Abstract
Central cholinergic structures within the brain of the even-toed hoofed Goettingen miniature domestic pig (Sus scrofa domesticus) were evaluated by immunohistochemical visualization of choline acetyltransferase (ChAT) and the low-affinity neurotrophin receptor, p75NTR. ChAT immunoreactive (-ir) perikarya were seen in the olfactory tubercle, striatum, medial septal nucleus, vertical and horizontal limbs of the diagonal band of Broca and the nucleus basalis of Meynert, medial habenular nucleus, zona incerta, neurosecretory arcuate nucleus, cranial motor nuclei III and IV, Edinger-Westphal nucleus, parabigeminal nucleus, pedunculopontine nucleus and laterodorsal tegmental nucleus. Cholinergic ChAT-ir neurons were also found within transitional cortical areas (insular, cingulate, and piriform cortices) and hippocampus proper. ChAT-ir fibers were seen throughout the dentate gyrus and hippocampus, in the mediodorsal, laterodorsal, anteroventral and parateanial thalamic nuclei, the fasciculus retroflexus of Meynert, basolateral and basomedial amygdaloid nuclei, anterior pretectal and interpeduncular nuclei, as well as select laminae of the superior colliculus. Double immunofluorescence demonstrated that virtually all ChAT-ir basal forebrain neurons were also p75NTR positive. The present findings indicate that the central cholinergic system in the miniature pig is similar to other mammalian species. Therefore, the miniature pig may be an appropriate animal model for preclinical studies of neurodegenerative diseases where the cholinergic system is compromised.
Keywords: acetylcholine, choline acetyltransferase, p75 low-affinity neurotrophin receptor, cholinergic basal forebrain, miniature pig, RRID: AB_2079751, RRID: AB_310649, RRID: AB_2314520, RRID: AB_2340612, RRID: AB_2307351, RRID: SCR_001775, RRID: SCR_014235, RRID: SCR_014329
Introduction
The distribution of cholinergic neurons in the central nervous system has been examined in numerous mammalian species (Armstrong et al., 1983; Mesulam et al., 1983a; Vincent and Reiner, 1987; Everitt et al., 1988; Maley et al., 1988; Mufson and Cunningham, 1988; Kordower et al., 1989; Alonso and Amaral, 1995; Ichikawa et al., 1997; Varga et al., 2003; Maseko and Manger, 2007; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010, 2012; Pieters et al., 2010; Calvey et al., 2013; Maseko et al., 2013; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c). These neurons play a key role in complex functions including learning, memory, attention, and sleep (Jouvet, 1972; Kodama and Honda, 1996; Hasselmo and McGaughy, 2004; Atri et al., 2004; Green et al., 2005; Klinkenberg et al., 2011). Cholinergic neurons are characterized by the presence of choline acetyltransferase (ChAT), the synthetic enzyme for the neurotransmitter acetylcholine (ACh), while its hydrolytic enzyme, acetylcholinesterase (AChE), is also present in cholinoceptive cells (Emson et al., 1979; Mesulam et al., 1984, Mufson et al., 1989; Woolf, 1991). Cholinergic neurons in the mammalian basal forebrain are found within the medial septum (MS) and vertical limb of diagonal band of Broca (VDB), the horizontal limb of diagonal band of Broca (HDB) and the magnocellular nucleus basalis of Meynert/substantia innominata complex (NBM/SI), which correspond to the Ch1, Ch2, Ch3 and Ch4 subregions, respectively (Mesulam et al., 1983b). The Ch4 complex has been subdivided into anteriomedial (Ch4am), intermediate dorsal (Ch4id) and ventral (Ch4iv), and posterior (Ch4p) subfields (Mesulam et al., 1983b; Liu et al., 2015). Ch1-2 neurons innervate the hippocampus, Ch3 the olfactory bulb and visual cortex (Mesulam et al., 1983b; Rye et al., 1984), while Ch4 neurons provide the major cholinergic innervation to the cortical mantle and amygdala (Armstrong et al., 1983; Mesulam et al., 1983b; Saper, 1984; Kasa, 1986; Eckenstein et al., 1988; Brückner et al., 1992; Semba, 2000). Within the epithalamus cholinergic neurons are found within the medial habenula (Ch7) (Mesulam et al., 1986; Mufson and Cunningham, 1988), which innervates the interpeduncular nucleus (Woolf and Butcher, 1985; Woolf et al., 1990; Woolf, 1991). More caudally, cholinergic neurons are found in the pedunculopontine (Ch5), laterodorsal tegmental (Ch6) nuclei and parabigeminal nucleus (Ch8), which project to the thalamus and superior colliculus, respectively (Mesulam et al., 1986; Mufson et al., 1986). Cholinergic basal forebrain neurons also express the low affinity pan-neurotrophin p75NTR receptor for nerve growth factor (Mufson et al., 1989) and depending upon the mammalian species examined, the ACh inhibitor, galanin (GAL) (Fisone et al., 1987; Kordower and Mufson, 1990; Benzing et al., 1993a; Perez et al., 2001).
