Summary
Visceral leishmaniasis (VL) is a serious public health problem on the Indian subcontinent, causing high morbidity and mortality. The governments in the region have launched a VL elimination initiative since 2005. We review current knowledge gaps and Research priorities. Key challenges include low health services coverage of those most at risk, drug resistance, the lack of a vaccine and the complex biology of the sand fly-human host transmission cycle. Vector control is an essential component, but innovation in this field is critically lacking. Significant progress has been made in the area of diagnostic, therapeutic and vaccine development, but there are still many hurdles to overcome. For VL elimination to become a reality, effective deployment of these existing and new tools is essential. A strong commitment at community level is imperative, and appropriate diagnostic and treatment services as well as effective epidemiological surveillance need to be ensured.
Keywords: Elimination, Visceral leishmaniasis, Post kala-azar dermal leishmaniasis, R&D, drugs, diagnostics, vector control, L donovani, di-chloro-di-phenyl-trichloro-ethane (DDT)
1. Background
Among the world’s poorest people, more than 1 billion are affected by one or more neglected tropical diseases (NTDs)(1, 2). Visceral leishmaniasis (VL) is one the most important disorders in this group, caused by intracellular protozoan parasites of the Leishmania (L.) donovani complex. VL is ranked second in mortality and fourth in morbidity among NTDs, with 20,000 to 40,000 deaths annually (3). Over 90% of the VL cases occur in India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil; and the disease has been a serious impediment to socioeconomic development in the affected areas. VL has never featured as a high priority in the drug development programs funded by the pharmaceutical industry because it is unlikely to yield good returns on research and development costs.
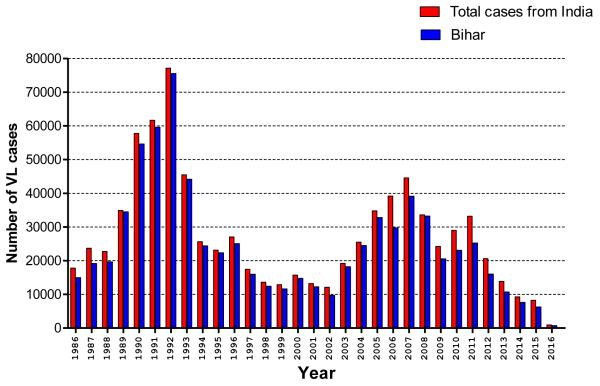
In 2005, the governments of India, Bangladesh and Nepal launched a regional initiative to eliminate VL by the year 2015(4). Elimination was defined as reducing VL incidence to a level where it would cease to be of public health importance, i.e. <1 per 10,000 inhabitants per year at sub-district levels (block level in India and Nepal, upazila level in Bangladesh). Elimination of VL was considered at the time an achievable goal for the following reasons: i) L. donovani, the causative species is transmitted in a human-to-human cycle in this region, without animal reservoir, ii) There is only a single sand fly vector species, P.argentipes and it is susceptible to insecticides, iii) Transmission is geographically restricted to a well-defined number of districts, iv) Recent breakthroughs in diagnosis and treatment: a rapid diagnostic test and an oral drug, miltefosine (5). At the time of committing to the elimination strategy, the annual incidence of VL was as high as 22 per 10,000 population in some endemic districts of Bihar, India. A peak was reached in 2007 when 44,533 cases were reported, after which there has been a decreasing trend (Figure-1). To date, more than 70% of endemic blocks have achieved the elimination target (7, 8). Bangladesh has achieved the elimination targets in 90% of their endemic upazilas and has so far been able to sustain these low levels, with the number of VL endemic upazilas decreasing from 140 initially to 16 in 2012 and 6 in 2014. (9). In Nepal, elimination has been reached at district level, and has been sustained for the past two years (8).
Figure1. Number and trends of VL cases reported per year in India and Bihar state (from 1986 to February, 2016).
(Source: adapted from National Vector-Borne Disease Control Programme, Directorate General of Health Services (DGHS), Ministry of Health and Family Welfare, New Delhi, Government of India; and world Health Organization.)
