/severe | 64 | 16.3 | 70 | 18.8 | 59 | 15.9 | 71 | 19.0 | |
| Body mass index, kg/m2, median (IQR) | 29.1 (25.2, 33.1) | 28.9 (25.3, 34.2) | 28.9 (24.9, 33.4) | 29.4 (25.3, 34.0) | 0.69 | ||||
| Smoking. % | 0.15 | ||||||||
| Never | 216 | 55.5 | 176 | 47.7 | 206 | 55.7 | 213 | 57.0 | |
| Past | 128 | 32.9 | 151 | 40.9 | 130 | 35.1 | 124 | 33.2 | |
| Current | 45 | 11.6 | 42 | 11.4 | 34 | 9.2 | 37 | 9.9 | |
| Hysterectomy, % | 175 | 44.4 | 160 | 42.6 | 158 | 42.4 | 157 | 41.5 | 0.87 |
| Bilateral oophorectomy, % | 71 | 18.5 | 68 | 19.0 | 54 | 15.0 | 54 | 15.0 | 0.30 |
| Medical treatment, % | |||||||||
| Treated for diabetes | 21 | 5.3 | 30 | 8.0 | 23 | 6.2 | 28 | 7.4 | 0.46 |
| Hypertensive or BP ≥140/90 | 164 | 43.4 | 182 | 49.3 | 164 | 46.7 | 169 | 47.5 | 0.43 |
| High cholesterol levels (medication) | 42 | 10.7 | 53 | 14.1 | 37 | 9.9 | 44 | 11.6 | 0.30 |
| Statin use at baseline | 20 | 5.1 | 32 | 8.5 | 17 | 4.6 | 24 | 6.3 | 0.11 |
| Aspirin use (≥80mg/d) at baseline | 71 | 18.0 | 62 | 16.5 | 58 | 15.5 | 72 | 19.0 | 0.59 |
| Medical History, % | |||||||||
| Myocardial infarction | 8 | 2.0 | 4 | 1.1 | 5 | 1.3 | 9 | 2.4 | 0.48 |
| Angina | 18 | 4.6 | 14 | 3.8 | 13 | 3.5 | 22 | 5.9 | 0.39 |
| CABG/PCI2 | 6 | 1.5 | 3 | 0.8 | 1 | 0.3 | 6 | 1.6 | 0.24 |
| Stroke | 8 | 2.0 | 7 | 1.9 | 5 | 1.3 | 1 | 0.3 | 0.15 |
| Deep vein thrombosis or pulmonary embolism | 3 | 0.8 | 4 | 1.1 | 2 | 0.5 | 3 | 0.8 | 0.88 |
| Family history of breast cancer | 53 | 14.6 | 51 | 15.0 | 63 | 17.9 | 50 | 13.9 | 0.46 |
| > High school degree/GED | 278 | 70.9 | 253 | 68.2 | 269 | 72.9 | 268 | 71.3 | 0.56 |
| Family income ≥ $50,000, % | 97 | 26.2 | 82 | 23.4 | 102 | 29.2 | 96 | 26.4 | 0.39 |
| LDL-C (mg/dL), mean (SD) | 140.3 | (36.8) | 136.1 | (34.1) | 140.4 | (34.2) | 140.0 | (35.1) | 0.28 |
| HDL-C (mg/dL), mean (SD) | 56.0 | (14.1) | 55.5 | (13.9) | 57.3 | (14.6) | 56.4 | (14.3) | 0.36 |
| Triglyceride (mg/dL), median (IQR) | 128.0 (95.0, 169.0) | 126.0 (98.0, 174.0) | 127.0 (93.5, 176.5) | 128.0 (93.0, 173.0) | 0.90 | ||||
| Total cholesterol (mg/dL) mean (SD) | 225.9 | (39.8) | 220.4 | (37.6) | 226.7 | (36.7) | 225.8 | (38.8) | 0.10 |
| Glucose (mg/dL), median (IQR) | 96.0 (89.0, 105.0) | 95.5 (89.0, 106.0) | 94.0 (88.5, 104.0) | 96.0 (89.0, 106.0) | 0.37 | ||||
| Insulin (uIU/ml), median (IQR) | 10.6 (7.9, 15.6) | 10.9 (7.6, 15.9) | 10.3 (7.5, 14.3) | 10.5 (7.4 15.6) | 0.47 | ||||
| Systolic BP (mm Hg), mean (SD) | 127.6 | (17.2) | 127.0 | (16.9) | 129.6 | (17.4) | 129.0 | (18.1) | 0.15 |
| Diastolic BP (mm Hg), mean (SD) | 76.1 | (9.1) | 76.6 | (9.5) | 76.5 | (8.6) | 76.0 | (9.6) | 0.78 |
| Weight (kg), mean (SD) | 77.7 | (16.8) | 78.7 | (18.5) | 76.9 | (17.7) | 77.2 | (18.5) | 0.53 |
| Height (cm), mean (SD) | 160.9 | (7.7) | 161.1 | (7.4) | 160.8 | (6.8) | 159.9 | (7.4) | 0.09 |
| Waist/hip ratio, median (IQR) | 0.82(0.77, 0.87) | 0.83 (0.78, 0.88) | 0.82 (0.77, 0.88) | 0.82 (0.78, 0.87) | 0.46 | ||||
| Waist (cm), mean (SD) | 90.3 | (13.2) | 91.0 | (14.2) | 89.5 | (13.6) | 90.6 | (14.4) | 0.51 |
Required a 3-month washout prior to randomization
CABG/PCI=coronary artery bypass graft/percutaneous coronary intervention
HORMONE THERAPY=Hormone therapy,
Figure 2.