Despite the large number of studies of the cholinergic system in mammals, to our knowledge, no study has examined the central cholinergic system in the Goettingen domestic miniature pig, a member of the species Sus scrofa domesticus, and order Artiodactyla. However, cholinergic profiles have been evaluated in other members of this order including even-toed ungulates such as sheep (Ferreira et al., 2001), giraffe (Bux et al., 2010), river hippopotamus (Dell et al., 2016a), and cetaceans including the harbor porpoise (Dell et al., 2016b) and minke whale (Dell et al., 2016c). The Goettingen miniature pig is the result of crossbreeding the Minnesota miniature pig, the Vietnamese potbelly pig, and the German Landrace pig (Köhn et al., 2007; Simianer and Köhn, 2010). The miniature pig has a well-defined genetic background, and its organ physiology is similar to humans (Dolezalova et al., 2014). The pig brain is gyrencephalic with gray/white matter proportions closer to humans than the rodent (Howells et al., 2010). Additionally, swine are more cost-effective than non-human primates and have a shorter gestation period (Dolezalova et al., 2014) making the Goettingen miniature pig well-suited for human disease-related translational research including myocardial infarction and stroke (Schuleri et al., 2008; Dolezalova et al., 2014), Alzheimer’s (Kragh et al., 2009; Fjord-Larsen et al., 2009; Søndergaard et al., 2012; Jakobsen et al., 2013) and Parkinson’s (Mikkelsen et al., 1999; Glud et al., 2011) disease. The latter disorders are associated with cholinergic basal forebrain (CBF) neuronal degeneration (Mufson et al., 1991; Gilmor et al., 1999). The aim of the present study was to immunohistochemically define the distribution of cholinergic profiles within the brain of the Goettingen miniature pig, a potential animal model for studies of the cholinergic system during aging and disease.
Materials and Methods
Tissue Processing
In the current study, six sexually mature female Goettingen miniature pigs (4-month-old, 10kg mean body mass, 67g mean brain mass) were anesthetized with isoflurane (1–2%) prior to intubation, and maintained on the drug until the administration of an intravenous lethal injection of Beuthanasia-D (Merck Animal Health, Madison, NJ). Pigs were perfused under anesthesia via the carotid artery with 0.9% cold saline followed by Zamboni’s solution (consisting of picric acid and 4% paraformaldehyde) and brains were removed from the calvarium and post-fixed for 48 hours in the same fixative. Brains were cryoprotected in 30% sucrose, and sectioned in either the coronal or horizontal plane at 40 microns on a sliding freezing microtome. Tissue provided for this study extended from the frontal cortex to the level of the brain stem pontomesencephalic nuclei. The lower brain stem and medulla were not available for analysis. Sections were stored in a cryoprotectant solution (30% glycerol, 30% ethylene glycol, 40% phosphate buffer) at −20° C until processing for immunohistochemistry. The use of these animals and the indicated anesthesia/perfusion protocol was approved by the Animal Care and Use Committees of the Department of Neuroscience at Rush University, Chicago, IL and NsGene Inc, Providence, RI.
