Abstract
Objective
Greater body mass index (BMI) and body fat are associated with vasomotor symptoms (VMS). Thus, weight loss may prevent VMS. We analyzed whether concurrent BMI or waist circumference and/or changes in weight or waist circumference predicted incident VMS and whether these relations differed by menopause stage or race/ethnicity.
Methods
Data from 10 follow-up visits for 1546 participants in the Study of Women's Health Across the Nation who reported no VMS at baseline were modeled for time to first symptomatic visit in relation to concurrent BMI and waist circumference and change in weight and waist circumference during early and late menopause using discrete survival analyses, adjusting for covariates.
Results
Greater concurrent BMI and waist circumference were significantly related to greater any and frequent (≥6 days in the last two weeks) incident VMS in early menopause and lower VMS risk in late menopause. Percentage weight change since baseline and since the prior visit were unrelated to incident any VMS in either menopause stage. Percentage weight change since baseline had a significant shallow U-shaped association with incident frequent VMS in early menopause (p=0.02), a shallow inverse U-shape in late menopause (p=0.02), and a significant interaction with menopause stage (p=0.004) but not with race/ethnicity. Recent weight change was unassociated with incident VMS in either menopause stage. Results were similar for waist change.
Conclusions
Concurrent BMI and waist circumference were positively related to incident VMS in early menopause and negatively related in late menopause. Maintaining healthy weight in early menopause may help prevent VMS.
Keywords: vasomotor symptoms, hot flashes, menopause, race/ethnicity, weight, waist circumference, body mass index
INTRODUCTION
Up to 80% of midlife women experience vasomotor symptoms (VMS, hot flashes and/or night sweats) during the menopause transition1-3. Early observations in postmenopausal women were that greater estrone production in peripheral fat from aromatization of androstenedione4-6 was associated with less symptom reporting. However, longitudinal and cross-sectional studies of women during the menopausal transition1, 7-9 have indicated that greater body mass index (BMI, weight in kg/[height in meters]2) is associated with greater reporting of VMS. In addition, increased percentage body fat and increases in body fat over time, measured by bioelectrical impedance, have also been associated with greater VMS reporting10, 11. These results were unexpected due to the older, prior observations cited above4-6 of greater weight being associated with less symptom reporting. This discrepancy may be explained by the hypothesis that in the earlier phases of the menopausal transition, higher BMI (and, as some have suggested, potentially its resulting greater heat insulation12) may predispose to increased VMS occurrence.
A better understanding of the impact of weight and weight change on VMS has important implications for women, if weight loss is a potential option to reduce VMS (which may have a long duration13), and for the health care system. VMS are one of the chief menopause-related problems for which midlife women seek medical care14, 15. Further, women with untreated VMS tend to have higher health care use and higher direct costs for care16. Research has not provided a clear understanding of the relationship of weight and body composition to VMS reporting. One recent study of midlife women found no relation between hot flashes and change in BMI and weight over one to five years of follow-up17. However, this study included largely only African American and White women, instead of a more diverse sample, and had a relatively short follow-up period. A small six-month weight-reduction trial found a reduction in self-reported hot flashes with weight loss but not in objectively measured hot flashes18. An additional report from the dietary intervention trial (reducing fat intake and increasing fruit, vegetables and whole grain intake and weight loss) of the Women's Health Initiative found that postmenopausal women with VMS at baseline who lost 10 or more pounds or 10% or more of their baseline body weight by one year were more likely to eliminate VMS compared with those who maintained weight, as were women assigned to the dietary intervention19. However, these analyses focused on postmenopausal women who reported VMS at baseline, rather than preventing their occurrence/incidence in peri- and postmenopausal women. It is possible that weight and weight gain may operate differently pre- and postmenopausally. Postmenopausal weight gain might result in more protection from VMS than premenopausal weight gain20 due to aromatization of testosterone producing relatively more estrogen in relatively estrogen-deficient postmenopausal women.
We undertook the present analyses to evaluate the following hypotheses: 1) greater concurrent BMI or waist circumference would be associated with greater incident VMS in pre- and early perimenopause (“early stage”) and less incident VMS in the late peri- and postmenopause (“late stage”); 2) in longitudinal analyses, increases in weight and waist circumference over time since baseline would be associated with greater incident reporting of VMS in the early stage and with less incident reporting of VMS in the late stage; 3) in longitudinal analyses, increases in weight and waist over time since the prior annual visit (recent increases) would be associated with greater incident VMS reporting; and 4) all of these relations would not differ by race/ethnicity.
METHODS
Study Participants
The Study of Women's Health Across the Nation (SWAN) is a seven-site, longitudinal cohort study of five racial/ethnic groups of women aged 42-52 years at enrollment in 1995-1997 who were pre- or early peri-menopausal. SWAN's initial cross-sectional study screened women for eligibility for the longitudinal study. Eligibility for the cross-sectional study were: resident in the geographical area of one of the sites, age 40-55 years, self-defined as one of the target racial/ethnic groups, and spoke English or one of the site-specific languages (Japanese in Los Angeles, Spanish in New Jersey, or Cantonese in Oakland). Details of recruitment for SWAN have been described elsewhere21.
To be included in the longitudinal cohort, participants had to have had at least one menstrual period in the prior 3 months and not been using exogenous sex steroids. Of the 16,065 cross-sectional participants, 6,521 were cohort-eligible, and 3,302 (50.6%) enrolled. Women were deactivated from the cohort only if they explicitly refused further contact. Those who had a hysterectomy or bilateral oophorectomy or initiated menopausal hormone therapy (HT) during follow-up continued to be followed.
Of the original SWAN study participants recruited at baseline (N=3,302), data from 65 women with a prevalent cancer diagnosis were omitted from all analyses – as this may have affected weight and/or VMS – as were data from 34 women who were missing information about baseline VMS or BMI. The remaining 3203 participants were included in cross-sectional analyses of baseline VMS frequency, 353 of whom had VMS on 6 or more days in the past 2 weeks (frequent VMS), an indicator of increased severity of VMS. Among the 1948 participants with no VMS at baseline, in longitudinal analyses of incident VMS, data from 102 women were omitted because at the first annual follow-up visit: 91 had initiated menopausal hormone therapy; 4 had incident cancer; 5 had a hysterectomy or bilateral oophorectomy; and 2 were pregnant or breastfeeding and reported VMS. Another 123 participants with no follow-up VMS data were omitted, as were 177 participants who had missing covariate data. Thus, the total analytic sample was 1546 participants for longitudinal analyses of incident VMS related to concurrent BMI and waist circumference and change in weight and waist. Study participants were followed for ten annual visits after the baseline visit; 65.0% of the analytic sample (N=1005) provided follow-up visit 10 data on VMS, concurrent BMI, and concurrent menopause status, and 60.2% (N=930) also provided all covariate data at follow-up visit 10.
Procedures
Participants in SWAN were followed with annual in-person clinic visits that included: signed, written informed consent; a standard protocol for measuring height with a stadiometer, weight with a balance beam scale and waist circumference at the level of the natural waist or the narrowest part of the torso from the anterior aspect; fasting morning blood draw on days 2-5 of the menstrual cycle for menstruating women or any day for non-menstruating women; and interviewer- and self-administered questionnaires, which contained questions on demographic characteristics, medical and reproductive history, medications, psychosocial measures, and symptoms. Common protocols were used at all sites. Trained, certified staff conducted the interviews with scripted questions and standard probes. The questionnaires were forward- and back-translated into Spanish, Cantonese, and Japanese and were administered by bilingual interviewers to women who preferred these languages. Institutional review boards at all sites approved the study protocols.
Outcome Variables
At each follow-up visit, participants were asked about a number of symptoms, including hot flashes and/or night sweats in the prior two weeks22-24. Prior analyses1 indicated that these two symptoms were highly correlated, thus providing justification for examination of them jointly. Any VMS was defined as reporting at least one day with hot flashes or night sweats within the previous two weeks, and frequent VMS was defined as having VMS on six or more days in the past two weeks1. For analyses of incident (newly reported) VMS, we excluded women who reported any VMS at baseline so as to examine relations of concurrent BMI and waist circumference and changes in weight and waist to newly-developing VMS during follow-up.
Independent Variables
The primary time-varying independent variables for incident VMS were concurrent BMI and percent change in weight since baseline, and concurrent waist circumference and percent change in waist circumference since baseline, for all visits in analyses, i.e., through the first visit at which VMS was reported or the last visit if no VMS was reported. Additional analyses substituted time-varying independent variables percent change in weight and waist circumference from the immediately prior – rather than baseline – visit, to assess the role of recent changes in weight and waist. BMI was computed as weight in kilograms/(height in meters)2 and categorized (<25, 25-29.9 and >=30). Waist circumference was assessed in quintiles. For analyses of change since baseline, percent change variables were categorized as: >5% loss, 1-5% loss, stable (<1% change), 1-5% gain, >5-≤10% gain, and >10% gain to allow for a non-linear association and to identify meaningful amounts of change with adequate numbers in each category of each variable. For analyses of change since the prior visit, because cell counts were small for the two highest categories of gain in weight or waist, these two categories were merged; results from sensitivity analyses keeping the two categories separate were consistent (data not shown).
Covariates
Time-invariant candidate covariates, measured at baseline included variables previously shown to be related to VMS1: educational level (less than high school, high school, some college, college or post-baccalaureate degree); race/ethnicity (self-designated white, African American, Japanese, or Chinese); baseline anxiety score (as a binary variable, using a cutoff value of >4 or ≤425 for a summed score of numbers of days in the past two weeks (ranging from 0=no days to 4=every day) of reporting irritability or grouchiness, feeling tense or nervous, heart pounding or racing, and feeling fearful for no reason)22; baseline depressive symptom score (elevated was score ≥16 on a 20-item Center for Epidemiology Studies – Depression scale)26; history of premenstrual symptoms; symptom sensitivity at first follow-up visit (using quartiles of a summed score of degree of awareness of loud noise, hot or cold, hunger, pain and things happening in one's body, with responses ranging from 1=not at all true to 5=extremely true)27; SWAN study site; passive smoke exposure (person hours/week)28; and physical activity score ( a summary physical activity score for occupational, household and caregiving, sports and exercise, and daily routine activities)29, 30. In our final multivariable models only baseline age, site, race/ethnicity, education, baseline depressive symptoms, history of premenstrual symptoms and symptom sensitivity scores were retained; other variables did not meet the retention criteria of the backward elimination approach described below in the analytic approach.
