Abstract
OBJECTIVE
Seizures are more frequent in patients with Alzheimer’s disease (AD) and can hasten cognitive decline. However, the incidence of subclinical epileptiform activity in AD and its consequences are unknown. Motivated by results from animal studies, we hypothesized higher than expected rates of subclinical epileptiform activity in AD with deleterious effects on cognition.
METHODS
We prospectively enrolled 33 patients (mean age 62 years) who met criteria for AD, but had no history of seizures, and 19 age-matched, cognitively normal controls. Subclinical epileptiform activity was assessed, blinded to diagnosis, by overnight long-term video-electroencephalography and a one-hour resting magnetoencephalography exam with simultaneous EEG. Patients also had comprehensive clinical and cognitive evaluations, assessed longitudinally over an average period of 3.3 years.
RESULTS
Subclinical epileptiform activity was detected in 42.4% of AD patients and 10.5% of controls (p = 0.02). At the time of monitoring, AD patients with epileptiform activity did not differ clinically from those without such activity. However, patients with subclinical epileptiform activity showed faster declines in global cognition, determined by the Mini-Mental State Examination (3.9 points/year in patients with epileptiform activity vs. 1.6 points/year in patients without, p = 0.006), and in executive function (p = 0.01).
INTERPRETATION
Extended monitoring detects subclinical epileptiform activity in a substantial proportion of patients with AD. Patients with this indicator of network hyperexcitability are at risk for accelerated cognitive decline and might benefit from antiepileptic therapies. These data call for more sensitive and comprehensive neurophysiological assessments in AD patient evaluations and impending clinical trials.
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disease and is associated with an increased risk of seizures. In an early report, Alois Alzheimer described epileptic attacks in a 56-year-old man with dementia.1 Since then, it has become evident that unprovoked seizures occur in AD patients at rates eight to tenfold higher than in the general population,2,3 and at even higher rates in autosomal-dominant and early-onset cases.4–6 AD patients with seizure disorders have more unpredictable symptoms, faster clinical decline,7 and greater neuronal loss8 than those without seizures.
A common indicator of seizures is epileptiform activity, which can be detected by neurophysiological recordings, even in patients without a history of clinical seizures. Epileptiform activity can acutely impair cognition in epilepsy patients.9 Moreover, chronic epileptiform activity has been implicated in the pathogenesis of synaptic and cognitive impairments in transgenic mouse models of AD.10,11 Transgenic mice that overexpress human amyloid precursor protein (hAPP) with mutations linked to familial AD have epileptiform activity, nonconvulsive seizures, and synaptic and cognitive dysfunction.12 Reducing tau or chronic treatment with the antiepileptic drug levetiracetam suppresses epileptiform activity and reduces synaptic, cognitive, and behavioral dysfunction in hAPP mice.11,13,14
How these findings relate to humans is unknown. While substantial data have been collected on clinically noticeable seizures in AD, studies on subclinical epileptiform activity have been limited to routine 20–30 minute electroencephalographic (EEG) recordings, which often fail to detect epileptiform activity even in patients with epilepsy.15 Therefore, it is unclear how many AD patients have subclinical epileptiform activity or whether it could affect their cognition.
Here, we tested the hypotheses that 1) subclinical epileptiform activity occurs more often in AD patients than in cognitively normal age-matched controls, and 2) the occurrence of subclinical epileptiform activity is associated with distinct clinical phenotypes or accelerates cognitive decline in these patients. We prospectively evaluated participants with two forms of sensitive neurophysiological monitoring – extended EEG and magnetoencephalography (MEG) – and characterized AD patients to determine whether those who have epileptiform activity differ clinically from those who do not.
METHODS
Participants
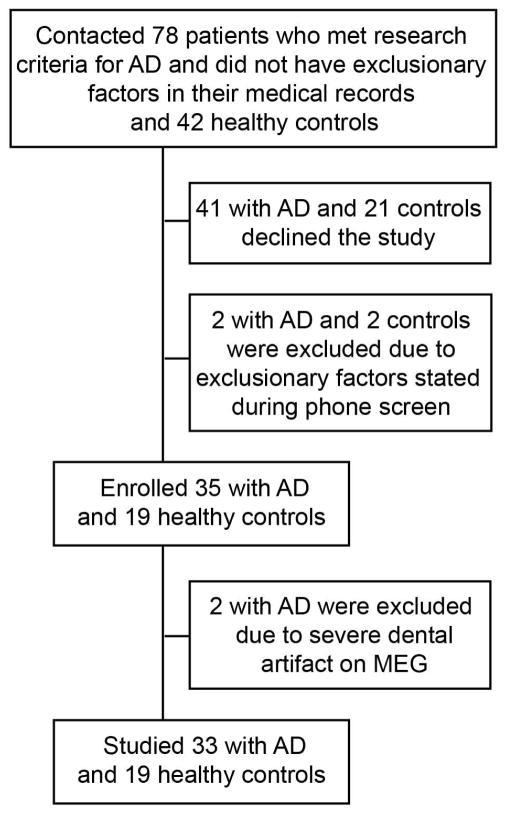
Between August 2008 and February 2015, we studied 33 patients who met the National Institute of Aging–Alzheimer’s Association criteria for probable AD16 and 19 controls without cognitive impairment. Recruitment details are listed in Fig 1. Controls were required to have a Mini-Mental State Examination score of ≥28, a Clinical Dementia Rating Sum of Boxes (CDR-SOB) score of 0, no cognitive concerns reported by themselves or their informants, normal results on magnetic resonance imaging (MRI) of the brain allowing for age-appropriate atrophy, and no neurological disorder affecting cognition. Exclusion criteria for all participants were a history of Korsakoff's syndrome, alcohol or substance abuse within five years of dementia onset, severe head trauma with persistent deficits, clinically significant brain lesions, seizure disorders, hydrocephalus, intracerebral hemorrhage, ischemic vascular dementia, multiple sclerosis or other demyelinating disease, encephalitis or meningitis, untreated B12 deficiency, untreated hypothyroidism, untreated syphilis, HIV infection, renal insufficiency requiring dialysis, symptomatic liver disease, severe periventricular white matter disease or white matter lesions greater than grade four,17 clinically significant lacunar infarcts, cortical stroke including cortical microbleed, respiratory condition requiring oxygen, significant systemic medical illness (e.g., end-stage cardiac insufficiency or cancer requiring chemotherapy), a pacemaker or other ferromagnetic material, and medications likely to affect CNS functions (e.g., benzodiazepines, typical antipsychotics, narcotics, and antihistamines) except drugs approved by the FDA for use in AD.
FIGURE 1.
Flowchart of participant recruitment and enrollment. AD = Alzheimer’s disease; MEG = magnetoencephalography.