Average group means (95%CI) during follow-up and corresponding Z-scores of treatment group effects. Group means for each randomization group were obtained by averaging the follow-up mesurements collected at years 3 and 6. HT indicates menopausal hormone therapy; CaD, calcium plus vitamin D; P, the p-value of the 3 degree-of-freedom test for the main-effect of randomization group; P-int, p-value of the 1 degree-of-freedom test for interaction.
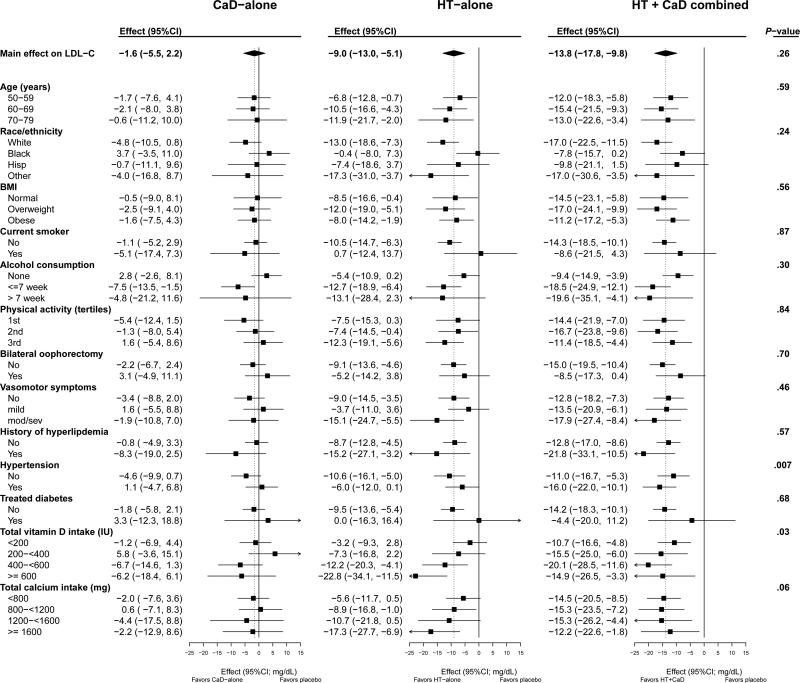
Figure 3.
Average group effect (treatement minus placebo; mg/dL; 95%CI) on LDL-C by subgroups. P-value corresponds to the test of a three-way interaction between HT randomizatoin group x CaD randomization group x subgroup. HT indicates menopausal hormone therapy; CaD, calcium plus vitamin D.
A sensitivity analysis was conducted to determine whether the calcium + Vitamin D + hormone therapy interaction depended on hormone therapy preparation by testing the three-way interaction calcium + Vitamin D + hormone therapy x cohort (estrogent plus progestagen vs. estrogen therapy alone). As expected, none of the three-way interactions provided statistical evidence against pooling the hormone therapy trials for any of the cardiovascular disease risk factors; all p-values > 0.30. The effect of hormone therapy (active vs. placebo) on LDL-C from baseline to year 1 was −16.8(−20.4, −13.2) and −18.4(−21.5−15.3) mg/dL among estrogen therapy and estrogen + progestegen therapy participants, respectively.