Antibody Characterization
Table 1 describes the characteristics of the primary antibodies used in the present study, including: an anti-choline acetyltransferase (ChAT) goat IgG antibody (1:1,000 dilution, Millipore, MA, AB144P, RRID: AB_2079751) raised against human placental ChAT (Hersh et al., 1978; Raghanti et al., 2008). According to the manufacturer western blot analysis of this antibody yields a single band at 70 kDa, and specifically stains cholinergic neurons (Mufson et al., 1989). Additionally, we used an anti-low affinity neurotrophin receptor p75NTR rabbit IgG antibody raised against a glutathione S-transferase (GST) fusion protein corresponding to the intracellular domain (residues 274–425) of rat p75NTR (1:3,000 dilution, Millipore, 07-476, RRID: AB_310649) (Matusica et al., 2013); and a rabbit polyclonal anti-galanin (GAL) antibody (1:1,000 dilution, gift from Dr. E. Theodorsson, Sweden, RRID: AB_2314520), whose specificity has been reported previously (Theodorsson and Rugarn, 2000; Perez et al., 2001; Diez et al., 2003; Kelley et al., 2011) and reacts with the central and C-terminal part of GAL but does not recognize the N-terminus in a radioimmunoassay (Theodorsson and Rugarn, 2000). Each of these antibodies have been used extensively in immunocytochemical investigations of the mammalian brain (Mufson et al., 1989; Obata et al., 1999; Theodorsson et al., 2000; Diez et al., 2003; McKeon-O’Malley et al., 2003; Ikonomovic et al., 2007; Motts and Schofield, 2009; Fu et al., 2010; Kelley et al., 2011).
Table 1.
Summary of Antibodies Used
| Antigen | Description of Immunogen | Source, Host Species, Cat. #, RRID | Dilution |
|---|---|---|---|
| Choline acetyltransferase (ChAT) | Human placental Choline acetyltransferase | Millipore, goat polyclonal, Cat# AB144P, RRID:AB_2079751 | Immunohistochemistry: 1:1,000 Immunofluorescence: 1:100 |
| Low affinity neurotrophin receptor (p75NTR) | GST fusion protein corresponding to the intracellular domain (residues 274–425) of rat p75 neurotrophin receptor | Millipore, rabbit polyclonal, Cat# 07-476, RRID:AB_310649 | Immunohistochemistry: 1:3,000 Immunofluorescence: 1:300 |
| Galanin (GAL) | Synthetic peptide: GWTLNSAGYLLGPHAIDNHRSFSDKHGLT-amide (rat Galanin 1–29) coupled to BSA via carbodiimide | Gift from E. Theodorsson, rabbit polyclonal, RatGal4, RRID:AB_2314520 | Immunohistochemistry : 1:1,000 |
ChAT, p75NTR and GAL Immunohistochemistry
Immunohistochemistry was performed as described previously (Mufson and Cuningham, 1988; Mufson et al., 1989; Benzing et al., 1993b; Perez et al., 2000). Briefly, sections were washed in phosphate buffer (3×10 minutes) and tris buffered saline (TBS, 3×10 minutes), to remove excess cryoprotectant, before a 20-minute incubation in 0.1 mol/L sodium metaperiodate (Sigma, St Louis, IL) in TBS to inactivate endogenous peroxidase activity. Tissue was then washed in a TBS solution containing 0.25% Triton X (ThermoFisher, Waltham, MA) (3×10 minutes), and placed in the same solution using 3% horse serum for 1 hour. Sections were then incubated with the appropriate primary antibodies (see Table 1) overnight in a 0.25% Triton X-100 and 1% horse serum solution. All washes and incubations were performed at room temperature on a shaker table. Sections were subsequently washed in TBS and 1% horse serum before incubation (3×10 minutes) with the appropriate biotinylated secondary antibody, horse anti-goat IgG, (1:200, Vector Laboratories, CA) or horse anti-rabbit IgG, (1:200, Vector), for one hour. After TBS washes, ChAT immunoreactivity was amplified by incubating the tissue using the Vectastain ABC kit (Vector) for 1 hour; rinsed in 0.2 mol/L sodium acetate, 1.0 mol/L imidazole buffer, pH 7.4; and developed in acetate-imidazole buffer containing 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma, St Louis, IL) and 0.0015% H2O2. Sections only immunostained for p75NTR or GAL were visualized with DAB and 1% nickel (II) ammonium sulfate hexahydrate and 0.0015% H2O2 resulting in a black reaction product. The histochemical reaction was terminated in acetate-imidazole buffer, tissue mounted on slides, dehydrated through graded alcohols (70%–95%–100%), cleared in xylene, and cover-slipped using DPX mounting medium (Biochemica Fluka, Buchs, Switzerland). The specificity of the secondary antibodies was determined by absence of immunocytochemical reaction in sections processed without the primary antibody. Additional sections were counterstained with cresyl violet to aid in cytoarchitectonic analysis. For each antibody, staining of all sections was performed at the same time to reduce batch-to-batch variability. A stereotaxic atlas of the pig brain was used for regional delineation (Félix et al., 1999), and abbreviations were taken from the atlas by Paxinos and colleagues (1999).