Patients were further characterized as having typical AD (predominantly memory-related symptoms) or atypical AD (predominantly language or visuospatial symptoms).18,19 A positive family history of dementia in a first-degree relative was present in 39.4% of cases (n = 13), and a strong family history suggestive of autosomal dominant pattern of inheritance in one case (patient 8), who was found to have a novel APP mutation (G to C mutation at base pair 1627, resulting in a glutamic acid to glutamine substitution at amino acid position 543), the significance of which is unknown. This patient did not contribute to the longitudinal data.
This study was approved by the University of California San Francisco (UCSF) Committee on Human Research. All participants or their assigned surrogate decision makers signed an informed written consent before enrollment.
Genetic Analysis
Apolipoprotein E (APOE)
DNA was purified from peripheral blood samples (Gentra PureGene Blood Kit, Qiagen) according to the manufacturer’s instructions. The primers GCATCTGCTCTCTGCATCTGTC (forward) and ACCTGCTCCTTCACCTCGTC (reverse) were used to straddle a 687-bp region spanning the APOE polymorphism encoding the ε2, ε3, or ε4 genotypes. Genomic DNA was amplified by standard PCR methods, and labeled and sequenced with an ABI Prism 3730 XL sequencer. Sequence data was analyzed with Sequencher (GeneCodes) and manually reviewed for accuracy. APOE alleles were defined by two genetic variants (reference SNP ID numbers rs429358 and rs7412).
Microtubule-Associated Protein Tau (MAPT)
DNA was isolated from peripheral blood, and exons 1–5, 7, and 9–13 were amplified with primer pairs complementary to the intronic regions of MAPT. Both strands of the PCR products were sequenced, and the H1 and H2 haplotypes of MAPT were identified with Sequencher software (reference SNP ID number rs9468).
Familial AD mutations
APP, PSEN1, and PSEN2 were analyzed by direct sequencing of amplified DNA in the patient with suspected autosomal dominant pattern of inheritance.
Prolactin Testing
Blood samples were drawn into plastic tubes coated with clot activator, inverted five times, allowed to clot for 60 minutes, and centrifuged at 1300 x g for ten minutes at 4ºC. The serum was aliquoted in volumes of 1 ml into cryovials and stored at –80ºC for analysis by immunoassay for prolactin concentration (Quest).
Procedures
Participants were evaluated at the Clinical and Translational Science Institute Clinical Research Center at Moffitt Hospital. Silver cup electrodes (Viasys) in the standard international 10–20 electrode array were placed for overnight long-term monitoring by video-electroencephalography (LTM-EEG) telemetry. Monitoring included three minutes of hyperventilation. The LTM-EEGs were a median length of 24.0 hours (interquartile range 18.6–25.8) for controls and 24.5 hours (interquartile range 21.2–26.9) for AD patients.
The next morning, a fasting blood sample was drawn and processed for genetic analysis and prolactin testing. Participants then underwent a one-hour resting-state MEG exam with simultaneous 21-lead EEG (M/EEG) in the Biomagnetic Imaging Laboratory. The MEG was recorded with a whole-head MEG system (CTF, Port Coquitlam, British Columbia, Canada) comprising 275 axial gradiometers (sampling rate=600 Hz). Fiducial coils were placed at the nasion and the left/right pre-auricular points to locate head position relative to the sensor array to co-register MEG data with magnetic resonance images of the brain. During recordings, participants were supine, with eyes closed, and encouraged to fall asleep. During the final ten minutes, participants hyperventilated for three minutes and then breathed normally for seven minutes. None of the participants had a contraindication for hyperventilation due to cardiopulmonary disease.
The LTM-EEG and M/EEG recordings were read by experienced epileptologists (P.A.G. for LTM-EEG; H.E.K. for M/EEG) and clinical neurophysiologists (K.G.R. for LTM-EEG; M.M. for M/EEG), blinded to the diagnosis. The entire recordings were reviewed frame-by-frame by visual inspection, and the determination of epileptiform activity was made by consensus between the clinicians who reviewed the recordings. The EEGs were reviewed with the following settings: montage: bipolar “double banana” including standard 10–20 electrode placements; sensitivity: 7 microvolts/mm; page speed: 30 mm/sec; high frequency filter: 70 Hz; low Frequency filter: 1.6 Hz; notch filter: on. Epileptiform activity was defined as paroxysmal sharp waveforms 20–200 ms, clearly distinct from ongoing background activity, with an associated subsequent slow wave. Additionally, the epileptiform discharges did not have spatial or morphological characteristics of normal variants, such as sharp transients, small sharp spikes (also known as benign epileptiform transients of sleep), rhythmic mid-temporal theta discharges, positive occipital sharp transients of sleep, or wicket spikes. The SpikeDensityV101 Calculation Engine in Persyst 11 EEG software was also used to help detect, locate, and count spikes and sharp waves on the LTM-EEG.20. To aid in identifying irritative zones on MEG, synthetic-aperture magnetometry (an automated spatial filtration technique) was used to quantify and locate background power changes in frequency bands of interest and to identify changes in spike-locked high-frequency power.21,22
Areas of cortical irritability on EEG recordings were the predominant regions of maximum electronegativity, corresponding to scalp electrodes as follows: frontal (Fp1, Fp2, F3, F4), central frontal (Fz), central (C3, Cz, C4), central parietal (Pz), frontotemporal (F7, F8), temporal (T3, T4, T5, T6), parietal (P3, P4), and occipital (O1, O2).23 For analysis of MEG tracings, equivalent current dipoles were fitted to the chosen spikes and sharp waves with software from CTF Systems (VSM MedTech, Port Coquitlam, British Columbia). To produce magnetic source images of dipoles superimposed on anatomic images, fiducials at the nasion and pre-auricular points were used to co-register dipoles to MRI scans. Spike dipoles were classified by location and orientation.24,25
Epileptologists also noted whether the awake, resting-state background activity on LTM-EEG or M/EEG was characterized by generalized, asymmetric, or focal slowing. None of the participants had skull defects or breach rhythm on EEG. Power spectra for each patient group and age-matched controls were generated from the MEG sensor data.26 The power in each frequency band was calculated by fast Fourier transformation of the time series data from posterior channels.
