Abstract
The mu opioid receptor gene undergoes extensive alternative splicing. Mu opioids can be divided into three classes based on the role of different groups of splice variants. Morphine and methadone require only full length 7 transmembrane (7TM) variants for analgesia, whereas IBNtxA (3’-iodobenzyol-6β-naltrexamide) needs only truncated 6TM variants. A set of endomorphin analogs fall into a third group that requires both 6TM and 7TM splice variants. Unlike morphine, endomorphin 1 and 2, DAPP (Dmt,D-Ala-Phe-Phe-NH2) and IDAPP (3’-iodo-Dmt-D-Ala-Phe-Phe-NH2) analgesia was lost in an exon 11 knockout mouse lacking 6TM variants. Restoring 6TM variant expression in a knockout mouse lacking both 6TM and 7TM variants failed to rescue DAPP or IDAPP analgesia. However, re-establishing 6TM expression in an exon 11 knockout mouse that still expressed 7TM variants did rescue the response, consistent with the need for both 6TM and 7TM variants. In receptor binding assays, 125I-IDAPP labeled more sites (Bmax) than 3H-DAMGO in wildtype mice. In exon 11 knockout mice 125I-IDAPP binding was lowered to levels similar to 3H-DAMGO, which remained relatively unchanged compared to wildtype mice. 125I-IDAPP binding was totally lost in an exon 1/exon 11 knockout model lacking all Oprm1 variant expression, confirming the drug was not cross labeling non-mu opioid receptors. These findings suggested that 125I-IDAPP labeled two populations of mu binding sites in wildtype mice, one corresponding to 7TM variants and the second dependent upon 6TM variants. Together, these data indicate that endomorphin analogs represent a unique, genetically defined and distinct class of mu opioid analgesic.
Keywords: Mu opioid receptor, Alternative splicing, Analgesia, G-Protein coupled receptor, Endorphin, Morphine
Introduction
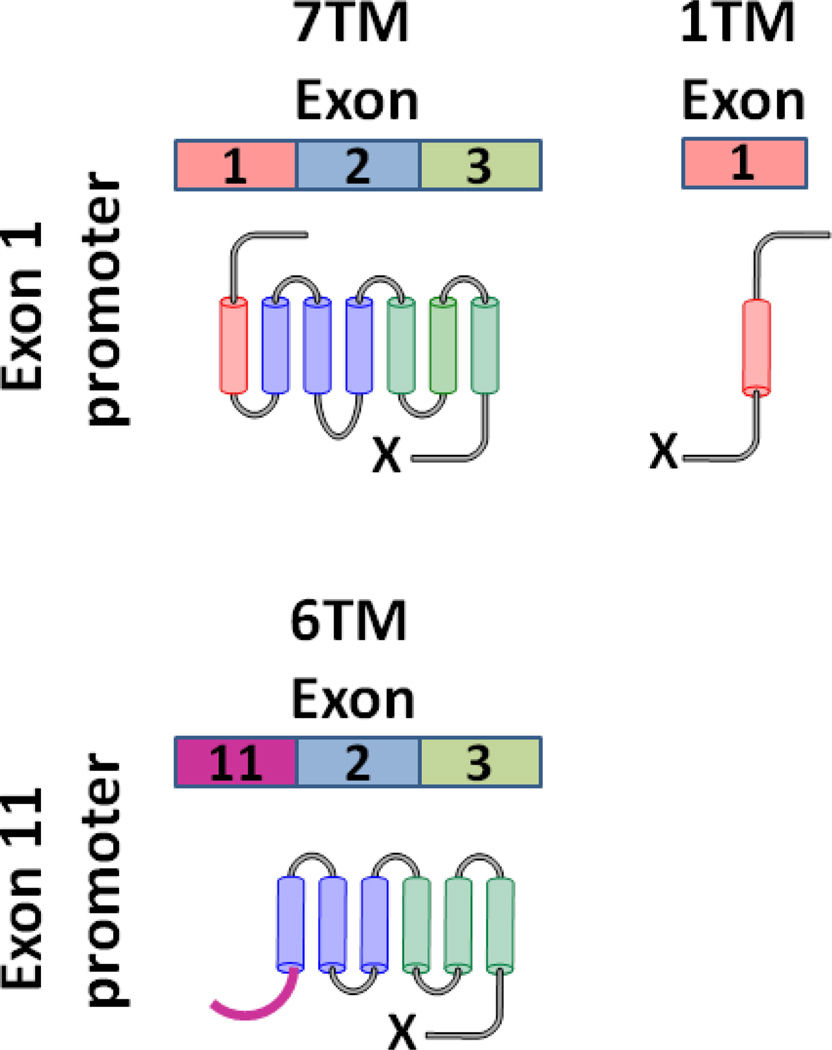
The vast majority of clinically used opioid analgesics produce their effects through mu opioid receptors generated from the Oprm1 gene (1). Yet, patients can have diverse responses to various mu analgesics (2, 3). These clinical observations are supported by preclinical models showing varied responses to these pain relievers among different mouse strains (1, 4–6). The differences might be explained by several potential mechanisms, including the possibility of multiple mu opioid receptor targets. The mu opioid receptor gene (Oprm1) undergoes extensive alternative splicing, producing three sets of structurally diverse splice variants (1). One set corresponds to traditional G-protein coupled receptors (GPCR) with 7 transmembrane (7TM) domains and alternatively spliced C-tails under the control of the promoter associated with exon 1 (Figure 1). The exon 1 promoter also generates a second set of truncated variants containing a single transmembrane domain (1TM). Although these variants are incapable of binding opioids, they impact opioid pharmacology through a chaperone activity with 7TM variants that stabilizes them, decreasing turnover and increasing overall expression (7). The last set of splice variants comprised of truncated 6 transmembrane domain (6TM) variants has proven increasingly interesting in recent years. This set is under different genetic control, being generated by a distinct promoter associated with exon 11 located approximately 30 kb upstream of exon 1 and generating a set of truncated 6TM variants with alternatively spliced C-tails.
Figure 1. Schematic of three categories of mouse Oprm1 splice variants.
Through alternative splicing, Oprm1 generates 7TM and 1TM variants through the exon 1 promoter and 6TM variants through the exon 11 promoter, located approximately 30 kb upstream of exon 1. Each class undergoes 3’ splicing to generate a diverse range of C-terminals (shown by ‘X’). The major class produces traditional 7TM receptors containing exons 1 (red), 2 (blue) and 3 (green) followed by unique collections of spliced exons yielding unique amino acid sequences for each variant. The 1TM variants are also associated with the exon 1 promoter and contain a single TM encoded by exon 1 with 3’ splicing to generate unique amino acid C-terminal sequences for each variant. The 6TM variants are associated with the exon 11 promoter and contain exons 11 (purple), 2 (blue), 3 (green) followed by unique collections of spliced exons yielding unique amino acid sequences for each variant. In the mouse, exon 11 encodes a unique sequence of 27 amino acids, with the exception of MOR-1K and mMOR-1L. Similar splicing schemes have been identified in both rats and humans.
Despite their atypical structure, these 6TM variants are pharmacologically relevant targets, as demonstrated with IBNtxA (3-iodobenzoyl-6β-naltrexamide)(8). Using knockout mouse models, IBNtxA analgesia was dependent upon 6TM variants and independent of 7TM variants. Biochemically, 125I-IBNtxA labeled a 6TM binding site that was clearly distinct from the classical 7TM mu receptors labeled by 3H-DAMGO ([D-Ala2,N-MePhe4,Gly(ol)5]-enkephalin) (8). The necessity of 6TM variants in IBNtxA analgesia was confirmed by rescue studies in which restoration of expression of a 6TM variant with a lentivirus vector in knockout mice lacking all 6TM and 7TM Oprm1 variants restored IBNtxA analgesia (9).
Endomorphin 1 and 2 are putative endogenous tetrapeptides lacking the canonical Tyr-Gly-Gly-Phe-Met/Leu structure present in all the other endogenous opioid peptides (10). They are very selective for mu receptors in binding studies, and lose their analgesic activity in knockout mice lacking 7TM variants (11–17). Yet, recent evidence suggests the possibility of different DAMGO and endomorphin mu analgesic mechanisms. Mice acutely tolerant to intracerebroventricular (i.c.v.) DAMGO failed to show cross tolerance to endomorphin 2 (18) and endomorphin 2 and DAMGO show differential sensitivities to the antagonists naloxonazine and 3-methoxynaltrexone (19). Here, we demonstrate at the genetic level the differences between DAMGO and endomorphin actions.
