Abstract
Background
To examine the yield of HIV partner services provided to persons newly diagnosed with acute and early HIV infection (AEH) in San Diego, United States.
Design
Observational cohort study.
Methods
The study investigated the yield (i.e. number of new HIV and AEH diagnoses, genetically linked partnerships, and high risk uninfected partners) of partner services (confidential contact tracing) for individuals with AEH enrolled in the San Diego Primary Infection Resource Consortium 1996 - 2014.
Results
A total of 107 of 574 persons with AEH (19%; i.e. index cases) provided sufficient information to recruit 119 sex partners. Fifty seven percent of the 119 recruited partners were HIV infected, and 33% of the 119 were newly HIV diagnosed. Among those newly HIV diagnosed, 36% were diagnosed during AEH. There were no significant demographic or behavioral risk differences between HIV infected and uninfected recruited partners. Genetic sequences were available for both index cases and partners in 62 partnerships, of which 61% were genetically linked. Partnerships in which both index case and partner enrolled within 30 days were more likely to yield a new HIV diagnosis (p=0.01) and to be genetically linked (p<0.01).
Conclusion
PS for persons with AEH within 30 days of diagnosis represents an effective tool to find HIV unaware persons, including those with AEH who are at greatest risk of HIV transmission.
Keywords: Acute and Early HIV infection, MSM, Contact Tracing, HIV transmission, epidemiology
INTRODUCTION
Universal HIV testing is a cornerstone in efforts to achieve epidemic control since HIV infected and unaware people are associated with the majority of HIV transmission events 1. In particular during acute and early HIV infection (AEH) people who are unaware of their HIV status, represent a subgroup with a disproportionate risk of HIV transmission due to high HIV viral loads (VL) 2-4, ongoing sexual risk behaviors 2, and greater per-contact infectivity 5.
The CDC recommends provision of confidential partner services (PS) to provide HIV risk reduction education and HIV testing to the recent sex or needle sharing partners of newly HIV diagnosed people 6. By linking recently exposed persons to testing and treatment, this public health intervention has been used to limit the spread of sexually transmitted infections (STI), such as syphilis and gonorrhea, since the early 20th century 7. In the setting of HIV, however, PS has had its limitations. In 2006, Katz et al. estimated that fewer than half of newly HIV-diagnosed persons received PS at public health departments across the United States 8. Reasons include that PS is not mandated by law for HIV infection, and, more importantly that HIV remains a highly stigmatizing condition with significant implications for direct or indirect disclosure. Not only is PS underutilized, but it can be limited in finding HIV unawares in the setting of newly diagnosed chronic HIV infection where persons are often required to recall partners from several years prior 7, 9. In 2007, the Task Force on Community Preventive Services, in reviewing the efficacy of PS, showed that 20% (range 14 – 26%) of all referred partners were newly diagnosed with HIV 10.
Persons with AEH likely represent a group particularly appropriate for PS, as recall of recent sexual or needle sharing partners may be more likely to identify putative transmission partners (i.e., as defined by similar HIV genetic sequence). Studies of PS in the setting of recent HIV infection are limited 11, 12, but demonstrate a greater yield of new HIV diagnoses in the setting of newly diagnosed acute HIV (AHI) as compared to PS provided to chronically HIV infected persons.
We examined the yield of HIV PS provided to persons newly diagnosed with AEH in San Diego for identification of HIV-unaware persons, individuals with AEH, genetically linked partners 13, 14, and HIV-uninfected individuals at high risk for acquiring HIV infection.
