Abstract
Purpose
To evaluate whether oral beta-blockers (BB) are associated with a decreased number of intravitreal injections in patients with incident neovascular age-related macular degeneration (nAMD).
Methods
A retrospective cohort study of subjects with a new diagnosis of nAMD was conducted using a medical claims database from a large national US insurer. Two cohorts were created for comparison consisting of patients with regular use of BBs or calcium channel blockers (CCB). The main outcome measured was the difference in the mean number of intravitreal injections administered between the two cohorts.
Results
After inclusion and exclusion criteria, 239 BB and 155 CCB subjects remained for analysis. Univariate analysis revealed that the mean number of injections in the BB cohort was 6.43 (95% CI 5.90-6.95) versus 6.55 (95% CI 5.85-7.25) in the CCB cohort (p=0.78). After multivariate adjustment, the mean number of injections in the BB group was 6.32 (95% CI 5.77-6.87) versus 6.71 (95% CI 6.02-7.40) in the CCB group. The overall difference between the two groups was -0.39 (95% CI difference -1.29-0.51; p=0.40).
Conclusion
The use of oral beta-blockers is not associated with a decreased number of intravitreal injections in incident nAMD patients.
Keywords: Age-related macular degeneration, beta-blocker, choroidal neovascularization
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in older individuals in developed countries1. Advanced stages of AMD, including choroidal neovascularization (CNV), are responsible for the majority of vision loss associated with AMD1. Prior to the advent of intravitreal anti-vascular endothelial growth factor (VEGF) injections, effective treatment options for neovascular AMD (nAMD) were limited2,3. Anti-VEGF agents have revolutionized nAMD treatment by not only preserving, but also potentially restoring visual function in many patients4. Treatment duration effect, however, remains somewhat variable and frequent office visits for surveillance and treatment are often necessary. This places a major burden not only on patients, but caregivers, physicians, and the health care system as well. Retina specialists are now faced with the challenge of maximizing visual outcomes, while minimizing treatment burden and treatment-related complications.
Several studies have explored other treatment modalities that may augment and possibly extend the effect of intravitreal anti-VEGF injections in order to mitigate the heavy burden they pose5-7. Various pathophysiologic mechanisms to reduce treatment burden have been proposed, including inflammatory mediators, components of the renin-angiotensin system, and beta-adrenergic blockade5-7. Studies in murine models revealed that beta-adrenergic blockade reduced expression of VEGF and induced regression of CNV7. A recent retrospective study did not reveal a protective effect of BB use between matched subjects with neovascular and non-neovascular AMD8. While this study offered evidence that BBs do not prevent the initiation of CNV, it did not investigate the findings established by the murine models, namely its role in inducing regression of CNV7,8. A report by Montero et al.9 gave clinical credence to the theory of augmented CNV regression by reporting that over a 2-year period, patients on systemic beta-blockers (BB) received an average of 5.2 (SD=2.4) anti-VEGF injections compared to 7.9 (SD=3.4) in those who were not on BBs. The study was a small case series of 46 eyes that used patient self-report of concomitant systemic medications to evaluate a differential in CNV response to treatment9. The limited sample size and lack of a refined control group makes the generalization of the study difficult, but its findings warrant additional investigation. The aim of this study is to use a large national insurance claims database to determine whether oral beta-adrenergic blocking agents decrease the number of intravitreal injections in patients with incident nAMD.
Methods
Data Set
The Clinformatics™ Data Mart Database (OptumInsight, Eden Prairie, MN) contains de-identified medical claims of beneficiaries from a large insurance network in the United States. The database contains all outpatient medical claims, claim-associated diagnoses, pharmaceutical prescriptions filled, and demographic data of beneficiaries during their enrollment in the insurance plan. The subset data of interest for this study included all patients diagnosed with nAMD on or after July 2006 to December 2013. The University of Pennsylvania's Institutional Review Board declared this study exempt from review due to the de-identified nature of the data.