RESULTS
In vivo studies knockout models
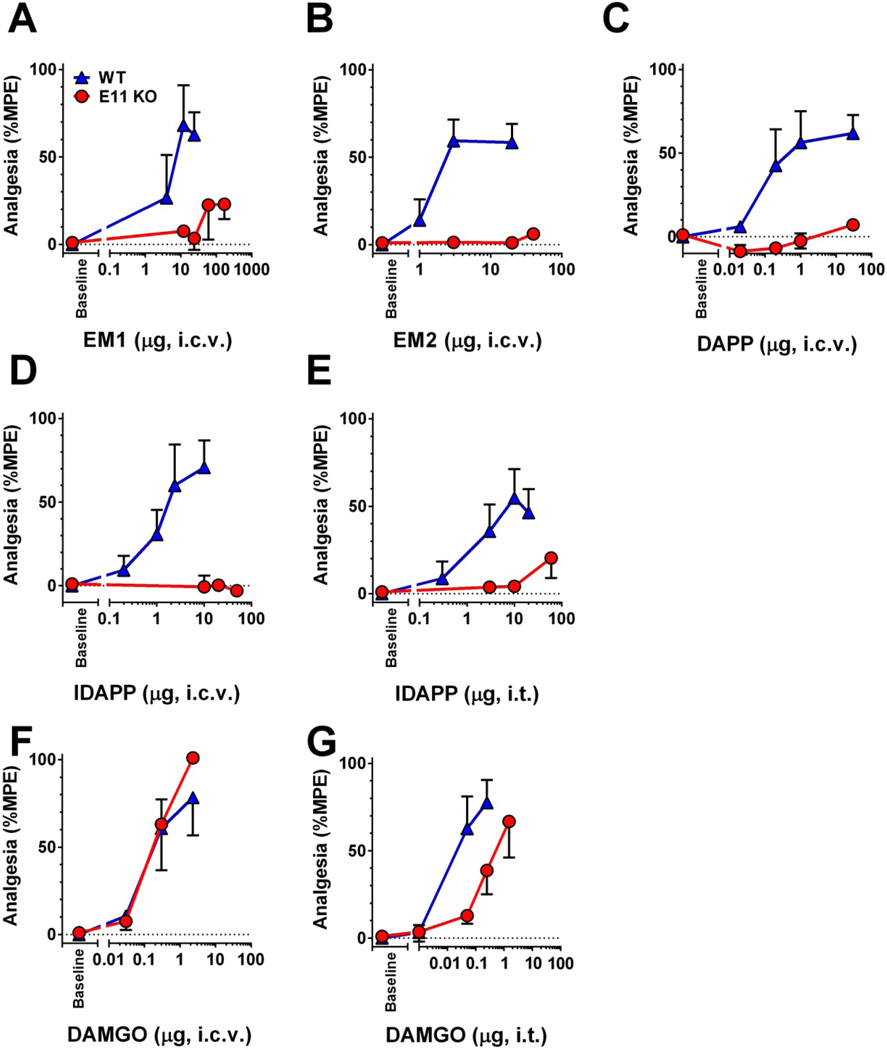
Endomorphin 1 and 2 are mu selective tetrapeptides (10). Morphine analgesia is independent of the E11 associated 6TM splice variants of the mu opioid receptor gene Oprm1, maintaining full analgesic activity in the E11 KO (20). Similarly, the mu opioid peptide DAMGO when given supraspinally retained full analgesic activity in E11 KO mice (Fig. 2f). At the spinal level, DAMGO still elicited a full response, but with a slightly lower potency (Fig. 2g). In contrast, loss of the 6TM variants eliminated the analgesic response of both supraspinal endomorphin 1 and endomorphin 2 (Fig. 2a,b). We next studied two synthetic endomorphin 2 analogs, DAPP (2’,6’-Dmt-D-Ala-Phe-Phe-NH2) and IDAPP (3-iodo-2’,6’-Dmt-D-Ala-Phe-Phe-NH2) (Fig. 2c,d,e). Both analogs displayed high selectivity and affinity for MOR-1 (Table 1). In vivo, DAPP and its iodinated analog IDAPP showed no activity in E11 KO mice, even at doses greater than 25-fold their wild type ED50 (Figure 2c–e). These findings clearly established the importance of E11 splice variants in endomorphin 1 and 2 and DAPP and IDAPP analgesic actions and differentiated them from both DAMGO and morphine.
Figure 2. Endomorphin and DAMGO analgesia in wildtype and exon 11 knockout mice.
Groups of C57Bl/6 wild type (WT) and exon 11 KO (E11 KO) (n=5–15) mice received either supraspinal (i.c.v.) or spinal (i.t.) injections of the indicated drugs at the indicated doses. All experiments were performed 2–3 times to ensure reproducibility and groups were pooled to determine responses. ED50 (95% confidence limits) are reported. The analgesic responses were insufficient to determine ED50s in the E11 KO mice for all compounds with the exception of DAMGO. Comparisons between wildtype and E11 KO for all drugs with the exception of DAMGO were made at a single drug dose and P values were determined by Students t-test. (a) Endomorphin 1 (EM1, i.c.v.) WT ED50 3.39 µg (1.9, 6.3), p=0.0014 (b) Endomorphin 2 (EM2, i.c.v.) WT ED50 1.14 µg (0.21, 5.8), p=0.0001 (c) DAPP (i.c.v.) WT ED50 0.10 µg (0.01, 0.83), p=0.0004 (d) IDAPP (i.c.v.) WT ED50 1.15 µg (0.44, 3.14), p=0.009 (e) IDAPP (i.t.) WT ED50 1.72 µg (0.09, 32.25), p=0.026 (f) DAMGO (i.c.v.) WT ED50 0.13 µg (0.02, 0.75) versus E11 KO ED50 0.23 µg (0.10, 0.49), p=0.24.(g) DAMGO (i.t.) WT ED50 0.02 µg (0.001, 0.21) versus E11 ED50 0.27 µg (0.04, 1.81), p<0.05.
Table 1.
Competition assays versus 125I-IBNtxA.
| Ki (nM) | |||||
|---|---|---|---|---|---|
| Peptide | Structure | 6TM/E11 | mMOR-1 | mDOR-1 | mKOR-1 |
| DAMGO | Tyr-D-Ala-Gly-N-MePhe-Gly-OH | >500 | 1 ± 0.3 | >500 | >500 |
| Endomorphin 1 | Tyr-Pro-Trp-Phe-NH2 | >500 | 7.6 ± 0.6 | >500 | >500 |
| Endomorphin 2 | Tyr-Pro-Phe-Phe-NH2 | >500 | 17 ± 6 | >500 | >500 |
| Dermorphin | Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2 | >500 | 4 ± 0.5 | 97 ± 40 | >500 |
| TAPP | Tyr-D-Ala-Phe-Phe-NH2 | 130 ± 16 | 3 ± 0.2 | 3 ± 0.1 | >500 |
| DAPP | Dmt-D-Ala-Phe-Phe-NH2 | 19 ± 6 | 0.2 ± 0.1 | 2 ± 0.2 | 3 ± 0.6 |
| IDAPP | Dmt(I)-D-Ala-Phe-Phe-NH2 | 16 ± 8 | 1 ± 0.2 | 58 ± 0.4 | 298 ± 6 |
| TIPP | Tyr-Tic-Phe-Phe-OH | 163 ± 12 | >500 | 33 + 3 | >500 |
| TAPS | Tyr-D-Ala-Phe-Sar-NH2 | 50 ± 8 | 6 ± 0.7 | 35 ± 2 | >500 |
| TAPA | Tyr-D-Ala-Phe-βAla-NH2 | 40 ± 7 | 11 ± 0.3 | 48 ± 1 | >500 |
| TAPPV | Tyr-D-Ala-Phe-Phe-Val-NH2 | >500 | >500 | 426 ± 23 | 178 ± 53 |
| TAPPI | Tyr-D-Ala-Phe-Phe-Ile-NH2 | 100 ± 10 | 0.2 ± 0.2 | 6 ± 0.1 | 69 ± 3 |
| DAPPI | Dmt-D-Ala-Phe-Phe-Ile-NH2 | 45 ± 11 | 0.3 ± 0.1 | 0.3 ± 0.2 | 7 ± 0.4 |
Peptides were screened in competition binding assays against 125I-IBNtxA to determine their relative potencies and selectivity for mMOR-CHO, mDOR-CHO, mKOR-CHO, and the 6TM-containing binding site labeled by 125I-IBNtxA with DAMGO, DPDPE, and U50,488 as blockers in mouse brain. Ki values were determined by nonlinear regression analysis (GraphPad Prism), and the means ± SEM of at least 3 the replicates are reported.
Lentivirus rescue of DAPP and IDAPP analgesia in knockout mice
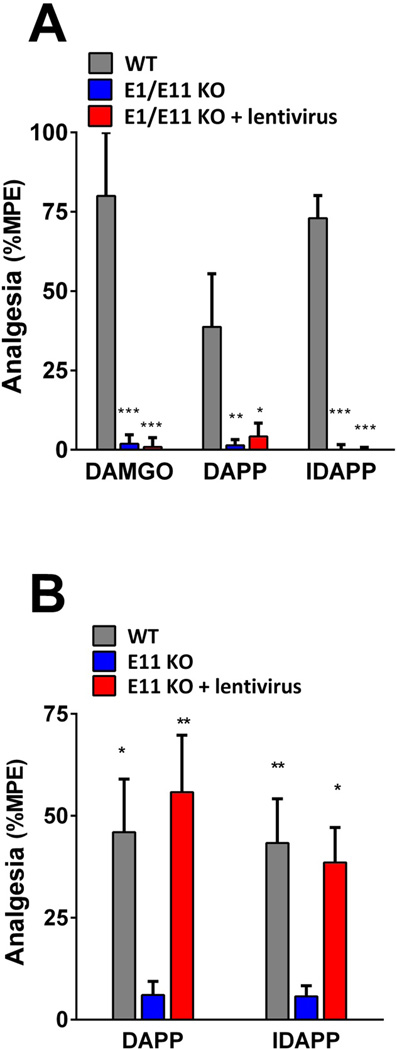
To confirm the importance of the 6TM variants in endomorphin analgesia, we attempted to rescue the response in knockout mice by reconstituting the expression of a 6TM E11 variant, mMOR-1G. This approach utilized a lentivirus construct, with a second control virus containing only the marker and lacking the mMOR-1G cassette, as previously described (9, 21). DAPP, IDAPP, and DAMGO were inactive in the in the exon 1/exon 11 (E1/E11) knockout mouse in which both exon 11 and exon 1 have been disrupted (9). These mice lack all Oprm1 variants. Despite being able to rescue IBNtxA (9), restoring mMOR-1G expression in these mice did not rescue DAPP, IDAPP or DAMGO analgesia (Fig. 3a), suggesting that all three required 7TM variants for activity. Both DAPP and IDAPP also were inactive in the E11 KO mouse (Fig. 3b, Fig. 2c,d) while DAMGO retained activity (Fig. 2f). Expression of mMOR-1G in the E11 KO rescued DAPP and IDAPP analgesia, restoring the response to wildtype levels and indicating that that they required both a 6TM and a 7TM for activity.
