Abstract
Objective
The objective of the study was to assess the relationship of depression at antiretroviral therapy (ART) initiation with mortality and clinical outcomes among Tanzanian women living with HIV.
Design
We conducted a prospective cohort study of 1,487 women who initiated ART in Dar es Salaam, Tanzania.
Methods
Symptoms of depression and anxiety were assessed using a Tanzanian adapted and validated version of the Hopkins Symptom Checklist (HSCL-25). Participants attended monthly clinic visits during the first two years of ART and CD4 T-cell counts were assessed every four months. Proportional hazard models were used to assess the relationship of depression with mortality and clinical outcomes.
Results
Symptoms consistent with depression were prevalent among 57.8% of women at ART initiation. After multivariate adjustment including social support and stigma, depression at ART initiation was associated with increased risk of mortality (HR: 1.92; 95% CI: 1.15-3.20; p=0.01) and incidence of severe anemia (hemoglobin <8.5 g/dL) (HR: 1.59; 95% CI: 1.07-2.37; p=0.02). Under the assumption of causality, we estimate 36.1% (95% CI: 13.6-55.1%) of deaths among the study cohort were attributable to depression and its consequences. Depression was not significantly associated with trajectory of CD4 T-cell reconstitution or the risk of immunologic failure (p-values >0.05).
Conclusions
Elimination of depression may reduce mortality during the first two years of ART by one-third in our study cohort. Randomized trials and rigorous implementation studies are needed to evaluate the individual- and population-level effects of integrated mental health interventions and HIV treatment approaches in resouce-limited settings.
Keywords: HIV, Antiretroviral Therapy, Highly Active, Depression, Mental Health, Africa
Introduction
Access to antiretroviral therapy (ART) has expanded rapidly in resource-limited settings during the last decade and in 2014 over 10 million people living with HIV (PLH) in sub-Saharan Africa were receiving treatment [1]. Despite marked increases in ART coverage, PLH initiating treatment in sub-Saharan Africa continue to experience high rates of morbidity and mortality as compared to PLH in high-income settings, particularly during the initial months of ART [2, 3]. In the Dar es Salaam, Tanzania HIV care and treatment program, adult men and women initiating ART have a 1-year mortality risk of 13.1% [4]. As a result, low-cost ART adjunct interventions are urgently needed to improve survival and quality of life for PLH in sub-Saharan Africa. In particular, treatment for depression may constitute one of the strategies to improve outcomes among PLH [5].
The risk of depression is at least two-fold as great among PLH as compared to the general population in both high and low-income settings [6, 7]. Multiple observational studies in high income countries have linked depression to increased risk of HIV disease progression and mortality; however, studies examining the relationship in resource-limited settings are much more limited [8, 9]. The mechanisms underlying the relationship of depression with mortality among people living with HIV are multifactorial and include poorer adherence to ART and altered immune responses [8, 10]. To the best of our knowledge, no studies have examined the relationship of depression with mortality among PLH who have access to ART in a resource-limited setting. In one of the few studies, our research group determined Tanzanian pregnant women living with HIV reporting symptoms consistent with depression had significantly increased risk of HIV disease progression and mortality; however, this study was conducted prior to the availability of ART [11].
In order to address the paucity of data on the relationship of depression with mortality and clinical outcomes among individuals receiving ART in resource-limited settings, we present a prospective cohort study of adult women living with HIV who initiated ART in Dar es Salaam, Tanzania. In this study we first examine sociodemographic and biological factors associated with symptoms consistent with depression at ART initiation and then assess the prospective relationship of depression with mortality, morbidity, and immunologic outcomes at the individual- and population-level. These analyses are intended to determine if symptoms of depression remain associated with poor clinical outcomes among women living with HIV in Tanzania in the era of ART as well as to inform the potential population-level impact of interventions to reduce the prevalence of depression among women in the ART program.
Methods
Study Population
This prospective cohort study included women living with HIV who were enrolled in a randomized and double-blind clinical trial conducted in Dar es Salaam, Tanzania during 2006–2010. This trial examined the effect of daily oral supplements of B-complex, C, and E vitamins at high versus the standard recommended dietary allowances (RDA) on HIV disease progression (Clinicaltrials.gov NCT00383669) [12]. There was no difference in mortality, morbidity, or immunologic outcomes between groups randomized to the multivitamin regimens [12]. Individuals were eligible for the trial if they were 18 years of age or older, initiated ART at trial enrollment, and intended to reside in the city of Dar es Salaam for the next two years. Women who were pregnant or lactating were excluded from the study. At the time of the trial, PLH with World Health Organization (WHO) HIV disease stage IV, CD4 T-cell count <200 cells/μL, or with WHO HIV stage III disease and CD4 T-cell count <350 cells/μL were initiated on ART [13].
Baseline Depression and Covariate Assessment
At the trial enrollment visit which occurred at ART initiation, the Hopkins Symptom Checklist (HSCL-25) was administered to assess symptoms of depression and anxiety. The HSCL-25 includes a 10-item anxiety scale and a 15-item depression scale [14]. We have previously validated the HSCL-25 among Tanzanian women living with HIV against diagnosis of major depressive disorder by a trained psychiatrist using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [15, 16]. Based on the validation study results, we define symptoms consistent with depression as an average score >1.06 for 8 questions which had demonstrated 88% sensitivity and 89% specificity compared with a DSM-IV diagnosis of major depressive disorder [16].