MATERIALS AND METHODS
Adults and adolescents (13 years of age or older) were offered confidential and free of charge screening for acute, early and established HIV infection at multiple community-based sites in San Diego as part of the San Diego Primary Infection Resource Consortium (SD PIRC) from 1996 to 2014 15, 16. Before 2007, a quantitative HIV RNA (Amplicor HIV Monitor, Roche Diagnostic Systems, Indianapolis, IN) was performed in HIV antibody (Ab) negative persons presenting with signs or symptoms of AEH and behavioral risks for HIV infection (i.e. risk based screening for AHI but universal screening for HIV). Beginning in 2007, HIV nucleic acid testing (NAT) (Procleix HIV-1/HCV Assay: Chiron, Emeryville, California, and Genprobe, San Diego, California) was provided to all HIV Ab negative persons regardless of symptoms and exposures (i.e. universal screening for AHI) 16-20. AHI was defined by a negative or indeterminate HIV Ab test in the presence of detectable HIV-1 RNA, corresponding to Fiebig stages I-II. Early HIV infection was characterized by using one of the available assays to estimate recency (Vironostika HIV-1 enzyme immunoassay [EIA] 21, Less-Sensitive or Detuned Vitros 22, and Limiting Antigen (Lag) 23), and defined as HIV Ab+/detuned HIV Ab consistent with infection <170 days. Consenting antiretroviral (ART) naïve individuals with AEH were offered enrollment and longitudinal follow-up in the observational SD PIRC study. Prompt linkage to HIV primary care services was provided for all clients. Routine clinical laboratories and HIV drug resistance testing were performed at baseline; demographic and behavioral risk data were collected for all individuals. Longitudinal follow-up included visits at weeks 2, 4, 8, 12, and every 24 weeks thereafter.
HIV PS were offered to all AEH clients (Index cases) and included education and counseling to elicit information about recent sex or needle sharing partners 6. Index cases were offered “self-disclosure” (i.e. index case was trained to disclose their HIV status to their partners and refer their partners to our study for HIV testing), “dual-disclosure” (i.e. partners got notified by the index and one trained study staff member during an appointment), and “third-party notification” (i.e. partners got notified by trained study staff, identities of the index were not disclosed to the partners) for recruiting their recent sex or needle sharing contacts. Study staff providing PS received structured PS training by the California Department of Public Health (CDPH) or Centers for Disease Control and Prevention (CDC). These structured trainings (duration 2-3 days) were repeated by our study staff every 5 years. The trainings included how to elicit partners from index cases, including prompts and re-interviews, and delivering exposure notifications to partners. Privacy concerns were taken very seriously, in particular when an index case chose third party notification (e.g. for example index cases and partners were not scheduled on the same day for study visits). Partners successfully contacted (Recruited partners) were offered free of charge HIV testing and counseling through SD PIRC or a testing facility of their choice and linkage to prevention and treatment services. Those with positive HIV test results who reported unknown or negative HIV serostatus before HIV testing were defined as newly HIV diagnosed, while those who reported positive serostatus and/or found (by screening local clinical and research HIV repositories) to have been diagnosed previously were defined as previously diagnosed. All recruited partners who underwent HIV testing and counseling with the SD PIRC provided behavioral risk information and recruited partners identified with AEH were also offered enrollment into SD PIRC as index clients (with subsequent provision of PS). Partnerships were characterized as genetically-linked if the HIV population sequence from an index case and their recruited partner were less than or equal to 1.5% genetically different using the TN93 model 24. The study focused on sex or needle sharing partners recruited within six months of diagnosis of the index case.
Statistical analysis was performed using SPSS version 22 (IBM Corp., Amrok, NY, USA) and SAS 9.3 (SAS Institute, Cary, NC). The efficacy of PS provided to AEH clients was assessed by the number of Index cases needed to interview to identify recruited partners: 1) for HIV/STI testing, 2) newly diagnosed with HIV infection, 3) AEH infection, and 4) genetically linked index and recruited partners. We compared demographic and behavioral characteristics between HIV-infected and HIV-uninfected recruited partners by using two-tailed t-tests and two-tailed χ2 analyses. Because both index and recruited partners were occasionally represented in multiple different partnerships, mixed-effects logistic modeling was performed for genetic linkage and new HIV diagnoses.
The UCSD Human Research Protections Program approved the study protocol, consent and all study related procedures. All study participants provided voluntary, written informed consent before any study procedures were undertaken.
RESULTS
A total of 574 ART-naive individuals were newly diagnosed with AEH and offered PS between 1996 - 2014. Among those index clients 107 (18.6%) provided contact information sufficient to successfully identify and test partner(s) [6/87 (7%) index clients diagnosed with AEH between 1996-2000, 33/128 (26%) between 2001 and 2004, 41/192 (21%) between 2005 – 2009, and 27/167 (16%) between 2010 and 2014]. These 107 index cases identified 119 recruited partners and 128 distinct partnerships (Table 1). Only for two recruited partners needle-sharing was identified as the most likely mode of HIV transmission (Table 2). There were 9 individuals who served as both index case and recruited partner in distinct partnerships (Table 2).