While substantial progress has been made by the three countries (reviewed in ref. 9, 10), they clearly fell short of the elimination target. Consequently, as countries remain committed to the goal of VL elimination, the original date was recently extended from 2015 to 2017 (11). Why this delay? Were the initial assumptions flawed? Were the tools or the resources inadequate, and if so what are the R&D needs. What is the prospect for achieving elimination in this region by 2017? Furthermore, is there scope to extend the ambition from eliminating VL as a public health problem (i.e. reducing incidence below a specific threshold) to complete interruption of transmission? In this paper, we will assess the technical and operational aspects of VL elimination as a public health problem and try to address these questions.
2. Is VL elimination technically feasible?
2.1. Disease transmission and potential parasite reservoirs: the role of asymptomatically infected humans and animals
It is an understatement to say that several factors in the transmission of VL are not yet clearly understood today. The first assumption underlying the elimination initiative is that VL is an anthroponosis. Implicitly it is suggested that only clinical human VL cases are the source of pathogen transmission. This is not proven, and these assumptions are challenged in at least three ways, i.e. by the established role of Post kala-azar dermal leishmaniasis (PKDL) (12), the potential role of latent human carriers (13), and domestic animals (14). One of the key attributes making elimination and eradication of infectious diseases (e.g. polio) possible is the fact that every infection led to easily detectable, overt clinical disease(15), is lacking in VL.
Mathematical models suggest that current transmission intensity could not be sustained by clinical VL patient alone (16-19)), challenging the assumption that --unless they progress to overt disease-- sub-clinically infected individuals do not contribute to transmission. While the models suggest that they are less infectious than clinical VL patients, the role in transmission is likely to be important because of their sheer number, as it is assumed that for every clinical VL case, there are 8.9 cases of sub-clinical VL (13). . Therefore, the dynamics of asymptomatic VL infection and its role in disease transmission should urgently be elucidated. If infectiousness of asymptomatic carriers to the sand fly vector is confirmed, this would present a major challenge to VL elimination efforts and ultimately for eradication.
The presumed absence of an animal reservoir has been challenged by the repeated observations of antibody and PCR positivity in domestic animals (13, 19).The sand fly vector is an opportunistic feeder, and these mammals provide an attractive blood source in the peridomestic environment. Again, the infectiousness of these animals has not been established yet, but if confirmed, this would again present a formidable challenge. The further the human parasite reservoir is depleted, the more important a possible animal reservoir might become.
2.2. PKDL: an unresolved mystery
PKDL is a late complication of VL that usually appears as a macular, maculo-papular or popular rash in patients who have recovered from VL and are otherwise usually doing well: therefore not be inclined to seek treatment (21). PKDL patients may represent an important reservoir of infection that has so far been largely neglected. Particularly in East Africa, PKDL usually occurs within weeks to few months following treatment in up to 50% of people who have recovered from VL (20). On the Indian subcontinent (ISC), PKDL seems less common, though adequate data is lacking (22, 23). One study from Bangladesh reports a cumulative incidence of up to 17% in the first 5 years after VL (24). There is evidence for their infectiousness as sand flies exposed to nodular PKDL lesions developed high infection rates (12). There is thus an urgent need to establish how prevalent PKDL really is and which forms are occurring (macular, maculo-nodular or nodular). For each form xenodiagnosis should be used to assess its level of infectiousness to sand flies. Moreover, there is no clear treatment option for PKDL. Lack of animal models and low incidence of PKDL makes R &D as well as prospective studies challenging.
2.3. HIV-VL co-infection
HIV-infection dramatically increases the risk of progression from asymptomatic VL infection to disease, leading to atypical presentations of VL, PKDL and cutaneous leishmaniasis (25). Moreover HIV-infected VL patients are very difficult to treat, with high relapse and mortality rates reported (26). In southern Europe HIV-VL co-infection has posed problems both due to reactivation of pre-existing asymptomatic VL infections in HIV positives as well as through increased transmission by sharing of needles among intra-venous drug users. On the ISC HIV has not been assumed to be a major factor in the epidemiology of VL: whereas VL is a disease of the rural poor HIV is mostly an urban problem, and consequently overlap of the two infections has been limited (27). However in a recent study of Burza et al. among over 2,000 adult VL patients in Bihar, India, 5.6% were found to be HIV infected (26).
