Summary
Hermansky-Pudlak syndrome (HPS) encompasses disorders with abnormal function of lysosomes and lysosome-related organelles, and some patients who develop immunodeficiency. The basic mechanisms contributing to immune dysfunction in HPS are ill-defined. We analysed Natural Killer (NK) cells from patients diagnosed with HPS-1, HPS-2, HPS-4, and an unreported HPS subtype. NK cells from an HPS-2 and an unreported HPS subtype share a similar cellular phenotype with defective granule release and cytotoxicity, but differ in cytokine exocytosis. Defining NK cell activity in several types of HPS provides insights into cellular defects of the disorder and understanding of mechanisms contributing to HPS pathogenesis.
Keywords: Hermansky-Pudlak syndrome, NK cell, cytotoxicity, lytic granule exocytosis, immune deficiency
Hermansky-Pudlak syndrome (HPS) is a group of disorders with abnormal function of lysosomes and lysosome-related organelles (Huizing et al, 2008). To date, 10 subtypes of HPS have been described in humans, with mutations in HPS1, AP3B1 (HPS2), HPS3-HPS6, DTNBP1 (HPS7), BLOC1S3 (HPS8), BLOC1S6 (HPS9) and AP3D1 genes identified as causative of HPS-1–10, respectively. In general, patients with HPS do not develop clinical manifestations associated with immunological deficiency, except for patients with HPS-2 and HPS-10 who are susceptible to recurrent infections (Huizing et al, 2002; Ammann et al, 2016), and HPS-2 patients developing haemophagocytic lymphohistiocytosis (Jessen et al, 2013). The cellular processes and mechanisms contributing to immune dysfunction in HPS-2 remain elusive, although they are probably associated with impaired function of cytotoxic T cells and Natural Killer (NK) cells.
NK cells are lymphocytes involved in both innate and adaptive immune responses, best known for their ability to mediate cytotoxic elimination of abnormal or overactive cells (Krzewski & Coligan 2012). The killing of target cells is a multi-stage process, culminating in the localized secretion of lytic granules (secretory lysosomes), containing perforin and granzymes, at the immunological synapse (Krzewski & Coligan 2012). Given that NK cells require lysosomes to mediate target cell killing, and defective NK cell cytotoxicity has been postulated to be one of the major factors contributing to the progression of haemophagocytic syndromes (Krzewski & Coligan 2012), NK cells are a suitable model to study basic mechanisms of disease in HPS.
A total of 15 subjects with HPS were studied: 12 patients with HPS-1, 1 with HPS-2, 1 with HPS-4, and 1 with an unknown HPS subtype (Table S1). The latter patient had clinical features of HPS, but genetic testing did not reveal any mutations in genes associated with HPS, suggesting a new HPS subtype; we classified this patient as HPS-new. All patients had oculocutaneous albinism; 7 of 10 patients with HPS-1 and the HPS-2 patient had pulmonary fibrosis. None of the patients had a history of unusual infections or tumourigenesis except the subject with HPS-2, who was previously reported to have a history of severe infections (Huizing et al, 2002; Gochuico et al, 2012). Detailed Materials and Methods are described in Data S1.