Table 1.
Number of Index Cases, Recruited Partners, and their Unique Partnerships.
| Index Cases (n=107) | Unique Partnerships (n=128) | |
|---|---|---|
| Identified 1 Recruited Partner (n, %) Identified 2 Recruited Partners (n, %) Identified 3 Recruited Partners (n, %) |
90 (84%) 13 (12%) 26 (20%) |
90 (70%) 26 (20%) 12 (9%) |
| Recruited Partners (n=119) | Unique Partnerships (n=128) | |
| Identified by 1 Index Case (n, %) Identified by 2 Index Cases (n, %) Identified by 4 Index Cases (n, %) |
112 (94%) 6 (5%) 1 (1%) |
112 (88%) 12 (9%) 4 (3%) |
Table 2.
Baseline Demographic, Laboratory and Risk Behavior Characteristics for AEH Index Cases with Acute or Early HIV infection and Recruited Partners.
| Index Cases |
Recruited Partners |
Both Index Case and Recruited Partner |
p-Value for comparing Index Cases and Recruited Partners* |
|
|---|---|---|---|---|
|
| ||||
| n = 98 | n = 110 | n = 9 | ||
| Demographics | ||||
| Age (median, IQR; n) | 30 (24 – 37; 98) |
32 (26 – 39; 107) |
28 (23 – 39; 9) | 0.17 |
| Male sex (%, n) | 95.9 (93/97) | 95.2 (98/103) | 100.0 (9/9) | > 0.2 |
| Race/ethnicity | > 0.2 | |||
| White non-Hispanic | 58.8 (57/97) | 64.4 (65/101) | 77.8 (7/9) | |
| Black non-Hispanic | 5.2 (5/97) | 5.9 (6/101) | 0 (0/9) | |
| Hispanic | 32.0 (31/97) | 22.8 (23/101) | 0 (0/9) | |
| Other (including
multi- racial) |
4.1 (4/97) | 6.9 (7/101) | 22.2 (2/9) | |
| HIV transmission risk | ||||
| MSM (%, n) | 93.7 (89/95) | 92.2 (94/102) | 100.0 (9/9) | > 0.2 |
| IDU transmission Risk (%, n) | 3.1 (3/98) | 1 (1/100) | 11.1 (1/9) | > 0.2 |
| Laboratory Data at first visit | ||||
| CD4 (cells/μl), (median, IQR;
n) |
504 (378 – 672; 98) |
419(294 – 595; 23) |
543 (390 – 633; 9) | 0.045 |
| VL (HIV-1 RNA log10
copies/ml), (median, IQR; n) |
4.9 (4.0 – 5.6; 98) |
4.7 (3.8 – 5.2; 23) |
5.4 (4.7 – 6.0; 9) | 0.10 |
|
Self-Reported Risk Behavior
in prior 3 months |
||||
| Number of Partners (median, IQR; n) |
2 (1– 5; 85) | 3 (1 – 6; 88) | 5 (3 – 6; 7) | > 0.2 |
| Condom Use RAI (%, n) | > 0.2 | |||
| -Always (100%) | 11.7 (9/77) | 17.3 (9/52) | 33.3 (2/6) | |
| -Usually (50% - 99%) | 29.9 (23/77) | 25.0 (13/52) | 16.7 (1/6) | |
| -Sometimes (1% - 49%) | 20.8 (16/77) | 26.9 (14/52) | 16.7 (1/6) | |
| -Never (0%) | 37.7 (29/77) | 30.8 (16/52) | 33.3 (2/6) | |
| Methamphetamine use, any route (%, n) |
22.9 (8/35) | 21.1 (4/19) | 20 (1/5) | > 0.2 |
| Any drug use, any route † (%,
n) |
36.6 (15/41) | 31.6 (6/19) | 20 (1/5) | > 0.2 |
Persons who were both Index Case and Recruited Partner excluded from comparison
Excluding alcohol and cannabis
Abbreviations: IDU, injection drug use; IQR, interquartile ratio; MSM, men who have sex with men; RAI, receptive anal intercourse; RPR, rapid plasma regain; VL, viral load
Index case and recruited partner demographics were not significantly different. The majority of both, index cases and recruited partners were non-Hispanic white (59% and 64%, respectively) men (96% and 95% respectively), who had sex with men (94% and 92%, respectively). The median age of index cases and recruited partners was not significantly different (30 and 32 years of age, respectively) (Table 2). Behavioral risks were also not significantly different between AEH index cases and recruited partners (both HIV infected and uninfected). Additionally, there were no significant demographic or behavioral risk differences between HIV infected and uninfected recruited partners except for age (Table 3).
