Abstract
Objective
Women’s risk of obstructive sleep apnea (OSA) increases substantially during and after the menopause transition, when depression risk is also elevated, raising the possibility that estrogen withdrawal contributes to OSA vulnerability, in turn contributing to mood disturbance. We examined the association between estradiol levels and OSA in depressed peri- and postmenopausal women.
Methods
Thirty depressed peri/postmenopausal women (mean BMI 30.82 kg/m2) without known OSA completed routine polysomnography concurrent with serum estradiol levels. Estradiol in women with apnea-hypopnea indices (AHI)≥15 indicating moderate-to-severe OSA was compared against those with AHI <15 using logistic regression adjusting for age and body mass index (BMI).
Results
Thirteen women (43%) had AHI≥15 (median AHI 21.6). Estradiol levels were lower (p=0.02) in those with OSA (median 19, interquartile range [IQR] 9–25, pg/ml) than without OSA (median 29, IQR 19–66, pg/ml). On univariate analysis, higher estradiol was associated with reduced odds of OSA (odds ratio [OR] 0.95, 95% confidence interval [CI] 0.90–0.99, p=0.04). After adjusting for age and BMI, E2 levels remained associated with lower odds of OSA (OR 0.90), but the association was no longer statistically significant (95% CI 0.76–1.05, p=0.18). Montgomery Åsberg Depression Rating Scale scores did not differ between those with and without OSA.
Conclusions
These preliminary results suggest that, in addition to higher BMI and age, lower estradiol may be associated with increased OSA risk in depressed women during the peri- and postmenopause, raising the possibility that estradiol withdrawal associated with menopause influences upper-airway patency in women.
Keywords: sleep apnea, estradiol, menopause, depression, women
INTRODUCTION
Menopause is a period of increased risk for the development of sleep problems and mood disturbance. Sleep complaints increase from 12% in women younger than age 44 to 40% in women during the late 40s and early 50s.1–3 A common and under-recognized cause of such sleep complaints is obstructive sleep apnea (OSA). OSA is defined by repetitive episodes of complete (apnea) or partial (hypopnea) airway obstruction that are associated with transient oxygen desaturations and brief arousals from sleep. OSA leads to snoring and sleep fragmentation, which in turn can result in excessive daytime sleepiness, cognitive deficits, and mood disturbance.4 While often undiagnosed and not treated,5 it is important to identify and treat OSA because this condition can result in important health problems such as clinical depression, diabetes, cardiovascular disease, heart failure, hypertension, and stroke.1,4,6
While OSA is 2–3 times more prevalent in men than in women before age 50, this sex difference decreases with age because of the increased prevalence of OSA in women during and after the menopause transition.6–8 However women may be less likely to be referred for evaluation and diagnosed with OSA because they can present with a different symptom profile.4,5 Women are less likely to have witnessed apneic events and often have symptoms that are less clearly linked to OSA, such as insomnia and depression.4,6,9 For women, the risk of OSA is elevated during pregnancy, in those with polycystic ovarian syndrome, during the late menopause transition, and in the postmenopause.5,10–12 Other risk factors for OSA in women are increasing age, body mass index (BMI), waist circumference, and anatomical changes in the upper airway. Epidemiologic studies show that, relative to premenopausal women, women in the menopause transition and recent postmenopause are 3 times more likely to have OSA,12–14 after controlling for known OSA risk factors of increasing age and higher BMI.5 This observation raises the possibility that lower levels of estradiol might contribute to the increased prevalence of OSA during the menopause transition and early postmenopause.
Women with OSA are more likely than men with OSA to have depression as a presenting symptom.9 The prevalence of depression is also increased15,16 during the menopause transition and early postmenopause, and depressive symptoms are strongly associated with variability and withdrawal of estradiol. Depression is closely linked with sleep disturbance, including in this midlife population.17,18 Therefore it is plausible that changes in female reproductive hormones underlie the increased vulnerability to both OSA and depression during the menopause transition and early postmenopause. As such, this preliminary study examines the association between serum estradiol levels and OSA in peri- and early postmenopause women with depressive disorders to test the hypothesis that lower estradiol levels are linked with greater likelihood of OSA in depressed peri- and postmenopausal women.