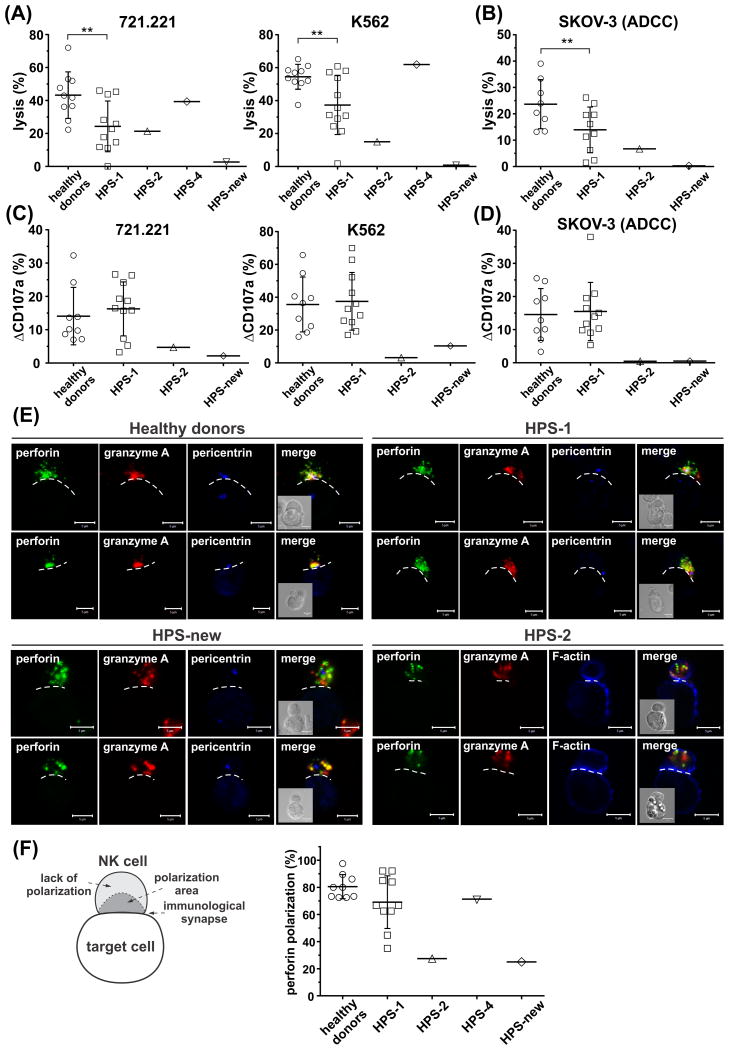
We tested the cytotoxicity of NK cells isolated from peripheral blood of HPS patients using two susceptible cell lines, 721.221 and K562. Compared to healthy individuals, cytotoxicity of HPS-1 NK cells was reduced (Fig 1A), suggesting that NK cell lytic function is less efficient in HPS-1; however, that reduction was mitigated with increased number of NK cells (data not shown), consistent with no predisposition to recurrent infections or malignancy in HPS-1. Moreover, the cytotoxicity of NK cells was not altered in the HPS-4 patient (Fig 1A), indicating that NK lytic capability is independent of HPS1 or the BLOC3 complex function. In contrast, cytotoxicity of NK cells isolated from the HPS-2 and HPS-new patients was severely impaired (Fig 1A). NK cells from HPS-2 patient showed a residual cytotoxicity toward 721.221, but not K562 cells. Killing of K562 cells is dependent mainly on NKG2D (KLRK1) and LFA1 (ITGAL) receptors, whereas cytotoxicity toward 721.221 cells relies on 2B4 receptor expression by NK cells (Chen et al, 2007). The altered cytotoxicity toward target cells could be due to differences in expression of ligands for NK activating receptors by those target cells (Chen et al, 2006), and/or varied expression levels of activating receptors on NK cells (Gil-Krzewska et al, 2016). Nevertheless, cytotoxicity of HPS-2 NK cells did not improve with increased ratio of NK to target cells (data not shown), indicating a profound impairment of their cytolytic function, in agreement with previous reports (Enders et al, 2006; Jessen et al, 2013). Furthermore, antibody-dependent cell-mediated cytotoxicity also revealed a failure to kill target cells by NK cells from HPS-2 and HPS-new patients (Fig 1B). The decreased lytic function of HPS-2 NK cells correlates with the reduced killing ability of cytotoxic T cells observed in these patients (Clark et al, 2003; Enders et al, 2006; Jessen et al, 2013), indicating that disruption of the AP-3 sorting complex has detrimental effects on the cytolytic activity of cytotoxic lymphocytes. We noted that HPS-2 and HPS-new NK cells had somewhat reduced ability to form conjugates with target cells (Fig S1), which probably contributed to the observed decrease in their cytotoxicity, but was unlikely to be the sole factor responsible for the inhibition of cytotoxicity observed in these NK cells. Therefore, we assessed the ability of HPS NK cells to degranulate, using a standard CD107a externalization assay. HPS-2 and HPS-new NK cells that conjugated with target cells showed reduced degranulation in response to different target cell lines, while HPS-1 NK cells showed normal degranulation (Fig 1C–D), indicating that defective lytic granule exocytosis is responsible for impaired cytotoxicity of HPS-2 and HPS-new NK cells. Our findings regarding HPS-2 NK cells support previous reports showing impaired degranulation from cytotoxic lymphocytes in HPS-2 patients, as well as HPS-2 animal models (Clark et al, 2003; Enders et al, 2006; Jessen et al, 2013).