Annually-measured time varying covariates included: menopause transition stage (premenopausal, indicating a menstrual period in the prior three months and no change in menstrual regularity in the prior year; early peri-menopausal, indicating menses in the prior three months and changes in regularity in the prior year; late peri-menopausal, indicating no menses in the prior three months but menses in the prior 11 months; and postmenopausal, indicating ≥12 months of amenorrhea; to maximize statistical power when examining possible effect modification by menopausal status, we combined pre- and early peri-menopausal categories (“early stage”) and late peri- and postmenopausal categories (“late stage”)); smoking31, categorized as never, past, and current; number of very upsetting life events (0, 1, 2+); and social support32. Final multivariable models omitted social support due to lack of statistical significance.
Data Analyses
At baseline, three VMS groups – none, infrequent (1-5 days), and frequent (6+ days) – were compared regarding baseline characteristics using chi-square and Kruskal-Wallis tests. Analyses of incident VMS – conducted separately for any VMS and frequent VMS – included only women reporting no VMS in the prior two weeks at baseline. Time to first symptomatic visit was modeled using discrete survival analyses33 due to the approximately annual nature of data collection. For ease of interpretation, associations are expressed as estimated percent with incident VMS at the concurrent visit, holding other factors fixed at their sample means34, in addition to hazard ratios. A participant's data were censored at initiation of hormone therapy, at hysterectomy or bilateral oophorectomy (which hindered assessment of menstrual/menopausal status or hormone status, respectively), or at her last visit, and visits concurrent with pregnancy or breastfeeding were omitted. Missing values of time-varying variables were interpolated based on prior and subsequent values for gaps of 1-2 visits, as in previous SWAN analyses35.
To assess hypothesis #1, we modeled incident VMS as a function of concurrent early (pre- and early perimenopause) versus late (late peri- and postmenopause) menopause stage, concurrent BMI or waist circumference, and tested the interaction between menopause stage and body composition indicator; parallel models were estimated substituting waist circumference for BMI. To address hypothesis #2 regarding long-term change in body composition, we expanded the model for hypothesis #1 to add within-woman percent change in weight since baseline and its interaction with concurrent menopause stage; parallel models were estimated adding percent change in waist circumference since baseline and its interaction with concurrent menopause stage. To address hypothesis #3, regarding recent change in body composition, we expanded the model for hypothesis #1 to add within-woman percent change in weight since the prior visit (approximately one year earlier) and its interaction with concurrent menopause stage; parallel models were estimated adding percent change in waist circumference since the prior visit and its interaction with concurrent menopause stage. Results adjusting waist circumference for height were similar (data not shown). Relative model fit, comparing hypothesis #2 models and hypothesis #3 models with hypothesis #1 models, was computed using the Akaike Information Criterion (AIC)36; values less than 1 indicated a better fit when change in body composition was included in the model; values greater than 1 indicated a better fit when change in body composition was not included, and a value of 1 indicated essentially the same fit. All fits were very close to 1. To address hypothesis #4, we included race/ethnicity and its interactions with concurrent and percent change in BMI or waist circumference as predictors in the above models.
In multivariable modeling, backward elimination (p<0.05 for retention) was employed to eliminate redundant or irrelevant predictors, forcing in the design variables of race/ethnicity and site as well as baseline age. Sensitivity analyses included: also censoring at the first visit with missing VMS; omitting participants from the New Jersey site, which had a several-year hiatus in data collection beginning with follow-up visit 06; and using a larger “stable” category (5% to ≤ 5% gain) for change in weight or waist. Results from all sensitivity analyses, except when noted, were similar to those presented here (data not shown). All analyses were conducted in SAS Version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Sample Characteristics
At baseline, 39.2% of participants reported any VMS, and 11.0% reported VMS for 6 or more days in the prior two weeks (frequent VMS). During follow-up visits, the proportion of women reporting any VMS ranged from 26.1% in the first year to a high of 51.1% in year 8, after which the proportion dropped to 48.8% by year 10; the corresponding figures for women reporting frequent VMS was 5.9% in the first year to 22.3% in year 8, after which the proportion dropped to 18.7% and 19.8% in years 9 and 10, respectively. At baseline, VMS were reported significantly more by women who were older, were African American or Hispanic, had less education, had a history of premenstrual symptoms, had higher waist circumference and BMI, were past or current smokers, were early perimenopausal, and had more passive smoke exposure, higher symptom sensitivity and anxiety scores, greater depressive symptoms, more upsetting life events and lower physical activity scores (Table 1).
Table 1.
Distribution of baseline characteristics for women by VMS reporting at baseline
| Number of Days of VMS in past 2 weeks | ||||
|---|---|---|---|---|
| Baseline Characteristics N=3,203 | None | 1-5 | 6+ | |
| N=1,948 | N=902 | N=353 | p-value(a) | |
| Age (years, mean(SD)) | 46.2 (2.7) | 46.4 (2.7) | 47.0 (2.8) | <0.0001 |
| Race/ Ethnicity, N (%) | <0.0001 | |||
| White | 957 (63.9) | 393 (26.2) | 148 (9.9) | |
| African American | 482 (53.5) | 281 (31.2) | 138 (15.3) | |
| Chinese | 168 (69.4) | 57 (23.6) | 17 (7.0) | |
| Hispanic | 151 (53.2) | 100 (35.2) | 33 (11.6) | |
| Japanese | 190 (68.4) | 71 (25.5) | 17 (6.1) | |
| Educational attainment, N (%) | <0.0001 | |||
| <High School | 111 (47.4) | 86 (36.8) | 37 (15.8) | |
| High School | 325 (57.0) | 173 (30.4) | 72 (12.6) | |
| Some College | 557 (55.1) | 320 (31.7) | 134 (13.3) | |
| College Degree | 424 (66.2) | 171 (26.7) | 46 (7.2) | |
| >College Education | 511 (71.3) | 145 (20.2) | 61 (8.5) | |
| History of premenstrual symptoms, N (%) | 0.079 | |||
| No | 223 (64.8) | 79 (23.0) | 42 (12.2) | |
| Yes | 1707 (60.3) | 813 (28.7) | 310 (11.0) | |
| Weight (kg, mean (SD)) | 73.0 (20.0) | 76.0 (20.8) | 81.6 (20.8) | <0.0001 |
| Waist circumference (cm, mean (SD)) | 84.6 (15.3) | 87.4 (16.3) | 92.6 (16.5) | <0.0001 |
| BMI (kg/m2, mean (SD)) | 27.6 (7.0) | 28.8 (7.4) | 30.7 (7.4) | <0.0001 |
| Smoking status, N (%) | <0.0001 | |||
| Never | 1191 (65.2) | 481 (26.3) | 155 (8.5) | |
| Past | 467 (58.1) | 235 (29.2) | 102 (12.7) | |
| Current | 275 (50.6) | 175 (32.2) | 93 (17.1) | |
| Passive smoking, N (%) | <0.0001 | |||
| 0 person-hours/week | 938 (65.5) | 362 (25.3) | 133 (9.3) | |
| 1 – 4 person-hours/week | 500 (59.7) | 250 (29.9) | 87 (10.4) | |
| 5+ person-hours/week | 497 (54.5) | 286 ( 31.4) | 129 (14.1) | |
| Menopause status, N (%) | <0.0001 | |||
| Pre | 1168 (68.4) | 406 (23.8) | 134 (7.9) | |
| Early Peri | 757 (52.1) | 481 (33.1) | 214 (14.7) | |
| Visit 1 symptom sensitivity score (mean (SD)) | 9.9 (3.6) | 10.5 (3.5) | 11.0 (3.6) | <0.0001 |
| Anxiety (≥4 on scale), N (%) | <0.0001 | |||
| No | 1677 (67.7) | 636 (25.7) | 164 (6.6) | |
| Yes | 271 (37.3) | 266 ( 36.6) | 189 (26.0) | |
| Depressive symptoms (≥16 on CESD), N (%) | <0.0001 | |||
| No | 1610 (66.3) | 625 (25.7) | 194 (8.0) | |
| Yes | 335 (43.5) | 276 (35.8) | 159 (20.7) | |
| Number of very upsetting negative life events, N (%) | <0.0001 | |||
| 0 | 1034 (64.8) | 414 (26.0) | 147 (9.2) | |
| 1 | 396 (59.7) | 198 (29.9) | 69 (10.4) | |
| 2+ | 512 (55.1) | 284 ( 30.5) | 134 (14.4) | |
| Physical activity score (mean (SD)) | 7.7 (1.8) | 7.6 (1.8) | 7.4 (1.7) | 0.0060 |
| Percentage weight change from baseline to last visit included in analyses of incident VMS, N (%):(b) | ||||
| Lost >5% | 159 (10.3) | |||
| Lost 1 – 5% | 242 (15.7) | |||
| Stable | 232 (15.0) | |||
| Gained 1 – 5% | 477 (30.9) | |||
| Gained >5 – 10% | 281 (18.2) | |||
| Gained > 10% | 154 (10.0) | |||
Chi-square test for categorical variables, Kruskal-Wallis test for continuous variables
1545 women included in analyses of incident VMS
First, we examined patterns of change in weight. About 28% of women had > 5% weight gain, and 10% had > 5% weight loss from baseline to the last visit included in analyses of incident any VMS (the first visit at which they reported VMS or the last non-censored visit). Those who gained at least 5% ranged from 23.0% in women who were obese at baseline to 28.6% in women with baseline BMI <25. Women who were currently obese were the most likely to have gained more than 10% since baseline, but the proportion with weight gain was fairly similar in other concurrent BMI categories, and the Spearman correlation was only 0.15, suggesting low collinearity of concurrent BMI and weight change. The percentages of women who lost weight ranged from 23.6% in those who were overweight (BMI 25-29.9) to 3% in women who were obese at baseline.
Association of Incident VMS with Concurrent BMI and Waist Circumference
Concurrent BMI
In unadjusted analyses, incidence of any VMS was positively related to concurrent BMI in the early stage (pre- and early peri-menopause, p<0.0001), while in the late stage (late peri- and postmenopause), obese women had a significantly lower incidence of any VMS (p=0.02); this effect modification by menopause stage was statistically significant (p<0.0001, Table 2, Model 1). Incident frequent VMS was also was significantly positively related to concurrent BMI during the early stage (p=0.0009). However, no association was observed in the late stage, and the interaction with menopause stage neared statistical significance (Table 3, Model 1). Adjustment for covariates resulted in little change in these associations (Table 2, Model 2 and Table 3, Model 2). However, the interactions with menopause stage were significant for any and frequent VMS (p<0.0001 and p=0.03, respectively). All of these relations were very similar when we examined baseline instead of concurrent BMI (data not shown).