METHODS
Data for this analysis derive from screening procedures completed as part of two clinical trials17 focused on 40–62 year-old women who (a) were in the menopause transition and postmenopause and (b) met criteria for a unipolar depressive disorder. Of the 30 women in this analysis, 23 were screened for one trial,17 and 7 were screened for the second clinical trial. Participants in this study comprise 20% and 29% of women enrolled in the first and second study, respectively. Details of those studies are available elsewhere.17 Briefly, women with untreated mild-to-moderate depressive symptoms and no known history of sleep apnea who appeared eligible for the studies provided a blood sample for estradiol and completed a polysomnographic (PSG) study to screen for OSA, which was a key exclusion criterion for the studies. Potential participants were recruited from hospital patients and the community using fliers and approved email circulars. All women who completed these procedures were included in this analysis, regardless of whether they met criteria for OSA or not. Serum estradiol levels in women who had an apnea-hypopnea index (AHI) of ≥15 on the PSG indicating at least moderate OSA5 were compared against those with an AHI <15. All study procedures were approved by the Partners HealthCare Institutional Review Board and completed at Massachusetts General Hospital between 2005 and 2010 after the women provided written informed consent.
Participants
Eligibility criteria for both of these studies were the same. Women were eligible to participate in these studies if they met the following criteria: 1) 40–62 years of age, 2) menopause transition or postmenopausal, as defined by the STRAW menstrual bleeding criteria,20 3) met criteria for a major depressive episode (MDE), dysthymia, or a minor depressive episode, 4) were experiencing mild to moderate depressive symptoms, as indicated by a score of 15–31 on the Montgomery Åsberg Depression Rating Scale (MADRS, range 0–60, higher score worse),21 and 4) in good general health. Five women who had undergone a hysterectomy without oophorectomy were classified as postmenopausal because they were ≥48 years, their serum estradiol levels were non-detectable, and their FSH levels were >50 IU/L. Exclusion criteria included: 1) known sleep disorder, including OSA; 2) psychiatric illness, including severe depression (MADRS score >31), bipolar disorder, and alcohol or substance-use disorder; 3) current or recent use of centrally active medication including antidepressants, anticonvulsants, or hypnotics; 4) use of systemic hormone medications in the past 3 months; 5) pregnancy; and 6) clinically significant abnormalities in screening laboratory studies. Additional inclusion criteria for one of the studies17 included the presence of hot flashes and sleep disturbance related to hot flashes.
Measures
Results of a single PSG study obtained for screening purposes was used for this analysis. All were conducted at Brigham and Women’s Hospital Sleep Health Centers in Boston and scored by registered polysomnographic technicians. Standard American Academy of Sleep Medicine (AASM)-recommended channels were recorded, including EEG, EOG, EMG, respiration (nasal pressure, oral thermistry, oxygen saturation [SaO2], and chest-abdominal motion), EKG, body position, snoring, and bilateral anterior tibialis electromyography. Respiratory events were scored using the Chicago criteria (AASM 1999). An AHI score ≥15 indicating at least moderate OSA was used to define the subgroup that had clinically meaningful levels of sleep apnea (n=13, 43%).