Figure 1. Defective cytotoxicity and granule polarization in Hermansky-Pudlak syndrome NK cells.
(A) Natural cytotoxicity. Ex vivo isolated NK cells were mixed with 721.221 or K562 target cells for 2 h at 37°C. The percentage of target cell lysis for at a 1:1 effector-to-target (E:T) ratio is shown for the indicated HPS types.
(B) Antibody-dependent cell-mediated cytotoxicity (ADCC). Ex vivo isolated NK cells, were incubated with SKOV-3 target cells, in the presence of anti-HER2 Ab (herceptin/trastuzumab). The percentages of target cell lysis at a 1:1 E:T ratio are shown for the indicated donor groups.
(C – D) NK cell degranulation ability. NK cells, cultured with Interleukin 2 (100 u/ml), were incubated for 60 min at 37°C alone (no target cells), or with 721.221, K562 (C), or SKOV-3 target cells in the presence of anti-HER2 monoclonal antibody (mAb) (D). After the incubation, the cells were stained with anti-CD56 and anti-CD107a mAb, and the cell-surface expression of CD107a was determined by flow cytometry. The graphs show relative change in CD107a externalization (ΔCD107a), determined after subtraction of background CD107a levels present on NK cells incubated without target cells.
Each symbol in (A – D) represents an individual donor. The graphs show data pooled from 5 - 8 experiments; each experiment in (A-B) was duplicated to ensure the reproducibility of the results. The horizontal lines indicate means, error bars represent standard deviation (SD); statistical significance is indicated by asterisks (** p < 0.01; unpaired t test with Welch's correction).
(E) Ex vivo isolated NK cells were activated by mixing with 721.221 target cells for 30 min at 37°C. The cells were next fixed, and stained with Ab against perforin (green), granzyme A (red), and pericentrin (MTOC marker; blue) or phalloidin (filamentous (F)-actin indicator; blue). The dashed line indicates the position of the immunological synapse. Scale bars represent 5 μm. The inserts show the differential interference contrast images, and illustrate the position of the cells. The images show representative results from 3 independent experiments.
(F) The percentage of perforin polarization to the immunological synapse in the indicated NK cells conjugated with 721.221 target cells, as in (E). Error bars represent SD, horizontal lines indicate means. The values were determined by evaluation of at least 25 conjugates for each donor in the indicated groups, in 2 – 3 separate experiments. The diagram on the left illustrates the scoring model. Perforin was regarded as polarized if it localized to a conical area limited by the centre of the cell and the edge points of the cell-cell interface (dark grey region in the diagram labelled as polarization area), and the MTOC (as determined by pericentrin staining) was adjacent to the cell-cell contact site.
NK cells isolated from healthy individuals or HPS-1 patients, had small lytic granules containing perforin and granzyme A that clustered around the microtubule organizing centre (MTOC) and polarized to the immunological synapse following stimulation with target cells (Fig 1E–F). HPS-2 and HPS-new NK cells had slightly or prominently enlarged granules, respectively, which were dispersed throughout the cell, and did not polarize to the immunological synapse upon contact with target cells (Fig 1E–F). Both HPS-2 and HPS-new NK cells had granules positive for perforin and granzyme A, suggesting that lytic enzyme trafficking was not affected. This is consistent with data showing that granzymes traffic to the lytic granules mainly through the mannose-6-phosphate receptor pathway (Griffiths & Isaaz 1993), whereas trafficking of perforin in NK cells has been reported to require the AP-1 sorting complex (Krzewski et al, 2013). Abnormal cytotoxicity of HPS-2 and HPS-new NK cells is then a result of defective motility of lytic granules, rather than their altered composition. Disruption of lytic granule movement in NK cells resembles the phenotype observed in HPS-2 T cells, and is probably related to faulty attachment and movement of lytic granules along microtubules (Clark et al, 2003).
