Abstract
Chest imaging plays a prominent role in blunt trauma patient evaluation, but indiscriminate imaging is expensive, may delay care, and unnecessarily exposes patients to potentially harmful ionizing radiation. To improve diagnostic chest imaging utilization, we conducted 3 prospective multicenter studies over 12 years to derive and validate decision instruments (DIs) to guide the use of chest x-ray (CXR) and chest computed tomography (CT).
The first DI, NEXUS Chest x-ray, consists of seven criteria (Age > 60 years; rapid deceleration mechanism; chest pain; intoxication; altered mental status; distracting painful injury; and chest wall tenderness) and exhibits a sensitivity of 99.0% (95% confidence interval [CI] 98.2-99.4%) and a specificity of 13.3% (95% CI, 12.6%-14.0%) for detecting clinically significant injuries.
We developed two NEXUS Chest CT DIs, which are both highly reliable in detecting clinically major injuries (sensitivity of 99.2%; 95% CI 95.4-100%). Designed primarily to focus on detecting major injuries, the NEXUS Chest CT-Major DI consists of six criteria (abnormal CXR; distracting injury; chest wall tenderness; sternal tenderness; thoracic spine tenderness; and scapular tenderness) and exhibits higher specificity (37.9%; 95% CI 35.8–40.1%). Designed to reliability detect both major and minor injuries (sensitivity 95.4%; 95% CI 93.6–96.9%) with resulting lower specificity (25.5%; 95% CI 23.5–27.5%), the NEXUS CT-All rule consists of seven elements (the six NEXUS CT-Major criteria plus rapid deceleration mechanism).
The purpose of this review is to synthesize the three DIs into a novel, cohesive summary algorithm with practical implementation recommendations to guide selective chest imaging in adult blunt trauma patients.
Background
Chest imaging is currently recommended in the evaluation of all blunt trauma patients who present with a concerning mechanism of injury. As a consequence, chest x-ray (CXR) imaging has become a reflexive test, often ordered regardless of clinical signs of injury.1-2 In fact, the CXR is the most common imaging study performed in blunt trauma patients.3
The use of chest computed tomography (CT) has increased in spite of stable injury prevalence rates.4-6 The push for head-to-pelvis CT (pan-scan) has similarly fueled an increased in utilization of CT in adult blunt trauma diagnostic protocols.7-10 Such routine ordering, however, may lead to expensive, low-yield, inefficient care and expose patients to unnecessary radiation exposure.11-17 For example, when performed after a normal CXR, chest CT diagnoses only one major injury for every 67 studies.13 This practice generates approximately $220,000 in radiographic charges and may induce as many as one lethal malignancy for every 11 major injury diagnoses.12-19
Order sets that include indiscriminate imaging have been shown to increase the costs, and in some cases, risk to patients.20 Median charges for chest x-ray in 2013 are $298 per examination, while median charges for chest CT are $3,294 per patient.13,21 Potential risks include harmful effects from ionizing radiation exposure, as well as potential renal injury and allergic reactions from intravenous contrast.22 While CXR delivers negligible amounts of radiation, chest CT exposes the patient to a considerable effective radiation dose (ERD) and cancer induction risk, especially for women. Chest CT delivers approximately 8.9 mSv, which is estimated to induce one lethal cancer among every 650 exposed 40 year old women.12 Although newer CT scan protocols may deliver lower ERD (approximately 5 mSv), lethal malignant transformation rates are higher among younger patients who comprise trauma populations.15,18,19
With these principles of cancer induction risk, expense, and resource utilization in mind, we have examined the issue of blunt trauma chest imaging in 5 prospective studies conducted over the past 12 years.3,13,23-26 With the ultimate goals of reducing unnecessary imaging and producing more efficient protocols for blunt trauma chest imaging, we prospectively enrolled over 24,000 adult blunt trauma victims at 10 Level 1 trauma centers. The purpose of this review of our previously published work is to synthesize the three resulting decision instruments into a cohesive summary algorithm with practical implementation recommendations to guide selective imaging in adult blunt chest trauma patients.
Methods
We began our chest imaging DI work in 2003, when chest CT was less commonly utilized. We therefore initially directed our efforts at reducing unnecessary CXR in a manner similar to the original NEXUS and Canadian cervical spine studies, which looked primarily at patients with plain radiography of the cervical spine.27,28 While deriving and validating a rule for selective CXR, we recognized the movement toward greater use of chest CT,7.8.9.10 and later sought to develop a rule for selective use of chest CT.3
Our DIs were developed as one-way directive instruments intended to reduce the reflexive, nearly universal use of chest imaging in blunt trauma patients. Adhering to the principles for clinical decision rule development put forth by Stiell, et al 29, we employed consistent core methodology in all of our pilot, derivation and validation studies.29,30 After our pilot study at 3 trauma centers, we conducted our 4 primary studies at 10 urban Level 1 trauma centers, prospectively enrolling patients with the following inclusion criteria: 1) age over 14 years, 2) presentation to the ED for blunt trauma that occurred within 6 hours of arrival, and 3) consistent with our objective to derive one-way rules--having chest imaging (either CXR or chest CT) ordered in the ED as part of their trauma evaluation.3,23,24 We focused our enrollment primarily to daytime hours according to research personnel availability.