Serum estradiol levels were obtained at the screening visit, which was scheduled within a median of 6 days from the overnight PSG laboratory study. Blood draws were not menstrually timed in women undergoing the menopause transition. Estradiol was assayed using either immunoassay or liquid chromatography-mass spectrometry. For one study (n=23),17 serum samples were analyzed using an automated, random access, microparticle enzyme immunoassay (AxSYM; Abbott Diagnostics, Inc, Abbott Park, IL) with an analytical sensitivity of 20 pg/mL, intra-assay coefficient of variation (CV) 2.1–4.5% and inter-assay CV 6.5–9.6%. For the other study (n=7), serum samples were analyzed using liquid chromatography-mass spectrometry (Mayo Clinic, Rochester, NY) with an analytical sensitivity of 10 pg/ml using an inter-assay CV of 8.6%.22 Given these detection limits, serum estradiol was assigned to be 19pg/mL (n=8) and 9 pg/mL (n=5) for assays with analytic sensitivity of 20 pg/mL and 10 pg/mL, respectively. In sensitivity analyses, estradiol levels <20 pg/mL obtained in the study with the more sensitive assay were assigned to 19 pg/mL. The prevalence of OSA was not associated with enrollment in a particular study (p=0.19).
Statistical Analysis
The correlation between the AHI and serum estradiol levels was analyzed using the Spearman correlation coefficient and the association between estradiol levels and OSA was analyzed using the non-parametric Wilcoxon rank sum test. Logistical regression models were built using OSA as the dependent measure and serum estradiol levels as the primary independent measure. Multivariate models were built to adjust for age and BMI, which are known predictors for OSA. Statistical significance was assumed at the two-sided α = 0.05 level. Parallel approaches were used for secondary analyses using estradiol 19 pg/mL for all women with estradiol levels <20 pg/mL. Descriptive data are reported on the proportion of apneic and hypopneic events occurring in rapid eye movement (REM) and non-REM and, similarly, when women were lying supine vs. non-supine among the subgroup of participants with AHI≥15, after standardizing to the amount of time spent in REM vs. non-REM and supine vs. non-supine.
RESULTS
Participant Characteristics
The mean age of all 30 women was 53.7 years (SD 4.4 years). Twenty-three (77%) were postmenopausal (median time from final menstrual period 66 months) and 7 (23%) women were in the menopause transition (4 late, 3 early transition). Serum estradiol levels for the group overall (median 24.5, IQR 19−53 pg/ml) were above the detectable threshold for the assay used in 17 (57%) participants. Undetectable levels were observed in 8 (35%) and 5 (71%) of those in the study with an estradiol threshold of 19 pg/ml and 9 pg/ml, respectively. Levels were higher among women in the menopause transition (median 48, IQR 19−84 pg/mL) than in the postmenopause (median 23, IQR 19–33 pg/ml, p=0.08). Approximately half of the women were obese (53%, BMI ≥30 kg/m2) while the remainder were overweight (37%, BMI 25−29.9 kg/m2) or of normal weight (10%, BMI 18.5−24.9 kg/m2). 67% of the women included in this study met criteria for MDE, 23% for dysthymia, and 10% for a minor depressive episode. The median AHI was 13.1 (IQR 6.9−19.1).