Neuropsychological Testing and Clinical Assessments
At the time of neurophysiological monitoring, patients’ informants completed two standardized questionnaires: the Clinician Assessment of Fluctuation (range 0–16) and One Day Fluctuation Assessment Scale (range 0–21), which assessed fluctuations over the previous month and day, respectively.27
Patients had neuropsychological and clinical assessments at the UCSF Memory and Aging Center between 2007 and 2015. Initial clinical evaluations for patients occurred 1.3 ± 1.7 years (mean ± SD) before the LTM-EEG and M/EEG exams. Patients were followed for 3.3 ± 2.2 years thereafter. Global cognition was assessed with the Mini-Mental State Examination (MMSE),28 and global functional performance was assessed with the CDR and CDR-SOB.29 A battery of neuropsychological tests was used to assess major domains of cognition.30
To create composite z scores of neuropsychological performance in cognitive domains based on age-matched norm datasets from the UCSF Memory and Aging Center, we used test components that were consistently collected in longitudinal examinations. The memory composite comprised 30-second and 10-minute verbal free recall on the California Verbal Learning Test and Benson visual free recall. The executive composite comprised design fluency, digit span forward, digit span backward, and learning efficiency on the California Verbal Learning Test. The language composite comprised lexical fluency (D-words), semantic fluency (animals), Boston Naming Test, and repetition. The visuospatial composite comprised visual constructional ability (Benson figure copy) and the number-location task of the Visual Object and Space Perception battery. Composite z scores were calculated as the average of the individual test z scores within a cognitive domain. At each testing session, neuropsychologists noted whether there were any quality issues due to any of the following reasons: motor difficulties, speech difficulties, hearing impairment, visual impairment, English as a second language, minimal education, lack of effort, unreliable informant, behavioral disturbances, or other reasons. None of the participants’ data contained any such quality concerns.
Voxel-Based Morphometry
Voxel-based morphometry (VBM) was performed in a subset of patients (n = 26) who underwent structural brain imaging with a 3-Tesla Siemens MRI scanner at the UCSF Neuroscience Imaging Center. The seven remaining patients had MRI scans that used different acquisition protocols and were suboptimal for inclusion in the VBM analysis. All images were obtained within 15 months of the MEG evaluation. Atrophy patterns were determined by comparison with images from 75 age- and sex-matched controls without cognitive impairments who were evaluated at the UCSF Memory and Aging Center.
Variations in the volume of gray matter in patients and controls were assessed with optimized voxel-based morphometry in the VBM8 toolbox of statistical parametric mapping version 8 (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/).26 The Expectation Maximization Segmentation tool in SPM8 was used for probabilistic mapping of gray and white matter. After tissue segmentation, probabilistic maps of gray matter were transformed into the standard Montreal Neurological Institute space. The probabilistic maps in their respective native space were then normalized to the population-based templates by nonlinear transformation. The volume differences in the spatially normalized gray-matter image were obtained by Jacobian modulation. The modulated images were smoothed with an 8-mm full-width-at-half-maximum isotropic Gaussian kernel for group analyses; differences between patient and control groups were analyzed with t-tests. Age was included as a covariate, and total intracranial volume was included as a nuisance covariate.
Statistical Analysis
Statistical analyses were done with SAS software, version 9.4 (SAS Institute). For pairwise comparisons of continuous variables, t-tests were used for those that were normally distributed and Wilcoxon-Mann-Whitney tests for those that were not. For comparisons of proportions of categorical variables in contingency tables, Pearson χ2 tests were used when expected cell values were ≥5 and Fisher exact tests when they were <5. Differences in the spectral power between patients and controls, for each frequency band, were tested by one-way analysis of variance and Dunnett’s post hoc test.
Longitudinal changes in cognitive performance and functional ability were compared in patients with or without epileptiform activity who had serial evaluations. To accommodate repeated data with variable observations, we used a linear mixed-effects model in the SAS Proc Mixed procedure.31 Starting from each patient’s first evaluation, we examined the longitudinal scores in MMSE, CDR-SOB, and composite scores in the four cognitive domains (executive, memory, language, and visuospatial). Patient identity was entered into the model as a repeated factor. Fixed effects were time from initial evaluation and presence or absence of epileptiform activity. A linear model was used because its log-likelihood ratio value was not significantly different from that of a quadratic model. A Holm correction was used for multiple comparisons to analyze the four cognitive domains. We report unadjusted p values and indicate those that are significant after correction for multiple comparisons. Cohen f2 effect sizes were calculated from the mixed-effects regression models using established methods.32
Because young age, male sex, higher education, and atypical focal presentations of AD can be associated with faster cognitive decline,33–35 significant associations derived from the linear model were confirmed by adding these additional factors into the model as covariates. All comparisons were two-tailed, and p values <0.05 indicated statistical significance.
RESULTS
Study Participants
The AD cohort was relatively young and in a mild stage of disease (Table 1). Patients carried the apolipoprotein (APOE) ε4 allele more frequently than controls. Disease biomarkers are listed in Supplementary Table S1.
TABLE 1.
Participant demographics and clinical characteristics.
| Characteristic | Controls (n = 19) | AD Patients (n = 33) | pa |
|---|---|---|---|
| Age (years) | 65.3 ± 5.6 | 61.7 ± 7.4 | 0.07 |
| Female sex | 11 (57.9%) | 21 (63.6%) | 0.68 |
| Whiteb | 18 (94.7%) | 32 (97.0%) | 1.0 |
| Education (years) | 18 (16–18) | 16 (14–18) | 0.052 |
| Right-handedness | 14 (73.7%) | 28 (84.8%) | 0.47 |
| APOE ε4 carrier | 5 (26.3%) | 18 (54.5%) | 0.048 |
| MMSEc | 30 (29–30) | 22 (18–24) | < 0.0001 |
| CDRd | 0 | 1 (0.5–1) | < 0.0001 |
| CDR-SOBd | 0 | 5 (4–7) | < 0.0001 |
Values for age are means ± SD, and values for education, MMSE, CDR, and CDR-SOB are medians with interquartile ranges in parentheses. Cognitive assessments were at visits closest to time of neurophysiological monitoring.
Statistical tests were t-test for age; Pearson χ2 test for sex and APOE ε4 carrier status; Fisher exact test for race and handedness; and Wilcoxon-Mann-Whitney test for education, MMSE, CDR, and CDR-SOB.
Race was self-reported.
Scores on the MMSE range from 0 to 30, with higher scores denoting better cognitive function.28
Scores on the CDR range from 0 to 3 and on the CDR-SOB from 0 to 18, with higher scores denoting more disability.29
AD = Alzheimer’s disease; APOE = apolipoprotein E; CDR = Clinical Dementia Rating; CDR-SOB = CDR Sum of Boxes; MMSE = Mini-Mental State Examination.