Candidate criteria
By reviewing literature and investigator consensus, we generated lists of DI candidate criteria and refined these lists into DIs through prospectively conducted derivation studies. We checked all criteria for inter-rater reliability using dual, independent assessments, ensuring that they met pre-defined kappa thresholds for agreement.3,23
Outcomes
We defined all injuries according to official radiologist interpretations. In order to assess the clinical impact of injuries, we convened a priori expert trauma clinician panels to classify injuries seen on chest and thoracic imaging into major, minor and no clinical significance categories. See Table 1 for this classification. We followed enrolled patients through their hospital course, abstracting outcome data by recommended chart abstraction guidelines and checked subsets of data to confirm inter-abstractor consistency and agreement.3,23,24,30
Table 1.
Trauma expert panel determination of clinical significance of injuries seen on chest imaging.
| Category | Injury |
|---|---|
| Major clinical significance | Aortic or great vessel injury (all considered major) |
| Ruptured diaphragm (all considered major) | |
| Pneumothorax: received evacuation procedure (chest tube or other procedure) | |
| Hemothorax: received drainage procedure (chest tube or other procedure) | |
| Sternal fracture: received surgical intervention | |
| Multiple rib fracture: received surgical intervention or epidural nerve block | |
| Pulmonary contusion: received mechanical ventilation (including non-invasive ventilation) primarily for respiratory failure within 24 h for management | |
| Thoracic spine fracture: received surgical intervention | |
| Scapular fracture: received surgical intervention | |
| Mediastinal or pericardial hematoma: received drainage procedure | |
| Esophageal injury: received surgical intervention | |
| Tracheal or bronchial injury: received surgical intervention | |
| Minor clinical significance | Pneumothorax: no evacuation procedure but observed as inpatient >24 h |
| Hemothorax: no drainage procedure but observed as inpatient for >24 h | |
| Sternal fracture: no surgical intervention | |
| Multiple rib fracture: no surgical intervention or epidural nerve block | |
| Pulmonary contusion or laceration: no mechanical ventilation but observed >24h | |
| Thoracic spine fracture: no surgical intervention | |
| Scapular fracture: no surgical intervention | |
| Mediastinal or pericardial hematoma: no surgical intervention | |
| Esophageal injury: no surgical intervention | |
| Tracheal or bronchial injury: no surgical intervention | |
| No clinical significance* | Hemothorax: no surgical intervention, no inpatient observation |
| Pneumothorax: no surgical intervention, no inpatient observation | |
| Pneumomediastinum without pneumothorax: no inpatient observation | |
| Pulmonary contusion or laceration: no mechanical ventilation, no surgical intervention, no inpatient observation |
This category was generated to account for those instances in which CT visualizes minute abnormalities that result in no changes in management.
Controls for bias
Systematically enrolling groups of patients who did not receive imaging or who were not admitted to the hospital, we controlled and checked our work extensively for the introduction of spectrum bias and follow-up bias. We also followed patients whose initial ED imaging was negative to see if they were later diagnosed with injury.3,24
Analyses
Considering that clinicians prefer directive rules over rating scales, we primarily focused on the development of DIs that rule out injury and eliminate the need for imaging (identification of patients who are at very low risk for injury seen on imaging). In terms of statistical analyses, we therefore used classification tree (binary recursive partitioning) techniques to derive our primary DIs.3,23 When determining acceptable DI sensitivity thresholds, we weighed the risks of missed injury against the costs and radiation exposure associated with imaging. Under this premise, we sought a very high sensitivity rule (very low miss rate) for all injuries for CXR. Given that the risks and costs of chest CT are exponentially greater, we accepted a lower DI sensitivity for clinically minor injuries in our CT DIs. Considering its high associated morbidity and mortality, we targeted a 100% sensitivity for aortic or great vessel injury in all our DIs, such that a missed aortic or great vessel injury would render any DI unacceptable.