Calcium and vitamin D + hormone therapy had a synergistic effect on LDL-C at low total intakes (dietary and supplements) of vitamin D (p-int = 0.03). In addition, the effect of hormone therapy-alone was more attenuated at lower levels of vitamin D intake. Calcium and vitamin D + hormone therapy had an additive (p-int = 0.06) effect at low intakes of calcium (Figure 3) and calcium + Vitamin D + hormone therapy had a synergistic effect (p-int=0.007) among hypertensive women (self-reported of treatment for hypertension or recorded blood pressure ≥ 140/90 mm Hg). The effect of calcium + Vitamin D + hormone therapy did not vary with age (p=0.59). An analysis of the 2 × 2 factorial for the main effects of calcium + Vitamin D and hormone therapy, without a calcium + Vitamin D + hormone therapy interaction term, is presented in Appendix 3. As previously shown in the full cohort (7, Hsia 2007), calcium + Vitamin D has a favorable effect on LDL-C with a mean decrease of 3.2 mg/dL (CI; −5.9, −0.5). In addition, we demonstrated a favorable effect on total cholesterol with a mean decrease of 3.2 mg/dL (CI: −6.2, −0.3).
Discussion
While there are data to suggest calcium + Vitamin D has some beneficial effects on cardiovascular disease risk factors 11,14,15, this has not been well-established, and there is a paucity of prospective data regarding the effect of calcium + Vitamin D on cardiovascular disease outcomes16,17. More recent data have suggested that estrogen therapy alone, in younger women closer to the time of menopause (the timing hypothesis) could have beneficial cardiovascular disease outcomes, particularly lower rates of myocardial infarction12,18-21, but no significant reduction was observed with younger women randomized to estrogen plus progestogen therapy9. While highly controversial, some observational data have raised questions about the safety of high doses of calcium supplements and potential cardiovascular disease risks22, although the WHI calcium + Vitamin D trial did not6. Our findings suggest that calcium combined with vitamin D is not detrimental, at least in regard to most cardiovascular disease risk factors.
The well decomunted7, 23 beneficial effect that hormone therapy has on cholesterol parameters aside from triglyceride is felt to be moderate compared to other cholesterol lowering therapies. Hormone therapy has been shown to have beneficial effects on other cardiovascular disease risks as well, like glucose24 and weight distribution25, but has increased mean systolic blood pressure in both WHI-HT trials26 and in other randomized clinical trials27. It would be helpful to know if the effects of hormone therapy or other interventions with a moderate effect on cardiovascular disease risk, would be additive or synergistic with calcium + Vitamin D. The data we present suggest an additive relationship with hormone therapy, which is modestly beneficial for some cardiovascular disease risk factors (e.g., LDL-C, HDL-C, total cholesterol, glucose, insulin, waist circumference, and the waist-hip-ratio), but modestly harmful for others (e.g., systolic and diastolic blood pressure, triglycerides), Figure 2.
In a similar study from this population, the effect of calcium + Vitamin D and hormone therapy on bone density was also additive1. A synergistic effect was identified, however, when the effect of hormone therapy and calcium + Vitamin D was studied on the primary outcome, fracture1. Hence, it is possible that calcium + Vitamin D and hormone therapy may have a synergistic effect for cardiovascular disease as a primary outcome. Calcium and vitamin D + hormone therapy has a greater effect on cardiovascular disease risk categories when compared with all other combinations. Moreover, for all endpoints except insulin, the effect of calcium + Vitamin D + hormone therapy and hormone therapy alone were in the same direction, but the magnitude of calcium + Vitamin D + hormone therapy was greater. Therefore, results suggest that the addition of calcium + Vitamin D supplementation to a hormone therapy regimen could enhance the effects of hormones. In contrast, for more than half of the endpoints, hormone therapy +calcium + Vitamin D and calcium + Vitamin D alone went in opposite directions, so the addition of hormone therapy may swamp the effect of Calcium and vitamin D supplementation, figure 2.