Subjects
Beneficiaries greater than or equal to 60 years of age with a new diagnosis of nAMD and at least one anti-VEGF injection were identified. The index date was considered the date of the incident diagnosis. Two cohorts were created for comparison consisting of patients with regular use of BBs or calcium channel blockers (CCB), defined as at least 80% daily coverage for a prescription in the respective class of medication during the observation period and no more than 30 days between refills. The number of intravitreal injections administered to each group was then evaluated after a 1-year observation period following the index date. CCBs were chosen as the comparison group because they have similar medical indications as BBs, but are not known to have any influence on VEGF levels or CNV growth. Exclusion criteria included a previous diagnosis of a disease that could lead to anti-VEGF use (cystoid macular edema, diabetic retinopathy, retinal vein occlusion, or sickle cell), less than 2 years of data prior to the index date, less than one year of data after the index date, or use of the other class of medication during the observation period (including topical BB use in the CCB group).
Main Outcome Measures
The primary outcome of interest was the difference in the mean number of intravitreal injections administered between the two cohorts. Covariates of interest included age, gender, race, year of diagnosis, and associated systemic diseases (history of diabetes mellitus, chronic kidney disease, transient ischemic attack, ischemic heart disease, chronic pulmonary disease, chronic liver disease, peripheral vascular disease, any malignancy, acute venous thrombosis, pulmonary embolism, atrial fibrillation, atrial flutter, chronic heart failure, and acute myocardial infarction). See Table 1 for complete list of ICD-9 codes used during this study.
Table 1. ICD-9 Codes Used in the Study.
| Diagnosis | ICD-9 Code |
|---|---|
| Neovascular AMD | 362.52 |
| Macular edema | 362.07, 362.53, 362.82, 362.83 |
| Proliferative retinopathy | 362.01, 362.02, 362.15, 362.16, 362.29, 364.42, 365.63, 365.89 |
| Sickle cell disease | 282.6 |
| Other retinopathies | 362.2x |
| Vein occlusion | 362.3x |
| Diabetes mellitus | 250.xx |
| Hypertension | 401.xx, 402.xx-405.xx |
| Chronic kidney disease | 582.xx, 583.xx, 585.1-585.4, 585.9, 586.xx, 587, 588.0 |
| Ischemic stroke/transient ischemic attack | 433.x1, 434.x1, 435, 436 |
| Ischemic heart disease | 421, 430 |
| Chronic liver disease | 456.0, 456.1, 456.2x, 572.2-572.8 |
| Chronic pulmonary disease | 416.8, 416.9, 490.x-505.x, 506.4, 508.1, 508.8 |
| Peripheral vascular disease | 093.0, 437.3, 440.x, 441.x, 443.1-443.9, 447.1, 557.1, 557.9, v43.4 |
| Malignancy | 140.x-172.x, 174.x-208.x, 238.6 |
| Acute venous thrombosis | 451.1x, 451.2, 451.81, 451.9, 453.1, 453.2, 453.4x, 453.6, 453.9 451.82, 451.83, 451.84, 451.89, 453.8x |
| Pulmonary embolism | 415.11, 415.12, 415.13, 415.19 |
| Atrial fibrillation | 427.31 |
| Atrial flutter | 427.32 |
| Congestive heart failure | 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4-425.9, 428.xx |
| Myocardial infarction | 410.xx, 411.xx, 412.xx |
| Arrhythmia | 427.xx |
Statistical Analysis
Demographic characteristics were obtained at the index date and analyzed using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Student t-tests were used for comparisons of continuous variables and chi-squared tests were used for categorical variables. An independent two-sample t-test was performed for the unadjusted comparison of the mean number of intravitreal injections between the two cohorts and a multiple linear regression was performed for the covariate-adjusted comparison. Statistical analysis was performed using SAS (version 9.4; SAS Institute Inc., Cary, NC).
Results
After inclusion and exclusion criteria, a total of 394 subjects remained for analysis (Figure 1), which consisted of 239 subjects in the BB group and 155 subjects in the CCB group. The majority of subjects in both groups were female (63.6% BB and 62.6% CCB; p=0.84), white (87.9% BB and 80.0% CCB; p=0.09), and older in age (78.0; SD=6.2 BB and 78.0; SD=5.9 CCB; p=0.94). Common associated systemic diseases other than hypertension in each group were an arrhythmia (44.3% BB and 33.6% CCB; p=0.03), chronic pulmonary disease (41.8% BB and 41.3% CCB; p=0.91), and diabetes (30.96% BB and 29.7% CCB; p=0.79). (See Table 2 for full list of baseline characteristics by cohort).