2.4. VL treatment
One of the main arguments for considering elimination of VL an attainable objective was the availability of an oral drug, Miltefosine. This drug was adopted as the first line treatment in 2002 to replace sodium stibogluconate, which needed to be administered intramuscularly and to which increasingly high levels of resistance were reported (29, 30). Unfortunately the failure rates of Miltefosine documented in a clinical cohort ten years after its introduction in India had doubled (31). Relapse rates of up to 20% have recently been reported in Nepal (32). The drug has a long half-life and needs to be taken for 28 days, factors that favor selection of resistant strains. Combination therapies is one possible approach to protect the drugs from failure due to non compliance or resistance and to prolong their clinically useful lives (33). More recently, liposomal amphotericin B (AmBisome®) treatment was shown to be highly effective and has now been adopted as treatment of choice in the regional VL elimination initiative, 34, 35). The drug is administered intravenously, since it is a single dose treatment the risk of emergence of drug resistance is greatly reduced. Results have so far been excellent (29,30). AmBisome® does require a cold chain though, where this cannot be guaranteed the combination of Miltefosine and Paromomycin is now recommended. Effective treatment regimens are still available but the initial assumption of having an effective oral drug that can easily be administered at the lowest levels of the healthcare system no longer holds.
2.5. Vector control and management
By convention it is assumed that the habitat of P. argentipes is restricted to areas in and around human homes(36). Indoor residual spraying (IRS) is therefore assumed to be an effective vector control measure and is a key component of the current VL elimination strategy. As a byproduct of massive DDT spraying in the malaria eradication campaigns of the 1950s and 1960s, VL disappeared from the Indian subcontinent for over a decade, until resistance to DDT emerged and became widespread (37, 38) (35, 36). In India IRS with synthetic pyrethroids has now been introduced in VL endemic district of Bihar (Muzaffarpur), and a recent survey, however confirmed excellent sucepatability of P. argentipes (7, 39). Bangladesh has already adopted deltamethrin, Nepal uses alpha-cypermethrin in its IRS program. However, even with highly effective insecticides the issue of proper performance of IRS remains crucial. In addition IRS will not affect outdoor sand fly populations. These may be more important than initially assumed. In a recent survey among 668 VL patients in Bihar, 93% reported sleeping outside during part of the year; the vast majority did so for 6 months (Richard Poché, personal communication). Furthermore, insecticide treated nets did reduce indoor sand fly density by 25% in a cluster randomized trial in India and Nepal, though no effect could be demonstrated on disease transmission (40,41). The fact that no reduction in VL transmission was observed despite a reduction in indoor vector density and although people were sleeping under insecticide treated nets raises the question whether infection could take place outdoors. Poche et al. found large numbers of P.argentipes sand flies in outdoor locations, blood meal analysis revealed that up to 90% of blood fed flies captured from palm tree canopies had fed on humans (42).
3. Is VL elimination operationally achievable?
VL control in the Indian sub-continent has always hinged on two strategies: early case detection and treatment, and vector control. Reaching the elimination target once has not much value in public health terms; the crux is in maintaining the incidence rate below that low threshold for the coming years. Case finding and surveillance activities will therefore need to be maintained for years. This will require community awareness and participation, for which vigorous information, education, communication activities are required to enable the affected communities to make informed decisions. The same full commitment will be required from health staff at all levels. At present, the apex of this vertical disease control program seems sometimes disconnected from field realities, where doctors and nurses working in resource limited settings do not necessarily focus on VL. They never received proper training in planning, communications, logistics, and are not very well aware of the objectives of the VL elimination program. Thus, we have to fill this knowledge vacuum with continued professional education, training and motivation, in line with the recent example of Pulse polio program success in India (43).