Table 2.
Unadjusted and covariate-adjusted(a) percentages and hazard ratios (HR) for incident any VMS associated with concurrent BMI and waist circumference, in women with no VMS at baseline; 5048 observations from 1546 women, 1023 incident any VMS
| Model | Unadjusted % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Model | Adjusted % (SE / HR (95% CI) |
p-value for interaction with menopause status | ||
|---|---|---|---|---|---|---|---|
| Early stage(b) | Late stage(c) | Early stage(b) | Late stage(c) | ||||
| Model 1: | Model 2: | ||||||
| Concurrent BMI (kg/m2) | <0.0001 | Concurrent BMI | <0.0001 | ||||
| <25 | 15.19 (0.84) / Reference | 33.50 (2.85) / Reference | 15.80 (0.91) / Reference | 34.21 (2.95) / Reference | |||
| 25-29.9 | 18.48 (1.13) / 1.27 (1.05, 1.53) | 34.19 (3.38) / 1.03 (0.71, 1.49) | 17.75 (1.12) / 1.15 (0.94, 1.41) | 34.74 (3.48) / 1.02 (0.70, 1.50) | |||
| ≥30 | 21.30 (1.22) / 1.51 (1.25, 1.82) | 23.66 (2.89) / 0.62 (0.42, 0.90) | 19.36 (1.22) / 1.28 (1.04, 1.57) | 19.86 (2.67) / 0.48 (0.32, 0.71) | |||
| P value | <0.0001 | 0.02 | 0.053 | 0.0003 | |||
| Model 3: | Model 4: | ||||||
| Concurrent waist circumference (cm) | 0.0005 | Concurrent waist circumference | <0.0001 | ||||
| <72 | 13.15 (1.16) / Reference | 32.09 (3.97) / Reference | 14.29 (1.30) / Reference | 33.02 (4.16) / Reference | |||
| 72-77.9 | 15.93 (1.25) / 1.25 (0.96, 1.63) | 33.04 (4.22) / 1.05 (0.63, 1.73) | 16.25 (1.30) / 1.16 (0.89, 1.53) | 34.70 (4.40) / 1.08 (0.64, 1.80) | |||
| 78-85.9 | 17.69 (1.26) / 1.42 (1.10, 1.84) | 36.17 (4.29) / 1.20 (0.73, 1.97) | 17.07 (1.25) / 1.23 (0.94, 1.62) | 35.54 (4.33) / 1.12 (0.67, 1.86) | |||
| 86 -96.9 | 21.39 (1.42) / 1.80 (1.39, 2.32) | 35.42 (4.03) / 1.16 (0.72, 1.88 | 20.23 (1.40) / 1.52 (1.16, 1.99) | 35.26 (4.12) / 1.11 (0.67, 1.83) | |||
| 97+ | 21.08 (1.47) / 1.76 (1.36, 2.29) | 21.53 (3.03) / 0.58 (0.36, 0.95) | 18.92 (1.44) / 1.40 (1.05, 1.86) | 17.47 (2.71) / 0.43 (0.26, 0.72) | |||
| P value | <0.0001 | 0.02 | 0.03 | 0.0003 | |||
BMI, body mass index; CES-D, Center for Epidemiology Studies-Depression; HR, hazard ratio; SE, standard error; VMS, vasomotor symptoms.
Covariates included: Time-invariant - baseline age, race/ethnicity, site, V01 symptom sensitivity, baseline CES-D depression (yes/no), history of premenstrual symptoms, and 5-category educational level; Time-varying - smoking (never, past, current), number very upsetting life events (0, 1, 2+)
Early stage includes pre- and early perimenopausal
Late stage includes late peri- and postmenopausal
Table 3.
Unadjusted and covariate-adjusted(a) percentages and hazard ratios (HR) for incident frequent(b) VMS associated with concurrent BMI and waist circumference, in women with no VMS at baseline; 7586 observations from 1546 women, 545 incident frequent VMS
| Model | Unadjusted % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Model | Adjusted % (SE) / HR (95% CI) |
p-value for interaction with menopause status | ||
|---|---|---|---|---|---|---|---|
| Early stage(c) | Late stage(d) | Early stage(c) | Late stage(d) | ||||
| Model 1: Concurrent BMI (kg/m2) | 0.052 | Model 2: Concurrent BMI | 0.03 | ||||
| <25 | 3.85 (0.40) / Reference | 14.13 (1.44) / Reference | 4.17 (0.44) / Reference | 15.28 (1.58) / Reference | |||
| 25-29.9 | 4.83 (0.53) / 1.27 (0.93, 1.72) | 14.28 (1.61) / 1.00 (0.73, 1.37) | 4.24 (0.49) / 1.02 (0.75, 1.40) | 12.65 (1.51) / 0.80 (0.57, 1.12) | |||
| ≥30 | 6.42 (0.61) / 1.71 (1.29, 2.27) | 14.30 (1.61) / 1.03 (0.75, 1.41) | 5.05 (0.53) / 1.23 (0.91, 1.66) | 11.08 (1.40) / 0.69 (0.49, 0.98) | |||
| P value | 0.0009 | 0.996 | 0.34 | 0.10 | |||
| Model 3: Concurrent waist circumference (cm) | 0.008 | Model 4: Concurrent waist circumference | 0.007 | ||||
| <72 | 3.36 (0.57) / Reference | 17.20 (2.35) / Reference | 3.97 (0.68) / Reference | 19.25 (2.67) / Reference | |||
| 72-77.9 | 3.87 (0.59) / 1.16 (0.73, 1.82) | 11.27 (1.84) / 0.61 (0.38, 0.97) | 4.04 (0.62) / 1.02 (0.64, 1.61) | 11.92 (1.96) / 0.57 (0.35, 0.91) | |||
| 78-85.9 | 4.61 (0.60) / 1.39 (0.91, 2.13) | 15.05 (1.96) / 0.85 (0.56, 1.30) | 4.30 (0.57) / 1.09 (0.70, 1.68) | 14.22 (1.91) / 0.70 (0.45, 1.08) | |||
| 86 -96.9 | 5.36 (0.64) / 1.63 (1.07, 2.47) | 14.70 (1.83) / 0.83 (0.55, 1.25) | 4.47 (0.56) / 1.13 (0.74, 1.74) | 12.61 (1.67) / 0.61 (0.39, 0.94) | |||
| 97+ | 7.06 (0.78) / 2.18 (1.45, 3.28) | 13.42 (1.75) / 0.75 (0.49, 1.13) | 5.42 (0.65) / 1.39 (0.90, 2.13) | 9.93 (1.46) / 0.46 (0.29, 0.73) | |||
| P value | 0.0006 | 0.31 | 0.46 | 0.02 | |||
BMI, body mass index; CES-D, Center for Epidemiology Studies-Depression; HR, hazard ratio; SE, standard error; VMS, vasomotor symptoms.
Covariates included: Time-invariant - baseline age, race/ethnicity, site, V01 symptom sensitivity, baseline CES-D depression (yes/no), history of premenstrual symptoms, and 5-category educational level; Time-varying - smoking (never, past, current), number very upsetting life events (0, 1, 2+)
Frequent was reporting having VMS 6+ days in the prior two weeks
Early stage includes pre- and early perimenopausal
Late stage includes late peri- and postmenopausal
Concurrent Waist Circumference
In unadjusted analyses, higher concurrent waist circumference was associated with higher incidence of any VMS in the early stage (p<0.0001) but was associated with the lowest incidence of any VMS in the late stage (p=0.02); the interaction with menopause stage was significant (p=0.0005, Table 2, Model 3). Similarly, incident frequent VMS also was positively associated with concurrent waist circumference in the early stage (p=0.0006), but no association was observed in the late stage; the interaction with menopause stage again was significant (p=0.008, Table 3, Model 3). Covariate adjustment produced similar associations of concurrent waist circumference and interaction with menopause stage for incident any VMS (Table 2, Model 4). However, for frequent VMS, the positive adjusted association in the early stage was smaller and no longer significant, and the negative association in the late stage was statistically significant (p=0.02); the interaction with menopausal stage remained significant (p=0.007, Table 3, Model 4).
Association of Incident VMS with Changes in Weight and Waist Circumference
Percent Weight Change Since Baseline
Adjusting only for concurrent BMI, percent weight change since baseline was not significantly related to incidence of any VMS for either menopausal stage, nor was its interaction with menopause stage statistically significant (Table 4, Model 1a). In contrast, the association of percent weight change since baseline with incident frequent VMS (Table 5, Model 1a) had a shallow U-shape in the early stage (p=0.02) and a shallow inverse U-shape in the late stage (p=0.02), and the interaction with menopause stage was significant (p=0.004). Unadjusted associations of concurrent BMI (or baseline BMI, data not shown) with incidence of any VMS (Table 4, Model 1a) and with incident frequent VMS (Table 5, Model 1a) were similar after adjustment for percent weight change since baseline. Differences in model fit compared with including only concurrent BMI (Table 2, Model 1 and Table 3, Model 1) were negligible for both any and frequent incident VMS, respectively, indicating essentially no improvement in predictive power with the addition of percent weight change.
Table 4.