Table 3.
Baseline Demographic, Laboratory and Risk Behavior Characteristics for Index Cases with Acute or Early HIV infection and Recruited Partners
| Recruited Partners HIV Uninfected n = 51 |
Recruited Partners HIV Infected n = 68 |
P-value* | |
|---|---|---|---|
| Demographics | |||
| Age (median, IQR; n) | 34 ( 28 – 41; 48) | 30 (26 – 35; 68) | 0.040 |
| Male sex (%, n) | 91.7 (44/48) | 98.4 (63/64) | 0.16 |
| Race/ethnicity (%, n) | > 0.2 | ||
| White non-Hispanic
Black non-Hispanic Hispanic Other (including multi-racial) |
68.9 (31/47) 8.9 (4/47) 17.8 (8/47) 4.4 (4/47) |
65.1 (41/63) 3.2 (2/63) 23.8 (15/63) 7.9 (5/63) |
|
| HIV transmission risk | |||
| MSM (%, N) | 87.5 (42/48) | 96.8 (61/63) | 0.074 |
| IDU transmission Risk (%, N) | 0 (0/46) | 3.2 (2/63) | > 0.2 |
|
Self-Reported Risk Behavior in
prior 3 months |
|||
| Number of Partners (median, IQR;
n) |
3 (1 – 5; 41) | 2 (1 – 6; 54) | > 0.2 |
| Condom Use RAI (%, N) | 0.031 | ||
| -Always (100%)
-Usually (50% - 99%) -Sometimes (1% - 49%) -Never (0%) |
5 (1/20) 25 (5/20) 45 (9/20) 25 (5/20) |
26.3 (10/38) 23.7 (9/38) 18.4 (7/38) 31.6 (12/38) |
|
| Methamphetamine use, any route (%, N) |
22.2 (2/9) | 20 (3/15) | > 0.2 |
| Any drug use, any route † (%, N) | 22.2 (2/9) | 26.7 (4/15) | > 0.2 |
Overlap excluded (2 persons in separate partnerships: once as an HIV uninfected partner and once as an HIV infected partner)
Excluding alcohol and cannabis
Abbreviations: IDU, injection drug use; IQR, interquartile ratio; MSM, men who have sex with men; RAI, receptive anal intercourse; RPR, rapid plasma regain; VL, viral load
Of the 128 distinct partnerships identified, 52 (40.6%) were HIV serodiscordant, and the remaining 76 (59.4%) were HIV seroconcordant. Paired HIV resistance test sequences were available in 62 of 76 (81.6%) seroconcordant partnerships and demonstrated genetic linkage in 38 (61.2%) of these partnerships. Genetic linkage between the index case and recruited partner was used to identify putative transmission pairs and was observed in 50% of recruited partners with AEH and 50% of recruited partners with chronic HIV infection. Behavioral risks were not significantly different between index cases who were part of a genetic cluster (i.e. ≥2 connected individuals) and those who were not (data not shown).
Evaluation of the time between identification of the index case and recruited partners showed that those recruited partners enrolled within 30 days of their index (72.7% of all partnerships, and 82.3% of seroconcordant partnerships) were significantly more likely to be newly diagnosed with HIV (p=0.01) and genetically linked to their index (p<0.01) than partners identified later. The results were robust to whether partnerships were treated as independent or were corrected for belonging to multiple partnerships in the mixed-effects framework.