For our CT DIs, we paid special attention to the issue of widely disparate viewpoints on the need to diagnose clinically minor injuries.10,31 Some clinicians believe that it is important to detect all (or nearly all) injuries, even if they do not result in interventions or significant changes in management.10 Others do not believe it is necessary to diagnose injuries that do not change management.31 Respecting both viewpoints, we developed two chest CT DIs—one with very high sensitivity for major and minor injury, and the other with very high sensitivity for major injury only.3
In our validation studies, we adhered strictly to recommended DI development guidelines and evaluated DI performance prospectively on separate, independent cohorts.29 We calculated screening performance characteristics for our DIs in these samples using the following 2 ×2 table definitions: True positive = one or more DI criteria, and injury present; True negative = no DI criteria, and no injury; False positive = one or more DI criteria present, but no injury; and False negative = no DI criteria, but injury present. In order to diminish the impact of variations in injury prevalence, we focused our analyses on sensitivity (high injury detection rate) and specificity (avoidance of non-diagnostic studies) instead of negative and positive predictive value.
Analyses for assistive DIs
Recognizing that clinicians may also seek risk stratification information, especially in patients who did not rule out for injury by the DIs, we analyzed data to provide assistive guidance for both CXR and chest CT. We determined the screening performance for injury of individual criteria and the prevalence of injury information when one or more criteria are present.26,32
Sample size considerations
Our validation sample size calculations were driven by the need to attain narrow confidence intervals around point estimates of high sensitivity thresholds for injury. For our initial CXR DI, we determined that we needed to enroll 9,718 patients and for our NEXUS Chest CT DIs, we needed 9,577 patients.
Results
We enrolled 24,010 patients in our four cohorts (2,628 for CXR derivation, 9,905 for CXR validation, 6,002 for CT derivation CT, and 5,475 for CT validation) and found similar patient characteristics in each group. See Table 2 for a summary of patient characteristics.3,23,24
Table 2.
Patient Characteristics of NEXUS Chest imaging studies
| Patient Characteristics | |||||
|---|---|---|---|---|---|
| Characteristic | CXR Derivation1 | CXR Validation2 | CT Derivation3 | CT Validation4 | TOTAL |
| Number | 2,628 | 9,905 | 6,002 | 5,475 | 24,010 |
| Gender: | |||||
| Male (%) | 1,698 (64.6) | 6,220 (62.8) | 3,583 (59.7) | 3,384 (61.8) | 14,885 (62.0) |
| Female | 930 (35.4) | 3,685 (37.2) | 2,419 (40.3) | 2,091 (38.2) | 9,125 (38.0) |
| Age, years, median (IQR) | 42 (28-57) | 45 (29-60) | 46 (29-62) | 45 (28-61) | 45 (29-61) |
| Mechanism of Injury: | |||||
| Motor vehicle crash (%) | 926 (35.2) | 3,463 (35.0) | 2,141 (35.7) | 1,945 (35.5) | 8,476 (35.3) |
| Motorcycle crash | 254 (9.7) | 878 (8.9) | 466 (7.8) | 594 (10.8) | 2,192 (9.1) |
| Pedestrian struck by vehicle | 418 (15.9) | 1,057 (10.7) | 498 (8.3) | 543 (9.9) | 2,516 (10.5) |
| Bicycle crash | 194 (7.4) | 624 (6.3) | 427 (7.1) | 408 (7.5) | 1,653 (6.9) |
| Fall | 535 (20.4) | 2,726 (27.5) | 1,781 (29.7) | 1,368 (25.0) | 6,410 (26.7) |
| Struck by object or assault | 177 (6.7) | 577 (5.8) | 416 (6.9) | 305 (5.6) | 1,475 (6.1) |
| Other or unknown | 124 (4.7) | 580 (5.9) | 273 (4.5) | 312 (5.7) | 1,289 (5.4) |
| GCS score, median (IQR) | 15 (15-15) | 15 (15-15) | 15 (15-15) | 15 (15-15) | 15 (15-15) |
| Admitted to hospital (%) | 5,173 (52.2) | 2,768 (46.1) | 2,733 (49.9) | 5,501 (47.9) | |
| Survival to discharge (%) | 4877 (94.3) | 2,599 (93.9) | 2,575 (94.2) | 5,174 (94.1) | |
| Hospital LOS, median (IQR) | 3 (1-5) | 3 (1-5) | 3 (1-5) | 3 (1-5) | |
| ISS, median (IQR) | 5 (1-10) | 5 (1-10) | 5 (1-10) | ||
| Imaging: | |||||
| CXR only (%) | 2,044 (78) | 4,817 (48.6) | 3,741 (62.3) | 2,555 (46.7) | 13,157 (54.8) |
| CXR and CT | 554 (21) | 4,828 (48.7) | 1,873 (31.2) | 2,628 (48) | 9,883 (41.2) |
| CT only | 30 (1) | 260 (2.6) | 388 (6.5) | 292 (5.3) | 970 (4.0) |
CXR = chest X-ray