Unadjusted and covariate-adjusted(a) percentages and hazard ratios (HR) for incident any VMS associated with percent change in weight or waist circumference since baseline and since prior visit, also adjusted for concurrent BMI and waist circumference, respectively; in women with no VMS at baseline; 5048 observations from 1546 women, 1023 incident any VMS
| Model | Unadjusted % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Relative model fit compared with only concurrent body composition(d) | Model | Adjusted(a) % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Relative model fit compared with only concurrent body composition(d) | ||
|---|---|---|---|---|---|---|---|---|---|
| Early stage(b) | Late stage(c) | Early stage(b) | Late stage(c) | ||||||
| Model 1a(e): Change in weight since baseline Concurrent BMI (kg/m2): | 0.0002 | 1.0033 | Model 1b: Change in weight since baseline | <0.0001 | 1.0029 | ||||
| <25 | 15.29 (0.85) / Reference |
33.40 (2.88) / Reference |
15.89 (0.93) / Reference |
34.07 (2.99) / Reference |
|||||
| 25-29.9 | 18.32 (1.12) / 1.24 (1.03, 1.51) |
33.95 (3.42) / 1.03 (0.71, 1.49) |
17.57 (1.12) / 1.13 (0.92, 1.38) |
34.40 (3.51) / 1.02 (0.69, 1.49) |
|||||
| ≥30 | 21.11 (1.23) / 1.48 (1.23, 1.79) |
23.18 (2.96) / 0.60 (0.41, 0.89) |
19.18 (1.23) / 1.26 (1.02, 1.55) |
19.39 (2.72) / 0.47 (0.31, 0.71) |
|||||
| P value | 0.0002 | 0.02 | 0.10 | 0.0003 | |||||
| % change in weight since baseline: | 0.56 | 0.58 | |||||||
| Lost >5% | 20.51 (2.16) / 1.33 (0.96, 1.84) |
39.61 (5.51) / 1.66 (0.90, 3.08) |
19.37 (2.11) / 1.27 (0.91, 1.76) |
38.33 (5.55) / 1.66 (0.88, 3.10) |
|||||
| Lost 1-5% | 15.90 (1.35) / 0.98 (0.74, 1.28) |
21.13 (3.91) / 0.68 (0.37, 1.27) |
15.18 (1.31) / 0.94 (0.72, 1.24) |
20.00 (3.80) / 0.67 (0.36, 1.25) |
|||||
| Stable | 16.24 (1.38) / Reference |
28.28 (4.50) / Reference |
15.94 (1.38) / Reference |
27.29 (4.47) / Reference |
|||||
| Gained 1-5% | 18.07 (1.06) / 1.14 (0.90, 1.44) |
34.88 (3.60) / 1.36 (0.81, 2.29) |
18.05 (1.08) / 1.16 (0.91, 1.48) |
34.03 (3.63) / 1.37 (0.81, 2.33) |
|||||
| Gained >5-10% | 18.11 (1.43) / 1.14 (0.87, 1.50) |
30.26 (3.74) / 1.10 (0.64, 1.90) |
17.86 (1.43) / 1.15 (0.87, 1.51) |
29.57 (3.74) / 1.12 (0.64, 1.95) |
|||||
| Gained >10% | 18.68 (2.22) / 1.19 (0.83, 1.68) |
31.70 (5.00) / 1.18 (0.63, 2.19) |
17.84 (2.18) / 1.15 (0.80, 1.64) |
31.00 (4.47) / 1.20 (0.64, 2.25) |
|||||
| P value | 0.42 | 0.14 | 0.39 | 0.08 | |||||
| Model 2a: Change in weight since prior visit Concurrent BMI (kg/m2): | <0.0001 | 1.0079 | Model 2b: Change in weight since prior visit | <0.0001 | 1.0077 | ||||
| <25 | 15.25 (0.85) / Reference |
33.89 (2.89) / Reference |
15.87 (0.92) / Reference |
34.64 (3.00) / Reference |
|||||
| 25-29.9 | 18.32 (1.13) / 1.25 (1.03, 1.51) |
34.13 (3.40) / 1.01 (0.70, 1.46) |
17.57 (1.12) / 1.13 (0.92, 1.38) |
34.62 (3.49) / 1.00 (0.68, 1.46) |
|||||
| ≥30 | 21.25 (1.23) / 1.50 (1.24, 1.81) |
23.68 (2.91) / 0.61 (0.41, 0.89) |
19.28 (1.23) / 1.27 (1.03, 1.56) |
19.89 (2.68) / 0.47 (0.31, 0.70) |
|||||
| P value | <0.0001 | 0.02 | 0.08 | 0.0003 | |||||
| % change in weight since prior visit (~1 year): | 0.85 | 0.93 | |||||||
| Lost >5% | 18.82 (2.27) / 1.15 (0.83, 1.61) |
27.22 (5.53) / 0.94 (0.50, 1.76) |
17.78 (2.20) / 1.11 (0.80, 1.54) |
26.44 (5.49) / 0.94 (0.50, 1.77) |
|||||
| Lost 1-5% | 17.29 (1.26) / 1.04 (0.82, 1.32) |
29.67 (3.69) / 1.06 (0.67, 1.68) |
16.79 (1.25) / 1.04 (0.82, 1.32) |
28.82 (3.67) / 1.06 (0.66, 1.69) |
|||||
| Stable | 16.75 (1.18) / Reference |
28.50 (3.48) / Reference |
16.25 (1.18) / Reference |
27.73 (3.48) / Reference |
|||||
| Gained 1-5% | 18.30 (1.02) / 1.11 (0.90, 1.37) |
34.37 (3.10) / 1.31 (0.87, 1.98) |
18.17 (1.03) / 1.14 (0.93, 1.42) |
33.31 (3.11) / 1.30 (0.86, 1.97) |
|||||
| Gained >5% | 17.71 (1.65) / 1.07 (0.81, 1.41) |
30.46 (5.54) / 1.10 (0.69, 2.01) |
17.26 (1.64) / 1.08 (0.82, 1.42) |
29.68 (5.55) / 1.10 (0.60, 2.03) |
|||||
| P value | 0.85 | 0.64 | 0.77 | 0.68 | |||||
| Model 3a: Change in waist since baseline Concurrent waist circumference (cm): | 0.0005 | 1.0081 | Model 3b: Change in waist since baseline | <0.0001 | 1.0087 | ||||
| <72 | 13.40 (1.20) / Reference |
33.10 (4.18) / Reference |
14.51 (1.33) / Reference |
34.08 (4.38) / Reference |
|||||
| 72-77.9 | 16.04 (1.26) / 1.23 (0.94, 1.61) |
33.09 (4.25) / 1.00 (0.60, 1.67) |
16.34 (1.31) / 1.15 (0.88, 1.51) |
34.62 (4.43) / 1.02 (0.61, 1.73) |
|||||
| 78-85.9 | 17.60 (1.26) / 1.38 (1.06, 1.79) |
35.83 (4.32) / 1.13 (0.67, 1.89) |
16.96 (1.25) / 1.20 (0.92, 1.58) |
35.20 (4.34) / 1.05 (0.62, 1.78) |
|||||
| 86 -96.9 | 21.01 (1.42) / 1.72 (1.32, 2.23) |
34.32 (4.08) / 1.06 (0.64, 1.76) |
19.95 (1.40) / 1.47 (1.11, 1.94) |
34.15 (4.17) / 1.00 (0.59, 1.70) |
|||||
| 97+ | 20.82 (1.47) / 1.70 (1.30, 2.22) |
20.69 (3.03) / 0.53 (0.32, 0.88) |
18.72 (1.44) / 1.36 (1.02, 1.81) |
16.78 (2.69) / 0.39 (0.23, 0.67) |
|||||
| P value | 0.0002 | 0.01 | 0.07 | 0.0002 | |||||
| % change in waist since baseline | 0.68 | 0.68 | |||||||
| Lost >5% | 18.16 (2.05) / 1.15 (0.83, 1.61) |
30.66 (5.32) / 1.22 (0.63, 2.38) |
17.53 (2.02) / 1.11 (0.79, 1.55) |
29.59 (5.34) / 1.26 (0.64, 2.28) |
|||||
| Lost 1-5% | 16.39 (1.31) / 1.02 (0.78, 1.33) |
32.29 (4.37) / 1.32 (0.73, 2.39) |
15.92 (1.30) / 0.99 (0.75, 1.29) |
31.55 (4.37) / 1.38 (0.76, 2.53) |
|||||
| Stable | 16.17 (1.39) / Reference |
26.55 (4.66) / Reference |
16.10 (1.40) / Reference |
25.00 (4.55) / Reference |
|||||
| Gained 1-5% | 18.24 (1.10) / 1.16 (0.91, 1.47) |
28.51 (3.38) / 1.10 (0.63, 1.93) |
18.08 (1.11) / 1.15 (0.90, 1.47) |
28.62 (3.43) / 1.20 (0.68, 2.11) |
|||||
| Gained >5-10% | 17.78 (1.40) / 1.12 (0.85, 1.47) |
36.80 (4.14) / 1.61 (0.91, 2.86) |
17.34 (1.39) / 1.09 (0.83, 1.44) |
35.85 (4.15) / 1.68 (0.94, 3.00) |
|||||
| Gained >10% | 19.94 (2.22) / 1.29 (0.92, 1.82) |
34.78 (5.17) / 1.48 (0.78, 2.81) |
18.80 (2.16) / 1.21 (0.85, 1.71) |
33.06 (5.15) / 1.48 (0.77, 2.85) |
|||||
| P value | 0.63 | 0.54 | 0.73 | 0.58 | |||||
| Model 4a: Change in waist since prior visit (~ 1 year earlier) Concurrent waist circumference (cm): | 0.0001 | 1.0039 | Model 4b: Change in waist since prior visit (~1 year earlier) | <0.0001 | 1.0041 | ||||
| <72 | 13.15 (1.17) / Reference |
33.41 (4.13) / Reference |
14.26 (1.31) / Reference |
34.31 (4.32) / Reference |
|||||
| 72-77.9 | 15.89 (1.25) / 1.25 (0.96, 1.63) |
33.43 (4.28) / 1.00 (0.60, 1.67) |
16.20 (1.30) / 1.16 (0.89, 1.53) |
35.09 (4.46) / 1.04 (0.61, 1.74) |
|||||
| 78-85.9 | 17.74 (1.27) / 1.42 (1.10, 1.85) |
36.15 (4.34) / 1.13 (0.68, 1.87) |
17.08 (1.26) / 1.24 (0.94, 1.63) |
35.59 (4.38) / 1.06 (0.63, 1.78) |
|||||
| 86 -96.9 | 21.17 (1.42) / 1.77 (1.37, 2.30) |
34.72 (4.08) / 1.06 (0.64, 1.74) |
20.06 (1.40) / 1.51 (1.15, 1.99) |
34.70 (4.17) / 1.02 (0.61, 1.71) |
|||||
| 97+ | 21.19 (1.48) / 1.78 (1.37, 2.31) |
20.61 (2.99) / 0.52 (0.31, 0.85) |
19.03 (1.46) / 1.41 (1.06, 1.88) |
16.73 (2.66) / 0.39 (0.23, 0.65) |
|||||
| P value | <0.0001 | 0.01 | 0.04 | 0.0001 | |||||
| % change in waist since prior visit (~1 year) | 0.14 | 0.11 | |||||||
| Lost >5% | 19.68 (2.08) / 1.21 (0.89, 1.65) |
36.63 (5.38) / 1.32 (0.75, 2.31) |
18.74 (2.03) / 1.15 (0.84, 1.57) |
35.38 (5.40) / 1.22 (0.69, 2.16) |
|||||
| Lost 1-5% | 17.40 (1.24) / 1.04 (0.82, 1.32) |
27.05 (3.61) / 0.85 (0.52, 1.38) |
16.88 (1.23) / 1.01 (0.80, 1.29) |
25.68 (3.54) / 0.77 (0.47, 1.26) |
|||||
| Stable | 16.82 (1.24) / Reference |
30.48 (3.83) / Reference |
16.70 (1.24) / Reference |
30.97 (3.93) / Reference |
|||||
| Gained 1-5% | 18.28 (1.09) / 1.11 (0.89, 1.38) |
29.08 (3.18) / 0.94 (0.60, 1.46) |
17.98 (1.09) / 1.09 (0.88, 1.37) |
28.19 (3.16) / 0.88 (0.56, 1.38) |
|||||
| Gained >5% | 16.71 (1.44) / 0.99 (0.76., 1.29) |
41.53 (4.98) / 1.62 (0.96, 2.74) |
16.24 (1.43) / 0.97 (0.74, 1.25) |
40.71 (5.02) / 1.53 (0.90, 2.61) |
|||||
| P value | 0.68 | 0.10 | 0.76 | 0.10 | |||||
BMI, body mass index; CES-D, Center for Epidemiology Studies-Depression; HR, hazard ratio; SE, standard error; VMS, vasomotor symptoms.