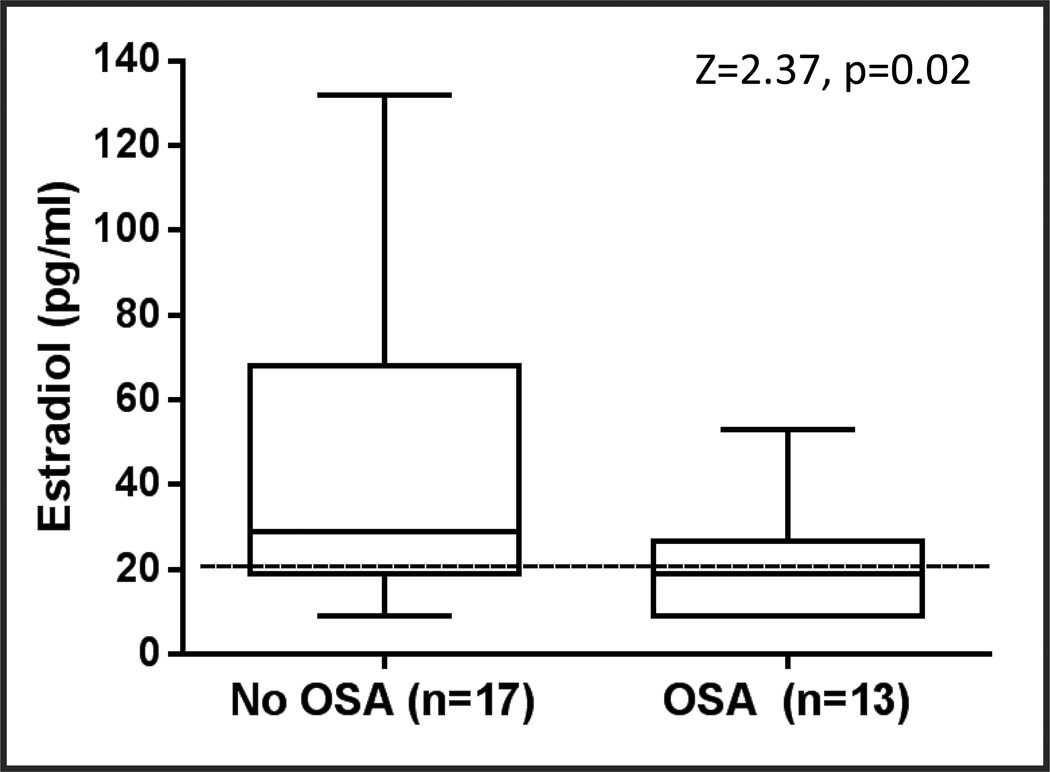
AHI scores were ≥15 in 13 (43%) women, whose median AHI was 21.6 (IQR 16.5−33.4). For those without OSA (AHI <15), the median AHI was 7.6 (IQR 4.1−10.2). Table 1 shows study characteristics of the group with and without OSA. Estradiol levels were lower (p=0.02; Figure 1) in those with OSA (median 19, interquartile range [IQR] 9–25, pg/ml) than without OSA (median 29, IQR 19–70, pg/ml). Women with OSA were more likely to be overweight or obese than those without OSA (p=0.01). The groups differed significantly in age (p<0.001) with women in the OSA group being older than those in the no OSA group. There were no differences between those with and without OSA in race, menopause status, or depressive disorder diagnosis. Median depressive symptom scores on the MADRS did not differ between OSA groups (21, interquartile range [IQR] 18−25, vs. 20, IQR 17−23, p=0.61).
Table 1.
Characteristics of Depressed Peri- and Postmenopausal Women According to Presence or Absence of Obstructive Sleep Apnea (OSA) based on Apnea-hypopnea Index (AHI) ≥15
| All Women (N = 30) |
No OSA (N = 17) |
OSA (N = 13) |
|
|---|---|---|---|
| Age, years (Median, IQR)a | 54 (51–57) | 52 (49–54) | 57 (54–59) |
| Race/Ethnicity, N (%) | |||
| White | 18 (60.0) | 11 (64.7) | 7 (53.8) |
| African-American | 10 (33.3) | 4 (23.5) | 6 (46.2) |
| Other | 2 (6.7) | 2 (11.8) | 0 (0.0) |
| Body Mass Index (BMI), kg/m2, N (%)a | |||
| Normal (BMI 18.5–24.9) | 3 (10.0) | 3 (17.6) | 0 (0.0) |
| Overweight (BMI 25–29.9) | 11 (36.7) | 8 (47.1) | 3 (23.1) |
| Obese (BMI ≥ 30) | 16 (53.3) | 6 (35.3) | 10 (76.9) |
| Menopausal Status, N (%) | |||
| Perimenopausal | 7 (23.3) | 5 (29.4) | 2 (15.4) |
| Postmenopausal | 23 (76.7) | 12 (70.6) | 11 (84.6) |
| Apnea Hypopnea Index (Median, IQR)a | 13.1 (6.9–19.1) | 7.6 (4.1–10.2) | 21.6 (16.5–33.4) |
| Estradiol, pg/ml (Median, IQR)a,b | 25 (19–53) | 29 (19–70) | 19 (9–25) |
| ≤ 19 pg/ml, N (%) | 13 (43%) | 5 (29%) | 8 (62%) |
| Depressive Disorders, N (%) | |||
| Major Depressive Episode | 20 (66.7) | 10 (58.8) | 10 (76.9) |
| Dysthymia | 7 (23.3) | 1 (5.9) | 2 (15.4) |
| Minor Depressive Episode | 3 (10.0) | 6 (35.3) | 1 (7.7) |
| Perceived Sleep Quality Index score, Median (IQR)c | 12 (10–15) | 11 (9–14) | 15 (10–16) |
| Daily Hot Flash frequency, Median (IQR)d | 5.8 (4.0–10.0) | 6.0 (3.1–15.4) | 5.3 (4.2–10.0) |
Continuous variables presented as median and interquartile range (IQR). Categorical data presented as N (%).