To measure functional dimensions of social support, a scale based on the Duke University–University of North Carolina Functional Social Support Questionnaire was also administered [17]. Women were then classified as having low social support if their total score was less than the 50th percentile. In order to assess enacted HIV stigma participants were asked if they had faced any of the following as a result of their HIV status: (1) excluded from a social gathering; (2) sent away/left by spouse, partner, or family; (3) gossiped, teased, insulted, sworn at; (4) lost respect or standing in family or community; (5) lost a job; (6) had property taken away; or (7) had her child taken away [18]. Participants were also asked if and to whom they had disclosed their HIV status.
A full clinical examination was also conducted at the baseline trial visit and a structured interview was completed to collect information on demographic characteristics. Study physicians also performed a complete medical examination and assessed HIV disease stage in accordance with the WHO guidelines, and collected blood specimens. Absolute CD4 T-cell count (FACSCalibur flow cytometer, Becton Dickinson, San Jose, CA) and complete blood count (AcT5 Diff AL analyzer, Beckman Coulter, Miami, FL) were also determined. Height/weight measurements were obtained by trained research assistants using standardized procedures and calibrated instruments [12].
Follow-up Assessments and Outcome Definitions
During the trial follow-up period participants were followed at monthly clinic visits. Participants who missed a clinic visit were followed at home where relatives or neighbors were asked about vital status of the participant. At each follow-up visit, study physicians performed a clinical examination, assessed WHO HIV disease stage, and diagnosed and treated any illness per standard of care [13]. Pulmonary tuberculosis was diagnosed according to Tanzanian National Tuberculosis and Leprosy Programme guidelines [19]. Height and weight were measured by clinic study nurses at each follow-up clinic visit. Wasting was defined as a body mass index of <18.5 kg/m2 and weight loss of >10% from baseline was based on the definition of HIV-related wasting [20]. Absolute CD4 T-cell count and a complete blood count were taken at clinic visits every 4 months. We analyzed two definitions of immunologic failure: (i) the Tanzanian national criteria of a CD4 T-cell count less than ART initiation CD4 count after at least 6 months of therapy and (ii) WHO 2006 definition of a CD4 T-cell count of 100 cells/μl or less after at least 6 months on therapy [21, 22]. Severe anemia was defined as a hemoglobin concentration of <8.5 g/dL[23].
Statistical Analysis
We first examined the cross-sectional association of sociodemographic and biologic factors associated with symptoms consistent with depression at ART initiation using binomial regression models in order to obtain risk ratio estimates [24, 25]. Variables included in this cross-sectional analysis included: age, marital status, education, having children, wealth tertile, engagement in an income generating activity, smoking status, body mass index (BMI), WHO HIV disease stage, social support, HIV disclosure and experience of stigma. In order to produce a parsimonious multivariate model, any variable with a p-value <0.20 in univariate models was included in the final multivariate model.
The prospective relationship of symptoms consistent with depression at ART initiation and mortality was investigated using proportional hazard models [26]. Proportional hazard models were also used to investigate the relationship of depression and first occurrence of pulmonary tuberculosis, oral thrush, severe anemia (hemoglobin <8.5 g/dL), wasting (BMI <18.5 kg/m2), >10% weight loss from ART initiation, and immunologic failure. For these morbidity outcomes, we excluded women who received a prevalent diagnosis of the event of interest at ART initiation. Confounders for all multivariate analyses were determined a priori by the study team based on potential associations with both depression and HIV treatment outcomes with the additional requirement that the variable was not a mediator of the relationship of interest. Confounders included in multivariate models included baseline age, marital status, presence of living children, education, wealth tertile, income generating activity, smoking status, CD4 count, WHO HIV disease stage, BMI, social support, HIV disclosure, experience of stigma due to HIV status, and randomized multivitamin regimen (high dose or single dose RDA). In addition we present sensitivity analyses defining symptoms consistent with depression using the standard (initially validated in the US) cutoff of 1.75 [14].
We then calculated the partial population-attributable risk percentage (PAR%) for depression [27]. Partial PAR%s were used to estimate the percent of deaths that would not have occurred in our study population if a hypothetical intervention eliminated depression, but other baseline risk factors for mortality did not change as a result of the intervention. All prevalence and effect sizes for other risk factors for death included in the multivariate model were assumed to remain constant in calculation of partial PAR%.
The association of symptoms consistent with depression at ART initiation with CD4 T-cell reconstitution was analyzed using generalized estimating equations (GEE) with stepwise restricted cubic splines to account for nonlinearity in CD4 T-cell trajectories [28, 29]. Change in CD4 T-cell count between clinic visits was modeled as a longitudinal continuous outcome while depression, time since ART initiation, and confounders were included as explanatory variables. An m-dependent working correlation matrix (m = 1) was used. In order to assess the statistical significance of differences in CD4 T-cell trajectory by depression at ART initiation, we included the interactions of depression with time post ART initiation and its splines and the statistical significance assessed using the robust score test.