CT = computed tomography
SD = standard deviation
IQR = interquartile range
GCS = Glasgow coma scale
LOS = length of stay
ISS = Injury severity score
= July 2007-August 2009;
= December 2009-January 2012;
= September 2011-December 2012;
= February 2013-May 2014
For selective initial chest imaging (CXR), we derived and validated NEXUS Chest, which consists of 1) age > 60; 2) rapid deceleration mechanism (explicitly defined as fall > 20 feet or motorized vehicle accident > 40 miles per hour); 3) chest pain; 4) intoxication; 5) distracting injury; 6) tenderness to chest wall palpation; and 7) abnormal alertness/mental status.23,24 In the separate validation cohort, NEXUS Chest had a sensitivity for major injury of 99.7% (95% confidence interval [CI] 98.2-100.0%) and for all injuries of 99.0% (95% CI 98.2-99.4%). NEXUS Chest specificity, however, was low at 13.3% (95% CI, 12.6%-14.0%).24
For selective chest CT we considered trauma providers’ varied minor injury diagnosis thresholds and therefore derived and validated two decision instruments.10,31 For clinicians who believe it is essential to diagnose all (or nearly all) injuries, we developed NEXUS Chest CT-All, which is highly sensitive for all thoracic injury and consists of 1) abnormal CXR, 2) rapid deceleration mechanism, 3) distracting injury, 4) chest wall tenderness, 5) sternal tenderness, 6) thoracic spine tenderness, and 7) scapular tenderness.3,10 For those who only seek to detect injuries that result in interventions or other significant changes in patient management, we developed NEXUS Chest CT-Major, which has the same criteria minus rapid deceleration mechanism3,31. NEXUS Chest CT-Major retains the very high sensitivity for injuries of major clinical significance 99.2% (95%CI, 95.4%-100%), with a slightly lower minor injury sensitivity of 90.7% (95%CI, 88.3%-92.8%), and a higher specificity of 37.9% (95%CI, 35.8%-40.1%), allowing clinicians to forego CT in a greater percentage of patients.
Of note, our three DIs would have detected all of the 38 patients with aortic or great vessel injury (sensitivity 100%; 95% CI 90.8-100%). See Table 3 for summary validation screening performance characteristics of our three chest imaging DIs.
Table 3.
Injuries seen on NEXUS Chest imaging studies
| Injury | CXR Derivation N = 2628 |
CXR Validation N = 9905 |
CT Derivation N = 6002 |
CT Validation N = 5475 |
TOTAL N = 24,010 |
|---|---|---|---|---|---|
| Patients with Injury (%) | 271 (10.3) | 1478 (14.9) | 777 (12.9) | 811 (14.8) | 3,337 (13.9) |
| ≥2 Rib fractures | 199 (7.6) | 996 (10.1) | 407 (6.8) | 446 (8.1) | 2,048 (8.5) |
| Pneumothorax | 103 (3.9) | 590 (6.0) | 207 (3.4) | 216 (3.9) | 1,116 (4.6) |
| Pulmonary contusion | 79 (3.0) | 527 (5.3) | 186 (3.1) | 249 (4.5) | 1,041 (4.3) |
| Hemothorax | 41 (1.6) | 207 (2.1) | 75 (1.2) | 60 (1.1) | 383 (1.6) |
| Sternal fracture | 24 (0.9) | 212 (2.1) | 112 (1.9) | 124 (2.3) | 472 (2.0) |
| Ruptured diaphragm | 14 (0.5) | 6 (0.1) | 1 (0.0) | 3 (0.1) | 24 (0.1) |
| Aorta/great vessel injury | 2 (0.1) | 15 (0.2) | 11 (0.2) | 10 (0.2) | 38 (0.2) |
| Spinal fracture* | 149 (2.5) | 90 (1.6) | 239 (2.1) | ||
| Scapular fracture* | 68 (1.1) | 66 (1.2) | 134 (1.2) | ||
| Mediastinal or pericardial hematoma* | 34 (0.6) | 40 (0.7) | 74 (0.6) | ||
| Pneumomediastinum* | 33 (0.5) | 20 (0.4) | 53 (0.5) | ||
| Other* | 13 (0.2) | 4 (0.1) | 17 (0.1) |
CXR = chest X-ray
CT = computed tomography
Injury types collected for CT study only
In terms of our assistive guidance for chest CT, we found that abnormal CXR was, by far, the best screening criterion for injury seen on subsequent chest CT (sensitivity for major injury 73.7% [95% CI 68.1-78.6%], specificity 83.9% [95% CI 83.6-84.2]).26,32 Distracting injury (11.5%), rapid deceleration mechanism (9.0%), and abnormal CXR (3.1%) were the criteria that most frequently occurred in isolation. The presence of any single clinical criterion (other than abnormal CXR) was associated with low prevalence of injury 16.8% (95% CI 15.2-18.6) and clinically significant injury 1.1% (95% CI 0.1-1.8). Injury prevalence increased with an increasing number of criteria present. See Table 4 for likelihood of injury with individual and combinations of criteria.26,32
Table 4.