Figure 3. Lentivirus rescue of DAPP, IDAPP and DAMGO analgesia in E1/E11 and E11 knockout mice.
a) Groups of E1/E11 KO knockout mice and littermate control 129/C57Bl/6 wild type (WT) mice (n=5–10) were tested for analgesia following i.c.v. injections of DAMGO (1.2 µg), DAPP (3 µg), or IDAPP (12 µg). The third group comprised E1/E11 KO mice (n=3–4) previously treated with a lentivirus containing a cassette to express the 6TM variant mMOR-1G (E1/E11 KO + lentivirus) given i.c.v. at least 6 weeks previously (9, 21). Significance was determined with two-way ANOVA (F2,49=46.33, p<0.0001) with Bonferroni post hoc analysis. The two knockout groups were significantly different from wildtype, but not from each other. *p<0.05, **p<0.01, ***p<0.0001
b) Groups of E11 KO and C57Bl/6 wild type (WT) mice (n=6–10) were tested for analgesia after receiving i.c.v. DAPP (3 µg) or IDAPP (12 µg). The third group comprised E11 KO mice (n=8) previously treated with a lentivirus containing a cassette to express the 6TM variant mMOR-1G (E11 KO + lentivirus) given i.c.v. at least 6 weeks previously (9, 21). Significance was determined with two-way ANOVA (F2,43=12.32, p<0.001) with Bonferroni post hoc analysis. For both DAPP and IDAPP the E11 KO groups were significantly different from both the WT and from the E11 KO + lentivirus groups, which were not different from each other. *p<0.05, **p<0.01
Antisense effects on DAPP and IDAPP
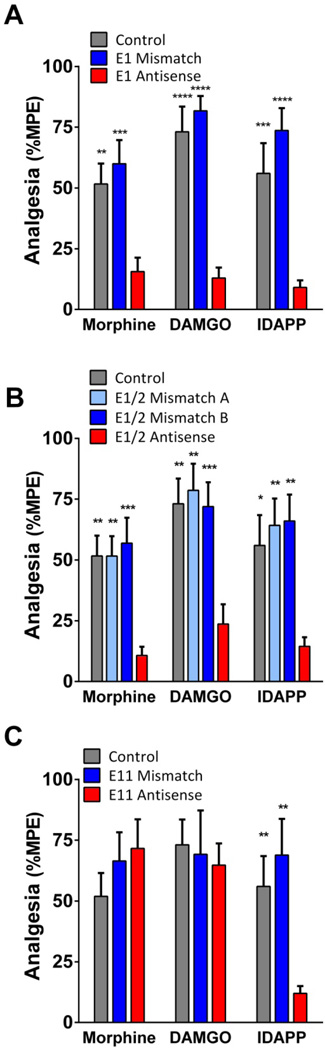
To confirm a role for 7TM variants in DAMGO and IDAPP analgesia, we used an antisense oligodeoxynucleotide mapping paradigm (22–24). Targeting exon 1, the antisense oligodeoxynucleotide lowered the analgesic actions of morphine, reproducing earlier studies (22). Similarly, the responses of DAMGO and IDAPP were lowered (Fig. 4a). The specificity of the response was established by the inactivity of the control mismatch.
Figure 4. Effect of antisense knockdown of 7TM and 6TM variants on IDAPP analgesia.
Groups of CD-1 mice (n=9–24) were injected with antisense oligodeoxynucleotides targeting exon 1 (E1 Antisense) (a), the splice junction of exons 1 and 2 (E1/2 Antisense) (b), exon 11 (E11 Antisense) (c), or mismatched (Mismatch) control oligodeoxynucleotides i.c.v. on days 1, 3, and 5. On day 6, mice received i.c.v. morphine (0.75 µg), DAMGO (0.6 µg), or IDAPP (1.2 µg) and were tested for analgesia. Control mice only received the opioid. All experiments were performed 3 times independently to ensure reproducibility and results pooled for analysis. Significance was determined by two-way ANOVA (E1: F2,115=48.76, p<0.0001; E1/2: F3,136=19.8, p<0.0001; E11: F2,98=2.3, p=0.10) with Bonferroni post hoc analysis. Asterisks indicate significance from antisense groups. Control and mismatch controls did not significantly differ from each other. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Since both the 7TM and the 1TM variants contain exon 1, this oligodeoxynucleotide antisense probe downregulates both sets of variants. To distinguish between them, we next employed an oligodeoxynucleotide antisense targeting the exon 1/exon 2 splice site junction (Supplemental Table 3) (7). Since the 1TM variants do not contain this sequence, the antisense selectively downregulates only 7TM variants and lowers morphine analgesia (7). In the current studies, the E1/E2 junctional antisense lowered morphine, DAMGO and IDAPP while neither mismatch probe had an effect (Fig. 4b). Finally, we examined an antisense probe targeting exon 11 (21) (Fig. 4c). These results were consistent with the knockout models, with the exon 11 antisense lowering only IDAPP but not morphine nor DAMGO. The mismatch was inactive. Together, these findings confirm the importance of E11-associated 6TM variants in IDAPP, but not morphine or DAMGO analgesia. Equally important, they indicated that IDAPP analgesia required expression of both 6TM and 7TM variants. The similar results in the antisense and knockout models argues that the E11 KO effects were not related to developmental/compensatory mechanisms since the antisense approaches involved adult mice with normal development.
Cross tolerance
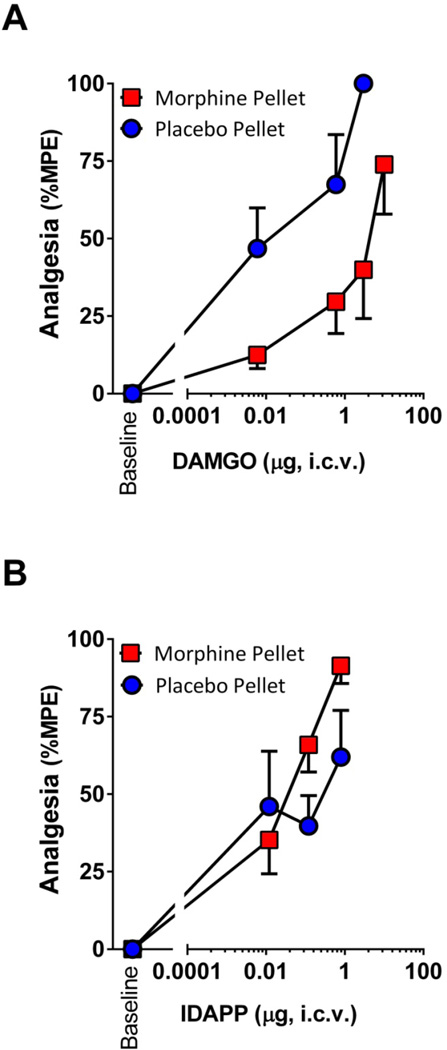
Cross tolerance is a sensitive measure of whether or not drugs share a common mechanism of action. Prior work demonstrated the lack of cross tolerance between DAMGO and endomorphin 2 (18). Since morphine, DAMGO, DAPP and IDAPP all require 7TM Oprm1 variants, the lack of cross tolerance raised the possibility of differences between the 7TM mechanisms involved with traditional mu opioids including morphine and DAMGO and the endomorphins. We used a morphine pelleting paradigm that has been extensively validated (25–28). DAMGO was cross tolerant with morphine, displaying more than a 35-fold shift in its ED50 in the morphine pelleted mice (Fig. 5). In contrast, IDAPP analgesia was unaffected. Indeed, at the higher doses, IDAPP was slightly more effective in morphine pelleted animals than in the placebo pellet controls.
Figure 5. DAMGO and IDAPP analgesia in morphine tolerant mice.
Groups of CD-1 mice (n=15–20) were implanted with morphine (75 mg free base) or placebo pellets. At 72 hr tail flick latencies returned to baseline values of 2–3 s, confirming the development of morphine tolerance at which point mice received i.c.v. a) DAMGO or b) IDAPP at the indicated dose and were tested for analgesia. Data are presented as %MPE at indicated doses, and ED50 values (mean ± SEM of 3 replicate experiments) were compared using Student’s t-tests. ED50 values for DAMGO were 0.04 ± 0.02 µg and 1.5 ± 0.47 µg for placebo and morphine groups, respectively (p<0.05). ED50 values for IDAPP were 0.11 ± 0.06 µg and 0.05 ± 0.01 µg for placebo and morphine groups, respectively (n.s.).