Increased Incidence of Subclinical Epileptiform Activity in AD
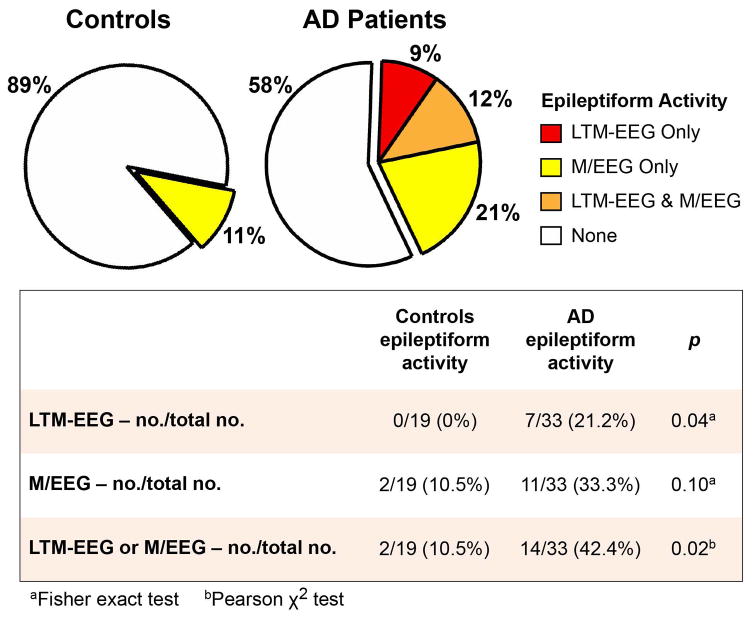
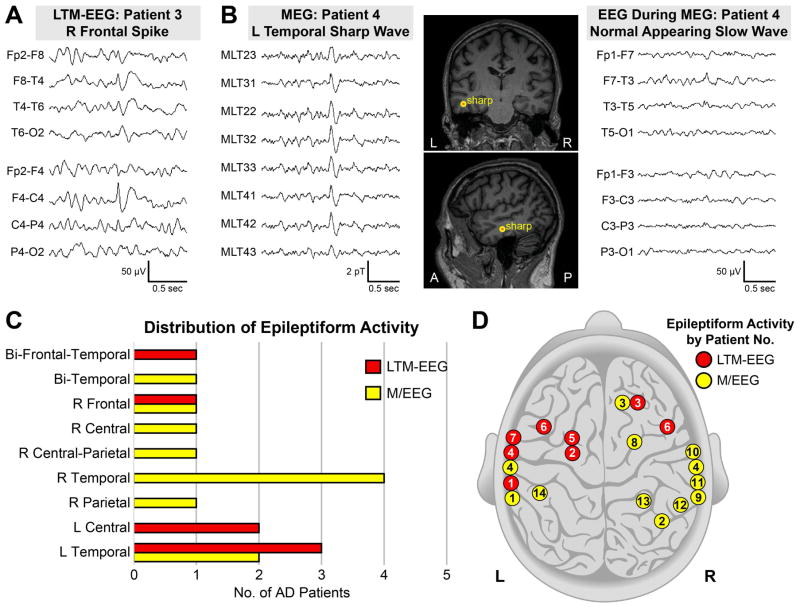
We detected subclinical epileptiform activity in four times as many AD patients as controls (Fig 2, 42.4% vs. 10.5%). LTM-EEG and M/EEG measures provided complementary information; in several cases, a single modality identified epileptiform activity (Fig 2 and Fig 3; Supplementary Table S2). Epileptiform discharges in patients were most often detected in the temporal lobes (Fig 3), and those that were lateralized were more frequently left-sided when detected by LTM-EEG and right-sided when detected by M/EEG (p = 0.04). The frequency of spikes and sharp waves in patients with epileptiform activity was 0.03 to 5.18 per hour on LTM-EEG and 1 to 20 per hour on M/EEG (Supplementary Table S2). Overall, 9.9% of epileptiform discharges in AD patients occurred during wakefulness, 25.7% during sleep stage 1, and 64.4% during sleep stages ≥2. Nine of the 14 AD patients with epileptiform activity (64.3%) had epileptiform discharges exclusively during sleep, and 5 of 14 (35.7%) had epileptiform discharges only during sleep stages ≥2. Examination of data obtained during hyperventilation did not increase the number of participants with epileptiform activity.
FIGURE 2.
Proportion of participants with subclinical epileptiform activity. Subclinical epileptiform activity in patients with Alzheimer’s disease (AD) and age-matched controls detected by long-term monitoring with video-EEG (LTM-EEG, overnight), magnetoencephalography with simultaneous EEG (M/EEG), or both. EEG = electroencephalogram.
FIGURE 3.
Subclinical epileptiform activity in Alzheimer’s disease (AD) patients. (A) Subclinical epileptiform spike detected by overnight long-term monitoring with video-EEG (LTM-EEG). The maximum negativity is isopotential between the Fp2 and F4 electrodes, corresponding neuroanatomically with the right frontal lobe (D, patient 3). (B) Subclinical epileptiform sharp wave observed on magnetoencephalography (MEG, left panel), localized in the left temporal lobe (B, middle panel and D, patient 4), and corresponding slow wave on EEG (right panel). (C and D) Distribution of predominant regions of subclinical epileptiform activity by recording modality and patient. Patients 4 and 6 had bilateral localization of epileptiform activity. A = anterior; EEG = electroencephalogram; L = left; μV = microvolts; P = posterior; pT = picotesla; R = right.
Background activity was characterized by generalized slowing in more patients than controls but did not show differences in asymmetric or focal slowing (Supplementary Tables S3 and S4). Additionally, spectral density analysis of MEG data showed that patients had lower power in alpha, beta, and gamma oscillations than controls (Fig 4). However, neither background slowing nor spectral power differed in patients with or without epileptiform activity (Fig 4 and Supplementary Tables S3 and S4).
FIGURE 4.
Averaged power spectral density estimates for different frequency bands during resting magnetoencephalography. Alzheimer’s disease (AD) patients with (n = 14) or without (n = 19) epileptiform activity had decreased power of alpha (α), beta (β), and gamma (γ) oscillations compared to age-matched controls (n = 19). p < 0.05 for each AD group vs. controls at each frequency band by ANOVA and Dunnett’s test. Spectral data were derived from posterior-based sensors over 60 seconds. Shaded areas are standard error of the mean. dB = decibels; Hz = hertz.
Subclinical Epileptiform Activity and its Relation to Other Patient Characteristics
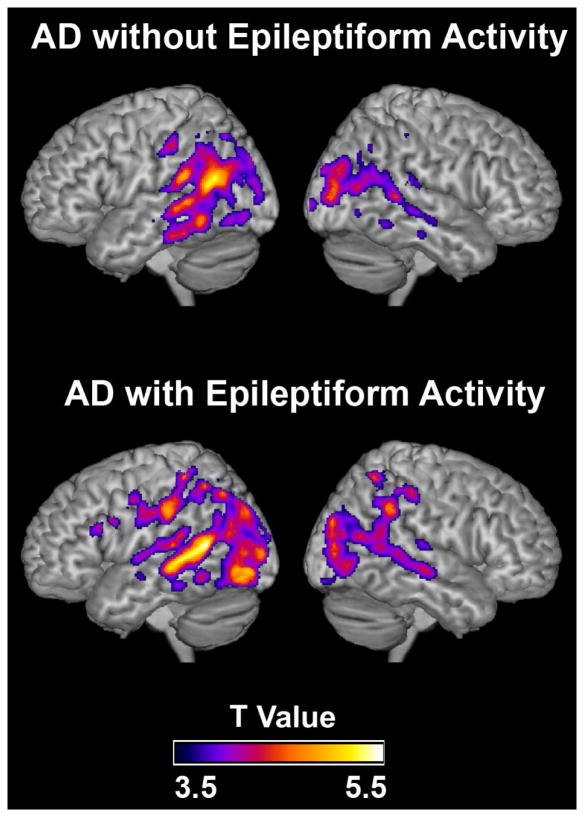
Subclinical epileptiform activity in AD was not related to differences in carrier status for the APOE ε4 allele, the major genetic risk factor for AD, or to MAPT (Tau) haplotype (Table 2). Nor was it related to age, duration of disease symptoms, atypical presentations, history of mild head trauma or myoclonus, clinical fluctuations, blood prolactin levels, or concomitant medications (Table 2), although there was a trend toward younger age in the patients with epileptiform activity. Two of the patients had a single first-degree relative with epilepsy; both patients were in the nonepileptiform group. Consistent with the subclinical nature of the detected epileptiform activity, only one patient with such activity had an elevated prolactin level (27.8 ng/mL; reference range 2–20). At the time of LTM-EEG and M/EEG monitoring, global cognitive, domain-specific cognitive, and functional measures did not differ between patients with or without epileptiform activity (Supplementary Table S5). Gray matter atrophy revealed by voxel-based morphometry was most prominent in the hippocampi and temporoparietal lobes of patients but did not differ between those with or without epileptiform activity (Fig 5).