Covariates included: Time-invariant - baseline age, race/ethnicity, site, V01 symptom sensitivity, baseline CES-D depression (yes/no), history of premenstrual symptoms, and 5-category educational level; Time-varying - smoking (never, past, current), number very upsetting life events (0, 1, 2+)
Early stage includes pre- and early perimenopausal
Late stage includes late peri- and postmenopausal
Values below 1 indicate superior fit, above 1 indicate worse fit
Percentages for concurrent BMI computed at observed distribution for % change in weight, and percentages for % change in weight computed at observed distribution for concurrent BMI
Table 5.
Unadjusted and covariate-adjusted(a) percentages and hazard ratios (HR) for associations of incident frequent(b) VMS to percent change in weight and waist circumference since baseline and since prior visit, adjusted for concurrent BMI and waist circumference, respectively, in women with no VMS at baseline; 7586 observations from 1546 women, 545 incident frequent VMS
| Model | Unadjusted % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Relative model fit compared with only concurrent body composition(c) | Model | Adjusted(a) % (SE) / HR (95% CI) |
p-value for interaction with menopause status | Relative model fit compared with only concurrent body composition(d) | ||
|---|---|---|---|---|---|---|---|---|---|
| Early stage(b) | Late stage(c) | Early stage(b) | Late stage(c) | ||||||
| Model 1a(e): Change in weight since baseline Concurrent BMI (kg/m2): | 0.16 | 0.9954 | Model 1b: Change in BMI since baseline | 0.13 | 0.9948 | ||||
| <25 | 3.90 (0.41) / Reference |
13.86 (1.44) / Reference |
4.20 (0.45) / Reference |
14.96 (1.58) / Reference |
|||||
| 25-29.9 | 4.71 (0.53) / 1.22 (0.89, 1.66) |
14.61 (1.66) / 1.06 (0.77, 1.46) |
4.14 (0.48) / 0.98 (0.72, 1.36) |
12.89 (1.55) / 0.84 (0.60, 1.18) |
|||||
| ≥30 | 6.35 (0.61) / 1.67 (1.25, 2.23) |
15.05 (1.72) / 1.10 (0.80, 1.52) |
4.99 (0.52) / 1.20 (0.88, 1.63) |
11.79 (1.51) / 0.76 (0.53, 1.09) |
|||||
| P value | 0.002 | 0.84 | 0.37 | 0.31 | |||||
| % change in weight since baseline: | 0.004 | 0.004 | |||||||
| Lost >5% | 7.85 (1.23) / 2.05 (1.28, 3.27) |
13.59 (2.47) / 0.86 (0.50, 1.48) |
6.68 (1.09) / 1.86 (1.16, 2.99) |
12.19 (2.31) / 0.81 (0.47, 1.41) |
|||||
| Lost 1-5% | 4.05 (0.65) / 1.01 (0.64, 1.61) |
10.04 (1.96) / 0.61 (0.35, 1.06) |
3.64 (0.59) / 0.98 (0.62, 1.56) |
8.86 (1.78) / 0.57 (0.32, 0.99) |
|||||
| Stable | 4.00 (0.66) / Reference |
15.51 (2.56) / Reference |
3.70 (0.62) / Reference |
14.64 (2.47) / Reference |
|||||
| Gained 1-5% | 4.36 (0.49) / 1.10 (0.73, 1.63) |
19.05 (2.06) / 1.28 (0.82, 1.99) |
4.18 (0.48) / 1.13 (0.76, 1.70) |
18.29 (2.05) / 1.30 (0.83, 2.05) |
|||||
| Gained >5-10% | 4.85 (0.65) / 1.23 (0.80, 1.89) |
14.33 (1.83) / 0.91 (0.57, 1.45) |
4.60 (0.63) / 1.26 (0.81, 1.94) |
13.59 (1.78) / 0.92 (0.57, 1.47) |
|||||
| Gained >10% | 5.83 (1.01) / 1.49 (0.91, 2.43) |
11.14 (1.89) / 0.68 (0.41, 1.15) |
5.25 (0.93) / 1.44 (0.88, 2.37) |
9.70 (1.71) / 0.63 (0.37, 1.06) |
|||||
| P value | 0.02 | 0.02 | 0.07 | 0.004 | |||||
| Model 2a: Change in weight since prior visit Concurrent BMI (kg/m2): | 0.02 | 1.0017 | Model 2b: Change in weight since prior visit | 0.01 | 1.0029 | ||||
| <25 | 3.77 (0.40) / Reference |
14.34 (1.46) / Reference |
4.05 (0.43) / Reference |
15.51 (1.61) / Reference |
|||||
| 25-29.9 | 4.78 (0.53) / 1.28 (0.94, 1.74) |
14.26 (1.61) / 0.99 (0.72, 1.36) |
4.20 (0.49) / 1.04 (0.76, 1.43) |
12.63 (1.51) / 0.79 (0.56, 1.10) |
|||||
| ≥30 | 6.47 (0.62) / 1.77 (1.33, 2.35) |
14.02 (1.60) / 0.97 (0.71, 1.34) |
5.09 (0.53) / 1.27 (0.94, 1.72) |
10.87 (1.39) / 0.67 (0.47, 0.94) |
|||||
| P value | 0.0004 | 0.99 | 0.24 | 0.07 | |||||
| % change in weight since prior visit (~1 year): | 0.10 | 0.09 | |||||||
| Lost >5% | 8.06 (1.36) / 1.74 (1.13, 2.69) |
14.50 (2.80) / 0.99 (0.60, 1.63) |
7.02 (1.22) / 1.66 (1.07, 2.57) |
12.47 (2.52) / 0.90 (0.54, 1.50) |
|||||
| Lost 1-5% | 4.90 (0.62) / 1.02 (0.72, 1.46) |
11.59 (1.65) / 0.76 (0.52, 1.13) |
4.53 (0.59) / 1.04 (0.73, 1.49) |
10.65 (1.56) / 0.75 (0.51, 1.12) |
|||||
| Stable | 4.79 (0.58) / Reference |
14.65 (1.80) / Reference |
4.36 (0.54) / Reference |
13.65 (1.73) / Reference |
|||||
| Gained 1-5% | 4.23 (0.46) / 0.88 (0.63, 1.22) |
15.39 (1.60) / 1.06 (0.76, 1.49) |
4.00 (0.44) / 0.91 (0.66, 1.27) |
14.61 (1.57) / 1.08 (0.77, 1.53) |
|||||
| Gained >5% | 4.25 (0.75) / 0.88 (0.57, 1.36) |
15.33 (2.78) / 1.06 (0.65, 1.71) |
3.95 (0.70) / 0.90 (0.59, 1.39) |
13.94 (2.61) / 1.02 (0.63, 1.68) |
|||||
| P value | 0.03 | 0.51 | 0.08 | 0.43 | |||||
| Model 3a: Change in waist since baseline Concurrent waist circumference (cm): | 0.03 | 1.0011 | Model 3b: Change in waist since baseline | 0.02 | 1.0010 | ||||
| <72 | 3.52 (0.60) / Reference |
17.30 (2.45) / Reference |
4.12 (0.71) / Reference |
19.31 (2.78) / Reference |
|||||
| 72-77.9 | 3.96 (0.60) / 1.13 (0.72, 1.79) |
11.31 (1.85) / 0.61 (0.38, 0.98) |
4.10 (0.63) / 1.00 (0.63, 1.58) |
11.98 (1.98) / 0.57 (0.35, 0.92) |
|||||
| 78-85.9 | 4.61 (0.60) / 1.33 (0.86, 2.04) |
15.12 (1.99) / 0.85 (0.55, 1.32) |
4.28 (0.57) / 1.04 (0.67, 1.62) |
14.31 (1.94) / 0.70 (0.44, 1.10) |
|||||
| 86 -96.9 | 5.15 (0.63) / 1.49 (0.97, 2.29) |
15.15 (1.91) / 0.85 (0.55, 1.32) |
4.30 (0.55) / 1.05 (0.67, 1.63) |
13.14 (1.76) / 0.63 (0.40, 1.002) |
|||||
| 97+ | 6.92 (0.77) / 2.04 (1.34, 3.10) |
13.83 (1.84) / 0.77 (0.49, 1.19) |
5.33 (0.64) / 1.31 (0.85, 2.03) |
10.28 (1.54) 0.48 (0.30, 0.77) | |||||
| P value | 0.004 | 0.33 | 0.56 | 0.04 | |||||