Immunofluorescence
Immunofluorescence was conducted on additional forebrain sections using a modification of a previously reported protocol (Oh et al., 2010). Briefly, sections were double labeled for ChAT (1:100) and p75NTR (1:300) overnight at room temperature, and then incubated for 1 hour using Cy2-conjugated donkey anti-rabbit (1:200, Jackson ImmunoResearch, RRID: AB_2340612) and Cy3-conjugated donkey anti-goat IgG (1:200, Jackson ImmunoResearch, RRID: AB_2307351) as secondary antibodies. Cy2 and Cy3 fluorescence was detected using FITC (excitation light= 490 nm) and TRITC (excitation light= 550 nm) filters, respectively.
Charting the distribution of cholinergic neurons in the pig brain
Schematic drawings of the distribution of ChAT-ir profiles were outlined manually under 1× and 10× lenses (numerical aperture (NA) 0.04 and 0.25, respectively) with the aid of a Nikon Optiphot-2 microscope using the Neurolucida Neuron tracing program (MBF Bioscience, VT, RRID: SCR_001775). Chartings were adjusted and edited using the CorelDraw Graphics Suite X7 software (Corel Corporation, Ottawa, ON, Canada, RRID: SCR_014235). Cholinergic cell diameter was determined by manually outlining twenty ChAT-ir neurons from 4 separate sections containing each cholinergic subgroup using the Nikon NIS-element software (Nikon, Melville, NY, RRID: SCR_014329). Measurements are presented as mean diameter ± standard deviation. Immunohistochemistry and immunofluorescence were visualized with the aid of a Nikon Eclipse 80i microscope.
Results
Telencephalon
Cerebral cortex and Olfactory Tubercle
ChAT-ir fibers were observed throughout the cortical areas examined, including the cingulate, insular and piriform cortices (mean diameter: 16 ± 1.73 μm) (Fig. 1A–D). In the cingulate cortex small multipolar and bipolar ChAT positive neurons were found mainly in layer VI (Fig. 1A), whereas in the insular cortex cholinergic neurons were observed scattered within layers III and V (Fig. 1B) and within layer III of the piriform cortex (Fig. 1C). These cortical areas also displayed a few small, bipolar p75NTR-ir neurons distributed in a pattern similar to that seen for ChAT-ir neurons (Fig. 1E). Small, spindle shaped ChAT- and p75NTR-ir neurons were scattered throughout the more rostral aspect olfactory tubercle (Fig. 1F).
Figure 1.
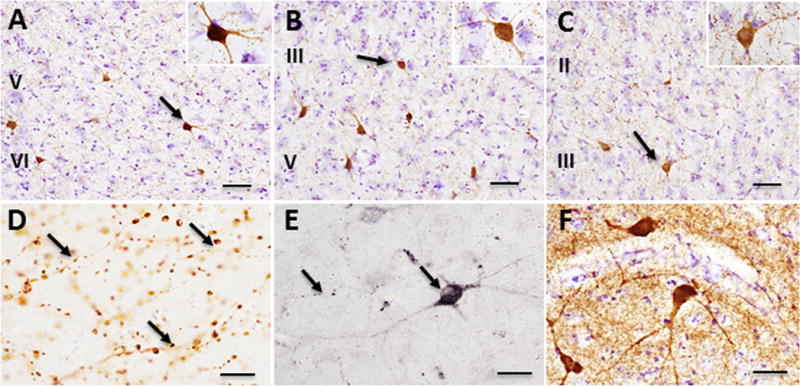
ChAT-ir profiles in the cortex and olfactory tubercle of Sus scrofa domesticus: ChAT-ir multipolar neurons (black arrows) within layers V and VI of the cingulate cortex (A), layers III and V of insular cortex (B), and layer III of the piriform cortex (C). Varicose ChAT-ir fibers within piriform cortex (black arrows) (D). P75NTR-ir neuron and fibers (black arrows) in the cingulate cortex (E). ChAT-ir neurons and neuropil within the olfactory tubercle (F). Tissue in panels A-C and F were counterstained with cresyl violet. Scale bar: 100 μm in A, B, and C; 25 μm in D, E, F. Insets: 40x A, B, C.