Receptor binding
Receptor binding studies further illustrated differences between the drugs 125I-IDAPP and 3H-DAMGO. In competition binding assays, DAPP showed high affinity for mu receptors and moderate affinity for delta and kappa receptors (Table 1). The incorporation of iodine into DAPP to generate IDAPP slightly lowered its mu affinity, but markedly increased its mu selectivity by lowering its delta and kappa affinity. DAPP and IDAPP also competed binding to the E11 binding site (8) with moderate affinity, further distinguishing them from DAMGO and the endomorphins.
125I-IDAPP binding was high affinity, with similar subnanomolar KD values in both brain and spinal cord tissue in all groups of mice (Table 2). Competition studies of 125I-IDAPP in mouse brain confirmed its mu selectivity, with delta and kappa ligands inactive (Table 3). Despite its mu selectivity and its saturation binding consistent with a single site, the shallow Hill slopes of 125I-IDAPP in the competition studies raised the possibility of binding heterogeneity. This extended to both agonists and the antagonist CTAP. The 125I-IDAPP results contrasted with the CTAP competitions of 3H-DAMGO binding in C57 brain tissue and CHO cells expressing MOR-1 where CTAP had IC50 values of 4.1 ± 0.2 and 4.8 ± 0.4 nM and Hill co-efficients of −1.1 ± 0.1 and −1.7 ± 0.09, respectively.
Table 2.
125I-IDAPP and 3H-DAMGO binding in exon 11 KO mice
| Brain | Spinal Cord | ||||
|---|---|---|---|---|---|
| Radioligand | Strain | WT | E11 KO | WT | E11 KO |
| BMAX (fmol/mg) | |||||
| 125I-IDAPP | C57 | 210 ± 5 | 112 ± 18**** | 206 ± 9 | 69 ± 5**** |
| 3H-DAMGO | C57 | 130 ± 7 | 103 ± 13 | 73 ± 5 | 71 ± 2 |
| 125I-IDAPP | 129 | 107 ± 14 | 33 ± 9*** | 226 ± 12 | 78 ± 16*** |
| 3H-DAMGO | 129 | 31 ± 0.1 | 33 ± 5 | 76 ± 1 | 78 ± 1 |
| KD (nM) | |||||
| 125I-IDAPP | C57 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| 3H-DAMGO | C57 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.2 |
| 125I-IDAPP | 129 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| 3H-DAMGO | 129 | 0.5 ± 0.01 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.02 |
Binding was assessed in E11 KO mice bred on C57Bl/6 and 129 backgrounds. Experiments were replicated at least 3 times. BMAX and KD were determined by nonlinear regression analysis (GraphPad Prism), and the means ± SEM of the replicates are reported. Significant difference was calculated by two-way ANOVA with Bonferroni post hoc analysis. (BMAX brain: F3,16=8.35, p=0.0014; KD brain: F3,16=0.61, p=0.62; BMAX spinal cord: F3,16=50.78, p<0.0001; KD spinal cord F3,16=2.66, p=0.08)
p<0.001,
p<0.0001
Table 3.
Competition of 125I-IDAPP binding in mouse brain
| Compound | Ki (nM) | Hill Slope |
|---|---|---|
| Morphine | 0.8 ± 0.2 | −0.6 ± 0.1 |
| Methadone | 1.4 ± 0.4 | −0.7 ± 0.1 |
| M6G | 0.7 ± 0.3 | −0.6 ± 0.1 |
| Buprenorphine | 0.2 ± 0.1 | −0.6 ± 0.1 |
| CTAP | 1.1 ± 0.3 | −0.5 ± 0.1 |
| DAMGO | 1.2 ± 0.5 | −0.9 ± 0.1 |
| Endomorphin 1 | 1.6 ± 0.5 | −0.7 ± 0.1 |
| Endomorphin 2 | 2.1 ± 0.8 | −0.5 ± 0.1 |
| SNC80 | >1,000 | |
| Naltrindole | >1,000 | |
| U50,488H | >1,000 | |
| NorBNI | >1,000 |
Competition assays were performed at 0.1 nM 125I-IDAPP in C57/Bl6 brain homogenates in the absence of blockers. Data represent mean ± SEM of at least 3 determinations of the Ki value. Ki values and Hill slopes were calculated using GraphPad Prism.
The Bmax of 125I-IDAPP binding far exceeded that of 3H-DAMGO in both C57 and 129 wildtype mouse brains, with even greater differences in spinal cord tissue (Table 2). 3H-DAMGO binding levels on both backgrounds did not appreciably differ between E11 KO and wildtype mice. In contrast, 125I-IDAPP binding was significantly lower in the E11 KO mice (Fig. 6a; Table 2), with levels in the E11 KO brain and spinal cord membranes approximating those seen with 3H-DAMGO (Table 2). These results suggested that wildtype 125I-IDAPP binding was comprised of two distinct populations, one of which was dependent on E11 variants while the other, with levels similar to 3H-DAMGO binding, likely reflected labeling to traditional full length 7TM mu receptor variants.
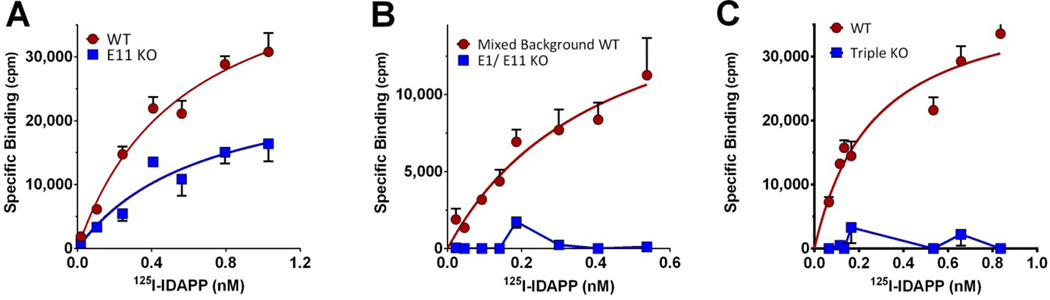
Figure 6. 125I-IDAPP saturation binding studies in E11, E1/E11 and triple KO brain.
125I-IDAPP saturation studies were carried out on brain membranes from a) wild type (WT) and exon 11 KO (E11 KO) mice, b) E1/E11 KO and control mixed background mice, and c) triple KO and control wildtype mice. Only specific binding is reported. Experiments were replicated at least 3 times. KD and Bmax were determined by nonlinear regression analysis (GraphPad Prism), and the means ± SEM of replicates were determined. KD values were best fit with a single site. a) Wild Type C57Bl/6: KD 0.5 ± 0.1 nM, BMAX 210 ± 5 fmol/mg; Exon 11 KO: KD 0.5 ± 0.1 nM, BMAX 112 ± 18 fmol/mg (t-test, p<0.01); B) Wild type 129/C57Bl/6: KD 0.4 ± 0.1 nM, BMAX 89 ± 27 fmol/mg, Exon 1/Exon 11 KO: KD and BMAX Not detectible; C) Wild type C57Bl/6: KD = 0.4 ± 0.03 nM, Bmax = 203 ± 13 fmol/mg, Triple KO: KD and BMAX Not detectible.
The complete loss of all I-IDAPP binding in the combined E1/E11 knockout (Fig. 6b) confirmed that both populations were totally dependent on Oprm1 gene products and did not involve simple cross labeling of delta or kappa receptors, consistent with the insensitivity of 125I-IDAPP binding to delta or kappa ligands (Ki > 1 µM) (Table 3). In addition, 125I-IDAPP binding was totally lost in a triple KO mouse (Fig. 6c). The triple KO mice (29) were generated by crossing an exon 1 mu knockout mouse (16) with delta (30) and kappa receptor KO mice (31). Loss of exon 1 of the Oprm1 gene removes all the full length 7TM and the 1TM MOR-1 variants, leaving the expression of only the exon 11 variants. Crossing these E1 KO mice with delta and kappa KO mice eliminated all the traditional mu opioid receptors, leaving the expression of only the E11-associated 6TM variants. The elimination of all 125I-IDAPP binding in the triple KO mice implied that both populations of 125I-DAPP binding sites required 7TM sites.