Predictive performance of individual and combinations of criteria
| Occurrence | PPV for major injury [95% CI] | PPV for major or minor injury [95% CI] | PLR for maj or or min or injury [95 % CI] | |
|---|---|---|---|---|
| Positive for 0 of 7 criteria# | 813 (18.1%) | 2 (0.2%) [0.1-0.7%] | 56 (6.9%) [5.3-8.8] | 0.17 [0.1 3-0.26] |
| Positive for 1 criterion (not abnormal CXR) # | 1523 (29.5%) | 17 (1.1%) [0.1-1.8] | 256 (16.8%) [15.2-18.6%] | 0.50 [0.4 4-0.56] |
| Only abnormal CXR# | 140 (3.1%) | 18 (12.9%) [8.3-19.4] | 85 (60.7%) [52.2-68.6%] | 3.59 [2.5 4-5.08] |
| Positive for 2 criteria (not abnormal CXR) # | 1040 (20.1%) | 33 (3.2%) [2.3-4.4%] | 265 (25.5%) [23.1-28.0%] | 0.84 [0.7 4-0.96] |
| Positive for abnormal CXR + 1 other criterion* | 254 (5.6%) | 47 (18.5%) [14.2- 23.7%] | 166 (65.4%) [59.3-71.0%] | 4.38 [3.3 9-5.68] |
| Positive for 3 criteria (not abnormal CXR) # | 513 (11.4) | 14 (2.7%) [1.6-4.5%] | 179 (34.9%) [31.0-39.0] | 1.32 [1.1 2-1.66] |
| Positive for abnormal CXR + 2 other criteria* | 251 (5.6%) | 62 (24.7%) [19.8-30.4] | 205 (81.7%) [76.4-86.1%] | 10.3 5 [7.5 0-14.3 6] |
PPV = positive predictive value; MCI = major clinical injury; CXR = chest x-ray; CI = confidence interval
Calculations for these criteria were based on all patients who had CT (N = 5169).
Calculations for these criteria were based on all patients who had both CXR and CT (N = 4501).
7 criteria = Sternal tenderness, Scapular tenderness, Chest wall tenderness, Thoracic spine tenderness, Distracting injury, Rapid deceleration, abnormal CXR.
Limitations
Although we used convenience (daytime) sampling, non-enrollment hour patient characteristics were similar and nighttime sampling would be unlikely to change our findings.23 All study sites were Level 1 trauma center teaching hospitals with many resident physician trauma providers. Nonetheless, all of our criteria are very simple elements of physical exam and history that demonstrated high levels of inter-rater reliability and can likely be assessed easily by a broad range of clinicians. Similarly, our algorithms are straightforward with an application mode that is familiar to trauma clinicians and has been successfully implemented with other well-known decision rules.
The low specificity of our original NEXUS Chest rule limits the potential resource savings from its application. When gauging the risk/benefit ratio of missed thoracic injury versus avoidance of the ionizing radiation of one CXR, we sought very high sensitivity, such that practitioners of all specialties would consider the DI acceptable for use. Our NEXUS Chest CT rules have better specificity, thereby allowing more patients to be effectively ruled out for injury.
One of the primary obstacles to broad acceptance and implementation of our DIs (and all trauma imaging decision rules) is the fundamental disagreement regarding the need to diagnose minor injuries. Practitioners commonly disagree about the significance of non-interventional injuries and generally fall into two camps: those who believe that nearly all injuries should be detected, and those who believe that only management-changing injuries are important.10,31 We attempted to address part of this controversy by convening expert trauma clinician consensus panels (balanced between EM physicians and trauma surgeons), who classified all injuries seen on imaging according to their clinical significance. Understanding that there is no “right” or “wrong” in this debate, we have presented summary algorithms that offer choices to suit the beliefs and preferences of individual practitioners and institutions. Furthermore, addressing this issue from the standpoint of patient-centered practice in one of our satellite studies, we demonstrated that patients wish to discuss risks and costs of CT and are willing to accept a small risk of missed injury in lower risk scenarios.33
Summary Recommendations and Discussion
We present our summary algorithm for blunt trauma chest imaging in Figure 1. We wish to emphasize several points from the standpoint of practical implementation. First, it is important to note that although our DIs were derived and validated in broad groups of patients (including severely injured patients), they are generally intended for use in awake, non-intubated, hemodynamically stable blunt trauma patients. In our work we did not address or refute the findings of several groups of investigators, who have reported improved outcomes with pan-scan in severely injured, poly-trauma patients.7,8,9,34 In order to use our NEXUS rules, clinicians must be able to assess whether patients have particular criteria (e.g. chest wall or sternal tenderness), and therefore they are not useful for unconscious patients.
Figure 1. NEXUS Chest algorithm to guide blunt trauma chest imaging.