Figure 1.
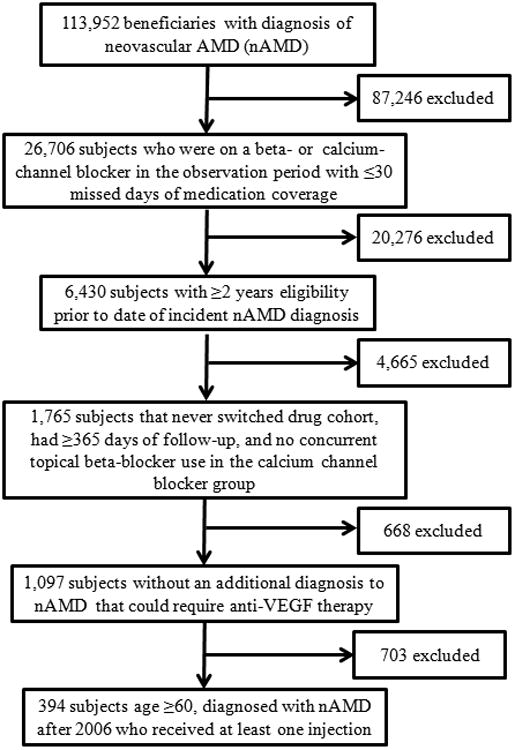
Flow chart demonstrating the number of patients excluded at each criteria and the final inclusion cohort used for the study.
Table 2. Baseline Characteristics of Cohorts.
| Characteristic | Beta-Blocker (n = 239) | Calcium Channel Blocker (n = 155) | P-Value |
|---|---|---|---|
| Age, mean (IQR) | 78 (75 – 82) | 78 (75 – 82) | 0.94 |
| n (%) | n (%) | ||
| Female | 152 (63.6) | 97 (62.6) | 0.84 |
| Race | 0.09 | ||
| White | 210 (87.9) | 124 (80.0) | |
| Black | 6 (2.5) | 10 (6.45) | |
| Hispanic | 7 (2.9) | 10 (6.45) | |
| Asian | 3 (1.3) | 4 (2.6) | |
| Other/unknown | 13 (5.4) | 7 (4.5) | |
| Hypertension | 230 (96.2) | 151 (97.4) | 0.52 |
| Diabetes mellitus | 74 (30.96) | 46 (29.7) | 0.79 |
| Chronic kidney disease | 47 (19.7) | 25 (16.1) | 0.75 |
| Transient ischemic attack | 36 (15.1) | 37 (23.9) | 0.03 |
| Ischemic heart disease | 2 (0.84) | 4 (2.6) | 0.17 |
| Chronic liver disease | 6 (2.5) | 0 (0) | 0.05 |
| Chronic pulmonary disease | 100 (41.8) | 64 (41.3) | 0.91 |
| Peripheral vascular disease | 70 (29.3) | 46 (29.7) | 0.93 |
| Malignancy | 44 (18.4) | 32 (20.7) | 0.66 |
| Acute venous thrombosis | 29 (12.1) | 14 (9.0) | 0.88 |
| Pulmonary embolism | 5 (2.1) | 2 (1.3) | 0.56 |
| Atrial fibrillation | 70 (29.3) | 38 (24.5) | 0.30 |
| Atrial flutter | 13 (5.4) | 8 (5.2) | 0.90 |
| Congestive heart failure | 71 (29.7) | 28 (18.1) | 0.01 |
| Myocardial infarction | 54 (22.6) | 21 (13.6) | 0.03 |
| Arrhythmia | 106 (44.3) | 52 (33.6) | 0.03 |
Univariate analysis revealed that the mean number of injections in the BB group was 6.43 (95% CI 5.90-6.95) versus 6.55 (95% CI 5.85-7.25) in the CCB group, for a mean difference of 0.12 less in the BB cohort (95% CI -0.98-0.74; p=0.78). After multivariate adjustment, the mean number of injections in the BB group was 6.32 (95% CI 5.77-6.87) versus 6.71 (95% CI 6.02-7.40) in the CCB group. The overall mean difference in injections between the two groups was again 0.39 less in the BB cohort (95% CI -1.29-0.51; p=0.40). In multivariate model testing the only disease history that was significantly associated with the number of injections was a history of transient ischemic attack (p<0.01). All other associations tested were not statistically significant (p>0.05).