TABLE 2.
Characteristics of Alzheimer’s disease patients with and without subclinical epileptiform activity.
| Characteristic | AD without Epileptiform Activity (n = 19) | AD with Epileptiform Activity (n = 14)a | pb |
|---|---|---|---|
| Age (years) | 63.1 (57.7–68.5) | 58.3 (54.8–62.8) | 0.08 |
| Duration of disease symptoms (years) | 5.4 (4.6–7.4) | 4.8 (3.5–6.3) | 0.15 |
| Atypical AD | 5 (26.3%) | 6 (42.9%) | 0.46 |
| APOE ε4 carrier | 10 (52.6%) | 8 (57.1%) | 0.80 |
| MAPT (Tau) H1 allele frequency – no./total no. | 29/38 (76.3%) | 19/26 (73.1%) | 0.77 |
| Mild head trauma | 8 (42.1%) | 2 (14.3%) | 0.13 |
| Myoclonus | 5 (26.3%) | 7 (50.0%) | 0.16 |
| Clinician Assessment of Fluctuationc | 0 (0–6) | 0 (0–3) | 0.33 |
| One Day Fluctuation Assessmentc | 2 (0–6) | 0 (0–3) | 0.30 |
| Prolactin level (ng/mL)d | 10.0 (7.9–12.4) | 8.6 (6.7–10.4) | 0.14 |
| Acetylcholinesterase inhibitor | 9 (47.4%) | 9 (64.3%) | 0.33 |
| Memantine | 1 (5.3%) | 0 (0.0%) | 1.0 |
| Acetylcholinesterase inhibitor and memantine | 8 (42.1%) | 3 (21.4%) | 0.28 |
| Antidepressants or anxiolytics | 11 (57.9%) | 7 (50.0%) | 0.65 |
| Atypical antipsychotics | 1 (5.3%) | 0 (0%) | 1.0 |
Values for age, duration of disease symptoms, Clinician Assessment of Fluctuation, One Day Fluctuation Assessment, and prolactin levels are medians with interquartile ranges in parentheses.
n = 13 for MAPT (Tau) H1 allele frequency, Clinician Assessment of Fluctuation, and One Day Fluctuation Assessment.
Statistical tests were Wilcoxon-Mann-Whitney test for age, duration of disease symptoms, Clinician Assessment of Fluctuation, One Day Assessment of Fluctuation; Pearson χ2 test for APOE ε4 carrier status, MAPT (Tau) H1 allele frequency, myoclonus, acetylcholinesterase inhibitor, and antidepressants or anxiolytics; and Fisher exact test for atypical AD, mild head trauma, memantine, acetylcholinesterase inhibitor and memantine, and atypical antipsychotics.
Scores on the Clinician Assessment of Fluctuation range from 0 to 16 and scores on the One Day Fluctuation Assessment range from 0 to 21, with higher scores indicating more clinical fluctuations.27
Serum was analyzed for all participants except for one patient with AD and epileptiform activity whose prolactin level was measured in plasma.
AD = Alzheimer’s disease; APOE = apolipoprotein E; MAPT = microtubule-associated protein tau.
FIGURE 5.
Subclinical epileptiform activity and brain atrophy in Alzheimer’s disease (AD). Patterns of brain atrophy in AD patients without (top images) or with (bottom images) subclinical epileptiform activity revealed by voxel-based morphometry. Atrophy maps are based on comparisons with age- and sex-matched controls from the UCSF Memory and Aging Center database. Regions of gray matter atrophy are shown on the 3-dimensional rendering of the Montreal Neurological Institute standard template brain. n = 16 AD patients without epileptiform activity, n = 10 AD patients with epileptiform activity, and n = 75 controls. The images were thresholded at p < 0.001, uncorrected.
Subclinical Epileptiform Activity is Associated with Faster Cognitive Decline in AD
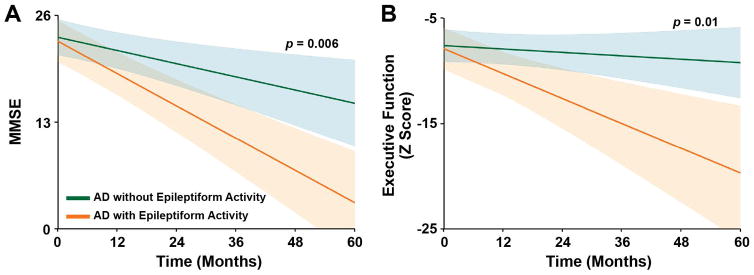
In AD patients evaluated longitudinally, subclinical epileptiform activity was associated with a faster decline in global cognition, determined by the MMSE (3.9 points/year in patients with epileptiform activity vs. 1.6 points/year in patients without, p = 0.006), and in a composite measure of executive function (2.4 z score points/year in patients with epileptiform activity vs. 0.3 z score points/year in patients without, p = 0.01) (Fig 6). These associations remained significant after accounting for age, education, sex, and presence of atypical AD as additional covariates. The Cohen f2 effect size for MMSE was 0.33 and for composite executive function 0.39. LTM-EEG and M/EEG were each effective at selecting patients with epileptiform activity who had significantly faster rates of decline in MMSE than those without epileptiform activity on either modality (LTM-EEG p < 0.02, M/EEG p < 0.006). There was a trend toward subclinical epileptiform activity being associated with faster progression of memory loss in AD patients (p = 0.07), and no significant associations were found with the rate of functional decline measured by the CDR-SOB or with the rate of language or visuospatial decline.
FIGURE 6.