The mean number of AEH index subjects needed to interview (NNTI) to successfully recruit a partner for testing (HIV positive or negative) was 5 (574/119), and 15 (574/39) to identify a newly HIV diagnosed partner. Overall, 68/119 (57.1%) recruited partners were HIV infected, with 39/68 (57.3%) newly HIV diagnosed and the remaining 29 (43%) previously aware of their HIV infected status. Of the 39 newly HIV diagnosed partners, 36% (n = 14) were identified with AEH infection (NNTI = 41), and 64% (n = 25) had established HIV infection. Of the 29 recruited partners that were already aware of their HIV diagnosis, 72% (n = 21) had established HIV, while 28% (n = 8) were still in acute or early stage when presenting for the study (Figure 1).
Figure 1.
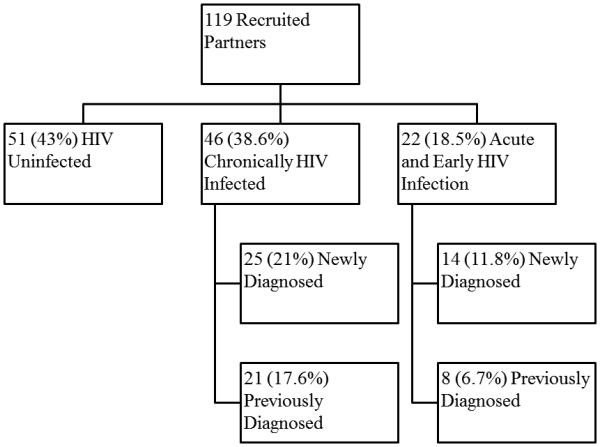
Partner Services Yield in individuals diagnosed with Acute or Early HIV infection in San Diego from 1996 - 2014
DISCUSSION
We found that PS for persons with AEH represents an effective tool to find HIV unaware persons, particularly when PS is performed within 30 days of diagnosis. Importantly more than a third of the newly HIV diagnosed recruited partners were still in the acute and early phases of HIV infection, i.e. the phase with the greatest risk of HIV transmission. PS also identified putative transmission partners, with genetically linked partners representing 61% of the seroconcordant partnerships. Finally, PS identified a high risk HIV uninfected cohort, whose risk behaviors did not differ from those newly diagnosed with HIV infection.
The HIV epidemic is propagated by HIV unawares, particularly during the phase of AEH. We demonstrated that HIV screening within the sexual contact network of persons diagnosed with AEH is an effective strategy to identify HIV unawares in early stages of HIV infection. In this study 1 out of 3 recruited partners was newly diagnosed with HIV infection and 1 out of 7 with AEH. This was 12 times higher than the overall yield of voluntary community based HIV screening of MSM with the SD PIRC (1 out of 41 tests positive for HIV and 1 out of 87 positive for AEH), the HIV screening program used to identify the index subjects in this study 25. Also, the recruited partners identified in this study represented a more high-yield cohort than previously documented 11, 12. In two prior studies of PS in acute HIV 11, 12, 7 – 10% of all recruited partners identified were newly diagnosed with HIV, as compared to 33% in this study. PS might contribute to broader public health goals to end the epidemic. While we found a decrease over time in the number of recruited partners (26% of index cases identified partners between 2001 and 2004, while only 16% identified partners between 2010 and 2014), which may be explained in part by the success of anonymous, internet-based sexual networks 26, PS continued to be high yield in terms of identifying HIV positive individuals (67% of partners identified between 2010 and 2014 were HIV positive).
Another key finding was that the immediacy of partner services was essential. Partners identified in the first 30 days of a new AEH diagnosis were more likely to yield a new HIV diagnosis (p=0.01), and a putative transmission link to the index case (p<0.01). Additionally, 29% of genetic linkages occurred in partnerships where the recruited partner also had AEH, showing that PS coupled with phylogenetic analysis could potentially be an effective tool in identifying and targeting real-time transmission outbreaks among AEH persons.
The HIV-uninfected recruited partners in this study reported behavioral risks that were comparable to AEH infected index cases. Because they belonged to the sexual network of an individual with high infectivity, and because their risk behaviors did not differ from HIV-infected recruited partners, this group may represent ideal candidates for focused HIV-prevention services, including pre-exposure prophylaxis (PrEP).