As an exploratory analysis we examined whether the association of depression with all outcomes varied within strata of social status and HIV disease severity variables including: social support, stigma, WHO HIV stage, and CD4 T-cell count. In proportional hazard models the likelihood ratio test was used to test statistical significance of interaction, while the robust score test was used in GEE analyses. Multiple imputation was used in multivariate models to account for missing data [30]. All p-values were 2-sided and p<0.05 was considered statistically significant. Statistical analyses were performed using the SAS v 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study was approved by the institutional review boards of the Harvard School of Public Health, Muhimbili University of Health and Allied Sciences, Tanzania Food and Drugs Authority, and National Institute of Medical Research.
Results
A total of 1,487 women initiating ART in the parent trial had the HSCL-25 administered at ART initiation. Socio-demographic, clinical, depression, disclosure, and stigma attributes of the cohort at ART initiation are presented in Table 1. At ART initiation, 859 women (57.8%) had prevalent symptoms consistent with depression utilizing the Tanzanian adapted and validated HSCL-25 cut-off. In Supplementary Table 1, we present a response summary of all individual HSCL-25 items. The most common HSCL-25 items endorsed with ‘at least a little’ were loss of sexual interest or pleasure (37.1%), feeling low in energy/slowed down (35.3%), worrying too much (34.4%), and blaming oneself about things (31.9%). Of note,236 women (15.9%) reported they felt like ending their life ‘at least a little’. At ART initiation 122 women (8.2%) reported experience with HIV enacted stigma and 80 women (5.4%) had not disclosed their HIV status to anyone.
Table 1. Characteristics of HIV-infected Tanzanian women (n=1,487) administered the HSCL-25 at ART initiation.
| Characteristic | Frequency (%) |
|---|---|
| Age (years) | |
| <30 | 272 (18.3) |
| ≥30 and <40 | 743 (50.0) |
| ≥40 | 471 (31.7) |
| Missing | 1 (0.1) |
| Marital status | |
| Married | 457 (35.4) |
| Single | 201 (15.6) |
| Divorced | 333 (25.8) |
| Widow | 299 (23.2) |
| Other | 107 (7.2) |
| Has at least one living child | |
| Yes | 1279 (87.2) |
| No | 188 (18.8) |
| Education | |
| None/Primary | 815 (54.8) |
| Secondary plus | 484 (32.5) |
| Missing | 188 (12.6) |
| No income generating activity | 525 (35.3) |
| Report current smoking | 31 (2.1) |
| Report alcohol use in last 30 days | 22 (1.5) |
| CD4 T-cell count (cells/μL) | |
| <50 | 284 (19.1) |
| ≥50 and <100 | 269 (18.1) |
| ≥100 and <200 | 569 (38.3) |
| ≥200 | 324 (21.8) |
| Missing | 41 (2.8) |
| WHO HIV disease stage | |
| I/II | 338 (22.7) |
| III | 888 (59.7) |
| IV | 150 (10.1) |
| Missing | 111 (7.5) |
| Body mass index (kg/m2) | |
| <18.5 | 355 (23.9) |
| ≥ 18.5 and <25.0 | 855 (57.5) |
| ≥ 25.0 | 277(18.6) |
| Antiretroviral regimen | |
| d4T, 3TC, NVP | 895 (6.2) |
| d4T, 3TC, EFV | 150 (10.1) |
| AZT, 3TC, NVP | 132 (8.9) |
| AZT, 3TC, EFV | 309 (20.8) |
| Missing | 1 (0.1) |
| Symptoms consistent with depression | 859 (57.8) |
| Has told no one of HIV status | 80 (5.4) |
| Experienced stigma due to HIV status | 122 (8.2) |
In Table 2 we present baseline factors associated with symptoms consistent with depression at ART initiation. In parsimonious multivariate models, women with low social support (< median level) had 1.32 times (95% CI: 1.32-1.21-1.45; p<0.01) the prevalence of depression at ART initiation as compared to those with high social support (≥ median level). The only other factor significantly associated with depression in multivariate models was experience of HIV enacted stigma. Women who reported experience with enacted stigma had 1.50 times (95% CI: 1.38-1.62; p<0.01) the prevalence of depression at ART initiation. In sensitivity analyses utilizing the 1.75 score cutoff to define symptoms consistent with depression (US-validated), social support and enacted stigma remained significantly associated with depression with the addition of current smoking as a significant factor (Supplemental Table 2).
Table 2.
Risk factors for symptoms consistent with depression at ART initiation among HIV-infected Tanzanian women (n=1,487).