p <0.05 for those with vs. without OSA.
Serum estradiol was assigned to be 19pg/mL (n=8) and 9 pg/mL (n=5) for assays with analytic sensitivity of 20 pg/mL and 10 pg/mL, respectively.
Data available for 22 women (No OSA, n=15; OSA n=7)
Data calculated from average number of hot flashes per day reported on a 7-day diary were available for 25 women (No OSA, n=14; OSA n=11).
Figure 1.
Serum estradiol levels in 30 women with depression differed significantly in those with and without obstructive sleep apnea (OSA, defined as AHI ≥15)
Analyses conducted using Wilcoxon rank sum test.
Regression analyses
The odds of meeting criteria for moderate-to-severe OSA was reduced by 5% for every 1 pg/ml increase in the estradiol level (OR 0.95, 95% CI 0.90−0.99, p=0.04). Although a greater proportion of postmenopausal women than of those in menopause transition had OSA (47.8% vs. 28.5%, respectively), menopause status was not significantly associated with OSA (p=0.38). Models restricted to postmenopausal women (n=23) were consistent, showing a marginal protective association between higher estradiol and odds of OSA (OR 0.95, 95% CI 0.90−1.01, p=0.11). Estradiol tended to be lower in those with compared to those without OSA (median estradiol 19 pg/ml vs. 26 pg/ml in OSA vs. non-OSA, p=0.08).
AHI was positively correlated with higher BMI (rs=0.48, p=0.01) and increasing age (rs=0.40, p=0.03). Bivariate logistical regression analyses with moderate-to-severe OSA as the dependent measure revealed that lower estradiol (OR 0.91, 95% CI 0.84−0.99, p=0.04) and higher BMI (OR 1.46, 95% CI 1.07−1.99, p=0.02) were significantly associated with OSA. In parallel bivariate logistical regression analyses adjusting for both estradiol and age, age (OR 1.39, 95% CI 1.03−1.89, p=0.03), but not estradiol levels (OR 0.96, 95% CI 0.91–1.01, p=0.16) was significantly associated with OSA. Multivariate analyses adjusting simultaneously for estradiol, age, and BMI similarly show that estradiol levels were not significantly associated with OSA (OR 0.90, 95% CI 0.76−1.05, p=0.18), while older age (OR 2.29, 95% CI 1.09−4.80, p=0.03) and higher BMI (OR 2.34, 95% CI 1.11−4.95, p=0.03) were significant correlates.
Exploratory analyses
Results of secondary analyses substituting estradiol 19 pg/mL for all estradiol levels <20 pg/mL were consistent with primary analyses (OR 0.95, 95% CI 0.90−1.00, p=0.055). In additional exploratory analyses, the REM:non-REM AHI ratio was 1.97 and the supine:non-supine AHI ratio was 1.53, after accounting for the proportion of time spent in REM vs. non-REM sleep and in the supine vs. non-supine position, respectively.