Limitations of this study included the observational study design and the convenience sampling used to identify the study cohort. Further, this study was performed among MSM and in San Diego, among whom the HIV epidemic may differ from other areas of the world. Despite the fact that new HIV diagnoses within this studies were based on laboratory findings, self-report (i.e. previous unknown or negative HIV serostatus), and also checked against local HIV clinical and research databases, we cant rule out that a proportion of recruited partners may have been diagnosed with HIV before. Also, our study participants identified fewer recruited partners when compared to two prior studies of PS (NNTI of 5 as compared to NNTI of 2 11 and 2.2 12). This is most likely because field-services (i.e. actually knocking on the doors of identified partners, in case the index case chose third party notification and the partners could not be reached by phone) were not provided in this study, as compared to the two prior studies where PS was performed by the local public health departments 11, 12.
In conclusion, our study indicates that provision of PS to persons with AEH within the first 30 days of diagnosis represents an effective tool for finding HIV unaware persons, including those with AEH who are at greatest risk of HIV transmission. Additionally, PS in this setting identifies HIV–uninfected partners who may greatly benefit from targeted prevention services, such as PrEP. These findings may suggest that in settings where time and funding are too limited to perform PS in all new HIV diagnoses, PS should be focused on individuals diagnosed with AEH and performed within 30 days of diagnosis. Increased focus of PS on individuals with AEH in these settings may potentially improve: a) PS delivery by clinicians and public health departments; b) identification of HIV-unawares and persons during AEH and c) identification of a high risk HIV-uninfected cohort appropriate for prioritized prevention services and PrEP.
Taken together, these could translate into a larger impact on HIV epidemic control than PS has had to date. Modeling studies evaluating the downstream effects of targeted PS, i.e. the effects of combined identification and treatment of high transmission risk persons, PrEP in those found to be HIV-uninfected, and also real-time identification of AEH outbreaks are needed. These studies would further elucidate the impact of PS in persons with AEH on epidemic control.
Acknowledgements/Funding
This work was supported by funds from the following: Interdisciplinary Research Fellowship in NeuroAIDS (R25-MH081482), Developmental grant from the UC San Diego Center for AIDS Research (NIAID 5 P30 AI036214), TMARC pilot study (P50DA026306), the California HIV/AIDS Research Program Grant F13SD321 and the Bettencourt-Schueller Foundation, and grants from the National Institutes of Health: AI007036, AI106039, AI043638, AI074621, AI036214, AI108351, and MH100974. The funders had no role in study design and conduct of the study, nor collection, management, analysis and interpretation of the data, nor preparation, review or approval of the manuscript.
The authors wish to thank David A. Rodriguez, testing manager at the antiviral Research Center (AVRC), for his valuable advice and input.
Footnotes
Original data of this manuscript have been presented in part at CROI 2016 in Boston, USA (Poster number 16-1773)
Authors contributions: NG and SJL designed the study, NG and MH analyzed and interpreted the data and drafted the manuscript. AC and CMA provided the data, CMA also performed statistical data analysis and AC performed part of the statistical analysis. SK performed network analysis. DS provided ideas and content critical to this manuscript, and participated in the drafting of the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Conflicts of Interest
Dr. Hoenigl served on the speakers’ bureau of Merck. Dr. Smith reported receiving grant funding from ViiV Healthcare (Pfizer joint venture) and having served as a consultant for Genprobe and Testing Talent Services. Dr. Little reported funding from Gilead Sciences, Inc. All other authors no conflicts.