In the subgroup analysis of total vitamin D intake, the effect of hormone therapy-alone had an impressive decreasing effect on LDL-C as the intake of total vitamin D increased (CI: −3.2, −7.3, −12.2, −22.8), (p=0.03), implying a synergistic relationship (figure 3, Hormone Therapy-alone column for Vitamin D effect). Looking at the effect of hormone therapy + calcium + Vitamin D on LDL-C, the effect (CI: −10.7, −15.5, −20.1, −14.9); p=0.03, seems to progressively increase until the total vitamin D intake exceeds 600 IU (figure 3, hormone therapy + calcium + Vitamin D column for Vitamin D effect). This implies a threshold phenomenon where total vitamin D intake is more beneficial to hormone therapy and calcium + Vitamin D up to a certain point (or threshold). Based on the above findings, for women on estrogens and who have low intake of vitamin D, one should consider supplementation to lower LDL-C that may decrease the risk of heart disease.
A major strength of the study is the double-blinded, randomized, placebo-controlled design in a well-characterized population. Given the numbers and demographic diversity of this cohort, the findings should be generalizable to the U.S. population. This is a large study where women were randomized to calcium + Vitamin D, hormone therapy, or both with nearly 400 women in each arm. Several studies have suggested that vitamin D may have a therapeutic window phenomenon with detriment at the extremes and benefit at midlevel's28,29. This may explain why hormone therapy and calcium + Vitamin D seemed to be synergistic at lower calcium + Vitamin D intakes. Limitations, therefore, include the 400IU of vitamin D, which is typically used to prevent rickets, but may be inadequate to lower LDL-C. Women were allowed to continue their own calcium supplements because it would have been unethical to prohibit concurrent calcium use in a long-term, placebo-controlled trial. Also, the supplement trial used a combination of calcium + Vitamin D so that the effects of either nutrient alone cannot be ascertained. We were not able to further explore the observations, that calcium + Vitamin D + hormone therapy had a synergistic effect on LDL-C at low total intakes (dietary and supplements) of vitamin D and calcium, by correlating blood concentration of vitamin D and calcium with total intake, since only a small percentage of women had these serum markers measured.
In summary, with the exception of insulin, the absolute effect of calcium + Vitamin D and hormone therapy on cardiovascular disease risk factors was larger compared to hormone therapy alone or calcium + Vitamin D alone, including LDL-C our primary endpoint. For clinicians and most patients deciding to begin calcium + Vitamin D supplementation, current use of hormone therapy should not influence that decision. However, based on the above findings, for women on estrogens and who have low intake of vitamin D, one should consider calcium + Vitamin D supplementation to lower LDL-C that may decrease the risk of heart disease.
Supplementary Material
ACKNOWLEDGMENTS
The research on which this publication is based was supported by R01 HL083326 (to Dr. Mackey) from the National Heart, Lung, & Blood Institute. The Women's Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. Information about the WHI investigators, their academic centers, the program office, and the clinical coordinating center can be found online at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
Erin LeBlanc has received grants from Amgen Inc, Bristol Meyers Squibb, and Astrezeneca (unrelated to the current research). Dr. Payne's effort was supported by an NIH Building Interdisciplinary Research Careers in Women's Health (BIRCWH) K12 grant (HD043446).
The research reported in this article was supported by the research budget of the Reading Health System. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
Footnotes
Presented as a poster from September 30 to October 3, 2015 at the 26th Annual Scientific Meeting of the North American Menopause Society in Las Vegas, Nevada.
Financial Disclosure:
The other authors did not report any potential conflicts of interest.
Clinical Trial Registration: ClinicalTrials.gov, https://clinicaltrials.gov, NCT00000611.