Discussion
Intravitreal anti-VEGF therapy has shifted the therapeutic paradigm for nAMD4. What was once considered a blinding disease with minimal treatment options for visual rehabilitation has now become a treatable chronic condition. However, significant variability in treatment response continues to pose a major challenge. Reasons for this variability have been attributed to tachyphylaxis, genetic differences, and concurrent topical or systemic medications9-12. Unfortunately for our patients, many of these theories often do not stand up to rigorous clinical inquiry. This study did not find oral BB use to be associated with a decreased number of intravitreal anti-VEGF injections in incident nAMD patients.
Although several basic science and animal studies have argued for a role of BBs in CNV response5-7, the few existing human studies have displayed contradictory results8, 9, 13, 14. First, the findings from the Beaver Dam Eye study14 and Thomas et al.8 argued against a protective effect of oral BB use in developing CNV. They postulated that this discrepancy between human response and animal models may be secondary to higher medication doses and alternate routes of administration in animals8. Next, findings in a small prospective interventional study suggested that topical dorzolamide-timolol administered as adjuvant therapy improved treatment response in nAMD subjects with incomplete response to intravitreal anti-VEGF injections13. The exact pathophysiology for this response is not clear, but may include its potent aqueous suppressant properties, which would theoretically increase the injected anti-VEGF's half-life, prolonging its length of action in the eye13. The effect of the individual drug components also cannot be ignored. The authors theorize that topical BBs may result in higher intraocular concentrations than oral formulations, improving ocular bioavailability and potentially enhancing its effect13.
Finally, Montero et al.9 demonstrated that those taking BBs were found to have received 2.7 less injections over roughly 2 years compared to non-users. Several differences exist between that study and the current study, which may explain the contradicting results. First, and likely most pertinent, the previous study used a control group made up of non-users of BBs. This may have introduced an indication bias into their study, in which the results being attributed to the BBs were actually due to the underlying indications for the BB usage. Our study attempted to address this bias by selecting a comparator medication class with similar disease indications for use. Additionally, we further controlled for many of these medical indications beyond just hypertension in the multivariate analysis. Lastly, despite 2 years of treatment, the BB group in the previous study only received 5.5 bevacizumab injections. This is far less than the 14.1 average seen over 2 years in the pro re nata (PRN) bevacizumab arm of the Comparison of AMD Treatments Trials (CATT), suggesting that there may be some underlying differences in the study population compared to other nAMD patients15.
In addition to those already mentioned, several other strengths to our study exist. The patients for this study were drawn from a database that is comprised of all beneficiaries enrolled in a large national insurance network in the United States, not those simply treated by one physician or office. This, in addition to the availability of sociodemographic and detailed medical claims data, allowed us to control for multiple potentially confounding variables yet still maintain a meaningful sample size. Furthermore, pharmacy records rather than patient report determined medication usage, eliminating any component of recall bias.
Several study limitations should also be noted. First, we are unable to identify recommended treatment patterns based on claims data (i.e. monthly, PRN, or treat and extend). However, in order for this limitation to impact the reported results, these patterns would have needed to be different based on BB or CCB usage, which is unlikely to have occurred. Also, to further control for potential changes in types of treatment patterns over time, we included year of diagnosis into the model, which showed no association with number of injections. Next, despite the national cohort used to find study patients, the database consists of beneficiaries from one insurance network, which may not generalize to other patient populations. Additionally, diagnoses were based purely on ICD-9 codes from medical claims data and are unable to be confirmed by review of medical records. Furthermore, the drug cohorts were chosen carefully for their similar medical indications of use, but the exact indication for the BB or CCB use could not be identified from the database. Similarly, this study abstracted diagnoses by patient and not individual eye, meaning an exact number of patients receiving bilateral treatment was unable to be calculated. This limitation would only have impacted the outcome if significantly more BB patients had bilateral disease than CCB patients, which although possible, is unlikely. Also, our study only examined 1 year of injections. It is possible that with a longer duration of observation a differential effect in medication class could be seen. Finally, we assume medication compliance based on pharmacy data and prescription refill dates, but this cannot be further verified.