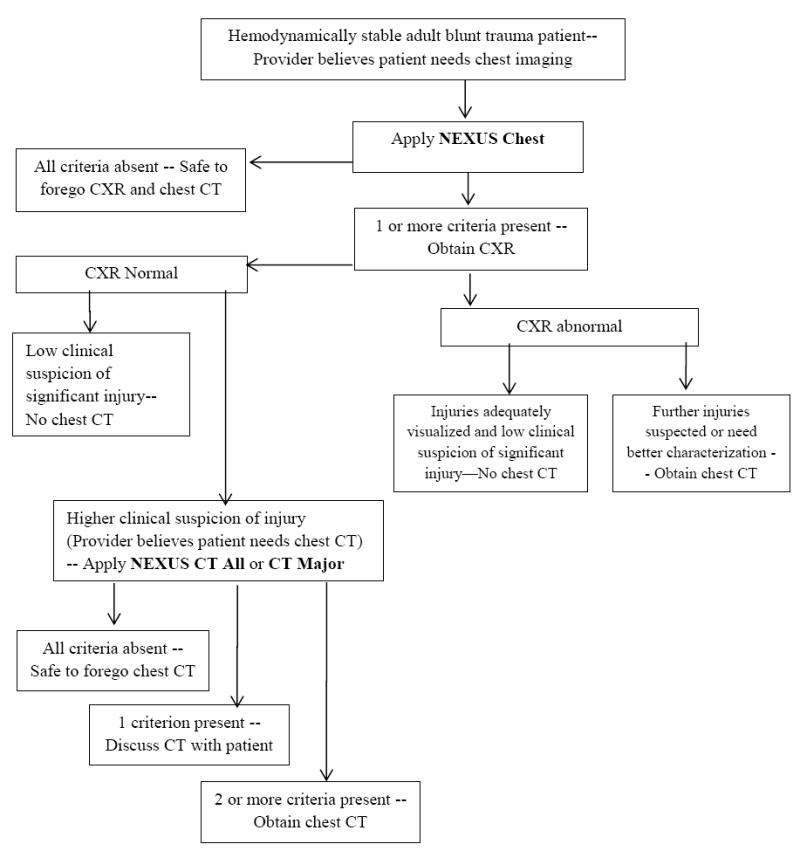
NEXUS chest = 1) age > 60; 2) rapid deceleration mechanism (fall > 20 feet or motorized vehicle accident > 40 miles per hour); 3) chest pain; 4) intoxication; 5) distracting injury; 6) tenderness to chest wall palpation; and 7) abnormal alertness/mental status.
NEXUS CT Major = 1) distracting injury; 2) chest wall tenderness; 3) sternal tenderness; 4) thoracic spine tenderness; and 5) scapular tenderness.
NEXUS CT All = above CT Major criteria + rapid deceleration mechanism
Second, we recommend the application of NEXUS Chest in tandem with the NEXUS Cervical Spine rule, with which it shares the common criteria of distracting injury, intoxication and altered level of alertness.3,27 If a patient has one of these three criteria, they cannot be ruled out for either cervical spine or chest injury. Similarly, chest wall tenderness should be considered analogous to the midline cervical spine tenderness criterion of the NEXUS Cervical Spine rule.
Third, when developing our DIs, we were very cognizant about time pressures and the mental overload that can occur with the need to memorize multiple decision rules. All of our criteria are intuitive elements of trauma history and physical exam, requiring little if any extra provider time or effort. The four physical exam criteria of NEXUS Chest CT (chest wall, sternal, thoracic spine and scapular tenderness) are all elements that are evaluated as part of the typical trauma exam, and for ease of remembering, can be grouped together as thoracic wall tenderness.
Fourth, as emphasized in our methods section, we deliberately developed these DIs as one-way rules to identify patients who are at very low risk of injury, and for whom imaging may be safely omitted. These rules are not intended to be used as primary screening tools to determine the need for imaging, but are designed to be used in conjunction with clinical judgment to reduce imaging. In this regard, we recommend that clinicians first decide whether they would order chest imaging according to their clinical impression and initial assessment. If imaging seems appropriate, the DI should be applied to determine whether imaging can be safely omitted. The presence of DI criteria does not mandate chest radiography in patients who would otherwise not be considered for chest imaging -- misapplication of these DIs to other patients who they were not going to image may paradoxically result in increased CXR and chest CT utilization.
Patients who are deemed low risk for injury (all criteria absent) by NEXUS Chest are effectively cleared for all chest imaging – clinicians may safely forego both CXR and chest CT. In our studies we found that CXR was the best screening criterion for injury seen on chest CT and we therefore advocate for the continued use of portable CXR as the most logical initial modality of chest imaging for most blunt trauma patients.3 If injuries are well characterized by CXR and there is not suspicion for other major injury, further imaging is unnecessary.