References
- 1.Robbins JA, Aragaki A, Crandall CJ, Manson JE, Laura Carbone, Jackson R, et al. Women's health initiative clinical trials: Interaction of calcium + Vitamin D with hormone therapy. Menopause. 2014 Feb;21(2):116–123. doi: 10.1097/GME.0b013e3182963901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnatz PF, Marakovits KA, O'Sullivan DM, Ethun K, Clarkson TB, Appt SE. Response to an adequate dietary intake of vitamin D3 modulates the effect of estrogen therapy on bone density. Journal of Women's Health. 2012;12(8):858–64. doi: 10.1089/jwh.2011.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCurdy RJ, Jiang X, Clark M, Nudy M, Schnatz PF. Vitamin D and Conjugated Equine Estrogen: The Association with Coronary Artery Atherosclerosis in Cynomolgus Monkeys. Menopause. 2016;23(5):481–7. doi: 10.1097/GME.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 4.Cheema C, Grant BF, Marcus R. Effects of estrogen on circulating “free” and total 1,25-dihydroxyvitamin D and on the parathyroid-vitamin D axis in postmenopausal women. J Clin Invest. 1989 Feb;83(2):537–42. doi: 10.1172/JCI113915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liel Y, Shany S, Smirnoff P, Schwartz B. Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology. 1999 Jan;140(1):280–5. doi: 10.1210/endo.140.1.6408. [DOI] [PubMed] [Google Scholar]
- 6.Hsia J, Heiss G, Hong Ren H, et al. Calcium/Vitamin D Supplementation and Cardiovascular Events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principle results from the Women's Health Initiative randomized control trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, Limacher M, Assaf AR, et al. (for the Women's Health Initiative Steering Committee) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 10.Hoenig JM, Heisey DM. The abuse of power. The American Statistician. 2012 [Google Scholar]
- 11.Bacchetti Peter. Peer review of statistics in medical research: the other problem. BMJ. 2002 May 25;324(7348):1271–1273. doi: 10.1136/bmj.324.7348.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, et al. Health Outcomes After Stopping Conjugated Equine Estrogens Among Postmenopausal Women With Prior Hysterectomy: A Randomized Controlled Trial. JAMA. 2011;305(13):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnatz PF, Jiang X, Vila-Wright S, Aragaki AK, Nudy M, O'Sullivan DM, Jackson R, LeBlanc E, Robinson JG, Shikany JM, Womack CR, Martin LW, Neuhouser ML, Vitolins MZ, Song Y, Kritchevsky S, Manson JE. 25(OH) Calcium/Vitamin D (Calcium + Vitamin D) Supplementation, Serum 25(OH) Vitamin D Concentrations, and Cholesterol Profiles in the Women's Health Initiative Calcium + Vitamin D Randomized Trial. Menopause. 2014;21(8):823–33. doi: 10.1097/GME.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu GF, et al. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Statistics in medicine. 28.20(2009):2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 15.Fitzmaurice Garrett M., Laird Nan M., Ware James H. Applied longitudinal analysis. Vol. 998. John Wiley & Sons; 2012. [Google Scholar]
- 16.Schnatz PF, Manson JE. Vitamin D and Cardiovascular Disease: An Appraisal of the Evidence. Clinical Chemistry. 2014;60(4):600–9. doi: 10.1373/clinchem.2013.211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnatz PF, Nudy M, Jiang X, Demko JE, Appt SE. Vitamin D Deficiency and Cardiovascular Disease in Post-Menopausal Women: Contributions from Human and Non-Human Primate Studies. Menopause. 2015;22(5):554–63. doi: 10.1097/GME.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, et al. Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women's Health Initiative Randomized Trials. JAMA. 2013;310(13):1353–68. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 20.Schnatz PF. Hormonal Therapy: Does it Increase or Decrease Cardiovascular Risk? Obstet Gynecol Surv. 2006;61(10):673–81. doi: 10.1097/01.ogx.0000238674.98471.bb. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson TB, Meléndez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20(3):342–53. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Manson JE, Sesso HD. Calcium intake and risk of cardiovascular disease. Am J of Cardiovasc Drugs. 2012 Apr 1;12(2):105–16. doi: 10.2165/11595400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Writing Group for the PEPI Trial Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 24.Kim JH, 1, Cho HT, Kim YJ. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014 Nov 28;61(11):1055–67. doi: 10.1507/endocrj.ej14-0262. Epub 2014 Aug 9. [DOI] [PubMed] [Google Scholar]
- 25.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010 Oct;122(1-3):65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimbo D, Wang L, Lamonte MJ, Allison M, Wellenius GA, et al. The effect of hormone therapy on mean blood pressure and visit-to-visit blood pressure variability in postmenopausal women: results from the Women's Health Initiative randomized controlled trials. J Hypertens. 2014;32(10):2071–81. doi: 10.1097/HJH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006 Oct;8(5):368–76. doi: 10.1007/s11906-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 28.Sato M, Lu J, Iturria S, et al. A nonsecosteroidal vitamin D receptor ligand with improved therapeutic window of bone efficacy over hypercalcemia. J Bone Miner Res. 2010 Jun;25(6):1326–36. doi: 10.1002/jbmr.15. [DOI] [PubMed] [Google Scholar]
- 29.Querfeld U, Mak RH. Vitamin D deficiency and toxicity in chronic kidney disease: in search of the therapeutic window. Pediatr Nephrol. 2010 Dec;25(12):2413–30. doi: 10.1007/s00467-010-1574-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.