Functional cellular assays
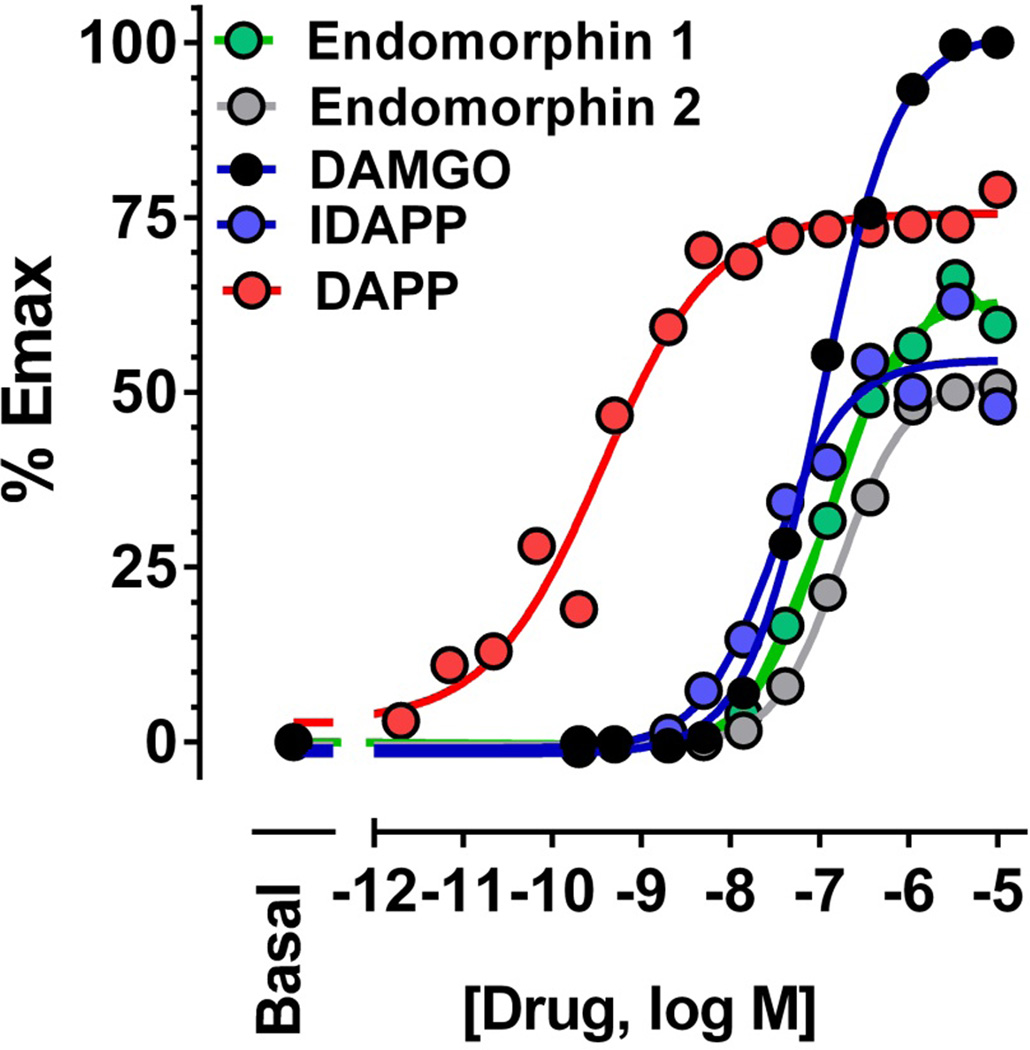
The endomorphins and IDAPP were potent and efficacious agonists in 35S-GTPγS binding assays (Table 4). In the transfected cell lines, they were quite efficacious against MOR-1, with little effect in the kappa and delta cell lines. In E1/E11 KO brain membranes none of the compounds stimulated 35S-GTPγS binding, consistent with the absence of 125I-IDAPP binding and confirming their inability to stimulate 35S-GTPγS binding through delta or kappa receptors. In the brain, DAMGO stimulated binding equally well in both wildtype and E11 KO mice. The maximal stimulation of IDAPP and of endomorphin 1 in wildtype mice exceeded DAMGO and was lowered by almost 50% and by 80%, respectively, in the E11 KO membranes. These results were similar to the receptor binding studies. In the E11 KO brain membranes, the maximal stimulation by all the compounds was similar. In the spinal cord, we saw a greater stimulation of 35S-GTPγS binding by the endomorphins and IDAPP compared to DAMGO. In addition to stimulating 35S-GTPγS binding, all the peptides stimulated β-arrestin −2 recruitment (Fig. 7), although they all showed lower maximal values than DAMGO. DAPP stood out based upon its more than 100-fold lower ED50 than the others, which was similar to its far greater potency stimulating 35S-GTPγS binding (ED50 0.45 ± 0.13; maximal increase over basal 186±3%.
Table 4.
35S-GTPγS binding in exon 11 KO, exon 1/exon 11 KO, mMOR-CHO, mDOR-CHO, and mKOR-CHO membranes.
| Brain | Spinal Cord | CHO | ||||||
|---|---|---|---|---|---|---|---|---|
| Peptide | WT | E11 KO |
E1/E11 KO |
WT | E11 KO |
mMOR | mDOR | mKOR |
| Max stimulation (% over basal) | ||||||||
| DAMGO | 15 ± 2 | 19 ± 6 | −6 ± 1 | 11 ± 2 | 7 ± 4 | 396 ± 21 | −2 ± 7 | −16 ± 8 |
| IDAPP | 31 ± 3 | 17 ± 4* | −2 ± 2 | 38 ± 2 | 21 ± 3* | 399 ± 6 | 42 ± 2 | 44 ± 3 |
| EM1 | 39 ± 2 | 23 ± 1** | −5 ± 3 | 38 ± 4 | 44 ± 2 | 322 ± 19 | −3 ± 1 | 22 ± 5 |
| EM2 | 35 ± 1 | 24 ± 2** | 1 ± 1 | 45 ± 3 | 36 ± 4 | 285 ± 10 | −3 ± 2 | 7 ± 4 |
| EC50 (nM) | ||||||||
| DAMGO | 190 ± 8 | 220 ± 13 | N.D. | 67 ± 8 | 50 ± 10 | 9 ± 1 | N.D. | N.D. |
| IDAPP | 60 ± 9 | 78 ± 5 | N.D. | 51 ± 2 | 83 ± 8 | 17 ± 1 | >500 | >500 |
| EM1 | 222 ± 31 | 17 ± 7** | N.D. | 316 ± 64 | 330 ± 30 | 44 ± 8 | N.D. | 216 ± 48 |
| EM2 | 151 ± 43 | 103 ± 8 | N.D. | 345±69 | 226 ± 14 | 10 ± 0.1 | N.D. | >500 |
35S-GTPγS binding in brain, spinal cord, and CHO cells stably expressing opioid receptors. Maximal stimulation (% increase over basal in the absence of drug) and EC50 values were calculated using nonlinear regression analysis with GraphPad Prism and are presented as mean ± SEM of at least 3 replicates. ANOVA analysis of the maximal stimulation in brain was significant for each drug (See Supplemental Figure 4). Bonferroni posthoc analysis revealed that the E1/E11 KO group was significantly different from both the other groups for all drugs. The WT groups were significantly different from the E11 groups for IDAPP, EM1 and EM2, but not for DAMGO. The EC50 values in the WT and the E11 KO groups were not significantly different by Student t-test for DAMGO, IDAPP or EM2, but the values for EM1 were significantly different. Comparison of maximal stimulation in spinal cord revealed a significant drop between the WT and E11 KO group for IDAPP, but not for the other drugs. No differences in EC50 values were observed.
p<0.05,
p<0.01,
p<0.001
Figure 7. Recruitment of β-arrestin-2 by opioid peptides.
β-arrestin-2 recruitment was determined using the DiscoveRx PathHunter enzyme complementation assay as described in Methods. At least 3 independent experiments were performed with figures representing mean ± SEM of independent determinations.
Discussion
The Oprm1 gene is genetically complex, contributing to the variable efficacy and side effects of different mu selective ligands. Endomorphin 1 and 2 are the only putative mu-selective endogenous opioids (10). Prior reports suggested different mu receptor mechanisms for endomorphin 1 and 2 and for DAMGO (18, 32, 33). Our results support these differences and extend them to DAPP and IDAPP.
Mu opioids were initially classified by their sensitivity towards antagonists (1, 34–37). However, the availability of a panel of knockout mice now offers the ability to define opioid actions at the level of the gene (Supplemental Table 1) (9, 16, 20, 30, 31). Morphine acts through classical 7TM receptors, as demonstrated first by antisense approaches (22, 38) and by a series of knockout mice (13, 15, 16, 39). However, morphine and methadone retain full analgesic activity in exon 11 knockout mice that lack truncated 6TM receptors but still express 7TM mu opioid receptors (Supplemental Table 1) (20).
DAMGO displays a selectivity profile similar to morphine, retaining full activity in the E11 KO mouse and showing cross tolerance to morphine. On the other hand, the endomorphins and their analogs had a very different profile, losing their analgesic activity in the E11 KO mice. IDAPP also failed to show cross tolerance to morphine. Despite the importance of E11 variants in their activity, the peptides also are readily distinguished from IBNtxA (8, 9, 40, 41). IBNtxA is a potent analgesic acting through E11 mechanisms. Its actions are independent of other opioid receptors, including delta, kappa and 7TM MOR-1 variants, as demonstrated by its continued activity in triple KO mice. In contrast, endomorphin 1 and 2, DAPP and IDAPP all require expression of exon 1-associated 7TM variants. Whereas IBNtxA analgesia can be rescued in E11 KO mice by a lentivirus expressing the 6TM variant mMOR-1G (9), neither DAPP nor IDAPP were rescued. However, the same lentivirus could rescue the DAPP and IDAPP response in E11 KO mice that continued to express 7TM variants. Antisense approaches further illustrated the need for both 6TM and 7TM variants for IDAPP activity. Antisense downregulation of exon 1-containing variants lowered morphine, DAMGO and IDAPP analgesia. The importance of full length 7TM variants was further established by the similar activity of the probe targeting the exon 1/2 splice junction. However, only IDAPP was affected by downregulation of the E11 variants. Thus, endomorphin 1 and 2, DAPP and IDAPP do not fit into either the morphine or IBNtxA category of mu opioids, implying a third category.