3.1. Population at risk and surveillance system
Operational challenges in VL elimination include the development and deployment of effective surveillance systems for delivering effective Leishmania prevention and treatment. Geographically the spread of VL on the Indian Subcontinent is limited. In India 54 out of a total of 676 districts are affected, including 34 districts in the state of Bihar that account for 70% of the total VL caseload on the subcontinent. In Bangladesh 34 out of 64 districts are affected but over 90% of cases are reported from just 10 districts, and 50% from a single district (Mymensingh) (44). In Nepal 11 districts out of 75 are affected, all situated in the north eastern Terai region (7, 8). Recently however clusters of VL cases have also been reported from some of the hilly districts previously considered non-endemic, with evidence of local transmission (45).
Unfortunately, the complexity of the VL transmission cycle does not help In endemic areas, infections tend to cluster into small foci, related to environmental, climatic, and ecological suitability for vectors and transmission(46). At the hamlet level, attack rates can be ten-fold higher than in surrounding areas for a number of years. Eventually such clusters are saturated and the disease shifts to other areas (47, 48). The exact determinants and of such clustering in VL are not fully understood, but need to be elucidated in order to have an effective control program.
3.2. Drug availability and supply
The adequate supply and delivery of existing medicines has to become a universal reality, not an inconsistently achieved objective. Many VL patients first present to unqualified medical practitioners. These practitioners often provide inappropriate treatment regimens, in particular low doses and at irregular intervals(49). It is essential that anti-leishmanial drugs are provided free of charge in VL endemic areas considering the fact that patients cannot afford to purchase and complete a full course of treatment. Even though Miltefosine had been adopted as first line treatment in 2005, a survey among VL patients treated in public health services in Bihar in 2008 still found that most of them were treated with SSG (50). Treatment success rates were low and many patients sought additional treatment in hospitals or private facilities. As a result they incurred substantial costs. AmBisome® is an excellent choice since it is a single dose treatment, no longer necessitating patients to be admitted to hospital with associated expenditures and opportunity costs. But the drug still needs to be made more widely available and routine monitoring of anti-leishmanial drug resistance remains essential.
3.3. Cost of VL elimination and financial support
In the countries affected VL has a cost not only at individual or household level but also at societal level. Uranw et al. in Nepal found that despite the availability of free treatment in public health facilities, 51% of households affected by VL incurred costs that were above the catastrophic threshold of 10% of annual household income (50). Adhikari and Supakankuti conducted a cost benefit analysis of VL elimination in Nepal from a societal perspective. They conclude that major benefits can be expected from increased productivity and resources saved once VL incidence has been reduced. They suggest that every rupee invested in VL control in Nepal at present (2010) will yield 71 rupees in future. (51). Yet investments are required, and strong commitments from political stakeholders as well as funding agencies are crucial to achieve the elimination goal. After the governments in the region led the way, the international donor community has now stepped in. In September 2014, the Bill & Melinda Gates Foundation (BMGF initiated a high level meeting of VL partners in London to unveil a road map for tackling kala-azar elimination in South Asia. It was agreed to work collaboratively, sharing the expertise and assessing the programmatic progress every three months in order to implement new strategies to ensure that success is not only achieved but also sustained continuously (8). This is a very promising development that introduced a new dynamics in the VL control programs in the region.
4. What are the possible solutions: Way Forward?
Essentially, successful and sustained VL elimination will depend on: i) a better understanding of transmission and; ii) optimal use of existing tools and iii) development of new, more effective tools with which to interrupt it. Understanding the dynamics and epidemiology of anthroponotic transmission will hold a clear importance in deciding whether or not adjustments should be made to the current VL control strategy. Thus, xenodiagnosis studies are required to establish the relative importance of VL patients, asymptomatically infected and PKDL patients in sustaining transmission. Xenodiagnosis studies are also required to investigate the potential role of domestic animals..