| Characteristic | No. depressed / Total (%) | UnivariateRR (95% CI) | p-value | MultivariateRR (95% CI) | p-value |
|---|---|---|---|---|---|
| Age (years) | |||||
| <30 | 159 / 272 (58.5) | Ref. | |||
| ≥30 and <40 | 438 / 743 (59.0) | 1.01 (0.90-1.13) | |||
| ≥40 | 261 / 471 (55.4) | 0.95 (0.83-1.08) | 0.20a | ||
| Marital status | |||||
| Married | 254 / 457 (55.6) | Ref. | Ref. | ||
| Single | 126/ 201 (62.7) | 1.10 (0.97-1.25) | 0.13 | 1.09 (0.96-1.23) | 0.20 |
| Divorced | 186 / 333 (55.9) | 0.98 (0.87-1.11) | 0.78 | 0.95 (0.85-1.07) | 0.40 |
| Widow | 182 / 299 (60.9) | 1.07 (0.96-1.20) | 0.23 | 1.03 (0.92-1.15) | 0.65 |
| Has children | |||||
| Yes | 123 / 208 (59.1) | Ref. | |||
| No | 736 / 1279 (57.5) | 0.95 (0.84-1.07) | 0.66 | ||
| Education | |||||
| None/Primary | 457 / 815 (56.1) | 0.91 (0.83-1.00) | 0.05 | 0.93 (0.85-1.02) | 0.12 |
| Secondary plus | 298 / 484 (61.6) | Ref. | Ref. | ||
| Wealth tertile | |||||
| Poorest tertile | 220 / 394 (55.8) | 0.97 (0.87-1.09) | 0.55a | ||
| Middle tertile | 335 / 569 (58.9) | 1.03 (0.93-1.14) | |||
| Richest tertile | 281 / 490 (57.4) | Ref. | |||
| Income generating activity | |||||
| Yes | 558 / 962 (58.0) | Ref. | |||
| No | 301 / 525 (57.3) | 0.99 (0.90-1.09) | 0.85 | ||
| Smoking | |||||
| Current smoker | 20 / 31 (64.5) | 1.12 (0.86-1.46) | 0.41 | ||
| Not current smoker | 839 / 1456 (57.6) | Ref. | |||
| CD4 T-cell count (cells/μL) | |||||
| <50 | 171 / 284 (60.2) | 1.08 (0.94-1.23) | 0.58a | ||
| ≥50 and <100 | 146 / 268 (54.3) | 0.97 (0.84-1.12) | |||
| ≥100 and <200 | 336 / 569 (59.1) | 1.06 (0.94-1.19) | |||
| ≥200 | 181 / 324 (55.9) | Ref. | |||
| WHO HIV disease stage | |||||
| I/II | 188 / 338 (55.6) | Ref. | Ref. | ||
| III | 510 / 888 (57.4) | 1.03 (0.92-1.15) | 1.01 (0.91-1.12) | ||
| IV | 94 / 150 (62.7) | 1.13 (0.96-1.32) | 0.16a | 1.08 (0.92-1.26) | 0.41a |
| Body mass index (kg/m2) | |||||
| <18.5 | 213 / 355 (60.0) | 1.04 (0.94-1.15) | 0.47 | ||
| ≥18.5 and <25.0 | 494 / 855 (57.8) | Ref. | |||
| ≥25.0 | 152 / 277 (54.9) | 0.95 (0.84-1.07) | 0.41 | ||
| Social support | |||||
| Low <50th percentile | 481 / 714 (67.4) | 1.38 (1.26-1.50) | <0.01 | 1.32 (1.21-1.45) | <0.01 |
| High ≥50th percentile | 378 / 773 (48.9) | Ref. | Ref. | ||
| HIV status disclosure | |||||
| Told no one of HIV status | 54 / 80 (67.5) | 1.18 (1.01-1.38) | 0.04 | 1.14 (0.98-1.33) | 0.10 |
| Disclosed HIV status | 805 / 1407 (57.2) | Ref. | Ref. | ||
| Enacted stigma | |||||
| Experienced stigma | 110 / 122 (90.2) | 1.64 (1.52-1.77) | <0.01 | 1.50 (1.38-1.62) | <0.01 |
| No experience of stigma | 749 / 1365 (54.9) | Ref. | Ref. |
p-value for trend
The median follow-up time post ART initiation for the cohort was 24.1 months (IQR: 15.7-35.1 months), and during this period 80 deaths (5.4%) were recorded among the cohort of 1,487 women. At the end of the trial, vital status was unknown for 7.0% of participants and was similar for those with (7.1%) and those without symptoms of depression (6.8%). Table 3 shows the univariate and multivariate relationship of symptoms consistent with depression at ART initiation and mortality. Among women with symptoms of depression at ART initiation a total of 57 of 859 women (6.6%) died, while only 23 of 628 women (3.7%) without symptoms of depression died. Multivariate analysis (including adjustment for stigma and social support) determined women with depression at ART initiation had 1.92 times (95% 1.15-3.20; p=0.01) the hazard of mortality as compared to women without depression. We estimate the percentage of death attributed to depression and its consequences (population attributable risk percent) in our study cohort was 36.1% (95%CI: 13.6-55.1%). We found no significant evidence of interaction for the depression and mortality relationship by social support, stigma, CD4 T-cell count, and WHO stage (p-values for interaction >0.10). In a sensitivity analysis, the magnitude of the relationship of symptoms consistent with depression as defined by the standard HSCL-25 score cutoff of 1.75 remained consistent (HR: 1.94; 1.17-3.23; p=0.01) (Supplemental Table 3).
Table 3.