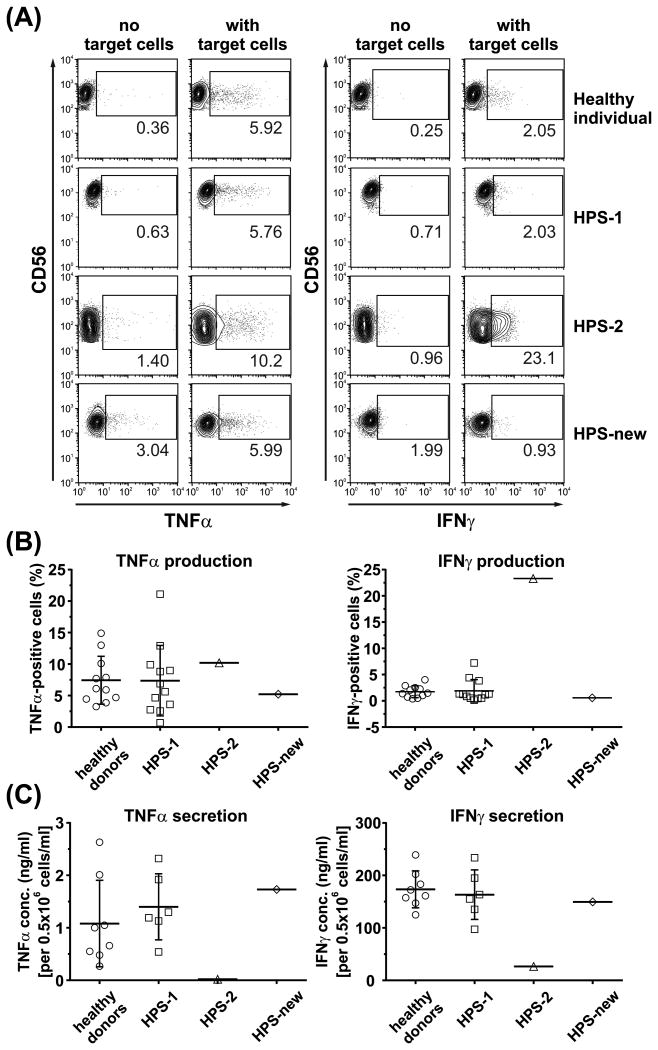
NK cells are known to produce cytokines, such as TNFα and IFNγ (Fauriat et al, 2010). Consequently, we assessed the ability of HPS NK cells to produce and secrete cytokines in response to cell activation. HPS-1 and HPS-new NK cells produced (Fig 2A–B) and secreted (Fig 2C) TNFα and IFNγ at levels comparable to normal NK cells, indicating that cytokine generation and exocytosis is normal in these cells. Conversely, HPS-2 NK cells had elevated intracellular levels (Fig 2A–B) and secreted only minute amounts of both cytokines (Fig 2C), indicating that HPS-2 NK cells retain the cytokines as a result of faulty exocytosis, and the AP-3 sorting complex is critical for exocytosis from NK cells, functioning both in regulated and constitutive secretory pathways.
Figure 2. NK cells in Hermansky-Pudlak syndrome subtype 2 fail to secrete cytokines in response to activation.
(A – B) Ex vivo isolated NK cells were either left unstimulated (no target cells) or were stimulated for 6 h by mixing with K562 target cells, in the presence of brefeldin A. The cells were then stained with anti-CD56 monoclonal antibody (mAb), fixed, permeabilized, and stained with anti-TNFα or anti-IFNγ mAb. The intracellular levels of cytokines were assessed by flow cytometry. The contour plots in (A) show a representative result, and illustrate gating strategy used to determine the population of TNFα- or IFNγ-positive cells; the gates were established based on the distribution of the unstimulated cell populations. The graph in (B) shows the cumulative data determined as in (A), and illustrates pooled results from 8 experiments; each symbol represents an individual donor.
(C) Ex vivo isolated NK cells were stimulated for 20 h with interleukin (IL) 12 (20 ng/ml), IL15 (100 ng/ml) and IL18 (100 ng/ml). The cell culture supernatants were collected and analysed for the presence of TNFα or IFNγ by enzyme-linked immunosorbent assay. Each symbol represents an individual donor. The horizontal lines indicate means, error bars represent standard deviation. The graphs show data pooled from 3 separate experiments; each experiment was duplicated to verify the reproducibility of the results.