Moreover, concerted efforts should be directed towards the development of highly sensitive, cheap and readily available rapid diagnostic and epidemiological tools to monitor L.donovani infection(52). Once elimination has been achieved, surveillance mechanisms will need to be maintained for many years to prevent another resurgence. Numerous tools have been developed in recent years such as DNA based diagnostic test (53), portable and field-friendly molecular testing kits that could identify all Leishmanias pecies at very low densities (54, 55) and a whole blood IFN-γ release assay (56). Some of these tools still require further validation, for others the main research question would be how they can be integrated in post-elimination surveillance.
Currently used single-dose drug regimen offers great perspectives better for control (57) but R&D for VL treatment should continue as no drug can be considered fail proof against resistance. New drugs are under development (58). Modeof action of the drugs and mechanisms involved in drug resistance need to be explored further for designing a better and effective drug regimen. Targeted vector control related research should be intensified, including the development of new insecticides to replace those to which resistance has developed or is developing (10). Vector control efforts need to be implemented in a systematic way and need to be well monitored. The local transmission patterns needs to be taken into account, because IRS and insecticide treated nets are unlikely to be successful where transmission occurs outside the house (40).
5. Concluding remarks
Despite many barriers and obstacles, substantial progress has been made over past years; and the VL elimination initiative in the ISC has already saved many lives. Keeping VL at bay will diminish the cycle of poverty in the community, we believe VL elimination as a public health problem is technically possible and operationally feasible, particularly following the renewed commitment by the three countries' governments as well as local and international stakeholders. Interrupting pathogen transmission totally from the region (eradication) is another game altogether.
Box-1.
Glossary
- Control
Reducing the disease incidence, prevalence, morbidity or mortality to a locally acceptable level at which it is no longer a major public health problem. However, continued measures are required to sustain the reduction (59).
- Elimination
Reduction of incidence to zero transmission in a defined geographical areas.. However, continued measures are required to prevent the reestablishment of transmission
- Eradication
Permanent reduction of disease, meaning zero transmission and zero cases globally, e.g. small pox.
Box-2. Key research priorities for VL elimination.
Development of innovative approach and preparation of micro plans to sustain surveillance system.
Development of new methods to measure transmission
Research on mathematical transmission modeling for public health to track 2017 elimination goal with the current strategies.
Research on epidemiology and transmission dynamics of VL
Research on the identification of sand fly breeding sites.
Strategies to improve the effectiveness of IRS
Research on direct xenodiagnosis to proof the disease spectrum and reservoir potential.
Development of sensitive non invasive diagnostic tools based on antigen detection.
Development of Pharmacovigilance capacity
Research in the area of drug resistance and insecticide resistance, and development of strategies to prevent of delay the resistance.
Research on co-infection and its mechanism
Developing research leadership in endemic areas
Research on current knowledge gaps in VL control program
Development of Product Development Partnerships (PDPs)
Acknowledgement
This work was supported by the Extramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (TMRC grant number P50AI074321) and grants from the Bill & Melinda Gates Foundation (OPP 1117011). The funders had no role in design, decision to publish, or preparation of the report.
Footnotes
Authors Contributions:
OPS and SS conceived the idea, and wrote the initial version of draft. EH and MB participated in writing and critical revision of draft. All authors took part in the review, preparation and final approval of draft.
Conflict of interest: We declare that we have no conflict of interest.
Contributor Information
Om Prakash Singh, Department of Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India..
Epco Hasker, Department of Public Health, Institute of Tropical Medicine Antwerp, Belgium.
Marleen Boelaert, Department of Public Health, Institute of Tropical Medicine Antwerp, Belgium..
Shyam Sundar, Department of Medicine, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India..
References
- 1.WHO Accelerating Work to Overcome the Global Impact of Neglected Tropical Dieases: A Roadmap for implementation. 2011 http://wwwwhoint/neglected_diseases/NTD_RoadMap_2012_Fullversionpdf.
- 2.Narain JP, Dash AP, Parnell B, Bhattacharya SK, Barua S, Bhatia R, et al. Elimination of neglected tropical diseases in the South-East Asia Region of the World Health Organization. Bull World Health Organ. 2010 Mar;88(3):206–10. doi: 10.2471/BLT.09.072322. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Regional Strategic Framework for Elimination of Kala-azar from the South-East Asia Region (2005-2015) 2005 Jan 01; http://appssearowhoint/pds_docs/b0211pdf. 2005.