Association of symptoms consistent with depression at HAART initiation with incidenta mortality, morbidity, weight loss, severe anemia, and immunologic failure among HIV-infected Tanzanian women (n=1,487).
| Outcome | Events / Total (%) | Crude HR(95% CI) | p-value | Multivariateb RR (95% CI) | p-value |
|---|---|---|---|---|---|
| Mortality | |||||
| Depression | 57 / 859 (6.6) | 1.84 (1.14-2.99) | 0.01 | 1.92 (1.15-3.20) | 0.01 |
| No Depression | 23 / 628(3.7) | Ref. | Ref. | ||
| Pulmonary tuberculosisa | |||||
| Depression | 38 / 778 (4.9) | 0.74 (0.47-1.17) | 0.20 | 0.77 (0.47-1.25) | 0.29 |
| No Depression | 35 / 576 (6.1) | Ref. | Ref. | ||
| Oral thrusha | |||||
| Depression | 119 / 857 (13.9) | 1.20 (0.89-1.62) | 0.24 | 1.15 (0.84-1.58) | 0.38 |
| No Depression | 66 / 626 (10.5) | Ref. | Ref. | ||
| Wasting(BMI <18.5 kg/m2)a | |||||
| Depression | 72 / 642 (11.2) | 1.37 (0.93-2.01) | 0.11 | 1.22 (0.81-1.83) | 0.34 |
| No Depression | 40 / 442 (8.3) | Ref. | Ref. | ||
| Weight loss >10% from HAART initiation | |||||
| Depression | 188 / 859 (21.9) | 1.19 (0.95-1.50) | 0.14 | 1.05 (0.83-1.35) | 0.67 |
| No Depression | 119 / 628 (19.0) | Ref. | Ref. | ||
| Severe Anemia (Hb<8.5 g/dL)a | |||||
| Depression | 80 / 606 (13.2) | 1.57 (1.075-2.30) | 0.02 | 1.59 (1.07-2.37) | 0.02 |
| No Depression | 40 / 454 (8.8) | Ref. | Ref. | ||
| Immunologic failurec defined as a CD4 T-cell count <100 cells/μL after at least 6 month of ART | |||||
| Depression | 28 / 774 (3.6) | 0.94 (0.54-1.64) | 0.82 | 1.01 (0.56-1.80) | 0.99 |
| No Depression | 22 / 579 (3.8) | Ref. | Ref. | ||
| Immunologic failure defined as a CD4 T-cell count after at least 6 months of ART less than initiation CD4 count | |||||
| Depression | 53 / 774 (6.9) | 0.92 (0.62-1.38) | 0.68 | 0.84 (0.55-1.29) | 0.43 |
| No Depression | 43 / 579 (7.4) | Ref. | Ref. |
Excluding individuals who had event of interest at baseline
Adjusted for age (<30, ≥30 and <40, and ≥40 years), marital status (married, single, divorced, widowed), education (none/primary vs. secondary plus), has children (yes vs. no) wealth tertile, income generating activity (yes vs. no), smoking status (current smoker vs. not current smoker), CD4 count (<50,≥50 and <100, ≥100 and <200, and ≥200 cells/μL), WHO HIV disease stage (I/II, III, IV), body mass index (<18.5, ≥18.5 and <25.0, ≥25.0 kg/m2), social support (<50th percentile vs. ≥50th percentile), HIV disclosure (told no one vs. disclosed status), experience stigma due to HIV status (yes vs. no) and randomized multivitamin regimen (high dose or single RDA). Wasting and weight loss multivariate models not adjusted for baseline BMI.
Table 3 also presents the association of symptoms consistent with depression at ART initiation with morbidity and nutritional outcomes. Women with symptoms of depression had significantly increased hazard of incident severe anemia (HR: 1.59; 95% CI: 1.07-2.37; p=0.02). In addition, there was some indication that women with depression may also have increased risk of oral thrush (HR: 1.15; 0.84-1.58; p=0.38) and wasting (HR: 1.22; 95% CI: 0.81-1.83; p= 0.34), but results were not statistically significant. There was no significant evidence for interaction of any morbidity or nutrition association by any third variable (p-values for interaction >0.10). Sensitivity analyses using the 1.75 score cutoff for depression determined similar magnitudes of association for morbidity associations (Supplemental Table 3).
A total of 1,335 women (90%) had two or more CD4 T-cell count measurements during the study follow-up period. As expected change in CD4 T-cell count post ART initiation was non-linear with greater increases in CD4 T-cell count during the initial 6 months of treatment. Figure 1 presents the nearly identical trajectory of CD4 T-cell counts post ART initiation for women with symptoms of depression versus those without. There was no indication of a difference in mean CD4 T-cell counts at baseline (93 vs. 112 cells/μL), 4 months (150 vs. 145 cells/μL), 8 months (321 vs. 331 cells/μL), 12 months (356 vs. 353 cells/μL) and 16 months (396 vs. 391 cells/μL) for women with symptoms of depression as compared to those without, respectively (p-value for difference in trajectory =0.40). There was also no difference in CD4 trajectory by symptoms of depression status in multivariate models (p-value for difference in trajectory =0.38). We also found no significant relationship of symptoms of depression at ART initiation with immunologic failure using two standard definitions (Table 3). There was no significant evidence of interaction of any CD4 trajectory or immunologic failure relationship by any third variable (p-values for interaction >0.10) and there was no difference in associations using the 1.75 score cutoff to define symptoms consistent with depression (Supplemental Table 3 and Supplemental Figure 1).
Figure 1.