Striatum and Basal Forebrain
ChAT-ir perikarya were identified within the striatum (i.e., caudate and putamen) and in each CBF subfield (Fig. 2). The striatum displayed large multipolar ChAT-ir neurons and fibers (mean diameter: 32 ± 3.21 μm) (Fig. 3A, B), which were p75NTR immunonegative (Fig. 4A). Within the CBF, ChAT-ir neurons were found in the medial (MS, Ch1), VDB (Ch2)/HDB (Ch3), and the NBM (Ch4) (Figs. 2, 3A, C–E, F–H). The lateral septum (LS) also displayed small spindle and multipolar ChAT-ir neurons (mean diameter: 24 ± 2.21 μm) in its lateroventral aspect (Figs. 2C, D, 3A, C). A few scattered ChAT-ir neurons were observed in the nucleus accumbens (Fig. 2A, B) and bed nucleus of the stria terminalis (Fig. 3F). Ch1 contained small bipolar and multipolar ChAT-ir neurons with processes directed dorsally and ventrally, forming an elongated cluster situated along the midline (mean diameter: 28 ± 1.56 μm) (Fig. 3A, D, F). Ch2 displayed medium-sized, round or spindle-shaped ChAT-ir neurons (mean diameter: 30 ± 2.16 μm) (Figs. 2C, D, 3A, E), with larger neurons found more rostrally (Fig. 3E). Ch3 medium-sized cholinergic neurons (mean diameter: 31 ± 2.33 μm) were located ventral to the anterior commissure and lateral to Ch2 (Figs. 2C, D, 3A, F, G). More caudally, ChAT-ir somata were seen within the Ch4 subfield ventral to the internal capsule and globus pallidus, which extended to the level of the anterior amygdala (Figs. 2C, D, 3F, H). At the level of the decussation of the anterior commissure ChAT-ir neurons within Ch4 (NBM/SI) appeared multipolar (mean diameter: 35 ± 2.10 μm) (Fig. 3H), with processes that curved ventrally around the globus pallidus, extending into the internal capsule (Fig. 3F). Although GAL-ir neurons were not observed in any of the CBF subgroups, numerous scattered GAL-ir fibers were seen surrounding neurons in the septum (Fig. 3I) and Ch4 (Fig. 3J). Dual immunofluorescence revealed that virtually all ChAT-ir neurons within the Ch subfields were p75NTR immunopositive (Fig. 4B–E).
Figure 2.
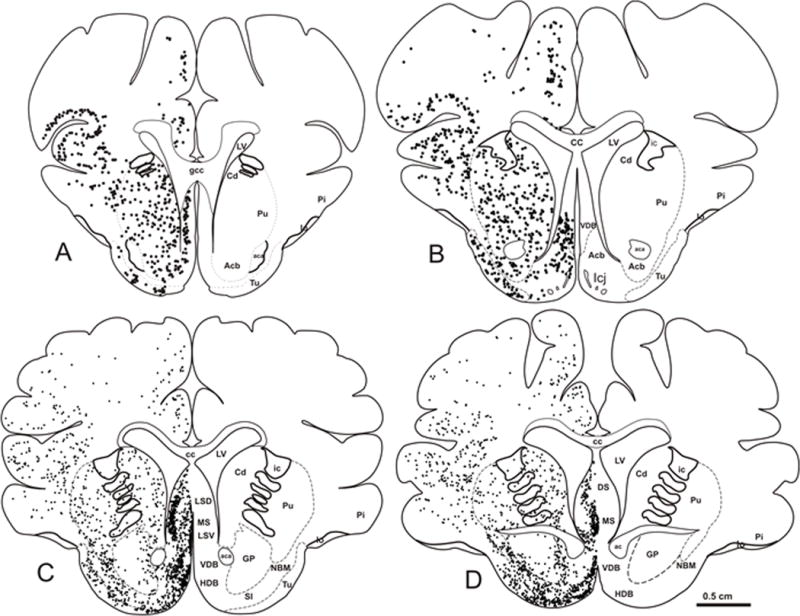
Schematic drawings of coronal sections (A-D) of the telencephalon of Sus scrofa domesticus depicting the distribution of ChAT-ir perikarya (black dots). Scale: 0.5 cm A, B, C, D.
Figure 3.