125I-IDAPP binding studies further supported the possibility of a distinct site associated with E11 variants. 125I-IDAPP binding levels in both brain and spinal cord wildtype membranes were significantly higher than 3H-DAMGO in the same membranes. However, their binding levels were equivalent in the E11 KO membranes, with 125I-IDAPP binding decreasing to 3H-DAMGO levels in the E11 KO mice. This is consistent with two populations of 125I-IDAPP sites, with one corresponding to the same classical mu sites labeled by 3H-DAMGO and a separate population dependent upon 6TM variants. Both populations of 125I-IDAPP binding sites depended on the mu opioid receptor gene Oprm1 since they both were lost in the E1/E11 KO brains.
Several lines of evidence argue against the possibility that these binding differences reflect agonist/antagonist conformational changes. The competition studies with 125I-IDAPP revealed shallow Hill coefficients for a range of agonists. While agonist competitions often are shallow against radiolabeled antagonists, 125I-IDAPP is an agonist so a slope of unity would be expected. Furthermore, the slope of the antagonist CTAP also was shallow (−0.5 ± 0.01), which would not be expected on the basis of agonist/antagonist conformations. To further support this, CTAP competitions of the agonist 3H-DAMGO in brain membranes from C57 mice and cell lines expressing MOR-1 revealed the expected steep slopes (−1.1 ± 0.1 and −1.7 ± 0.09, respectively).
Thus, both behavioral and binding studies indicate an E11-dependent target for IDAPP and the other endomorphin derivatives. How the 6TM and 7TM MOR-1 variants interact to mediate endomorphin analgesia is unclear. Several possibilities might be considered. In one, the 6TM may physically associate with the 7TM to form a heterodimer with a distinct pharmacology. Alternatively, the 6TM may act downstream from the 7TM within the analgesic circuit. This scenario could give similar binding results if 125I-IDAPP separately labeled both the 7TM and a second downstream 6TM target, which could even involve distant regions such as spinal/supraspinal pathways. The increased binding of 125I-IDAPP compared to 3H-DAMGO in wildtype mice and the reduction of 125I-IDAPP binding to 3H-DAMGO levels in the E11 KO mice would be consistent with either possibility. Prior work established the relevance of heterodimers involving single transmembrane domain (1TM) and 6TM variants (7). In transfected cell lines expressing both 6TM and 7TM variants, the two co-immunoprecipitate, indicating that they can heterodimerize. However, the ability of the variants to dimerize in transfected cells may not predict native conditions.
In conclusion, our findings extend our results implicating truncated Oprm1 6TM variants in analgesic mechanisms and suggest they may help explain many of the subtle, but important, clinical differences among mu opioids (Table 5). 6TM variants are important in differentiating different mu opioid receptor mechanisms. IBNtxA illustrates a compound dependent only upon 6TM Oprm1 variants, while morphine and methadone act only through 7TM targets. The tetrapeptides endomorphin 1 and 2, DAPP and IDAPP reveal a third category of mu opioid dependent upon both 6TM and 7TM variants. Prior work had established that endomorphin 2 analgesia was lost in an exon 2/3 mu receptor knockout mouse (17). Since both 6TM and 7TM variants contain exons 2 and 3, these mice presumably would lack both sets of variants. Our E11 KO mouse findings, along with the antisense mapping studies, extends this result to implicate both 6TM and 7TM variants. Thus, we predict three classes of mu opioid analgesics. However, the importance of 6TM variants extends beyond mu opioids (Table 5). Recent studies have implicated 6TM variants in the analgesic mechanisms of delta and kappa opioids, as well as α2 adrenergic compounds (21). Since these compounds also lose activity in knockouts of their respective prototypic receptors, their analgesic mechanisms require expression of both 6TM Oprm1 variants and their respective receptors. Like the endomorphin tetrapeptides, however, the molecular mechanisms remain unclear, with both direct dimerization or downstream interactions within a circuit remaining possibilities. While the focus of these studies has been on analgesia, 6TM variants may be involved in alternative actions, such as hyperalgesia (42). Thus, truncated Oprm1 variants provide a diversity of action beyond the traditional 7TM GPCRs.
Table 5.
Summary of the involvement of Oprm1 variants in mu opioid analgesia
| MOR -1 Variants required for analgesia |
Drug | Non-mu opioid receptor |
Reference |
|---|---|---|---|
| 7TM | Morphine | (8, 20) | |
| Methadone | (8, 20) | ||
| DAMGO | Figs. 2,3,4 | ||
| 7TM + 6TM | Buprenorphine | (54) | |
| Endomorphin 1 | Fig. 2 | ||
| Endomorphin 2 | Fig. 2 | ||
| DAPP | Figs. 2,3,4 | ||
| IDAPP | Figs. 2,3,4 | ||
| DPDPE | Delta opioid | (21) | |
| SNC80 | Delta opioid | (21) | |
| 6TM | IBNtxA | Unknown | (8, 40, 46) |
| Ketocyclazocine | Kappa opioid | (21) | |
| U50,488H | Kappa opioid | (21) | |
| Salvinorin A | Kappa opioid | (21) | |
| Clonidine | α2-adrenergic | (21) | |
| Dexmedetomidine | α2-adrenergic | (21) | |
Summary of the requirements of a series of analgesics for various classes of Oprm1 variants based upon analgesia in either knockout models and/or antisense models. Mu analgesics can be subclassified based on their requirements for MOR-1 splice variants. The first category includes drugs that require 7TM variants independent of 6TM variants. The second category requires both 6TM and 7TM variants to be expressed for activity. For the third class of drugs, 6TMs are both necessary and sufficient for activity (e.g. IBNtxA). No non-opioid receptor that is involved with IBNtxA analgesia has yet been identified, but this remains a possibility. A number of non-opioid analgesics that depend upon Oprm1 6TM variants for activity also require their respective non-mu opioid receptor, as indicated in the third column. Delta compounds are unique in that they require both 6TM and 7TM Oprm1 variants as well as delta opioid receptors. It is not clear whether the 6TM variants play a direct role in the targets for these non-mu analgesics (e.g. heterodimerization) or modulate the response through downstream mechanisms
METHODS
Mice
Male CD-1, 129, and C57Bl6/J mice (24–38 g) were purchased from Jackson Laboratories. Male and female mice from a variety of knockout mouse models were utilized. Triple KO mice (29) lacked delta, kappa and 7TM mu opioid receptors, but still expressed 6TM variants (Supplemental Table 1). They were obtained by cross breeding exon 1 mu receptor KO mice (16) with delta receptor knockout and kappa opioid receptor knockout mice (29, 30). Littermates served as controls. Mice with a targeted disruption of exon 11 (E11 KO) were generated and backcrossed over 10 generations into C57Bl/6 (20). Wildtype C57Bl/6 mice provided controls for this group. Mice lacking all variants generated from the mu opioid receptor gene Oprm1 were generated by targeting both exon 1 and exon 11 (E1/E11 KO). This group was on a mixed 129/C57Bl/6 background and littermates were used as controls (9). Mice were maintained on a 12 h light/dark cycle with Purina rodent chow and water available ad libitum and housed in groups of 4–5 until testing. Both male and female mice were included and KO and wild type control mice were matched for sex and age. Although not formally evaluated, no obvious sex differences were observed. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Memorial Sloan Kettering Cancer Center and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals in an AAALAC accredited facility.
Drugs and Chemicals
Opiates were obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD). Endomorphin 1 and 2 were purchased from Tocris. TIPP was purchased from Phoenix Pharmaceuticals. 2’,6’-Dmt-D-Ala-Phe-Phe-NH2 (DAPP), 3-iodo-Dmt-D-Ala-Phe-Phe-NH2 (IDAPP) (43) and analogs were synthesized by Celtek Peptides (Franklin, TN). Miscellaneous chemicals and buffers were purchased from Sigma-Aldrich.
3H-D-Ala2,N-MePhe4,Gly(ol)5]Enkephalin (DAMGO; 53.4 Ci/mmol) was received from PolyPeptide Group (San Diego, CA). 125I-BNtxA was synthesized as previously described (8, 44). 125I-IDAPP was synthesized (45) using chloramine T. DAPP was dissolved in sodium phosphate buffer (50 mM, pH 7.4). Chloramine T (10 µL of a 1 mg/ml solution) was added to 15 nmol DAPP (15 µl), sodium phosphate buffer (50 µ L, 50 mM, pH 7.4), and 1 mCi of Na125I (Perkin-Elmer). The iodination reaction was quenched after 30 s with 100 µL of 2 mg/mL cysteine in 50 mM sodium phosphate buffer. The contents of the reaction vial were immediately injected onto a reverse phase HPLC C18 column (Thermo Scientific, 150×4.6mm, 5um) and run using a water-0.1% trifluroacetic acid/acetonitrile gradient, from 5% to 35% acetonitrile. The retention time of the desired product (final yield 17%) was 11 min and confirmed with a nonradioactive IDAPP standard (Supplemental Figure 1). Binding was linear with tissue (Supplemental Figure 2) and mu selective based on competition and saturation assays performed in various KO mouse tissues.