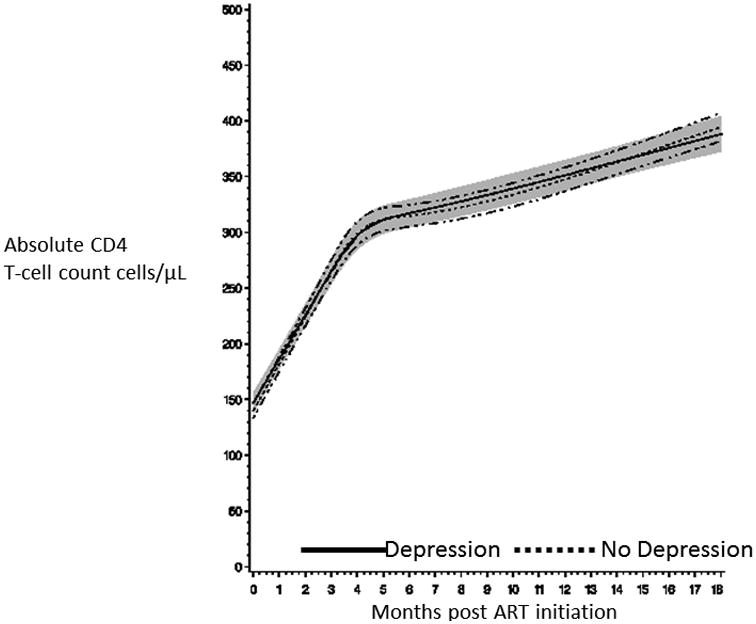
Absolute CD4 T-cell count trajectory after ART initiation for HIV-infected Tanzanian women with symptoms consistent with depression (solid line for mean CD4 T-cell count and shaded cloud for 95% confidence interval) and without (dotted line for mean CD4 T-cell count and hash-dot lines for 95% confidence interval).
Discussion
In this study we found over half of Tanzanian women in our study population had symptoms consistent with depression at ART initiation. Low social support and experience of stigma were significant risk factors for depression while markers of HIV disease severity (CD4 T-cell count, WHO stage, BMI) had no significant relationship. Prospectively, women with symptoms consistent with depression at ART initiation had almost twice the risk mortality as well as incidence of severe anemia. In terms of potential population-level impact, we estimate that one-third of deaths in the cohort were attributed to depression and its consequences. There was no difference in CD4 T-cell reconstitution trajectory or risk of immunologic failure during the first 2 years of ART by depression status.
At ART initiation we determined that over 50% of women living with HIV in urban Tanzania had symptoms consistent with depression at ART initiation. In a previous study of Tanzanian pregnant women living with HIV conducted in the same setting before the availability of ART, we found the prevalence of depression was 43% utilizing the same adapted HSCL-25 cut-off to define depression [11]. As a result, the prospect of access to ART may not have resulted in reductions in the prevalence of depression among women in urban Tanzania. In across-sectional analysis we found only social support and enacted stigma were associated with prevalent symptoms consistent with depression. These findings are consistent with a growing number of studies in sub-Saharan Africa that have shown social support is a key, if not primary, determinant of depression risk and quality of life for individuals on ART [31-33]. Our results are partially in contrast to studies of combined male and female PLH populations in rural South Africa and Uganda which found strong relationships of low CD4 T-cell counts with increased risk of depression [32, 34]. The lack of an association of markers of HIV disease severity, including CD4 T-cell count and WHO HIV stage, with symptoms of depression in our study population of women in Tanzania initiating ART may indicate the determinants and magnitudes of risk for depression among PLH may vary by sex, setting, and duration of ART. Specifically, our study population of women initiating ART based on standardized treatment guidelines is by definition more homogenous in terms of HIV disease severity as compared to population-based studies and may be underpowered to detect moderate to small differences in CD4 T-cell count. In addition our cross-sectional analysis may be prone to selection bias by differences in care seeking behaviors for women with depression.
In prospective analyses, we found women reporting symptoms consistent with depression at ART initiation had almost twice the risk of mortality during the first 2 years of treatment as compared to women without depression. To our knowledge, this is the first study linking depression to mortality for a population with access to ART in a resource-limited setting. In our previous study of Tanzanian pregnant women living with HIV before availability of ART, women with depression had 2.65 times the risk of mortality as compared to those without depression [11]. Accordingly, in the presence of ART availability, depression remained strongly associated with mortality among Tanzanian women, but the magnitude of the association may be slightly smaller as compared to the pre-ART era; however, these conclusions cannot be directly drawn from this study due to differences in time and pregnancy status of participants. Studies in the US and Europe have also determined that PLH with depression were at increased risk of mortality and poor clinical outcomes both before and after the discovery of highly active antiretroviral therapy [8, 35, 36]. A limitation of our study is that depression status was determined by a symptom checklist, which may lead to misclassification; however, we utilized a locally adapted version of the HSCL-25 with cut-off scores calibrated to the DSM-IV depression criteria to minimize potential bias and performed sensitivity analyses utilizing US-validated cutoffs. Nevertheless, potential non-differential misclassification of depression status would lead us to underestimate the magnitude of the true effect. In addition, we only had data on symptoms of depression at ART initiation and future studies should examine the relationship of change in symptoms of depression over time with mortality and HIV treatment outcomes.