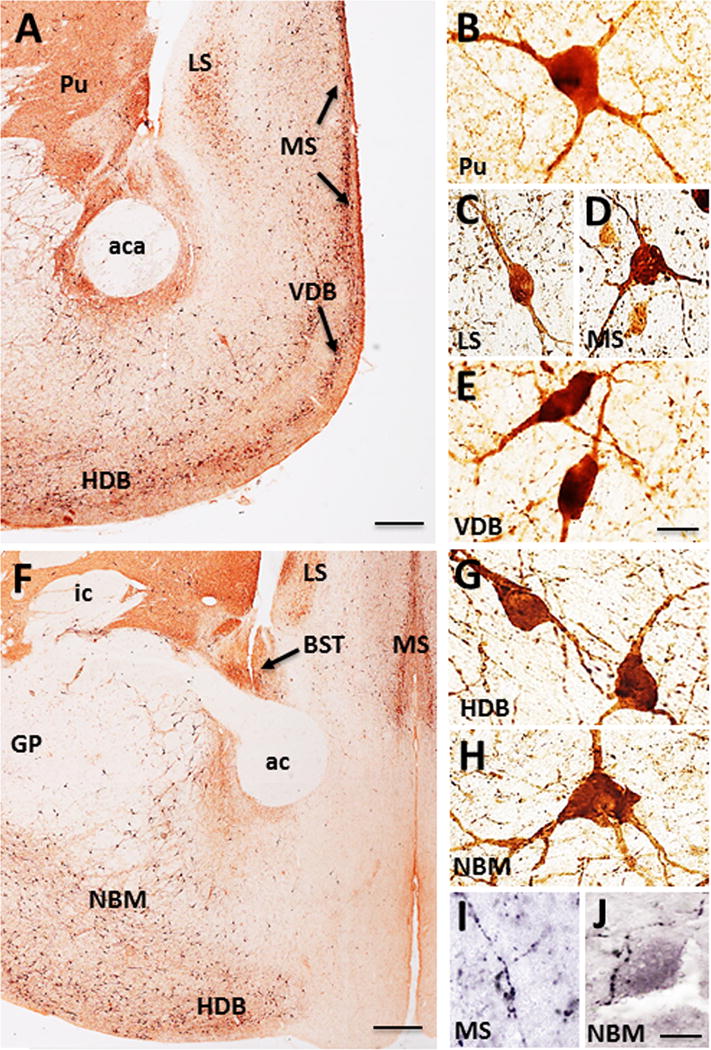
A–B. ChAT-ir perikarya and neuropil within the striatum and basal forebrain of Sus scrofa domesticus. ChAT-ir neurons (black arrows) and neuropil within the putamen (Pu), medial septum (MS), vertical and horizontal diagonal band (VDB, HDB), and lateral septum (LS) (A). High-power photomicrographs showing multipolar ChAT-ir neurons in the Pu (B), LS (C), MS (D), and bipolar VDB (E) neurons. Caudal section showing ChAT-ir profiles in the LS, MS, bed nucleus of the stria terminalis (BST, black arrow), and the nucleus basalis of Meynert (NBM) (F). High-power photomicrographs of multipolar HDB (G) and NBM (H) neurons and GAL-ir fibers surrounding a neuron within the MS (I) and in the NBM (J). Scale: 1000 μm A, F; 25 μm B-E, G-J.
Figure 4.