Binding Assays
Binding assays were performed in whole brain membrane homogenates or membranes from cell lines stably transfected with MOR-1, DOR-1 or KOR-1 as previously described (8). Assays were optimized to 25°C for 90 min in potassium phosphate buffer (50 mM, pH 7.4, 5 mM MgSO4) (Supplemental Figure 3) after which samples were filtered through glass-fiber filters (Whatman Schleicher & Schuell, Keene, NH) and washed three times with 3 mL of Tris-HCl, (50 mM, 0oC, pH 7.7) on a semiautomatic Brandel cell harvester. For 3H-DAMGO assays, filters were transferred into vials containing Liquiscent (3 ml, National Diagnostics, Atlanta, GA), and the radioactivity determined by scintillation spectroscopy in a Tri-Carb 2900TR counter (PerkinElmer Life and Analytical Sciences). Nonspecific binding was defined by levallorphan (8 µM) and was subtracted from total binding to yield specific binding. KD, Bmax, and Ki values were calculated by nonlinear regression analysis (GraphPad Prism, San Diego, CA). Data are reported as mean and S.E.M. of at least 3 independent replicates.
125I-IBNtxA binding in wild type mice examining at E11 binding sites was carried out in the presence of mu [D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)], kappa (U50,488H), and delta (DPDPE) blockers (200 nM each) as previously described (8, 44, 46). Binding on transfected cell lines used 125I-IBNtxA in the absence and presence of levallorphan (8 µM) to define nonspecific binding. Membranes were prepared from CHO cells stably transfected with mMOR-1, mDOR-1 or mKOR-1as previously described (47). Protein concentrations were determined using the Lowry method with BSA as the standard (48).
35S-GTPγS binding was performed on membranes prepared from brain or spinal cord in the presence and absence of the indicated peptide for 60 min at 30°C in the assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, and 10 mM NaCl) containing S-GTPγS (0.05 nM), leupeptin, pepstatin, aprotinin, and bestatin (2 µg/mL each) and GDP (30 µM), as previously reported (49). After the incubation, the reaction was filtered through glass-fiber filters (Whatman Schleicher & Schuell, Keene, NH) and washed three times with 3 mL of ice-cold 50 mM Tris-HCl, pH 7.7, placed into 3 mL of Liquiscent (National Diagnostics, Atlanta, GA), and the radioactivity determined by scintillation spectroscopy. Basal binding was determined in the presence of GDP and the absence of drug. Maximal stimulation and EC50 values were calculated by nonlinear regression analysis (GraphPad Prism, San Diego, CA). Data are reported as mean and S.E.M. of 3 independent replicates.
β-arrestin-2 assays
β-arrestin-2 recruitment was determined using the PathHunter enzyme complementation assay (DiscoveRx. Fremont, CA). Cells were plated at a density of 2500 cells/well in a 384-well plate as described in the manufacturer’s protocol. The following day, cells were treated with DAMGO, endomorphin 1, endomorphin 2, DAPP, or IDAPP for 90 minutes at 37°C followed by incubation with PathHunter© detection reagents for 60 minutes. Chemiluminescence was measured with an Infinite M1000 Pro plate reader (Tecan, Männedorf, Switzerland).
Analgesia Assays
Analgesia was determined using the radiant heat tail flick technique (Ugo Basile; model 37360). The intensity was set to achieve a baseline latency between 2 and 3 s. The latency to withdraw the tail from a focused light stimulus was measured electronically using a photocell. Baseline latencies were determined before experimental treatments for all mice. Post-treatment tail flick latencies were determined as indicated for each experiment, and a maximal latency of 10 s for tail flick was used to minimize tissue damage. Tail flick analgesia was assessed by percent maximal effect (%MPE) of tail flick latency according to the formula: %MPE = [(Observed latency - Baseline latency) / (10 - Baseline latency)] × 100. Similar results were obtained when data was analyzed quantally with analgesia defined as a doubling of the baseline latency. Analgesic ED50’s were determined using nonlinear regression analysis (Graphpad Prism). All experiments were replicated 2–3 times with similar results. Mice were allowed at least one week of wash out before being retested.
Peptides were delivered intracerebroventricularly (i.c.v.) as previously described (50). Briefly, a small scalp incision was made under isoflurane anesthesia and compounds (2–4 µL/mouse) were injected using a 10 µL Hamilton syringe fitted to a 27 gauge needle into the right lateral ventricle at the following coordinates: 2 mm caudal to bregma, 2 mm lateral to sagittal suture, and 2 mm in depth. Mice were tested for analgesia at peak effect (10 min) following i.c.v. injections. DAMGO and IDAPP were also administered intrathecally (i.t.) via lumbar puncture (1 µL) as previously described (51, 52). Mice were tested for analgesia at peak effect (15 min) following i.t. injections.
Cross tolerance
Cross tolerance was performed as previously described (8, 41, 53). Groups of mice (n=15–20) were implanted with subcutaneous morphine (75 mg) or placebo pellets while under isoflurane anesthesia. All morphine pelleted mice reached tail flick cutoff values (10 sec) on the day of implantation while none of the placebo pellet animals did. Morphine tolerance was seen 72 hours later when the tail flick returned to baseline latencies of 2–3 s despite the continued presence of the morphine pellets. The mice were randomly assigned to receive either supraspinal IDAPP or DAMGO and analgesic dose responses were determined.
Antisense treatments
Groups of mice received the stated antisense (5–10 µg) or mismatch (5–10 µg) oligodeoxynucleotide i.c.v. under isoflurane anesthesia on days 1, 3 and 5, as previously described (21, 22, 38). Analgesia was tested on day 6. The antisense oligodeoxynucleotides were based upon the mouse MOR-1 sequence and targeted either E1, the splice junction of exons 1 and 2, or exon 11 (22) (Supplemental Table 2). The downregulation pattern of the oligodeoxynucleotide antisense probes and a summary of their actions has been established previously (Supplemental Table 3) (7, 21–23, 38).
Lentiviral Injections
Reconstitution of a 6TM variant, mMOR-1G, was carried out using a lentivirus vector with eGFP (enhanced GFP) with or without the mMOR-1G cassette. Detailed methods have been previously described (9, 21). In brief, lentiviral particles (1.5 × 109 transducing units in 4 µl) were injected supraspinally to E11 KO mice on days 1, 3, and 5. Mice were tested for analgesia at least 6 weeks later when mMOR-1G was at peak expression (9, 21). Widespread expression of eGFP in the brain was observed 22 days after virus injection and 6TM variant mRNA expression persisted for at least a year at levels similar to that in wildtype brain (9, 21).
Supplementary Material
Acknowledgments
This work was supported by research grants from the National Institute on Drug Abuse of the National Institutes of Health (DA06241 and DA07242) to GWP and (DA034106) to SM, grants from the Peter F. McManus Charitable Trust and from the Harrington Discovery Institute, a Core Grant from the National Cancer Institute to MSKCC (CA08748), and the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1257284 to GFM. The authors thank Mingming Xu and Joan Subrath for their technical assistance with mouse breeding, binding, and chemistry.
Abbreviations
- DAMGO
[D-Ala2,MePhe4,Gly(ol)5]enkephalin
- DAPP
2’,6’-Dmt-D-Ala-Phe-Phe-NH2
- IDAPP
3-iodo-Dmt-D-Ala-Phe-Phe-NH2
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- IBNtxA
3-iodobenzoyl-6β-naltrexamide
- GPCR
G-protein coupled receptor
- 7TM
7 transmembrane domain receptor generated from the mu opioid receptor gene Oprm1
- 6TM
6 transmembrane domain receptor generated from the mu opioid receptor gene Oprm1
- MOR-1
variant generated from the mu opioid receptor gene Oprm1
- DOR-1
delta opioid receptor clone generated from the Oprd1 gene
- KOR-1
kappa opioid receptor clone generated from the Oprk1 gene
Footnotes
Supporting information:
Additional information summarizing the knockout models, antisense methods and receptor binding studies.