The mechanisms underlying the relationship of depression and HIV mortality are potentially a combination of behavioral and biological factors. There is a large literature linking depression to decreased ART adherence in both high- and low-income settings [37, 38]. In a recent review of twenty three studies presenting data on depression and ART adherence in sub-Saharan Africa, the pooled probability of achieving ‘good’ adherence to ART was 55% lower among PLH with depressive symptoms [10]. Unfortunately, in the current study we did not have measures of ART adherence and as a result we were unable to examine adherence as a potential mediator of the relationship of depression with mortality. There is also growing evidence that depression can have deleterious effects on immune system function, particularly cell-mediated responses [39]. Several studies have found PLH with depression have decreased cytotoxic CD8+ T-cell counts and decreased number and function of natural killer (NK) cells, which may lead to decreased lysis of HIV-infected cells and increased viral replication [40, 41]. Accordingly, interventions which only promote ART adherence for individuals with depression may not completely reduce the excess mortality risk due to underlying immunologic deficits resulting from depression.
In the current study we found no significant association of depression at ART initiation with CD4 T-cell reconstitution or risk of immunologic failure. Our findings are somewhat in contrast to prospective cohort studies in high-income settings which have found individuals with depression had poorer CD4 T-cell and viral load outcomes on ART, but evidence in resource-limited settings is much more limited [42-44]. In a longitudinal study of Ethiopian PLH, each one unit increase in depression severity as measured by the Center for Epidemiological Studies Depression Scale (CES-D) questionnaire was associated with a 10 cells/μL lower CD4 T-cell count at time points post ART initiation [45]. As a result, the contributions of biological and adherence mechanisms to the relationship of depression with CD4 T-cell reconstitution may also differ by sex, setting, and by duration of ART. ART adherence in also known to decrease over time and since our study examined the period immediately following ART initiation there may have been minimal variation in immunologic response by depression status [46]. As a result, we may have been underpowered to detect moderate to small differences in CD4 T-cell trajectory by depression status. Nevertheless, our CD4 T-cell trajectory and immunologic failure results may also be biased towards the null due to competing risk of death in our cohort. Women who died before 6 months of ART did not receive a follow-up CD4 T-cell count measurement eligible for determination of immunologic failure and therefore women who may have had the poorest CD4 T-cell trajectories were possibly excluded from our analyses.
The combination of the high prevalence and strong risk of mortality associated with symptoms of depression among women starting ART in Tanzania suggest providing effective psychosocial interventions may have a large population-level impact on improving survival and quality of life. Assuming a causal relationship, we estimated eliminating depression may avert about one-third of deaths in our study population. Nevertheless, due to the observational nature of the study our population attributable risk estimate should be interpreted with caution due to potential for confounding and bias in risk estimates. Nevertheless, our findings provide support for future large scale intervention studies of depression prevention and treatment for women living with HIV in Tanzania and similar resource-limited settings.
Cognitive behavioral therapy, interpersonal psychotherapy, and problem-solving therapy interventions have shown promise in terms of reducing depression among PLH; however, very few HIV programs have integrated mental health interventions into their routine ART services in resource-limited settings [5]. Our results strongly support randomized controlled trials and rigorous implementation evaluations of integrated mental health programs are needed to determine the effect of depression interventions on early mortality and clinical outcomes among HIV-infected women starting ART in resource-limited settings. Additional studies of depression are also needed among HIV-infected adult men, adolescents, childrenm and pregnant women on ART.
Supplementary Material
Acknowledgments
WWF, SK, FM, WWF, and SA designed and conducted the parent multivitamin trial. SK and MCSF designed and integrated the depression, social support and stigma questionnaires into the trial. CRS, NG, WWF, MCSF developed the depression analysis. CRS lead implementation of the statistical analysis and drafted the initial report. All authors contributed to and approved of the final manuscript. We thank the study participants, and field teams, including physicians, nurses, midwives, supervisors, laboratory, and the administrative staff, who made the study possible; and Muhimbili National Hospital, Muhimbili University of Health and Allied Sciences, City of Dar es Salaam Regional Office of Health and the National AIDS Control Program/Ministry of Health and Social Welfare for their institutional support. The parent trial for this study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD32257).