DISCUSSION
Results of this preliminary study provide initial evidence that undiagnosed obstructive sleep apnea occurs commonly in women with depression who are in the menopause transition and early postmenopause and suggest that lower levels of estradiol are associated with moderate-to-severe OSA in this population. The likelihood of having OSA was high (43% OSA prevalence) but not linked with severity of depressive symptoms. Given the cross-sectional analyses and the attenuation of the association after adjustment for age and BMI, we cannot conclude that lower estradiol levels are definitively associated with OSA. Because advancing age is associated with both declining estradiol levels and increasing BMI, our results suggest a need to further consider the roles of reproductive hormones in the context of BMI and age in modulating the vulnerability to OSA that emerges during the late menopause transition and postmenopause hormonal transition periods. Increasing age might also contribute to susceptibility to OSA through increases in weight gain, upper airway collapsibility, and sleep fragmentation. Taken together, these preliminary findings suggest that, in midlife women with depression, there is a high prevalence of undiagnosed OSA that is accompanied by lower estradiol levels.
While withdrawal of reproductive hormones has been hypothesized to contribute to the increased risk of OSA in women during the late menopause transition and postmenopause, few studies have examined the relationship between estradiol levels and OSA.23,24 Results of one small study of women referred to a sleep clinic for suspected OSA showed that estradiol levels were lower in 20 postmenopausal women with OSA as contrasted with 6 postmenopausal women without OSA (mean 12.9 vs. mean 30.8 pg/ml).23 Age and BMI were not statistically different between those with and without OSA, but their study results were not statistically adjusted for age or BMI. Our results are consistent with this earlier study, and extend the findings to a distinct population of women who had clinical depression. Our results are also consistent with studies demonstrating an inverse relationship between estradiol and OSA, which is indirectly suggested by reports that postmenopausal women using hormone therapy have a lower prevalence of OSA than those not taking hormone therapy.13,25 In contrast, findings from intervention studies using estrogen withdrawal or replacement suggest that there is no clear association between estradiol levels and OSA.24,26–29 Estradiol withdrawal did not induce OSA in a study of 12 healthy premenopausal women evaluated after 5 weeks of hypo-estrogenism precipitated by a gonadotropin releasing hormone agonist. In addition, results of studies administering estrogen therapy to postmenopausal women who have OSA are mixed, with several studies reporting that estrogen with or without progesterone has favorable effects on some, but not all, OSA symptoms and measures,26–28 while others found no significant improvement in OSA.24,27,28 While results of these intervention studies suggest that the link between estradiol levels and OSA is tenuous, it is notable that the interval in which the estrogen withdrawal or replacement occurred was limited to 5 and a maximum of 24 weeks, respectively. 24,26–29 It is plausible that any contribution of reduced estradiol levels to OSA vulnerability may not accrue until there is a substantial period of exposure to hypo-estrogenism.
Previous studies have linked OSA and reduced upper-airway activation with reduced levels of both estradiol and progesterone, and several hypotheses about the mechanisms through which reductions in female gonadal steroids are linked with OSA have been proposed.7 Results of a physiologic study conducted in women without OSA showed that activation of the pharyngeal dilating genioglossal muscle is lowest in postmenopausal women and lower in the mid-follicular than the mid-luteal phase among premenopausal women,30 which could render the pharyngeal airway more vulnerable to collapse during sleep. In contrast, upper airway resistance was not influenced by menopause status or gonadal steroid levels.30 While a limitation of our study was that progesterone was not measured, progesterone levels are exceedingly low in postmenopausal hypo-estrogenic women due to anovulation. It is also plausible that estradiol and progesterone may affect ventilatory control stability or the arousal threshold, but this hypothesis was not examined.
Like other populations of midlife women, our peri/postmenopausal study sample has a greater proportion of OSA events during REM than do men.31,32 We also observed our midlife female population to have a disproportionately greater number of apneic and hypopneic events in the supine position, consistent with studies in post-menopausal women and in men,32 and higher than that in younger women.33 Our findings add to the literature showing that, like men, postmenopausal women with OSA are more position sensitive and have more apneic events lying on their back.