CXR may miss injuries that are detected by CT, most notably sternal fractures, rib fractures and minor pulmonary contusions, and clinicians may feel the need to obtain the greater anatomic detail provided by chest CT. In such instances, clinicians should then apply the CT-All or CT-Major DI to determine whether advanced imaging is warranted. Ultimately, the decision regarding whether to employ CT-All or CT-Major is based on individual physician preference and risk tolerance. For clinicians who desire very high sensitivity for all injuries, we recommend use of NEXUS CT-All, which should detect nearly all minor and major injuries, but at the cost of increased imaging and its attendant risk and expense.3,10 Physicians who want to spare a greater percentage of patients from CT, and thereby reduce costs and radiation exposure, may elect to apply NEXUS CT-Major, which retains the same very high sensitivity for major injuries, but exhibits higher specificity and corresponding greater reduction in CT.3,31
Patients who exhibit none of the risk criteria in either CT-Major, or CT-All have a very low likelihood of having clinically significant injury. Patients who have normal plain chest radiographs and exhibit only one of the other clinical criteria still have a relatively low risk of injury and may be candidates for an imaging strategy that involves shared decision making. We have demonstrated that most patients prefer to discuss the risks and costs of CT whenever possible and that they will often choose to forego CT in scenarios in which there is a low risk of injury.33 The shared decision model is less suitable for patients who have two or more criteria, and who consequently have an increased risk of injury and greater benefit from diagnostic CT imaging.
Finally, we recommend the incorporation of the NEXUS Chest DIs and algorithm as checklists into chart templates and electronic medical records. Clinicians may thereby state that a “patient meets NEXUS Chest low risk criteria for injury” or “patient ruled out for chest injury by NEXUS Chest Imaging Algorithm”.
Overall, our work provides clinicians with evidence-based mechanisms to use basic physical exam and history findings, instead of routine imaging, to safely and efficiently rule out injury in appropriate patients. We believe that our DIs and algorithm will save provider and patient time by allowing for the safe avoidance of unnecessary imaging.
Acknowledgments
Funded by grants from the Centers for Disease Control and Prevention (RO-1/CE001589-01) and the University of California Center for Health Quality and Innovation (CHQI) (071:2011).
Footnotes
Author Contributions: All authors formulated the study design and contributed to the article composition. RMR takes responsibility for the paper as a whole.
All authors were required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American College of Surgeons. Advanced Trauma Life Support for Doctors: Student Course Manual. 7. Chicago, IL: American College of Surgeons; 2008. [Google Scholar]
- 2.Wisbach GG, Sise MJ, Sack DI, et al. What is the role of chest X-ray in the initial assessment of stable trauma patients? J Trauma. 2007;62(1):74–79. doi: 10.1097/01.ta.0000251422.53368.a3. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez RM, Langdorf MI, Nishijima D, et al. Derivation and Validation of Two Decision Instruments for Selective Chest CT in Blunt Trauma: A Multicenter Prospective Observational Study (NEXUS Chest CT) PLOS Medicine. 2015 doi: 10.1371/journal.pmed.1001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder J, Warshauer DM. Increasing utilization of computed tomography in the adult emergency department, 2000-2005. Emerg Radiol. 2006;13:25–30. doi: 10.1007/s10140-006-0493-9. [DOI] [PubMed] [Google Scholar]
- 5.Larson DB, Johnson LW, Schnell BM, Salisbury SR, Forman HP. National trends in CT use in the emergency department: 1995-2007. Radiology. 2011;258:164–73. doi: 10.1148/radiol.10100640. [DOI] [PubMed] [Google Scholar]
- 6.Korley FK, Pham JC, Kirsch TD. Use of advanced radiology during visits to US emergency departments for injury-related conditions, 1998-2007. JAMA. 2010;304:1465–71. doi: 10.1001/jama.2010.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campion EM, Mackersie RC. Recent developments in the assessment of the multiply injured trauma patient. Opin Crit Care. 2014;20:620–5. doi: 10.1097/MCC.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 8.Huber-Wagner S, Lefering R, Qvick LM, Körner M, Kay MV, Pfeifer KJ, et al. Effect of whole-body CT during trauma resuscitation on survival: a retrospective multicentre study. Lancet. 2009;373:1455–61. doi: 10.1016/S0140-6736(09)60232-4. [DOI] [PubMed] [Google Scholar]
- 9.Salim A, Sangthong B, Martin M, Brown C, Plurad D, Demetriades D. Whole body imaging in blunt multisystem trauma patients without obvious signs of injury. Arch Surg. 2006;141:468–75. doi: 10.1001/archsurg.141.5.468. [DOI] [PubMed] [Google Scholar]