Source of Funding: The parent trial for this study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD32257).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.UNAIDS. The gap report. Geneva: UNAIDS; 2014. [Google Scholar]
- 2.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 3.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalamilla G, Hawkins C, Okuma J, Spiegelman D, Aveika A, Christian B, et al. Mortality and treatment failure among HIV-infected adults in Dar Es Salaam, Tanzania. J Int Assoc Physicians AIDS Care (Chic) 2012;11:296–304. doi: 10.1177/1545109711406733. [DOI] [PubMed] [Google Scholar]
- 5.Kaaya S, Eustache E, Lapidos-Salaiz I, Musisi S, Psaros C, Wissow L. Grand challenges: Improving HIV treatment outcomes by integrating interventions for co-morbid mental illness. PLoS Med. 2013;10:e1001447. doi: 10.1371/journal.pmed.1001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 7.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 8.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 9.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS. 2012;26(Suppl 2):S117–135. doi: 10.1097/QAD.0b013e32835bde0f. [DOI] [PubMed] [Google Scholar]
- 10.Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16:2101–2118. doi: 10.1007/s10461-011-0087-8. [DOI] [PubMed] [Google Scholar]
- 11.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44:470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isanaka S, Mugusi F, Hawkins C, Spiegelman D, Okuma J, Aboud S, et al. Effect of high-dose vs standard-dose multivitamin supplementation at the initiation of HAART on HIV disease progression and mortality in Tanzania: a randomized controlled trial. Jama. 2012;308:1535–1544. doi: 10.1001/jama.2012.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. 2004. [Google Scholar]
- 14.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (February 1996 Final), SCID-I/P. Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- 16.Kaaya SF, Fawzi MC, Mbwambo JK, Lee B, Msamanga GI, Fawzi W. Validity of the Hopkins Symptom Checklist-25 amongst HIV-positive pregnant women in Tanzania. Acta Psychiatr Scand. 2002;106:9–19. doi: 10.1034/j.1600-0447.2002.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26:709–723. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Nyblade L, Pande R, Mathur S, MacQuarrie K, Kidd R, Banteyerga H, et al. Disentangling HIV and AIDS stigma in Ethiopia, Tanzania and Zambia. 2003 [Google Scholar]
- 19.Enarson DA, Rieder HL, Arnadottir T, Trébucq A. Management of tuberculosis: a guide for low income countries: International Union Against Tuberculosis and Lung Disease (IUATLD) 2000 [Google Scholar]
- 20.Polsky B, Kotler D, Steinhart C. HIV-associated wasting in the HAART era: guidelines for assessment, diagnosis, and treatment. AIDS Patient Care STDS. 2001;15:411–423. doi: 10.1089/108729101316914412. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organziation. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach-2010 revision. 2010 [PubMed] [Google Scholar]
- 22.The United Republic of Tanzania MoHaSW. National Guidelines for the Management of HIV and AIDS, National AIDS Control Programme (NACP) (Third) 2008 [Google Scholar]
- 23.World Health Organization. Nutritional anaemias: report of a WHO scientific group. World Health Organization; 1968. [PubMed] [Google Scholar]
- 24.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972:187–220. [Google Scholar]
- 27.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons; 2004. [Google Scholar]
- 31.Asante KO. Social support and the psychological wellbeing of people living with HIV/AIDS in Ghana. African journal of psychiatry. 2012;15:340–345. doi: 10.4314/ajpsy.v15i5.42. [DOI] [PubMed] [Google Scholar]
- 32.Yeji F, Klipstein-Grobusch K, Newell ML, Hirschhorn LR, Hosegood V, Barnighausen T. Are social support and HIV coping strategies associated with lower depression in adults on antiretroviral treatment? Evidence from rural KwaZulu-Natal, South Africa. AIDS Care. 2014;26:1482–1489. doi: 10.1080/09540121.2014.931561. [DOI] [PubMed] [Google Scholar]
- 33.Simbayi LC, Kalichman S, Strebel A, Cloete A, Henda N, Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med. 2007;64:1823–1831. doi: 10.1016/j.socscimed.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaharuza FM, Bunnell R, Moss S, Purcell DW, Bikaako-Kajura W, Wamai N, et al. Depression and CD4 cell count among persons with HIV infection in Uganda. AIDS Behav. 2006;10:S105–111. doi: 10.1007/s10461-006-9142-2. [DOI] [PubMed] [Google Scholar]
- 35.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 36.Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11:291–307. doi: 10.1007/s11904-014-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58:181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruess DG, Douglas SD, Petitto JM, Leserman J, Ten Have T, Gettes D, et al. Association of depression, CD8+ T lymphocytes, and natural killer cell activity: implications for morbidity and mortality in Human immunodeficiency virus disease. Curr Psychiatry Rep. 2003;5:445–450. doi: 10.1007/s11920-003-0083-4. [DOI] [PubMed] [Google Scholar]
- 40.Cruess DG, Douglas SD, Petitto JM, Have TT, Gettes D, Dube B, et al. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am J Psychiatry. 2005;162:2125–2130. doi: 10.1176/appi.ajp.162.11.2125. [DOI] [PubMed] [Google Scholar]
- 41.Evans DL, Folds JD, Petitto JM, Golden RN, Pedersen CA, Corrigan M, et al. Circulating natural killer cell phenotypes in men and women with major depression. Relation to cytotoxic activity and severity of depression. Arch Gen Psychiatry. 1992;49:388–395. doi: 10.1001/archpsyc.1992.01820050052009. [DOI] [PubMed] [Google Scholar]
- 42.Ironson G, O'Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther. 2005;10:53–61. [PubMed] [Google Scholar]
- 44.Ironson G, O'Cleirigh C, Kumar M, Kaplan L, Balbin E, Kelsch CB, et al. Psychosocial and Neurohormonal Predictors of HIV Disease Progression (CD4 Cells and Viral Load): A 4 Year Prospective Study. AIDS Behav. 2015;19:1388–1397. doi: 10.1007/s10461-014-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alemu H, Haile Mariam D, Tsui A, Ahmed S, Shewamare A. Effect of depressive symptoms and social support on weight and CD4 count increase at HIV clinic in Ethiopia. AIDS Care. 2012;24:866–876. doi: 10.1080/09540121.2011.648160. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