We identified moderate-to-severe OSA in 43% of midlife women seeking treatment for an episode of depression. This prevalence estimate is consistent with that in other clinic-based estimates in depressed individuals (median 48% prevalence, range 0–66%).34 We focused on moderate-to-severe OSA because this OSA threshold has been most strongly associated with important health outcomes. In addition, limited statistical power precluded use of a more liberal threshold that included women with much milder OSA (AHI≥5; n=24, 80% of sample). Levels of depressive symptoms did not differ between those with and without OSA, but our study design limited our ability to discriminate because the MADRS range was restricted to 15–31 for study eligibility, limiting our ability to assess the association with the full spectrum of depressive symptoms. However, the high prevalence of OSA highlights the importance of OSA screening in peri/postmenopausal women with depression who present with depression during midlife, particularly those who are obese or overweight. Approximately half of our study population was obese. Higher BMI and body fat in the neck region specifically are linked with OSA in women.35 However, higher BMI and body fat are linked with higher estradiol levels in postmenopausal women,36–38 highlighting the complex relationship of OSA with BMI and estradiol levels that warrant further exploration.
While limited by small sample size and a single cross-sectional measure of estradiol, this study provides preliminary evidence warranting further investigation in women because of the potential to identify women susceptible to both OSA and depression episodes during the menopause transition and postmenopause. The largely obese study population might also limit generalizability of our findings to the women with a lower mean BMI. The cumulative detrimental effects of OSA and depression, often in combination with increasing BMI, can lead to sleepiness, lethargy, and social withdrawal that perpetuates poor health and well-being in aging women.
CONCLUSION
These preliminary results suggest that risk factors for OSA in depressed women during the menopause transition and postmenopause may include lower estradiol levels as well as age and BMI. Although our study was not sufficiently large to disentangle the independent contributions of these factors, our findings raise the possibility that estradiol withdrawal associated with increasing age during the menopause transition and postmenopause may influence the predisposition to OSA in women. Women are more likely to experience depression in general and to have mood disturbance as a presenting symptom of an undiagnosed OSA, emphasizing the importance of screening for OSA in midlife women with depression who may or may not have other OSA features (e.g., sleepiness) or that is non-responsive to traditional interventions for depression. These studies have important implications for the diagnosis and treatment of co-morbid sleep disorders in midlife women with depression.
Acknowledgments
The authors would like to acknowledge the assistance of Aleta Wiley, MPH, for statistical consultation.
Dr. Joffe has received research funding from Merck and Cephalon/Teva and is a consultant/advisor to Mitsubishi-Tanabe, Merck, Noven, and NeRRe Therapeutics. Dr. Redline has received research funding from Jazz Pharmaceuticals. Dr. White is the Medical Officer for Apnicure Inc, a consultant for Phillips Repironics, and on the advisory board for NightBalance.
Source of Funding: This research was supported by the National Institute of Mental Health through grants to HJ: K23MH066978 (clinicaltrials.gov #: NCT01126801) and R01MH082922 (clinicaltrials.gov #: K23 NCT00227942).
Footnotes
Conflict of Interest/Disclosure Information: TG, JC, SK, and KS have no conflicts of interest to disclose.