- 5.Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res. 2006 Mar;123(3):195–6. [PubMed] [Google Scholar]
- 6.National Road map for kala-azar elimination, Delhi Directorate of National Vector Borne Disease Control Programme (NVBDCP) 2014 http://nvbdcp.gov.in/Doc/Road-map-KA_2014.pdf.
- 7.Kala-Azar Elimination Programme . Report of a WHO consultation of partners. Geneva, Switzerland: Feb 10-11, 2015. http://apps.who.int/iris/bitstream/10665/185042/1/9789241509497_eng.pdf?ua=1. [Google Scholar]
- 8.Chowdhury R, Mondal D, Chowdhury V, Faria S, Alvar J, Nabi SG, et al. How far are we from visceral leishmaniasis elimination in Bangladesh? An assessment of epidemiological surveillance data. PLoS Negl Trop Dis. 2014 Aug;8(8):e3020. doi: 10.1371/journal.pntd.0003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matlashewski G, Arana B, Kroeger A, Be-Nazir A, Mondal D, Nabi SG, et al. Research priorities for elimination of visceral leishmaniasis. Lancet Glob Health. 2014 Dec;2(12):e683–4. doi: 10.1016/S2214-109X(14)70318-3. [DOI] [PubMed] [Google Scholar]
- 10.Gurunath U, Joshi R, Agrawal A, Shah V. An overview of visceral leishmaniasis elimination program in India: a picture imperfect. Expert Rev Anti Infect Ther. 2014 Aug;12(8):929–35. doi: 10.1586/14787210.2014.928590. [DOI] [PubMed] [Google Scholar]
- 11.Health Ministers comit to eliminate kala azar [media advisory] New Delhi: WHO Regional Office for South - East asia. 2014 http://www.searo.who.int/mediacentre/release/2014/pr158/en/
- 12.Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ. 1992;70(3):341–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clin Infect Dis. 2014 May;58(10):1424–9. doi: 10.1093/cid/ciu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattarai NR, Van der Auwera G, Rijal S, Picado A, Speybroeck N, Khanal B, et al. Domestic animals and epidemiology of visceral leishmaniasis, Nepal. Emerg Infect Dis. 2010 Feb;16(2):231–7. doi: 10.3201/eid1602.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; Geneva: 1988. http://whqlibdoc.who.int/smallpox/9241561106.pdf. [Google Scholar]
- 16.Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, Rijal S, et al. Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis. 2011 Nov;5(11):e1405. doi: 10.1371/journal.pntd.0001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Matlashewski G, Bhunia GS, Kesari S, Das P. Asymptomatic Leishmania infections in northern India: a threat for the elimination programme? Trans R Soc Trop Med Hyg. 2014 Nov;108(11):679–84. doi: 10.1093/trstmh/tru146. [DOI] [PubMed] [Google Scholar]
- 18.Hasker E, Malaviya P, Gidwani K, Picado A, Ostyn B, Kansal S, et al. Strong association between serological status and probability of progression to clinical visceral leishmaniasis in prospective cohort studies in India and Nepal. PLoS Negl Trop Dis. 2014 Jan;8(1):e2657. doi: 10.1371/journal.pntd.0002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, et al. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis. 2011 Oct;5(10):e1284. doi: 10.1371/journal.pntd.0001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SP, Hasker E, Picado A, Gidwani K, Malaviya P, Singh RP, et al. Risk factors for visceral leishmaniasis in India: further evidence on the role of domestic animals. Trop Med Int Health. 2010 Jul;15(Suppl 2):29–35. doi: 10.1111/j.1365-3156.2010.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003 Feb;3(2):87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 22.Singh RP, Picado A, Alam S, Hasker E, Singh SP, Ostyn B, et al. Post-kala-azar dermal leishmaniasis (PKDL) in visceral leishmaniasis-endemic communities in Bihar, India. Trop Med Int Health. 2013 Dec 28; doi: 10.1111/tmi.12044. [DOI] [PubMed] [Google Scholar]