With the large number of patients and injections included in the study we had an 80% power to detect an effect difference of 1.1 injections between the two study cohorts, which our data clearly did not demonstrate. Although our study was not powered to detect smaller differences, an argument could be made that any effect that reduces injections by less then 1 injection per year is not likely to be clinically significant, even if a higher powered study could find a statistically significant difference.
In conclusion, this study did not find a difference in the mean number of intravitreal anti-VEGF injections administered to incident nAMD subjects taking either oral beta- or calcium channel blockers over a 1-year observation period. Additional studies are warranted to investigate other modifiable differences that may contribute to response variations in patients receiving anti-VEGF injections for nAMD.
Acknowledgments
Financial Support: National Institutes of Health K23 Award (1K23EY025729 - 01) and University of Pennsylvania Core Grant for Vision Research (2P30EY001583-41). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Funding from each of the above sources was received in the form of block research grants to the Scheie Eye Institute. None of the organizations had any role in the design or conduction of the study
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Portions of this data have been submitted for presentation at the 2016 Annual Retina Society Meeting
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, De Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Virgili G, Bini A. Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD004763.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Wormald R, Evans J, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD002030.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal Bevacizumab Versus Ranibizumab for Treatment of Neovascular Age-Related Macular Degeneration: Findings from a Cochrane Systematic Review. Ophthalmology. 2016;123:70–7. doi: 10.1016/j.ophtha.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campa C, Costagliola C, Incorvaia C, Sheridan C, Semeraro F, De Nadai K, Sebastiani A, Parmeggiani F. Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/546826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099–104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- 7.Lavine JA, Sang Y, Wang S, Ip MS, Sheibani N. Attenuation of choroidal neovascularization by β2-adrenoreceptor antagonism. JAMA ophthalmology. 2013;131:376–82. doi: 10.1001/jamaophthalmol.2013.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas AS, Redd T, Hwang T. Effect of systemic beta-blockers, ace inhibitors, and angiotensin receptor blockers on development of choroidal neovascularization in patients with age-related macular degeneration. Retina. 2015;35:1964–8. doi: 10.1097/IAE.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 9.Montero JA, Ruiz-Moreno JM, Sanchis-Merino E, Perez-Martin S. Systemic beta-blockers may reduce the need for repeated intravitreal injections in patients with wet age-related macular degeneration treated by bevacizumab. Retina. 2013 Mar 1;33:508–12. doi: 10.1097/IAE.0b013e3182695ba0. [DOI] [PubMed] [Google Scholar]
- 10.Brantley MA, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114:2168–73. doi: 10.1016/j.ophtha.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Heimes B, Lommatzsch A, Zeimer M, Gutfleisch M, Spital G, Bird AC, Pauleikhoff D. Foveal RPE autofluorescence as a prognostic factor for anti-VEGF therapy in exudative AMD. Graefe's Archive for Clinical and Experimental Ophthalmology. 2008;246:1229–34. doi: 10.1007/s00417-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 12.Tranos P, Vacalis A, Asteriadis S, Koukoula S, Vachtsevanos A, Perganta G, Georgalas I. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneration. Drug Des Devel Ther. 2013 Jan 1;7:485–90. doi: 10.2147/DDDT.S43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sridhar J, Hsu J, Shahlaee A, Garg SJ, Spirn MJ, Fineman MS, Vander J. Topical Dorzolamide-Timolol With Intravitreous Anti–Vascular Endothelial Growth Factor for Neovascular Age-Related Macular Degeneration. JAMA ophthalmology. 2016;134:437–43. doi: 10.1001/jamaophthalmol.2016.0045. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Myers CE, Klein BE. Vasodilators, blood pressure-lowering medications, and age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology. 2014 Aug 31;121:1604–11. doi: 10.1016/j.ophtha.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012 Jul 31;119:1388–98. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]