REFERENCES
- 1.Joffe H, Massler A, Sharkey KM. Evaluation and Management of Sleep Disturbance during the Menopause Transition. Semin Reprod Med. 2010 Sep;28(5):404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chumlea WC, Schubert CM, Roche AF, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003 Jan;111(1):110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 3.Cirignotta F, Mondini S, Zucconi M, Lenzi PL, Lugaresi E. Insomnia: an epidemiological survey. Clin Neuropharmacol. 1985;8(Suppl 1):S49–S54. doi: 10.1097/00002826-198508001-00007. [DOI] [PubMed] [Google Scholar]
- 4.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. American journal of epidemiology. 2013 Jul 1;178(1):70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006 Mar-Apr;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. American journal of respiratory and critical care medicine. 1994 Mar;149(3 Pt 1):722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 7.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep medicine reviews. 2008 Dec;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003 May 7;289(17):2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 9.Georgakis MK, Thomopoulos TP, Diamantaras A, Kalogirou EI, Skalkidou A, Daskalopoulou SS, Petridou ET. Age at menopause and duration of reproductive period in association with depression in postmenopausal women: a systematic review and meta-analysis. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2015.2653. In press. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004 Apr 28;291(16):2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 11.Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001 Mar;86(3):1175–1180. doi: 10.1210/jcem.86.3.7316. [DOI] [PubMed] [Google Scholar]
- 12.Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001 Jul;120(1):151–155. doi: 10.1378/chest.120.1.151. [DOI] [PubMed] [Google Scholar]
- 13.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. American journal of respiratory and critical care medicine. 2001 Mar;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine. 2003 May 1;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010 Jul;17(4):823–827. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 16.Bromberger JT, Kravitz HM. Mood and menopause: findings from the Study of Women's Health Across the Nation (SWAN) over 10 years. Obstetrics and gynecology clinics of North America. 2011 Sep;38(3):609–625. doi: 10.1016/j.ogc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011 Apr 27; doi: 10.1210/jc.2010-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joffe H, Soares CN, Thurston RC, White DP, Cohen LS, Hall JE. Depression is associated with worse objectively and subjectively measured sleep, but not more frequent awakenings, in women with vasomotor symptoms. Menopause. 2009 Jul-Aug;16(4):671–679. doi: 10.1097/gme.0b013e3181957377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effects of Estrogen and Hot Flashes in Postmenopausal Women Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01126801.
- 20.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012 Apr;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 22.Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013 Dec;36(12):1977–1985. doi: 10.5665/sleep.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netzer NC, Eliasson AH, Strohl KP. Women with sleep apnea have lower levels of sex hormones. Sleep & breathing = Schlaf & Atmung. 2003 Mar;7(1):25–29. doi: 10.1007/s11325-003-0025-8. [DOI] [PubMed] [Google Scholar]
- 24.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994 Jul;49(7):699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. American journal of respiratory and critical care medicine. 2003 May 1;167(9):1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 26.Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J Appl Physiol. 1989 Apr;66(4):1656–1661. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- 27.Hachul H, Bittencourt LR, Andersen ML, Haidar MA, Baracat EC, Tufik S. Effects of hormone therapy with estrogen and/or progesterone on sleep pattern in postmenopausal women. Int J Gynaecol Obstet. 2008 Dec;103(3):207–212. doi: 10.1016/j.ijgo.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Polo-Kantola P, Rauhala E, Helenius H, Erkkola R, Irjala K, Polo O. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstet Gynecol. 2003 Jul;102(1):68–75. doi: 10.1016/s0029-7844(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 29.D'Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005 Dec;2(4):238–245. doi: 10.1016/s1550-8579(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 30.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998 Mar;84(3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. American journal of respiratory and critical care medicine. 2000 May;161(5):1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqui F, Walters AS, Goldstein D, Lahey M, Desai H. Half of patients with obstructive sleep apnea have a higher NREM AHI than REM AHI. Sleep medicine. 2006 Apr;7(3):281–285. doi: 10.1016/j.sleep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Mohsenin V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep medicine. 2003 Nov;4(6):523–529. doi: 10.1016/s1389-9457(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 34.Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2015 Feb 15;11(2):165–175. doi: 10.5664/jcsm.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. Apr 1;33(4):467–474. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liedtke S, Schmidt ME, Vrieling A, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring Md) 2012 May;20(5):1088–1095. doi: 10.1038/oby.2011.383. [DOI] [PubMed] [Google Scholar]
- 37.Jones ME, Schoemaker M, Rae M, et al. Changes in estradiol and testosterone levels in postmenopausal women after changes in body mass index. J Clin Endocrinol Metab. 2013 Jul;98(7):2967–2974. doi: 10.1210/jc.2013-1588. [DOI] [PubMed] [Google Scholar]
- 38.Baglietto L, English DR, Hopper JL, et al. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009 May;115(1):171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]