Intriguingly, despite similarities at cellular level, the clinical phenotypes of HPS-2 and HPS-new are different. In general, patients with HPS-2 can experience disease manifestations related to immune deficiency. However, the HPS-new patient has mild symptoms and no clinical evidence of immunological defects. Our results suggest that features common to HPS-2 and HPS-new NK cells (i.e., enlarged granules, inhibited transport of lytic granules to the immunological synapse, impaired degranulation and cytotoxicity) are unlikely to be the primary mechanism underlying the immunological abnormalities associated with HPS-2. Thus, another mechanism(s) could underlie the differences in the immunological manifestations between HPS-2 and HPS-new. One of them could be altered cytokine secretion. Given that cytokines influence both the innate and adaptive immune response, one can speculate that poor cytokine secretion could affect overall activation and responses of the immune cells, which could contribute to immune deficiency. We found that, in contrast to HPS-new, HPS-2 NK cells showed severely impaired release of TNFα and IFNγ. Notably, dendritic cells from HPS-2 patients also show severely impaired cytokine and chemokine release (Prandini et al, 2016), indicating that faulty cytokine secretion could be one of major factors contributing to immunological deficiency observed in HPS-2, but not in HPS-1 or HPS-new, where cytokine secretion is most likely unaffected. As the levels of circulating cytokines in different HPS subtypes are still ill described, further studies are required to verify this hypothesis.
Supplementary Material
Acknowledgments
We thank the patients for their contributions to this research. This study was supported by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, and the National Human Genome Research Institute, National Institutes of Health.
Footnotes
Competing interests: The authors have no competing interests.
Author contributions: KK, JEC, WAG, BRG designed the study. AGK, YM, GP, KK performed the experiments and collected the data. AGK, YM, KK analysed the data. KK and BRG interpreted the data. BRG and WAG provided a clinical review of the study. KJO, MM, BRG provided clinical care to the patients. ARC provided essential resources, and critically revised the paper. KK, BRG, JEC wrote the manuscript and critically revised the paper.
References
- Ammann S, Schulz A, Krageloh-Mann I, Dieckmann NM, Niethammer K, Fuchs S, Eckl KM, Plank R, Werner R, Altmuller J, Thiele H, Nurnberg P, Bank J, Strauss A, von Bernuth H, Zur Stadt U, Grieve S, Griffiths GM, Lehmberg K, Hennies HC, Ehl S. Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood. 2016;127:997–1006. doi: 10.1182/blood-2015-09-671636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Stinchcombe JC, Day A, Blott E, Booth S, Bossi G, Hamblin T, Davies EG, Griffiths GM. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, Kontny U, Muller C, Nurden A, Rohr J, Henschen M, Pannicke U, Niemeyer C, Nurden P, Ehl S. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108:81–87. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Krzewska A, Wood SM, Murakami Y, Nguyen V, Chiang SC, Cullinane AR, Peruzzi G, Gahl WA, Coligan JE, Introne WJ, Bryceson YT, Krzewski K. Chediak-Higashi syndrome: Lysosomal trafficking regulator domains regulate exocytosis of lytic granules but not cytokine secretion by natural killer cells. J Allergy Clin Immunol. 2016;137:1165–1177. doi: 10.1016/j.jaci.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochuico BR, Huizing M, Golas GA, Scher CD, Tsokos M, Denver SD, Frei-Jones MJ, Gahl WA. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Molecular medicine. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GM, Isaaz S. Granzymes A and B are targeted to the lytic granules of lymphocytes by the mannose-6-phosphate receptor. The Journal of cell biology. 1993;120:885–896. doi: 10.1083/jcb.120.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, Gahl WA. Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr Res. 2002;51:150–158. doi: 10.1203/00006450-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annual review of genomics and human genetics. 2008;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen B, Bode SF, Ammann S, Chakravorty S, Davies G, Diestelhorst J, Frei-Jones M, Gahl WA, Gochuico BR, Griese M, Griffiths G, Janka G, Klein C, Kogl T, Kurnik K, Lehmberg K, Maul-Pavicic A, Mumford AD, Pace D, Parvaneh N, Rezaei N, de Saint Basile G, Schmitt-Graeff A, Schwarz K, Karasu GT, Zieger B, Zur Stadt U, Aichele P, Ehl S. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood. 2013;121:2943–2951. doi: 10.1182/blood-2012-10-463166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Frontiers in immunology. 2012;3:335. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood. 2013;121:4672–4683. doi: 10.1182/blood-2012-08-453738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prandini A, Salvi V, Colombo F, Moratto D, Lorenzi L, Vermi W, De Francesco MA, Notarangelo LD, Porta F, Plebani A, Facchetti F, Sozzani S, Badolato R. Impairment of dendritic cell functions in patients with adaptor protein-3 complex deficiency. Blood. 2016;127:3382–3386. doi: 10.1182/blood-2015-06-650689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.