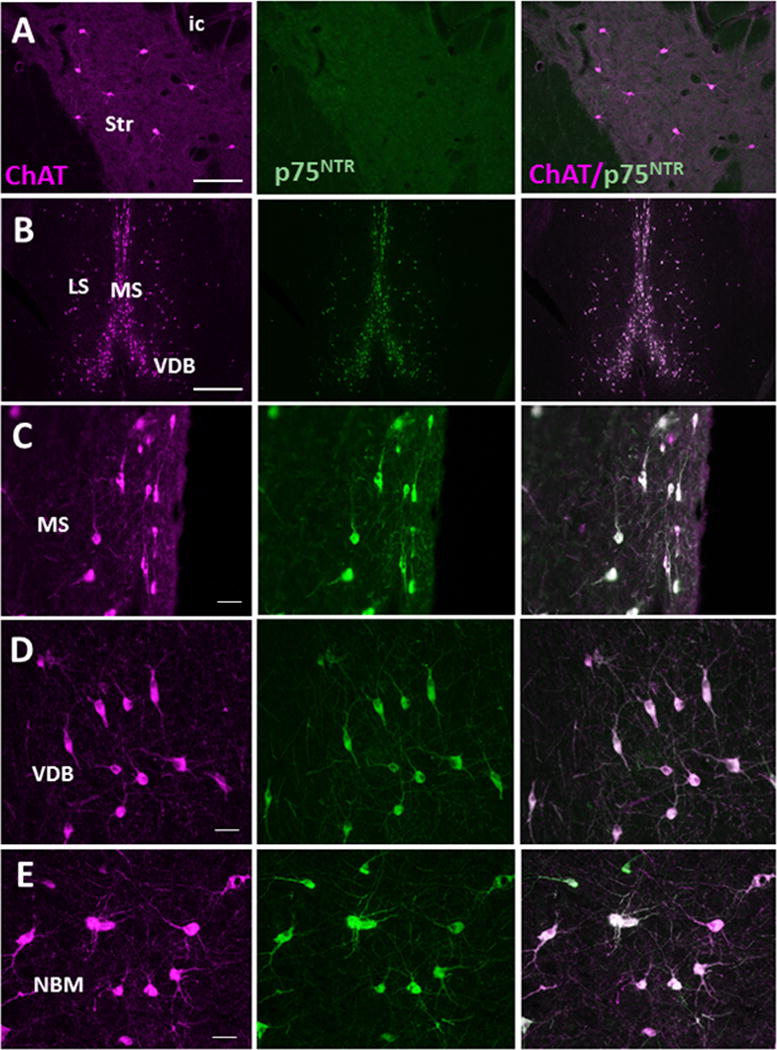
Immunofluorescent photomicrographs of ChAT (magenta), p75NTR (green) and merged (white) neurons and fibers in the striatum (A), MS (B, C), VDB (D), and NBM (E) of Sus scrofa domesticus. Note that virtually all ChAT-ir neurons and fibers are p75NTR positive in all basal forebrain cell groups, while striatal neurons were only ChAT immunopositive. Scale: 250 μm A, B; 25 μm C, D, E.
Hippocampal Formation and Amygdala
The hippocampal formation contained ChAT-ir neurons and fibers (Fig. 5A–E). Scattered, small bipolar ChAT-ir neurons were found dorsal to the CA1 pyramidal cell layer, mainly in the caudal aspect of the hippocampus (Fig. 5B, inset), but not in the dentate gyrus. Although numerous fine, beaded ChAT-ir fibers were observed throughout the hippocampus and dentate gyrus (Fig. 5A–C), the densest collection of fibers was observed within the polymorphic and molecular layers of the dentate gyrus, stratum oriens, and CA1-3 pyramidal cell layers (Fig. 5B–E).
Figure 5.
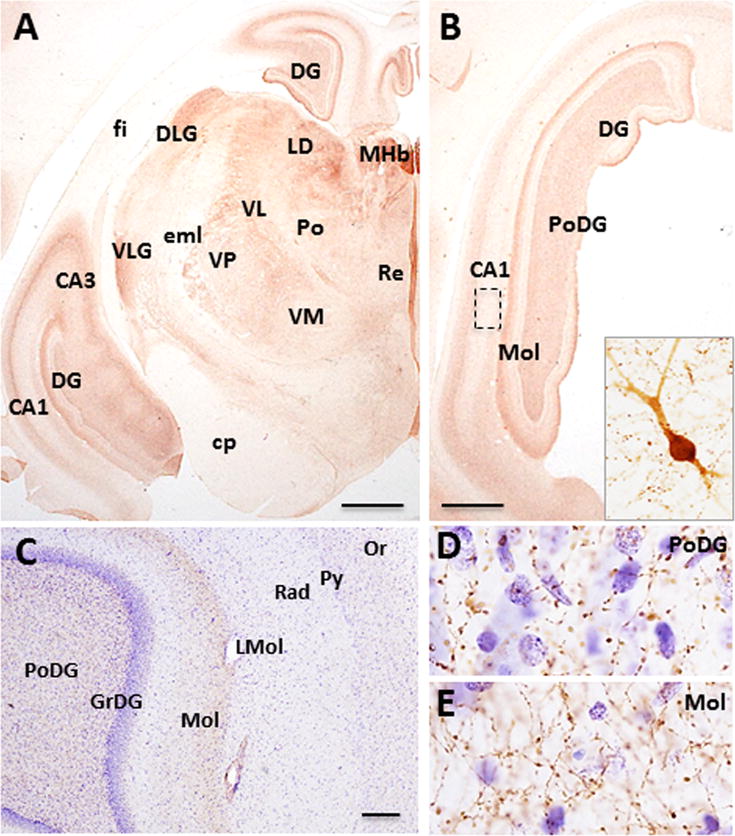
Photomicrographs showing the distribution of ChAT-ir profiles in the hippocampus and thalamus of Sus scrofa domesticus. Coronal sections (A, B) showing ChAT-ir labeling in the dorsal and temporal aspects of the hippocampal formation and dentate gyrus (DG) and thalamic nuclei. Insert in B shows a cholinergic bipolar hippocampal neuron. Low (C) and high power (D, E) photomicrographs of a Nissl counterstained section showing ChAT-ir fibers within the polymorphic (C, D) and molecular layer (C, E) of the dentate gyrus. Scale 1000 μm A, B; 100 μm C.