| % change in waist since baseline | 0.006 | 0.006 | |||||||
| Lost >5% | 6.21 (1.13) / 1.67 (1.00, 2.77) |
9.90 (2.28) / 0.53 (0.29, 0.98) |
5.58 (1.04) / 1.55 (0.93, 2.59) |
8.63 (2.06) / 0.50 (0.27, 0.93) |
|||||
| Lost 1-5% | 4.17 (0.62) / 1.10 (0.70, 1.72) |
16.11 (2.45) / 0.93 (0.56, 1.54) |
3.76 (0.57) / 1.03 (0.65, 1.62) |
15.11 (2.38) / 0.94 (0.56, 1.56) |
|||||
| Stable | 3.82 (0.65) / Reference |
17.13 (2.82) / Reference |
3.67 (0.63) / Reference |
15.97 (2.72) / Reference |
|||||
| Gained 1-5% | 4.75 (0.53) / 1.26 (0.84, 1.89) |
13.21 (1.68) / 0.74 (0.46, 1.17) |
4.49 (0.51) / 1.23 (0.82, 1.86) |
12.80 (1.66) / 0.77 (0.48, 1.24) |
|||||
| Gained >5-10% | 4.77 (0.64) / 1.26 (0.81, 1.95) |
17.27 (2.03) / 1.01 (0.64, 1.60) |
4.40 (0.60) / 1.21 (0.78, 1.88) |
15.99 (1.95) / 1.00 (0.63, 1.60) |
|||||
| Gained >10% | 6.38 (1.05) / 1.72 (1.05, 2.80) |
11.50 (1.34) / 0.63 (0.37, 1.06) |
5.70 (0.97) / 1.59 (0.97, 2.60) |
10.00 (1.75) / 0.59 (0.34, 0.997) |
|||||
| P value | 0.18 | 0.07 | 0.29 | 0.04 | |||||
| Model 4a: Change in waist since prior visit Concurrent waist circumference (cm): | 0.007 | 1.0063 | Model 4b: Change in waist since prior visit | 0.006 | 1.0058 | ||||
| <72 | 3.36 (0.57) / Reference |
17.53 (2.41) / Reference |
3.97 (0.68) / Reference |
19.65 (2.74) / Reference |
|||||
| 72-77.9 | 3.84 (0.58) / 1.15 (0.73, 1.81) |
11.44 (1.89) / 0.61 (0.38, 0.97) |
4.00 (0.61) / 1.01 (0.64, 1.60) |
12.19 (2.00) / 0.57 (0.35, 0.92) |
|||||
| 78-85.9 | 4.62 (0.60) / 1.39 (0.90, 2.14) |
15.21 (1.98) / 0.84 (0.55, 1.29) |
4.28 (0.57) / 1.08 (0.70, 1.68) |
14.36 (1.93) / 0.69 (0.44, 1.07) |
|||||
| 86 -96.9 | 5.24 (0.64) / 1.59 (1.04, 2.43) |
14.53 (1.83) / 0.80 (0.53, 1.21) |
4.36 (0.55) / 1.10 (0.71, 1.71) |
12.50 (1.67) / 0.58 (0.38, 0.91) |
|||||
| 97+ | 7.06 (0.78) / 2.19 (1.45, 3.30) |
13.37 (1.76) / 0.73 (0.48, 1.11) |
5.36 (0.65) / 1.37 (0.89, 2.12) |
9.77 (1.46) / 0.44 (0.28, 0.71) |
|||||
| P value | 0.0007 | 0.30 | 0.48 | 0.01 | |||||
| % change in waist since prior visit (~1 year prior) | 0.26 | 0.24 | |||||||
| Lost >5% | 5.49 (1.02) / 1.43 (0.89, 2.30) |
12.31 (2.37) / 0.74 (0.45, 1.21) |
4.73 (0.90) / 1.28 (0.79, 2.07) |
10.59 (2.12) / 0.65 (0.39, 1.07) |
|||||
| Lost 1-5% | 4.89 (0.61) / 1.27 (0.87, 1.86) |
12.50 (1.68) / 0.75 (0.51, 1.11) |
4.43 (0.57) / 1.19 (0.81, 1.75) |
11.42 (1.59) / 0.70 (0.47, 1.05) |
|||||
| Stable | 3.90 (0.55) / Reference |
15.99 (2.01) / Reference |
3.74 (0.53) / Reference |
15.50 (2.01) / Reference |
|||||
| Gained 1-5% | 5.15 (0.53) / 1.34 (0.94, 1.90) |
14.67 (1.66) / 0.90 (0.63, 1.29) |
4.80 (0.50) / 1.30 (0.91, 1.85) |
13.54 (1.59) / 0.85 (0.59, 1,23) |
|||||
| Gained >5% | 4.71 (0.71) / 1.22 (0.80, 1.84) |
15.20 (2.34) / 0.94 (0.61, 1.46) |
4.31 (0.66) / 1.16 (0.76, 1.76) |
14.15 (2.24) / 0.90 (0.57, 1.41) |
|||||
| P value | 0.51 | 0.57 | 0.69 | 0.33 | |||||
BMI, body mass index; CES-D, Center for Epidemiology Studies-Depression; HR, hazard ratio; SE, standard error; VMS, vasomotor symptoms.
Covariates included: Time-invariant - baseline age, race/ethnicity, site, V01 symptom sensitivity, baseline CES-D depression (yes/no), history of PMS, and 5-category educational level; Time-varying - smoking (never, past, current), number very upsetting life events (0, 1, 2+)
Early stage includes pre- and early perimenopausal
Late stage includes late peri- and postmenopausal
Values below 1 indicate superior fit, above 1 indicate worse fit
Percentages for concurrent BMI computed at observed distribution for % change in weight, and percentages for % change in weight computed at observed distribution for concurrent BMI
Adjusting for covariates had little impact on associations between weight change since baseline and incidence of any VMS (Table 4, Model 1b). However, the association of percent weight change since baseline with incident frequent VMS in the early stage was somewhat reduced (p=0.07, Table 5, Model 1b). The association in the late stage remained an inverse U-shape that was significant (p=0.004) as was the interaction by menopause stage (p=0.004). Other associations were similar before and after adjustment.
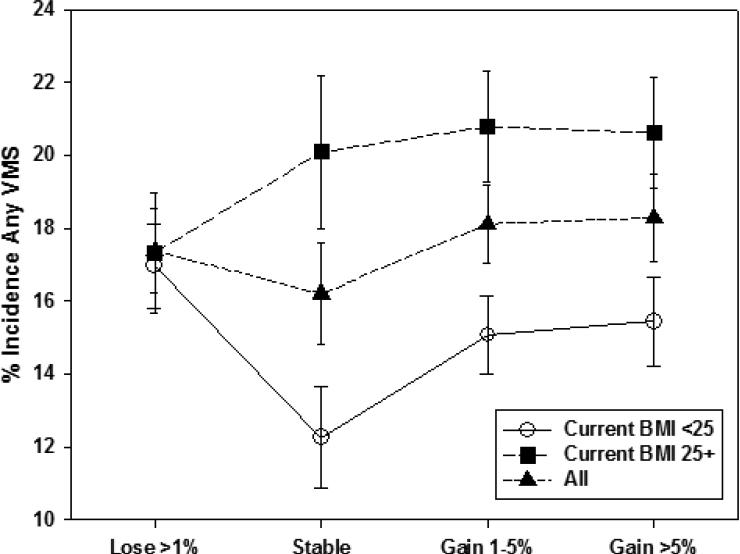
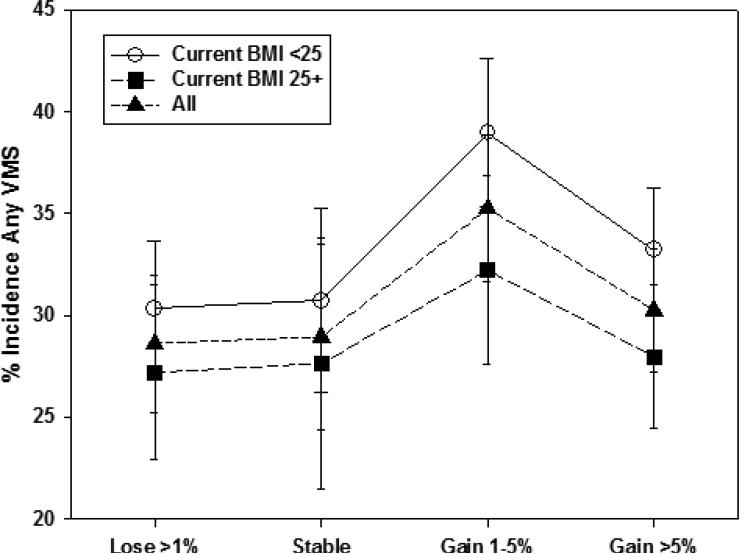
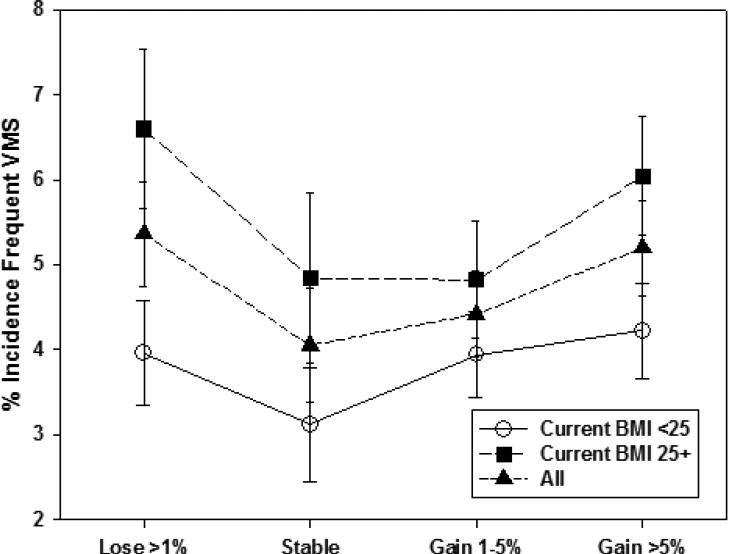
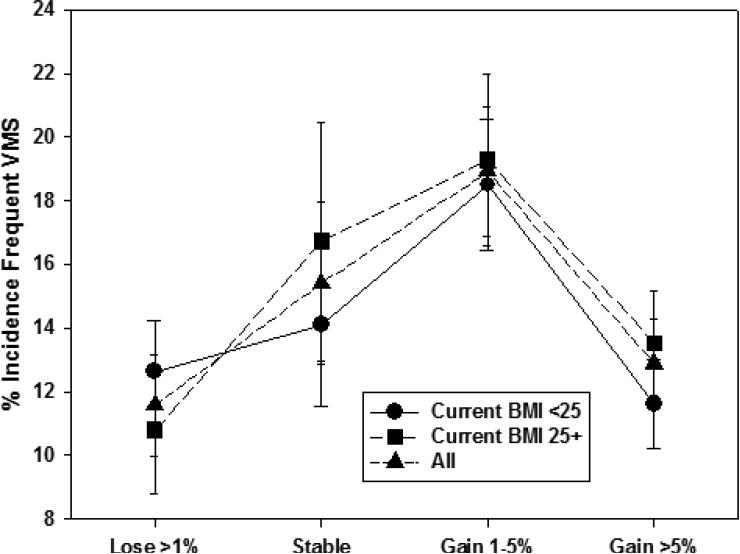
We also examined whether percent weight change since baseline modified the relation of concurrent BMI to incident VMS within menopause stage. Unadjusted patterns of associations of concurrent BMI with incident VMS were generally similar across categories of weight change. An exception was incident any VMS in the early stage (Figure 1a), with a smaller BMI-related difference in incidence at visits with greater than 1% weight loss and a larger BMI-related difference in incidence at visits with stable weight (p=0.002 for interaction between concurrent BMI and percent weight change). For incident frequent VMS in the early stage (Figure 1b), this interaction was not statistically significant (p=0.70). In the late stage, we did not observe interaction of concurrent BMI with weight change for either incident any VMS or incident frequent VMS (Figures 2a [interaction p-value=0.053] and 2b [interaction p-value=0.09], respectively). Thus, within both the early and late stage, weight change since baseline was not significantly associated with any VMS, but the patterns of association differed significantly by menopause status for frequent VMS, and weight change generally did not modify the effect of concurrent BMI.