Subclinical epileptiform activity and longitudinal change in cognition in Alzheimer’s disease (AD). Estimates from linear mixed-effects models of the longitudinal change in cognitive functions. (A) Mini-Mental State Examination (MMSE) score. n = 34–55 observations from 10–15 patients per group (7 observations included in the model occurred after 60 months, and these data are provided in Supplementary Table S6). (B) Composite measure of executive function (z score). n = 11–34 observations from 4–11 patients per group. Shaded areas are 95% confidence intervals.
DISCUSSION
In this prospective study, extended neurophysiological monitoring detected subclinical epileptiform activity in 42.4% of patients with AD. At the time of scanning, patients had no overt clinical or demographic features that distinguished between those with or without subclinical epileptiform activity. Nevertheless, over time, patients with subclinical epileptiform activity had faster declines in global cognition and executive function. The effect sizes in the longitudinal analysis were of a large magnitude that would be considered clinically meaningful in clinical trials.32 Thus, AD patients with subclinical epileptiform activity may have a more aggressive disease course than those without such activity. LTM-EEG and M/EEG are therefore important tests for identifying patients who may progress more rapidly and who might benefit from antiepileptic therapies. Additionally, screening for epileptiform activity could enhance balance and precision in impending clinical trials.
An estimated 10–22% of patients with AD have clinically obvious seizures, and rates vary widely because manifestations that prompt medical attention can depend on factors such as level of supervised care and stage of disease.4 In a retrospective study, we found that most seizures in AD are nonconvulsive and could easily go unrecognized.23 In light of the substantial proportion of patients with subclinical epileptiform activity identified in the current study, the extent of network hyperexcitability in AD has likely been greatly underestimated. Although no electrographic seizures were detected in this study, we cannot exclude the possibility of occult seizures occurring in deeper brain regions. For example, seizures that are confined to the mesial temporal lobes do not always show ictal activity on the scalp EEG,36,37 and such focal seizures often have little to no clinical manifestation.38 New algorithms that improve sensitivity of surface EEG or MEG to detect such events or more invasive EEG monitoring, if warranted clinically, would be needed to more thoroughly investigate this possibility.39
Three key aspects of our study enabled us to detect subclinical epileptiform activity with greater sensitivity and higher yield than in previous studies of AD patients, which reported rates as low as 2%.40 First, we combined information from overnight LTM-EEG and M/EEG obtained using a standardized epilepsy protocol that includes data collections while participants fall asleep, which is more sensitive than routine 20-minute EEG recordings in awake patients. A single routine EEG detects interictal epileptiform activity in only about half of patients with clinically confirmed seizures.15 Overnight recording captures all stages of sleep, when epileptiform abnormalities can be more evident than during wakefulness.41 Indeed, in nearly two-thirds of the AD participants who had epileptiform activity in the current study, we detected such activity exclusively during sleep. These data highlight the increased yield of obtaining a prolonged EEG because patients rarely reach stage 2 sleep during a routine 20-minute EEG. Furthermore, MEG often detects epileptiform activity not present on the EEG (Figs 2 and 3).25 Thus, the combination of LTM-EEG and M/EEG offers the most sensitive noninvasive methods to detect subclinical epileptiform activity. Second, we used a prospective blinded design with well-characterized participants and standardized measures; as a result, there were fewer potential sources of bias and confounders than in retrospective studies. Third, our disease cohort included relatively young AD cases, which are at higher risk for clinical seizures and epileptiform activity.4,5,40
Our study revealed striking similarities between patients with AD and transgenic animal models of the disease. The frequency of epileptiform activity that we observed on M/EEG in our patients (1–20 events/hour) is similar to that in hAPP mice.11 Interventions that suppress network hyperexcitability, such as tau reduction or antiepileptic treatment, improve synaptic and cognitive function in hAPP mice and other transgenic mouse models of AD.11,13,14,42–44 Furthermore, levetiracetam, at low doses, suppresses task-associated hippocampal hyperactivity and improves performance in a hippocampus-dependent pattern separation task in patients with amnestic mild cognitive impairment,45,46 often an early stage of AD.47
While animal studies suggest that epileptiform activity could contribute to cognitive decline in the context of elevated levels of hAPP/Aβ, epileptiform activity in human AD patients could also be a surrogate marker for a more aggressive form of underlying pathology causing more rapid cognitive decline. Interestingly, a small open-label trial demonstrated that treatment of young epilepsy patients with levetiracetam for 10 weeks suppressed interictal spikes and improved performance in attention, concentration, and memory.48 Ongoing studies are evaluating whether suppressing epileptiform activity with levetiracetam improves outcomes in AD patients with subclinical epileptiform activity (clinicaltrials.gov, NCT02002819).
Patients with subclinical epileptiform activity had faster declines in the MMSE and other cognitive measures. The MMSE is a widely used and well validated measure of global cognition. MMSE scores correlate well with AD Assessment Scale-Cognitive Subscale (ADAS-cog) scores.49 The MMSE also forms part of a composite test that is sensitive to amyloid-β-related cognitive decline and has been used in pertinent clinical trials.50 The CDR-SOB, a measure of global functional ability, failed to detect faster changes in AD patients with epileptiform activity. Other studies showed similar discrepancies between neuropsychological measures, such as MMSE and ADAS-cog, and the CDR-SOB.33 The CDR-SOB, which is informant-based and limited to multiple-choice ratings of the patient’s abilities, may be less sensitive to cognitive decline than neuropsychological tests.
Clinical fluctuations did not differ in patients with or without subclinical epileptiform activity (Table 2). Therefore, subclinical epileptiform activity may affect cognitive performance less through rapid alterations in network functions than through more chronic remodeling of neuronal circuits, as suggested by findings in transgenic mouse models.11 This idea is supported by our finding that longitudinal cognitive decline is accelerated in patients with subclinical epileptiform activity.
Apolipoprotein E4, the major risk factor for sporadic AD, is associated with synchronous high-voltage slow-wave activity and sharp waves induced by hyperventilation in nondemented individuals with a family history of AD.51 However, APOE ε4 carrier status was not associated with epileptiform activity in our patients. Conceivably, apolipoprotein E4, which has been associated with increased hippocampal atrophy in AD and hippocampal GABA-ergic dysfunction in animal models,52,53 may induce aberrant network activity in deeper brain structures that cannot be detected with scalp EEG and MEG recordings. The temporal discharges detected by MEG all localized to lateral temporal regions. Our study may not have completely separated AD patients with and without epileptiform activity in the hippocampus, and this could also explain why the association between epileptiform activity and memory decline did not reach statistical significance.