References
- 1.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175:588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 2.Colfax GN, Buchbinder SP, Cornelisse PG, Vittinghoff E, Mayer K, Celum C. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS (London, England) 2002;16(11):1529–35. doi: 10.1097/00002030-200207260-00010. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS (London, England) 2007;21(13):1723–30. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenigl M, Chaillon A, Little SJ. CD4/CD8 cell ratio in acute HIV infection and the impact of early antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63:425–6. doi: 10.1093/cid/ciw293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in adults in east africa and thailand. The New England journal of medicine. 2016;374(22):2120–30. doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR.Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2008;57(RR-9):1. 83; quiz CE1-4. [PubMed] [Google Scholar]
- 7.Frieden TR, Foti KE, Mermin J. Applying public health principles to the HIV epidemic--how are we doing? The New England journal of medicine. 2015;373(23):2281–7. doi: 10.1056/NEJMms1513641. [DOI] [PubMed] [Google Scholar]
- 8.Katz DA, Hogben M, Dooley SW, Jr, Golden MR. Increasing public health partner services for human immunodeficiency virus: Results of a second national survey. Sexually transmitted diseases. 2010;37(8):469–75. doi: 10.1097/OLQ.0b013e3181e7104d. [DOI] [PubMed] [Google Scholar]
- 9.Golden MR. HIV partner counseling and referral services: Finally getting beyond the name. American Journal of Preventive Medicine. 2007;33(2 Suppl):S84–5. doi: 10.1016/j.amepre.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. American Journal of Preventive Medicine. 2007;33(2 Suppl):S89–100. doi: 10.1016/j.amepre.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Ahrens K, Kent CK, Kohn RP, et al. HIV partner notification outcomes for HIV-infected patients by duration of infection, san francisco, 2004 to 2006. Journal of acquired immune deficiency syndromes (1999) 2007;46(4):479–84. doi: 10.1097/qai.0b013e3181594c61. [DOI] [PubMed] [Google Scholar]
- 12.Moore ZS, McCoy S, Kuruc J, Hilton M, Leone P. Number of named partners and number of partners newly diagnosed with HIV infection identified by persons with acute versus established HIV infection. Journal of acquired immune deficiency syndromes (1999) 2009;52(4):509–13. doi: 10.1097/QAI.0b013e3181ac12bf. [DOI] [PubMed] [Google Scholar]
- 13.Smith DM, May SJ, Tweeten S, et al. A public health model for the molecular surveillance of HIV transmission in san diego, california. AIDS (London, England) 2009;23(2):225–32. doi: 10.1097/QAD.0b013e32831d2a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoenigl M, Chaillon A, Kessler HH, et al. Characterization of HIV transmission in south-east austria. PloS one. 2016;11(3):e0151478. doi: 10.1371/journal.pone.0151478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoenigl M, Anderson CM, Green N, Mehta SR, Smith DM, Little SJ. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: A cohort study. BMC medicine. 2015;13:218. doi: 10.1186/s12916-015-0458-5. 015-0458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris SR, Little SJ, Cunningham T, Garfein RS, Richman DD, Smith DM. Evaluation of an HIV nucleic acid testing program with automated internet and voicemail systems to deliver results. Annals of Internal Medicine. 2010;152(12):778–85. doi: 10.1059/0003-4819-152-12-201006150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenigl M, Graff-Zivin J, Little SJ. Costs per diagnosis of acute HIV infection in community-based screening strategies: A comparative analysis of four screening algorithms. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(4):501–11. doi: 10.1093/cid/civ912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoenigl M, Weibel N, Mehta SR, et al. Development and validation of the san diego early test score to predict acute and early HIV infection risk in men who have sex with men. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(3):468–75. doi: 10.1093/cid/civ335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoenigl M, Green N, Camacho M, et al. Signs or symptoms of acute HIV infection in a cohort undergoing community-based screening. Emerging infectious diseases. 2016;22(3):532–4. doi: 10.3201/eid2203.151607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoenigl M, Chaillon A, Mehta SR, Smith DM, Graff-Zivin J, Little SJ. Screening for acute HIV infection in community-based settings: Cost-effectiveness and impact on transmissions. The Journal of infection. 2016 doi: 10.1016/j.jinf.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. Journal of acquired immune deficiency syndromes (1999) 2003;33(5):625–34. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Keating SM, Hanson D, Lebedeva M, et al. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1+2 assay for detection of recent HIV infections and incidence estimation. Journal of clinical microbiology. 2012;50(12):3968–76. doi: 10.1128/JCM.01454-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PloS one. 2012;7(3):e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular biology and evolution. 1993;10(3):512–26. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 25.Hoenigl M, Chaillon A, Morris SR, Little SJ. HIV infection rates and risk behavior among young men undergoing community-based testing in san diego. Scientific reports. 2016;6:25927. doi: 10.1038/srep25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown MJ, Pugsley R, Cohen SA. Meeting sex partners through the Internet, risky sexual behavior, and HIV testing among sexually transmitted infections clinic patients. Arch Sex Behav. 2015;44:509–19. doi: 10.1007/s10508-014-0463-3. [DOI] [PubMed] [Google Scholar]