References
- 1.Pasternak GW, Pan Y-X. Mu opioids and their receptors: Evolution of a concept. Pharmacol.Rev. 2013;65:1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley KM. Controlling the pain of cancer. Sci.Am. 1996;275:164–165. doi: 10.1038/scientificamerican0996-164. [DOI] [PubMed] [Google Scholar]
- 3.Payne R, Pasternak GW. Pain. In: Coyles SJEJT, editor. Pharmacological Management of Neurological and Psychiatric Disorders. New York: McGraw-Hill; 1998. pp. 429–457. [Google Scholar]
- 4.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc.Natl.Acad Sci U.S.A. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang A, Emmel DW, Rossi GC, Pasternak GW. Methadone analgesia in morphine-insensitive CXBK mice. European Journal of Pharmacology. 1998;351:189–191. doi: 10.1016/s0014-2999(98)00366-5. [DOI] [PubMed] [Google Scholar]
- 6.Neilan CL, King MA, Rossi G, Ansonoff M, Pintar JE, Schiller PW, Pasternak GW. Differential sensitivities of mouse strains to morphine and [Dmt1]DALDA analgesia. Brain Res. 2003;974:254–257. doi: 10.1016/s0006-8993(03)02590-3. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Xu M, Brown T, Rossi GC, Hurd YL, Inturrisi CE, Pasternak GW, Pan YX. Stabilization of the mu opioid receptor by truncated single transmembrane splice variants through a chaperone-like action. J.Bio.Chem. 2013;288:21211–21227. doi: 10.1074/jbc.M113.458687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar S, Grinnell S, Le RV, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc.Natl.Acad.Sci.U.S.A. 2011;108:19776–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Z, Xu J, Rossi GC, Majumdar S, Pasternak GW, Pan YX. Mediation of opioid analgesia by a truncated 6-transmembrane GPCR. J Clin Invest. 2015;125:2626–2630. doi: 10.1172/JCI81070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the µ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 11.Qiu CY, Sora I, Ren K, Uhl G, Dubner R. Enhanced δ-opioid receptor-mediated antinociception in µ-opioid receptor-deficient mice. European Journal of Pharmacology. 2000;387:163–169. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- 12.Monory K, Bourin MC, Spetea M, Tömböly C, Tóth G, Matthes HW, Kieffer BL, Hanoune J, Borsodi A. Specific activation of the µ opioid receptor (MOR) by endomorphin 1 and endomorphin 2. European Journal of Neuroscience. 2000;12:577–584. doi: 10.1046/j.1460-9568.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 13.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 14.Narita M, Mizoguchi H, Sora I, Uhl GR, Tseng LF. Absence of G-protein activation by µ-opioid receptor agonists in the spinal cord of µ-opioid receptor knockout mice. British Journal of Pharmacology. 1999;126:451–456. doi: 10.1038/sj.bjp.0702330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define µ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc.Natl.Acad.Sci.USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuller AG, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat.Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 17.Loh HH, Liu HC, Cavalli A, Yang WL, Chen YF, Wei LN. Mυ opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Molecular Brain Research. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 18.Wu HE, Hung KC, Mizoguchi H, Fujimoto JM, Tseng LF. Acute antinociceptive tolerance and asymmetric cross-tolerance between endomorphin-1 and endomorphin-2 given intracerebroventricularly in the mouse. J Pharmacol Exp Ther. 2001;299:1120–1125. [PubMed] [Google Scholar]
- 19.Sakurada S, Hayashi T, Yuhki M, Fujimura T, Murayama K, Yonezawa A, Sakurada C, Takeshita M, Sato T, Zadina JE, Kastin AJ, Sakurada T. Differential antagonism of endomorphin-1 and endomorphin-2 supraspinal antinociception by naloxonazine and 3-methylnaltrexone. Peptides. 2002;23:895–901. doi: 10.1016/s0196-9781(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 20.Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc.Natl.Acad.Sci.U.S.A. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrone GF, Grinnell SG, Lu Z, Rossi GC, Le Rouzic V, Xu J, Majumdar S, Pan YX, Pasternak GW. Truncated mu opioid GPCR variant involvement in opioid-dependent and opioid-independent pain modulatory systems within the CNS. Proc Natl Acad Sci U S A. 2016;113:3663–3668. doi: 10.1073/pnas.1523894113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi GC, Pan Y-X, Brown GP, Pasternak GW. Antisense mapping the MOR-1 opioid receptor: Evidence for alternative splicing and a novel morphine-6β-glucuronide receptor. FEBS Lett. 1995;369:192–196. doi: 10.1016/0014-5793(95)00757-z. [DOI] [PubMed] [Google Scholar]
- 23.Rossi GC, Pan Y-X, Cheng J, Pasternak GW. Blockade of morphine analgesia by an antisense oligodeoxynucleotide against the mu receptor. Life Sci. 1994;54:L375–L379. doi: 10.1016/0024-3205(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 24.Standifer KM, Chien C-C, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of δ opioid analgesia and binding by antisense oligodeoxynucleotides to a δ opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 25.Yoburn BC, Chen J, Huang T, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J.Pharmacol Exp.Ther. 1985;235:282–286. [PubMed] [Google Scholar]
- 26.Cicero TJ, Meyer ER. Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J Pharmacol Exp Ther. 1973;184:404–408. [PubMed] [Google Scholar]
- 27.Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- 29.Cox V, Clarke S, Czyzyk T, Ansonoff M, Nitsche J, Hsu MS, Borsodi A, Tomboly C, Toth G, Hill R, Pintar J, Kitchen I. Autoradiography in opioid triple knockout mice reveals opioid and opioid receptor like binding of naloxone benzoylhydrazone. Neuropharmacology. 2005;48:228–235. doi: 10.1016/j.neuropharm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhu YX, King MA, Schuller AGP, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 31.Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, Pintar JE. Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther. 2006;318:641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- 32.Wu HE, Darpolor M, Nagase H, Tseng LF. Acute antinociceptive tolerance and partial cross-tolerance to endomorphin-1 and endomorphin-2 given intrathecally in the mouse. Neurosci Lett. 2003;348:139–142. doi: 10.1016/s0304-3940(03)00748-1. [DOI] [PubMed] [Google Scholar]
- 33.Sakurada S, Hayashi T, Yuhki M, Orito T, Zadina JE, Kastin AJ, Fujimura T, Murayama K, Sakurada C, Sakurada T, Narita M, Suzuki T, Tan-No K, Tseng LF. Differential antinociceptive effects induced by intrathecally administered endomorphin-1 and endomorphin-2 in the mouse. Eur.J.Pharmacol. 2001;427:203–210. doi: 10.1016/s0014-2999(01)01238-9. [DOI] [PubMed] [Google Scholar]
- 34.Pasternak GW. Early studies of opiate binding. In: Pasternak GW, editor. The Opiate Receptors. Clifton, NJ: Humana Press Inc; 1988. pp. 75–93. [Google Scholar]
- 35.Cox BM. Pharmacology of opioid drugs. In: Pasternak GW, editor. The Opiate Receptors. 2nd. New York: Springer; 2011. pp. 23–58. [Google Scholar]
- 36.Ward SJ, Fries DS, Larson DL, Portoghese PS, Takemori AE. opioid receptor binding characteristics of the non-equilbrium µ antagonist, β-funaltrexamine (P-FNA) Eur. J.Pharmacol. 1985;107:323–330. doi: 10.1016/0014-2999(85)90257-2. [DOI] [PubMed] [Google Scholar]
- 37.Maurer R, Gaehwiler BH, Buescher HH, Hill RC, Roemer D. Opiate antagonistic properties of an octapeptide somatostatin analog. Proc.Natl.Acad.Sci.USA. 1982;79:4815–4817. doi: 10.1073/pnas.79.15.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi GC, Standifer KM, Pasternak GW. Differential blockade of morphine and morphine-6p–glucuronide analgesia by antisense oligodeoxynucleotides directed against MOR-1 and G-protein α subunits in rats. Neurosci.Lett. 1995;198:99–102. doi: 10.1016/0304-3940(95)11977-5. [DOI] [PubMed] [Google Scholar]
- 39.Kieffer BL. Opioids: first lessons from knockout mice. Trends in Pharmacological Sciences. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 40.Wieskopf JS, Pan YX, Marcovitz J, Tuttle AH, Majumdar S, Pidakala J, Pasternak GW, Mogil JS. Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene. Pain. 2014;155:2063–2070. doi: 10.1016/j.pain.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grinnell SG, Majumdar S, Narayan A, Le RV, Ansonoff M, Pintar JE, Pasternak GW. Pharmacologic characterization in the rat of a potent analgesic lacking respiratory depression, IBNtxA. J Pharmacol Exp Ther. 2014;350:710–718. doi: 10.1124/jpet.114.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oladosu FA, Maixner W, Nackley AG. Alternative Splicing of G Protein-Coupled Receptors: Relevance to Pain Management. Mayo Clin Proc. 2015;90:1135–1151. doi: 10.1016/j.mayocp.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lever JR, Allmon RL, Gustafson JL, Gallazzi F, Lever SZ. Synthesis and evaluation of a radioiodinated dermorphin analog for mu opioid receptors. J Nucl.Med. 2004;45S:441P. [Google Scholar]
- 44.Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. Generation of novel radiolabeled opiates through site-selective iodination. Bioorg.Med.Chem.Lett. 2011;21:4001–4004. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schetz JA, Mayr CA, Taylor JE, Rosenblatt M, Chorev M, Davis TP. Distribution and pharmacokinetics of a potent peptide antagonist of parathyroid hormone and parathyroid hormone-related protein in the rat. J Pharmacol Exp Ther. 1995;274:1456–1462. [PubMed] [Google Scholar]
- 46.Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, Ocampo J, Haselton N, Pasternak AR, Grinnell S, Pan Y-X, Pasternak GW. Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated mu opioid receptor (MOR-1) splice variants. J.Med.Chem. 2012;55:6352–6362. doi: 10.1021/jm300305c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan YX, Xu J, Bolan EA, Abbadie C, Chang A, Zuckerman A, Rossi GC, Pasternak GW. Identification and characterization of three new alternatively spliced mu opioid receptor isoforms. Mol.Pharmacol. 1999;56:396–403. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- 48.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J.Biol.Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 49.Bolan EA, Pan YX, Pasternak GW. Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse. 2004;51:11–18. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]
- 50.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injections of drugs in the conscious mouse. Br.J.Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul D, Bodnar RJ, Gistrak MA, Pasternak GW. Different µ receptor subtypes mediate spinal and supraspinal analgesia in mice. Eur.J.Pharmacol. 1989;168:307–314. doi: 10.1016/0014-2999(89)90792-9. [DOI] [PubMed] [Google Scholar]
- 52.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur.J.Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 53.Ling GSF, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. Separation of morphine analgesia from physical dependence. Science. 1984;226:462–464. doi: 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- 54.Grinnell SG, Ansonoff M, Marrone GF, Lu Z, Narayan A, Xu J, Rossi G, Majumdar S, Pan XY, Bassoni DL, Pintar J, Pasternak GW. Mediation of buprenorphine analgesia by a combination of traditional and truncated mu opioid receptor splice variants. Synapse. 2016 doi: 10.1002/syn.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.