- 23.Uranw S, Ostyn B, Rijal A, Devkota S, Khanal B, Menten J, et al. Post-kala-azar dermal leishmaniasis in Nepal: a retrospective cohort study (2000-2010) PLoS Negl Trop Dis. 2011 Dec;5(12):e1433. doi: 10.1371/journal.pntd.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam S, Kenah E, Bhuiyan MA, Rahman KM, Goodhew B, Ghalib CM, et al. Clinical and immunological aspects of post-kala-azar dermal leishmaniasis in Bangladesh. Am J Trop Med Hyg. 2013 Aug;89(2):345–53. doi: 10.4269/ajtmh.12-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008 Apr;21(2):334–59. doi: 10.1128/CMR.00061-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burza S, Mahajan R, Sinha PK, van Griensven J, Pandey K, Lima MA, et al. Visceral leishmaniasis and HIV co-infection in Bihar, India: long-term effectiveness and treatment outcomes with liposomal amphotericin B (AmBisome) PLoS Negl Trop Dis. 2014 Aug;8(8):e3053. doi: 10.1371/journal.pntd.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S. Changing trends in the epidemiology, clinical presentation, and diagnosis of Leishmania-HIV co-infection in India. Int J Infect Dis. 2014 Dec;29:103–12. doi: 10.1016/j.ijid.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 28.van Griensven J, Zijlstra EE, Hailu A. Visceral leishmaniasis and HIV coinfection: time for concerted action. PLoS Negl Trop Dis. 2014 Aug;8(8):e3023. doi: 10.1371/journal.pntd.0003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jha T, Singh N, Jha S. Therapeutic use of sodium stibogluconate in kala-alar from some hyperendemic districts of N. Bihar, India J Assoc Physicians India. 1992;40:868. [Google Scholar]
- 30.Sundar S, Singh A. What steps can be taken to counter the increasing failure of miltefosine to treat visceral leishmaniasis? Expert Rev Anti Infect Ther. 2013 Feb;11(2):117–9. doi: 10.1586/eri.12.170. [DOI] [PubMed] [Google Scholar]
- 31.Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis. 2012 Aug;55(4):543–50. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- 32.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis. 2013 Jun;56(11):1530–8. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- 33.Bryceson A. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health. 2001 Nov;6(11):928–34. doi: 10.1046/j.1365-3156.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 34.Lucero E, Collin SM, Gomes S, Akter F, Asad A, Das AK, et al. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl Trop Dis. 2015;9(4):e0003699. doi: 10.1371/journal.pntd.0003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect Dis Poverty. 2016 Mar 8;5(1):19. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008 Dec;45(4):255–72. [PubMed] [Google Scholar]
- 37.Coleman M, Foster GM, Deb R, Pratap Singh R, Ismail HM, Shivam P, et al. DDT-based indoor residual spraying suboptimal for visceral leishmaniasis elimination in India. Proc Natl Acad Sci U S A. 2014 Jul 14;112(28):8573–8. doi: 10.1073/pnas.1507782112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar V, Shankar L, Kesari S, Bhunia GS, Dinesh D, Mandal R, et al. Insecticide susceptibility of Phlebotomus argentipes & assessment of vector control in two districts of West Bengal, India. Indian J Med Res. 2015 Aug;142(2):211–5. doi: 10.4103/0971-5916.164260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh R, Kumar P. Susceptibility of the sandfly Phlebotomus argentipes Annandale and Brunetti (Diptera: Psychodidae) to insecticides in endemic areas of visceral leishmaniasis in Bihar, India. Jpn J Infect Dis. 2015;68(1):33–7. doi: 10.7883/yoken.JJID.2013.262. [DOI] [PubMed] [Google Scholar]