Figure 1a.
Unadjusted Any Vasomotor Symptom Incidence in the Early Menopause Stage by Percent Weight Change Since Baseline
Figure 1b.
Unadjusted Any Vasomotor Symptom Incidence in the Late Menopause Stage by Percent Weight Change Since Baseline
Figure 2a.
Unadjusted Frequenta Vasomotor Symptom Incidence in the Early Menopause Stage by Percent Weight Change Since Baseline
aFrequent vasomotor symptoms was reporting having those symptoms on 6+ days in the prior two weeks
Figure 2b.
Unadjusted Frequenta Vasomotor Symptom Incidence in the Late Menopause Stage by Percent Weight Change Since Baseline
aFrequent vasomotor symptoms was reporting having those symptoms on 6+ days in the prior two weeks
Percent Weight Change Since the Prior Visit
In analyses adjusting only for concurrent BMI (Table 4, Model 2a) or for all covariates (Table 4, Model 2b), percent weight change since the prior visit, approximately one year earlier, like weight change since baseline, was unrelated to incidence of any VMS in both the early and late stages, and the interaction with menopause stage was not significant. Incidence of frequent VMS was reduced with increasing percent weight gain since the prior visit only in the early stage (p=0.03, Table 5, Model 2b). In contrast to the corresponding association with percent weight change since baseline, this latter association was monotonic and negative, not U-shaped. Adjustment for covariates (Table 5, Model 2b) reduced the association of recent percent weight change with incident frequent VMS in the early stage, which still showed a monotonic reduction in VMS with increased weight gain but was no longer significant (p=0.08). Associations of concurrent BMI with incidence of any and of frequent VMS remained similar after adjustment for recent percent weight change (Table 4, Model 2b and Table 5, Model 2b, respectively). Moreover, adding recent percent weight change to the model with only concurrent BMI did not improve model fit for prediction of incident any or frequent VMS.
Percent Change in Waist Circumference Since Baseline
In analyses adjusting only for concurrent waist circumference, percent change in waist since baseline was not significantly related to incidence of any VMS (Table 4, Model 3a) or of frequent VMS (Table 5, Model 3a) in either the early or late menopause stages. Also, as for weight change, no obvious pattern or trend was observed, though the interaction with menopause stage was significant for frequent VMS (p=0.006). Adjusting for covariates, percent change in waist from baseline was unrelated to incident any VMS (Table 4, Model 3b) in either menopausal stage but was significantly related to incident frequent VMS (Table 5, Model 3b) in the late stage (p=0.04) with an inverse U-shaped pattern and a significant interaction with menopause stage (p=0.006). Relative model fit was close to 1 for both any and frequent VMS, indicating that, like with change in weight, the addition of percent change in waist since baseline added little predictive ability after accounting for concurrent waist circumference.
Percent Change in Waist Circumference Since the Prior Visit
In analyses adjusting only for concurrent waist circumference, percent change in waist since the prior visit was unrelated to incident any (Table 4, Model 4a) and frequent (Table 5, Model 4a) VMS in both the early and late menopause stages. Associations with concurrent waist circumference and model fit were similar to those without change in waist. Adjusting for covariates, the lack of association with change in waist since the prior visit remained, although if the wider stable category (5% loss to ≤5% gain) was used, the association was U-shaped for any incident VMS in the late stage (p=0.04) with significant interaction with stage (p=0.04) (data not shown). However, the positive association of concurrent waist circumference with any VMS in the early stage was somewhat weakened (p=0.04) (Table 4, Model 4b) and for frequent VMS became non-significant (Table 5, Model 4b), but a stronger and statistically significant negative association was observed for concurrent waist circumference with incident frequent VMS in the late stage (p=0.01) with a significant interaction for menopause status (p=0.006). Inclusion of recent percent change in waist circumference yielded a negligible worsening of model fit in the adjusted analyses and thus did not improve prediction of incident VMS beyond that for concurrent waist circumference.
Effect Modification by Race/Ethnicity
Only one interaction of race/ethnicity with concurrent weight or waist circumference or percent change in weight or waist was statistically significant in multivariate models (data not shown); given the large number of tests, this finding was likely to be spurious. The trends were similar, but because the numbers in some racial/ethnic subsets were fairly small, statistical significance was not observed.
DISCUSSION
The results of these longitudinal analyses have shown, first, that concurrent BMI and waist circumference, rather than the change in these measures, were most strongly related to incidence of VMS (Tables 4 and 5). That is, for a given concurrent BMI or waist circumference, a woman's incidence of VMS was essentially unaffected by whether her weight or waist circumference was stable or had changed in the long-term or short-term. Changes in weight and waist circumference essentially did not add predictive power to the models, nor did adjustment for change in these measures affect the associations of concurrent BMI or waist circumference with incidence of VMS. Women who gained the most or the least since baseline had a greater likelihood of incident frequent VMS in the early stage but less likelihood of incident frequent VMS in the late stage; these were the only instances for which we observed anything resembling a consistent pattern in adjusted models for incident VMS with changing weight or waist circumference. However, these associations may have been due to chance because we did not observe U-shaped associations for any VMS in either the early or late menopausal stage. These results may be due to the fact that most percent changes in weight and waist represented relatively small absolute changes and do not rule out that large absolute changes may be necessary to observe changes in risk of VMS.
The second important observation was that interactions with menopause status were statistically significant for all but one of the adjusted associations of any and frequent VMS with concurrent BMI and waist circumference (Tables 2 and 3), indicating that these effects differed in the early and late menopause stages. Concurrent BMI and waist circumference tended to be positively associated with VMS in the early stage and negatively associated in the late stage, although these trends were not always monotonic and not statistically significant for frequent VMS due to relatively small numbers of women in these categories. These interactions with menopause stage are consistent with early observations in postmenopausal women that greater estrone production in peripheral fat from aromatization of androstenedione4-6 was associated with less symptom reporting, while in the earlier phases of the menopause transition, higher BMI (potentially resulting greater heat insulation12) may predispose to increased VMS occurrence.
Our results are largely consistent with those of a recent study of midlife women showing that change in BMI and weight change over one to five years of follow-up were not related to reporting hot flashes17. However, our study went beyond this study by: including five racial/ethnic groups, compared to only two in that study; having a much longer follow-up period; examining both changes in weight and waist circumference; and examining relations of recent and long-term change in weight and waist circumference to developing VMS.
Our findings, however, differ from those reported from the dietary intervention trial of the Women's Health Initiative (WHI), the Women's Health in Lund Area study (WHLA) and the Australian Longitudinal Study on Women's Health (ALSWH). The WHI results indicated that postmenopausal women with VMS at baseline who lost 10 or more pounds or 10% or more of their baseline body weight in one year were more likely to “eliminate” VMS compared with those who maintained weight19. The WHLA and ALSWH found that VMS were reported more by women who had gained weight but not significantly less in those who lost weight9, 37. Some of these inconsistencies may be due to methodological differences between studies. Notably, the WHI focused on reduced prevalent VMS in postmenopausal women who reported VMS at baseline, unlike the present analyses, which focused on incidence of VMS in women who did not have these symptoms at baseline and included women in the early and late menopausal transition stages. The WHLA was large but cross-sectional and asked about weight gain over the past five years but did not report the time over which VMS were reported nor the racial/ethnic distribution37. The ALSWH was longitudinal over 15 years and asked about VMS in the last 12 months in a larger sample of women who were aged 45-50 years at baseline, but race/ethnicity was not reported9, while the present study examined VMS in the prior two weeks longitudinally over 10 years in association with recent and long-term weight change in women aged 42-52 years at baseline in five racial/ethnic groups. A prior paper on a subset of the SWAN cohort showed the same relation of concurrent weight to VMS by menopausal stage as observed in these analyses, but that paper had a smaller, less diverse sample in which postmenopausal women were relatively under-represented, and change in weight was not assessed20.
Our study had several strengths. First, we followed a large and diverse sample of midlife women from five racial/ethnic groups longitudinally for 10 years with nearly annual objective measures of weight and waist circumference, using the same protocol for all participants, thus reducing potential recall bias. Second, we used appropriate sophisticated analyses to accommodate the nature of the interval data and controlled confounding by multiple variables. These strengths provided adequate statistical power to detect meaningful associations, provided reasonable representativeness of midlife women which enhances the generalizability of the findings, used the same high quality measures for the independent and dependent variables (and covariates) for all participants and controlled potential confounding by a large and varied group of variables.
The study did, however, have some limitations. First, not all racial/ethnic groups were represented in our sample, somewhat compromising generalizability. Second, only a small percentage of women (<15%) lost weight or reduced their waist circumference or gained >10% (10%), making detection of meaningful associations of large weight changes with VMS somewhat more tenuous, particularly when examining racial/ethnic groups separately. Third, we made multiple statistical comparisons, so that some of the observed associations may have occurred by chance or represent other, uncontrolled factors; thus, caution must be used for interpreting marginally significant results and significant but modest associations. Fourth, VMS were not rare; so, if we had used odds ratios, they may have overestimated risk, but the use of percentage of women reporting VMS somewhat mitigated this concern. Fifth, because our study aims involved incident, rather than prevalent, VMS, those who had incident VMS in the early stage were omitted from analyses of the later stage and differed (fewer African American and overweight/obese women) from those who developed VMS in the late stage. Finally, differential loss to follow-up by BMI or waist circumference was possible, which could have affected our findings. However, while women who were heavier at baseline or at the prior visit were somewhat more likely to have missing data at the subsequent visit, the differences were small (9.3% of obese women had missing VMS data at the subsequent visit versus 6.0% of normal-weight women), so that the bias was likely to be small.