AD patients had significantly reduced resting-state alpha-, beta-, and gamma-band power, as reported.54 In hAPP mice, depletion of voltage-gated sodium channels in parvalbumin-positive inhibitory interneurons in the parietal cortex critically contributes to epileptiform activity, reduced power of gamma oscillations, and cognitive impairments; similar sodium channel depletions have been identified in patients with AD.55 Resting-state gamma activity did not differ in our AD patients with or without epileptiform activity, and thus is not a useful marker of subclinical epileptiform activity in AD. Unknown compensatory factors may explain why only some patients with reduced gamma activity develop detectable epileptiform activity. In regards to blood markers, transient increases in prolactin have been associated with interictal epileptiform discharges in epilepsy patients.56 However, the prolactin level was increased in only one of our patients with subclinical epileptiform activity. Thus, prolactin, which has a short half-life, is not a useful biomarker for intermittent subclinical network hyperexcitability in AD.
In previous studies, spontaneous epileptiform activity was reported in 0 to 6.6% of healthy adults of all ages.57 The higher rate of epileptiform activity in our controls – 10.5% – could reflect the more extensive neurophysiological monitoring, the use of more advanced MEG methods, and the older age of the population in our study than in previous investigations. Aging is associated with an increased risk of seizures, and a quarter of seizures in older adults have no known cause.58 It is unclear why epileptiform discharges detected by LTM-EEG were more left-sided and those detected by M/EEG were more right-sided (Fig 3D). Previous studies also detected epileptiform discharges on EEG more often in the left hemisphere in adults with or without epilepsy.59
The prospective and comprehensive design of our study has considerable strengths, yet there were also some limitations. Because of the time and resources required for extended neurophysiological monitoring, the sample sizes were not large enough to broadly search for genetic, environmental, and other factors that could promote neural network hyperexcitability. In addition, the frequency of epileptiform events in our patients detected by scalp recordings was too low to assess any acute effects of epileptiform discharges on cognition, as has been done with depth electrodes in epilepsy patients.9 Next, this study was not designed to determine inter-rater reliability between readers of the neurophysiological recordings; therefore, we used conservative criteria for determination of epileptiform activity and required consensus between clinicians reviewing the recordings. Finally, many of the patients at our tertiary referral center are relatively young and a high proportion present with non-memory predominant symptoms. Despite their phenotypic heterogeneity, our AD cohort overall was representative of sporadic AD, and an advantage to including younger patients was their low burden of comorbidities. The rates of cases with family history of AD in a first-degree relative (39.4%) and autosomal dominant pattern of inheritance (3.0%) and rates of APOE ε4 carriers (54.5%) were consistent with rates previously reported in patients presenting to dementia clinic.60 To expand on our investigation, additional studies will be needed to assess the relevance of our findings to later-onset AD in more diverse populations around the world.
In summary, we identified a population of AD patients who have subclinical epileptiform activity and decline faster cognitively than those without such activity. This previously under-recognized sign of neural network hyperexcitability requires greater attention, both in patient evaluations and in clinical trials. Our findings support investigating antiepileptic strategies in the comprehensive treatment approach to AD.
Supplementary Material
Acknowledgments
We thank Jeanne Paz for helpful comments on the manuscript; Mary Betinis for LTM-EEG oversight; the Clinical and Translational Science Institute (CTSI) Clinical Research Services for nursing, coordination, and biospecimen support; Averill Cantwell, Pia Ghosh, Matthew Growdon, Jung Jang, Baber Khan, and Teresa Wu for coordination support; Joe Hesse, Albert Lee, and Charlie Toohey for database support; Ryan Fitch for support with biospecimens; Joel Kramer for oversight of neuropsychological testing; Manu Hegde for assistance with MEG reading; Coleman Garrett for MEG acquisition support; EEG technicians for excellent technical support; Grisell Diaz-Ramirez, Clifford Anderson-Bergman, and Elissaios Karageorgiou for advice on statistical analysis; William Jagust and Gil Rabinovici for providing the amyloid imaging; Lea Grinberg, Eric Huang, and William Seeley for neuropathological assessments; Crystal Herron and Stephen Ordway for editorial assistance; Giovanni Maki and Benedicte Rossi for graphics support; and Monica Dela Cruz and Amy Cheung for administrative assistance. We are deeply grateful to all study participants and caregivers who contributed to this study. This study was supported by the National Institutes of Health (NIH) grants K23 AG038357 (KAV), P50 AG023501 and P01 AG19724 (BLM), R21 NS76171 (SSN), R01 DC010145 (SSN), NS066654 (SSN), NS64060 (SSN), University of California San Francisco Alzheimer’s Disease Research Center pilot project grant (KAV), National Science Foundation grant BCS-1262297 (SSN), a grant from the Alzheimer’s Association (PCTRB-13-288476) made possible by Part the Cloud™ (KAV), the John Douglas French Alzheimer’s Foundation (KAV), the S. D. Bechtel Jr. Foundation (LM), the McBean Family Foundation (KAV), and the Larry L. Hillblom Foundation (KGR). This study was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through University of California San Francisco–CTSI Grant Numbers UL1 RR024131 and UL1 TR000004.
Footnotes
AUTHORS’ CONTRIBUTIONS
K.A.V., D.E.B., E.D.R., B.L.M., L.M., and S.S.N. contributed to the conception and design of the study. K.A.V., K.G.R., A.J.B., D.M., S.M.H., A.F.D., S.M.D., V.V.B., A.M.K., G.C., M.M., P.E.G., H.E.K., and S.S.N. contributed to the acquisition and analysis of data. K.A.V., K.G.R., P.E.G., H.E.K, L.M., and S.S.N. contributed to writing the manuscript or preparing the figures.
POTENTIAL CONFLICTS OF INTERESTS
Nothing to report.