- 40.Picado A, Singh SP, Rijal S, Sundar S, Ostyn B, Chappuis F, et al. Longlasting insecticidal nets for prevention of Leishmania donovani infection in India and Nepal: paired cluster randomised trial. BMJ. 2010;341:c6760. doi: 10.1136/bmj.c6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picado A, Das ML, Kumar V, Kesari S, Dinesh DS, Roy L, et al. Effect of village-wide use of long-lasting insecticidal nets on visceral Leishmaniasis vectors in India and Nepal: a cluster randomized trial. PLoS Negl Trop Dis. 2010;4(1):e587. doi: 10.1371/journal.pntd.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poche D, Garlapati R, Ingenloff K, Remmers J, Poche R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2011 Mar;36(Suppl 1):S106–17. doi: 10.1111/j.1948-7134.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 43.Aylward B, Tangermann R. The global polio eracidation initiatives: Lessons learned and prospects for success. Vaccine. 2011 Dec 30;29(Suppl 4):D80–5. doi: 10.1016/j.vaccine.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006 Mar;123(3):275–88. [PubMed] [Google Scholar]
- 45.Ostyn B, Uranw S, Bhattarai NR, Das ML, Rai K, Tersago K, et al. Transmission of Leishmania donovani in the Hills of Eastern Nepal, an Outbreak Investigation in Okhaldhunga and Bhojpur Districts. PLoS Negl Trop Dis. 2015 Aug 7;9(8):e0003966. doi: 10.1371/journal.pntd.0003966. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasker E, Kansal S, Malaviya P, Gidwani K, Picado A, Singh RP, et al. Latent infection with Leishmania donovani in highly endemic villages in Bihar, India. PLoS Negl Trop Dis. 2013;7(2):e2053. doi: 10.1371/journal.pntd.0002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analyses for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. 2010;4(2):e599. doi: 10.1371/journal.pntd.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. Kathmandu Univ Med J (KUMJ) 2006 Oct-Dec;4(4):488–96. [PubMed] [Google Scholar]
- 49.Sundar S, Singh A, Singh OP. Strategies to overcome antileishmanial drugs unresponsiveness. J Trop Med. 2014;2014:646932. doi: 10.1155/2014/646932. Epub 2014 Apr 30. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasker E, Singh SP, Malaviya P, Singh RP, Shankar R, Boelaert M, et al. Management of visceral leishmaniasis in rural primary health care services in Bihar, India. Trop Med Int Health. 2010 Jul;15(Suppl 2):55–62. doi: 10.1111/j.1365-3156.2010.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uranw S, Meheus F, Baltussen R, Rijal S, Boelaert M. The household costs of visceral leishmaniasis care in south-eastern Nepal. PLoS Negl Trop Dis. 2013;7(2):e2062. doi: 10.1371/journal.pntd.0002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adhikari SR, Supakankunti S. A cost benefit analysis of elimination of kala-azar in Indian subcontinent: an example of Nepal. J Vector Borne Dis. 2010 Sep;47(3):127–39. [PubMed] [Google Scholar]
- 53.Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003 Feb 15;326(7385):377–82. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2010 Jan;105(1):1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deborggraeve S, Laurent T, Espinosa D, Van der Auwera G, Mbuchi M, Wasunna M, et al. A simplified and standardized polymerase chain reaction format for the diagnosis of leishmaniasis. J Infect Dis. 2008 Nov 15;198(10):1565–72. doi: 10.1086/592509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan MG, Bhaskar KR, Salam MA, Akther T, Pluschke G, Mondal D. Diagnostic accuracy of loop-mediated isothermal amplification (LAMP) for detection of Leishmania DNA in buffy coat from visceral leishmaniasis patients. Parasit Vectors. 2012;5:280. doi: 10.1186/1756-3305-5-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh OP, Sundar S. Whole blood assay and visceral leishmaniasis: Challenges and promises. Immunobiology. 2014 Apr;219(4):323–8. doi: 10.1016/j.imbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010 Feb 11;362(6):504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 59.Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitology. 2004 Aug;20(8):347–51. doi: 10.1016/j.pt.2004.06.004. [DOI] [PubMed] [Google Scholar]