- 10.Tillou A, Gupta M, Baraff LJ, Schriger DL, Hoffman JR, Hiatt JR, et al. Is the use of pan-computed tomography for blunt trauma justified? A prospective evaluation. J Trauma. 2009;67:779–87. doi: 10.1097/TA.0b013e3181b5f2eb. [DOI] [PubMed] [Google Scholar]
- 11.Choosing Wisely: Five things physicians should and patients should question. American College of Surgeons; [12/04/2015]. https://www.facs.org/~/media/files/education/acslist.ashx. [Google Scholar]
- 12.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de González A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez RM, Baumann BM, Raja AS, Langdorf MI, Anglin D, Bradley RN, Medak AJ, Mower WR, Hendey GW. Diagnostic yields, charges, and radiation dose of chest imaging in blunt trauma evaluations. Acad Emerg Med. 2014 Jun;21(6):644–50. doi: 10.1111/acem.12396. [DOI] [PubMed] [Google Scholar]
- 14.Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected Cancer Risks From Computed Tomographic Scans Performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 16.Fazel R, Krumholz HM, Wang Y, Ross JS, Chen J, Ting HH, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amis ES, Jr, Butler PF, Applegate KE, Birnbaum SB, Brateman LF, Hevezi JM, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4:272–284. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Hall EJ, Brenner DJ. Cancer risk from diagnostic radiology: the impact of new epidemiological data. Br J Radiol. 2012;85(1020):e1316–17. doi: 10.1259/bjr/13739950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez-Almazor ME, Belseck E, Russell AS, Mackel JV. Use of lumbar radiographs for the early diagnosis of low back pain: proposed guidelines would increase utilization. JAMA. 1997;277(22):1782–1786. [PubMed] [Google Scholar]
- 21.Ziegler K, Feeny JM, Desai C, Sharpio D, Marshall WT, Twohig M. Retrospective review of the use and costs of routine chest x rays in a trauma setting. Journal of Trauma Management & Outcomes. 2013;7:2–6. doi: 10.1186/1752-2897-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CI, Aims AH, Monico EP, Brink JA, Forman HP. Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology. 2004;231:393–8. doi: 10.1148/radiol.2312030767. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez RM, Hendey GW, Mower W, Kea B, Fortman J, Merchant G, et al. Derivation of a decision instrument for selective chest radiography in blunt trauma. J Trauma. 2011;71:549–53. doi: 10.1097/TA.0b013e3181f2ac9d. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez RM, Anglin D, Langdorf MI, Baumann BM, Hendey GW, Bradley RN, et al. NEXUS Chest: Validation of a decision instrument for selective chest imaging in blunt trauma. JAMA Surg. 2013;148:940–946. doi: 10.1001/jamasurg.2013.2757. [DOI] [PubMed] [Google Scholar]
- 25.Langdorf MI, Medak AJ, Hendey GW, et al. Prevalence and clinical import of thoracic injury identified by chest computed tomography but not chest radiography in blunt trauma: Multicenter prospective cohort study. Ann Emerg Med. 2015;66:589–600. doi: 10.1016/j.annemergmed.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja AS, Lanning J, Gower A, et al. Prevalence of chest injury with the presence of NEXUS Chest criteria: Data to inform shared decisionmaking about imaging use. Ann Emerg Med. 2015 Nov 19; doi: 10.1016/j.annemergmed.2015.09.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI National Emergency X-Radiography Utilization Study Group. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94–99. doi: 10.1056/NEJM200007133430203. [DOI] [PubMed] [Google Scholar]
- 28.Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001 Oct 17;286:1841–8. doi: 10.1001/jama.286.15.1841. [DOI] [PubMed] [Google Scholar]
- 29.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerge Med. 1999;33:437–47. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996;27:305–308. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 31.Gupta M, Schriger DL, Hiatt JR, Cryer HG, Tillou A, Hoffman JR, et al. Selective use of computed tomography compared with routine whole body imaging in patients with blunt trauma. Ann Emerg Med. 2011;58:407–416. doi: 10.1016/j.annemergmed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Raja AS, Baumann B, et al. Prevalence of Chest Injury with the Presence of NEXUS Chest Criteria: Data to Inform Shared Decision-Making Regarding Imaging Use. Ann Emerg Med. doi: 10.1016/j.annemergmed.2015.09.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez RM, Henderson TM, Ritchie AM, Langdorf MI, Raja AS, Silverman E, et al. Patient preferences and acceptable risk for computed tomography in trauma. Injury. 2014;45:1345–9. doi: 10.1016/j.injury.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Huber-Wagner S, Biberthaler P, Habarle S, Wierer M, Dobritz M, Rummeny E, et al. Whole-body CT in haemodynamically unstable severely injured patients--a retrospective, multicentre study. PLoS One. 2013 doi: 10.1371/journal.pone.0068880. [DOI] [PMC free article] [PubMed] [Google Scholar]