CONCLUSIONS
Concurrent BMI and waist circumference, rather than percentage changes in weight and waist, were significantly related to incident VMS during longitudinal follow-up. This suggests that maintenance of healthy weight in midlife women early in the menopausal transition may help to prevent VMS. However, it is important to note that we still lack a comprehensive understanding of the fundamental underlying physiologic mechanisms for VMS38, and findings have been inconsistent regarding the effects of weight gain or loss. Inconsistent findings may also reflect the menopausal status-by-weight interaction that we observed because most prior studies predominantly involved late-stage observations, and others may have had a mix without considering the interaction.
Additional research on the role and potential mechanisms of weight and waist circumference, which are modifiable risk factors, in incident VMS has the potential to inform appropriate preventive strategies and benefit many women who experience these prevalent and often bothersome and long-lasting VMS.
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Dr. Tepper previously received a research grant from Pfizer for a study of the use of novel anticoagulants among atrial fibrillation patients, which has ended.
Funding for this work was received from the National Institutes of Health
Footnotes
No other authors have any competing financial conflicts of interest.
REFERENCES
- 1.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation (SWAN). Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. PMID: 16735636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed SR, Lampe JW, Qu C, Copeland WK, Gundersen G, Fuller S, Newton KM. Premenopausal vaosmotor symptoms in an ethnically diverse population. Menopause. 2013;21:153–8. doi: 10.1097/GME.0b013e3182952228. PMID: 23760434. [DOI] [PubMed] [Google Scholar]
- 3.Williams RE, Kalilani L, DiBenedetti B, Zhou X, Granger AL, Fehnel SE, Levine KB, Jordan J, Clark RV. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. doi: 10.1080/13697130701744696. PMID: 18202963. [DOI] [PubMed] [Google Scholar]
- 4.Soule MR, Bremner WJ. The menopause and climacteric: endocrinologic basis and associated symptomatology. J Am Geriatrics Soc. 1982;3:547. doi: 10.1111/j.1532-5415.1982.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 5.Grodin JM, Siiteri PK, MacDonald PC. Extraglandular estrogen in the postmenopause. In: Ryan KJ, Gibson DC, editors. The Menopause and Aging. US Government Printing Office; Washington DC: 1973. p. 15. [Google Scholar]
- 6.Nimrod A, Ryan KJ. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab. 1975;40:367–372. doi: 10.1210/jcem-40-3-367. PMID: 234975. [DOI] [PubMed] [Google Scholar]
- 7.Gold EB, Sternfeld B, Kelsey JL, et al. The relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Amer J. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 8.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms in midlife women: Baseline results from the Study of Women's Health Across the Nation (SWAN). Amer J Epidemiol. 2004;159:1189–99. doi: 10.1093/aje/kwh168. PMID: 15191936. [DOI] [PubMed] [Google Scholar]
- 9.Herber-Gast G-CM, Mishra GD, van der Schouw YT, Brown WJ, Dobson AJ. Risk factors for night sweats and hot flushes in midlife: results from a prospective cohort study. Menopause. 2013;20:953–9. doi: 10.1097/GME.0b013e3182844a7c. PMID: 23531688. [DOI] [PubMed] [Google Scholar]
- 10.Thurston RC, Sowers MF, Chang Y, Sternfeld B, Gold EB, Johnston JM, Matthews KA. Adiposity and reporting of vasomotor symptom among midlife women: The Study of Women's Health Across the Nation. Amer J Epidemiol. 2008;167:78–85. doi: 10.1093/aje/kwm244. PMID: 17881385. [DOI] [PubMed] [Google Scholar]
- 11.Thurston RC, Sowers MF, Sternfeld B, Gold EB, Bromberger J, Chang Y, Joffe H, Crandall CJ, Waetjen LE, Matthews KA. Gains in body fat and vasomotor symptom reporting over the menopausal transition: The Study of Women's Health Across the Nation. Amer J Epidemiol. 2009;170:766–774. doi: 10.1093/aje/kwp203. PMID: 19675142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman RR. Core body temperature variation in symptomatic and asymptomatic postmenopausal women: Brief report. Menopause. 2002;9:399–401. doi: 10.1097/00042192-200211000-00004. PMID: 12439098. [DOI] [PubMed] [Google Scholar]
- 13.Avis NE, Crawford SL, Greendale GA, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, Thurston RC. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Int Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063. doi:10.1001/jamainternmed.2014.8063 PMID: 25686030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58(4):348–358. doi: 10.1016/j.maturitas.2007.09.006. PMID: 17964093. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson WK, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: A national study. Am J Obstet Gynecol. 2001;184(4):523–530. doi: 10.1067/mob.2001.111795. PMID: 11262448. [DOI] [PubMed] [Google Scholar]
- 16.Sarrel P, Portman D, Lefebvre P, Lafeuille MH, Grittner AM, Fortier J, Gravel J, Duh MS, Aupperle PM. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. Mar. 2015;22(3):260–6. doi: 10.1097/GME.0000000000000320. doi: 10.1097/GME.0000000000000320. PMID: 25714236. [DOI] [PubMed] [Google Scholar]
- 17.Gallicchio L, Miller SR, Kiefer J, Green T, Zacur HA, Flaws JA. Change in body mass index, weight and hot flashes: a longitudinal analysis from the midlife women's health study. J Women's Health. 2014;23:231–237. doi: 10.1089/jwh.2013.4526. PMID: 24341351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurston RC, Ewing LJ, Low CA, Christie AJ, Levine MD. Behavioral weight loss for the management of menopausal hot flashes: a pilot study. Menopause. 2015;22:59–65. doi: 10.1097/GME.0000000000000274. doi: 10.1097/GME.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke CH, Caan BJ, Stefanick ML, Anderson G, Brzyski R, Johnson KC, LeBlanc E, Lee C, LaCroix AZ, Park HL, Sims ST, Vitolins M, Wallace R. Effects of a dietary intervention and weight change on vasomotor symptoms in the Women's Health Initiative. Menopause. 2012;19:980–988. doi: 10.1097/gme.0b013e31824f606e. PMID: 22781782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston RC, Chang Y, Mancuso P, Matthews KA. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women's Health Across the Nation. Fertil Steril. 2013;100:793–800. doi: 10.1016/j.fertnstert.2013.05.005. doi: 10.1016/j.fertnstert.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowers MF, Crawford S, Sternfeld B, et al. In: Design, survey sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. Lobos R, Marcus R, Kelsey JL, editors. Academic Press; Menopause. New York: 2000. pp. 175–88. [Google Scholar]
- 22.Neurgarten BL, Kraines RJ. Menopausal symptoms in women of various ages. Psychosom Med. 1965;27:266–273. doi: 10.1097/00006842-196505000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Avis NE, McKinlay SM. A longitudinal analysis of women's attitudes toward the menopause: results from the Massachusetts Women's Health Study. Maturitas. 1991;13:65–79. doi: 10.1016/0378-5122(91)90286-y. PMID: 14327878. [DOI] [PubMed] [Google Scholar]
- 24.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Inter Med. 1994;154:2349–2355. PMID: 7944857. [PubMed] [Google Scholar]
- 25.Bromberger JT, Kravitz HM, Chang Y, Randolph JF, Jr, Avis NE, Gold EB, Matthews KA. Does risk for anxiety increase during the menopausal transition? Study of Women's Health Across the Nation. Menopause. 2013;20(5):488–95. doi: 10.1097/GME.0b013e3182730599. doi: 10.1097/GME.0b013e3182730599. PMID: 23615639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff LS. Applied Psychological Measurement. West Publishing Co; 1977. The CES-D scale: A self-report depression scale for research in the general population. pp. 385–401. [Google Scholar]
- 27.Barsky AJ, Goodson JD, Lane RS, Cleary PD. The amplification of somatic symptoms. Psychosom Med. 1988;50:510–519. doi: 10.1097/00006842-198809000-00007. PMID: 3186894. [DOI] [PubMed] [Google Scholar]
- 28.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130(4):696–704. doi: 10.1093/oxfordjournals.aje.a115391. PMID: 2773917. [DOI] [PubMed] [Google Scholar]
- 29.Sternfeld B, Ainsworth BA, Quesenberry CP., Jr Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. PMID: 10072751. [DOI] [PubMed] [Google Scholar]
- 30.Baecke JAH, Burema J, Fritjers JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. PMID: 7137077. [DOI] [PubMed] [Google Scholar]
- 31.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118:1–120. PMID: 742764. [PubMed] [Google Scholar]
- 32.Sherborne, Stewart The MOS Social Support Survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. PMID: 2035047. [DOI] [PubMed] [Google Scholar]
- 33.Allison PD. Survival analysis using SAS: a practical guide. SAS Institute Inc.; Cary, NC: 1995. [Google Scholar]
- 34.Long JS. Regression models for categorical and limited dependent variables. Sage Publications, Inc.; Thousand Oaks, CA: 1997. [Google Scholar]
- 35.Waetjen LE, Xing G, Feng W-Y, Johnson WO, Greendale GA, Gold EB. Serum estradiol levels are not associated with urinary incontinence in midlife women transitioning through menopause. Menopause. 2011;18(12):1283–1290. doi: 10.1097/gme.0b013e31821f5d25. PMID: 21785372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agresti A, Caffo B. Measures of relative model fit. Computational Statistics & Data Analysis. 2002;39:127–136. [Google Scholar]
- 37.Li C, Samsioe G, Borgfeldt C, Lidfeldt J, Agardh CD, Nerbrand C. Menopause-related symptoms: what are the background factors? A prospective population-based cohort study of Swedish women (The Women's Health in Lund Area study). Am J Obstet Gynecol. 2003;189:1646–53. doi: 10.1016/s0002-9378(03)00872-x. PMID: 14710092. [DOI] [PubMed] [Google Scholar]
- 38.Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, Santoro N, Simoncini T. Menopause. Nat Rev. 2015;1:1–19. doi: 10.1038/nrdp.2015.4. [DOI] [PubMed] [Google Scholar]