References
- 1.Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. (On certain peculiar diseases of old age) Zeitschrift für die gesamte Neurologie und Psychiatrie. 1911;4:356–85. [Google Scholar]
- 2.Scarmeas N, Honig LS, Choi H, et al. Seizures in Alzheimer disease: who, when, and how common? Arch Neurol. 2009;66:992–7. doi: 10.1001/archneurol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer's disease. Neurology. 1986;36:1226–30. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 4.Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs Aging. 2003;20:791–803. doi: 10.2165/00002512-200320110-00001. [DOI] [PubMed] [Google Scholar]
- 5.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–72. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 6.Cabrejo L, Guyant-Marechal L, Laquerriere A, et al. Phenotype associated with APP duplication in five families. Brain. 2006;129:2966–76. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- 7.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6:258–63. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 8.Forstl H, Burns A, Levy R, et al. Neurologic signs in Alzheimer's disease. Results of a prospective clinical and neuropathologic study. Arch Neurol. 1992;49:1038–42. doi: 10.1001/archneur.1992.00530340054018. [DOI] [PubMed] [Google Scholar]
- 9.Kleen JK, Scott RC, Holmes GL, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. 2013;81:18–24. doi: 10.1212/WNL.0b013e318297ee50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci U S A. 2012;109:E2895–903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 14.Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31:700–11. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldin E, Hauser WA, Buchhalter JR, et al. Yield of epileptiform electroencephalogram abnormalities in incident unprovoked seizures: a population-based study. Epilepsia. 2014;55:1389–98. doi: 10.1111/epi.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 18.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SB, Turner CA, Emerson RG, Scheuer ML. Spike detection II: automatic, perception-based detection and clustering. Clin Neurophysiol. 1999;110:404–11. doi: 10.1016/s1388-2457(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch HE, Robinson SE, Mantle M, Nagarajan S. Automated localization of magnetoencephalographic interictal spikes by adaptive spatial filtering. Clin Neurophysiol. 2006;117:2264–71. doi: 10.1016/j.clinph.2006.06.708. [DOI] [PubMed] [Google Scholar]
- 22.Guggisberg AG, Kirsch HE, Mantle MM, et al. Fast oscillations associated with interictal spikes localize the epileptogenic zone in patients with partial epilepsy. Neuroimage. 2008;39:661–8. doi: 10.1016/j.neuroimage.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vossel KA, Beagle AJ, Rabinovici GD, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–66. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiboriboon K, Nagarajan S, Mantle M, Kirsch HE. Interictal MEG/MSI in intractable mesial temporal lobe epilepsy: spike yield and characterization. Clin Neurophysiol. 2010;121:325–31. doi: 10.1016/j.clinph.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirsch HE, Mantle M, Nagarajan SS. Concordance between routine interictal magnetoencephalography and simultaneous scalp electroencephalography in a sample of patients with epilepsy. J Clin Neurophysiol. 2007;24:215–31. doi: 10.1097/WNP.0b013e3180556095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranasinghe KG, Hinkley LB, Beagle AJ, et al. Regional functional connectivity predicts distinct cognitive impairments in Alzheimer's disease spectrum. Neuroimage Clin. 2014;5:385–95. doi: 10.1016/j.nicl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker MP, Ayre GA, Cummings JL, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry. 2000;177:252–6. doi: 10.1192/bjp.177.3.252. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Littell RC, Milliken GA, Stroup WW, et al. SAS for Mixed Models. 2. Cary, NC: SAS Institute; 2006. [Google Scholar]
- 32.Selya AS, Rose JS, Dierker LC, et al. A Practical Guide to Calculating Cohen's f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider LS, Kennedy RE, Wang G, Cutter GR. Differences in Alzheimer disease clinical trial outcomes based on age of the participants. Neurology. 2015;84:1121–7. doi: 10.1212/WNL.0000000000001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt C, Wolff M, Weitz M, et al. Rapidly progressive Alzheimer disease. Arch Neurol. 2011;68:1124–30. doi: 10.1001/archneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 35.Roselli F, Tartaglione B, Federico F, et al. Rate of MMSE score change in Alzheimer's disease: influence of education and vascular risk factors. Clin Neurol Neurosurg. 2009;111:327–30. doi: 10.1016/j.clineuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Ebersole JS, Pacia SV. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia. 1996;37:386–99. doi: 10.1111/j.1528-1157.1996.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 37.Pacia SV, Ebersole JS. Intracranial EEG substrates of scalp ictal patterns from temporal lobe foci. Epilepsia. 1997;38:642–54. doi: 10.1111/j.1528-1157.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 38.Wennberg R, Arruda F, Quesney LF, Olivier A. Preeminence of extrahippocampal structures in the generation of mesial temporal seizures: evidence from human depth electrode recordings. Epilepsia. 2002;43:716–26. doi: 10.1046/j.1528-1157.2002.31101.x. [DOI] [PubMed] [Google Scholar]
- 39.Lam AD, Zepeda R, Cole AJ, Cash SS. Widespread changes in network activity allow non-invasive detection of mesial temporal lobe seizures. Brain. 2016 doi: 10.1093/brain/aww198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liedorp M, Stam CJ, van der Flier WM, et al. Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord. 2010;29:432–7. doi: 10.1159/000278620. [DOI] [PubMed] [Google Scholar]
- 41.Clemens Z, Janszky J, Szucs A, et al. Interictal epileptic spiking during sleep and wakefulness in mesial temporal lobe epilepsy: a comparative study of scalp and foramen ovale electrodes. Epilepsia. 2003;44:186–92. doi: 10.1046/j.1528-1157.2003.27302.x. [DOI] [PubMed] [Google Scholar]
- 42.Shi JQ, Wang BR, Tian YY, et al. Antiepileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice. CNS Neurosci Ther. 2013;19:871–81. doi: 10.1111/cns.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nygaard HB, Kaufman AC, Sekine-Konno T, et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer's disease mouse model. Alzheimers Res Ther. 2015;7:25. doi: 10.1186/s13195-015-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang MY, Zheng CY, Zou MM, et al. Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice. Neurobiol Aging. 2014;35:2713–25. doi: 10.1016/j.neurobiolaging.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–74. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakker A, Albert MS, Krauss G, et al. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–98. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 48.Mintz M, Legoff D, Scornaienchi J, et al. The underrecognized epilepsy spectrum: the effects of levetiracetam on neuropsychological functioning in relation to subclinical spike production. J Child Neurol. 2009;24:807–15. doi: 10.1177/0883073808330762. [DOI] [PubMed] [Google Scholar]
- 49.Verhey FR, Houx P, Van Lang N, et al. Cross-national comparison and validation of the Alzheimer's Disease Assessment Scale: results from the European Harmonization Project for Instruments in Dementia (EURO-HARPID) Int J Geriatr Psychiatry. 2004;19:41–50. doi: 10.1002/gps.1035. [DOI] [PubMed] [Google Scholar]
- 50.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–70. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging. 2008;29:819–27. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Agosta F, Vossel KA, Miller BL, et al. Apolipoprotein E epsilon4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A. 2009;106:2018–22. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–17. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Haan W, Stam CJ, Jones BF, et al. Resting-state oscillatory brain dynamics in Alzheimer disease. J Clin Neurophysiol. 2008;25:187–93. doi: 10.1097/WNP.0b013e31817da184. [DOI] [PubMed] [Google Scholar]
- 55.Verret L, Mann EO, Hang GB, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–21. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molaie M, Culebras A, Miller M. Effect of interictal epileptiform discharges on nocturnal plasma prolactin concentrations in epileptic patients with complex partial seizures. Epilepsia. 1986;27:724–8. doi: 10.1111/j.1528-1157.1986.tb03601.x. [DOI] [PubMed] [Google Scholar]
- 57.So EL. Interictal epileptiform discharges in persons without a history of seizures: what do they mean? J Clin Neurophysiol. 2010;27:229–38. doi: 10.1097/WNP.0b013e3181ea42a4. [DOI] [PubMed] [Google Scholar]
- 58.Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37:224–9. doi: 10.1111/j.1528-1157.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 59.Dean AC, Solomon G, Harden C, et al. Left hemispheric dominance of epileptiform discharges. Epilepsia. 1997;38:503–5. doi: 10.1111/j.1528-1157.1997.tb01743.x. [DOI] [PubMed] [Google Scholar]
- 60.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43:835–45. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.