Although ChAT-ir neurons were not observed in the amygdala, ChAT-ir fibers were found throughout the anterior part of the basolateral, basomedial, and dorsolateral nuclei (Fig. 6A), with the heaviest concentration observed within the anterior aspects of the basolateral amygdaloid nucleus (Fig. 6A).
Figure 6.
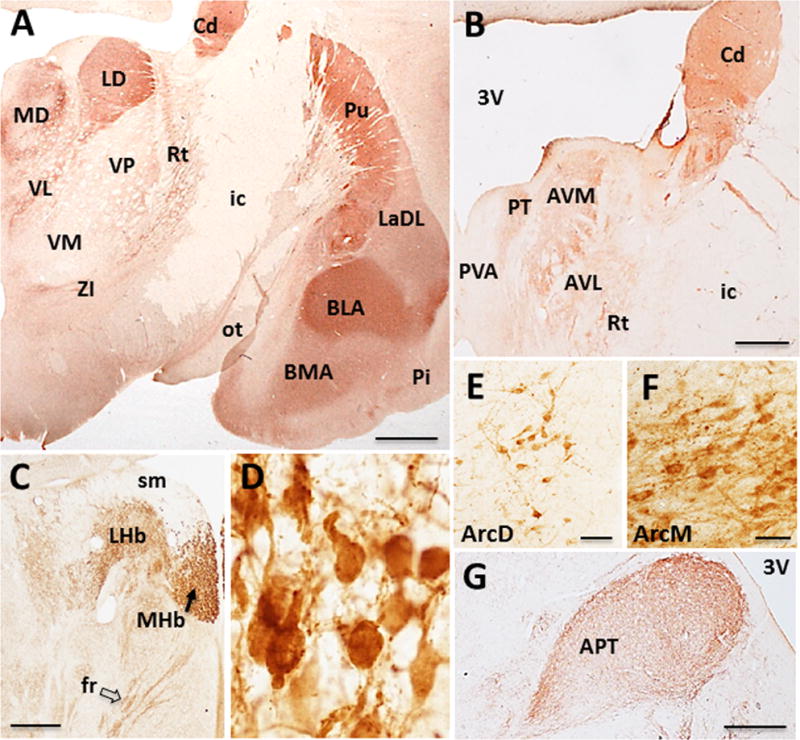
Photomicrographs showing ChAT-ir profiles in the epithalamus, thalamus, hypothalamus and amygdala of Sus scrofa domesticus. ChAT-ir fibers within the caudal (A) and rostral thalamus and amygdala (B; see text for detailed description). ChAT-ir profiles within the lateral (LHb) and medial (MHb) (black arrow) habenular nuclei and fasciculus retroflexus (fr; open arrow) (C) and cholinergic neurons within the MHb nucleus (D). ChAT-ir neurons and fibers within the dorsal (E) and medial (F) arcuate nuclei and neuropil staining within the anterior pretectal nucleus (APT) (G). Scale: 500 μm A; 1000 μm C, D, G; 25 μm E, F.
Diencephalon
Epithalamus
A group of small densely packed, oval-shaped ChAT-ir neurons were seen in the medial habenular nucleus (Ch7) (mean diameter: 15 ± 0.81 μm) (Fig. 6C, D). In contrast, only ChAT-ir fibers were observed in the lateral nucleus of the habenula, which coursed within the fasciculus retroflexus (Fig. 6C, open arrow), presumably projecting to the interpeduncular nucleus, which displayed a dense cholinergic innervation (Fig. 7C).
Figure 7.
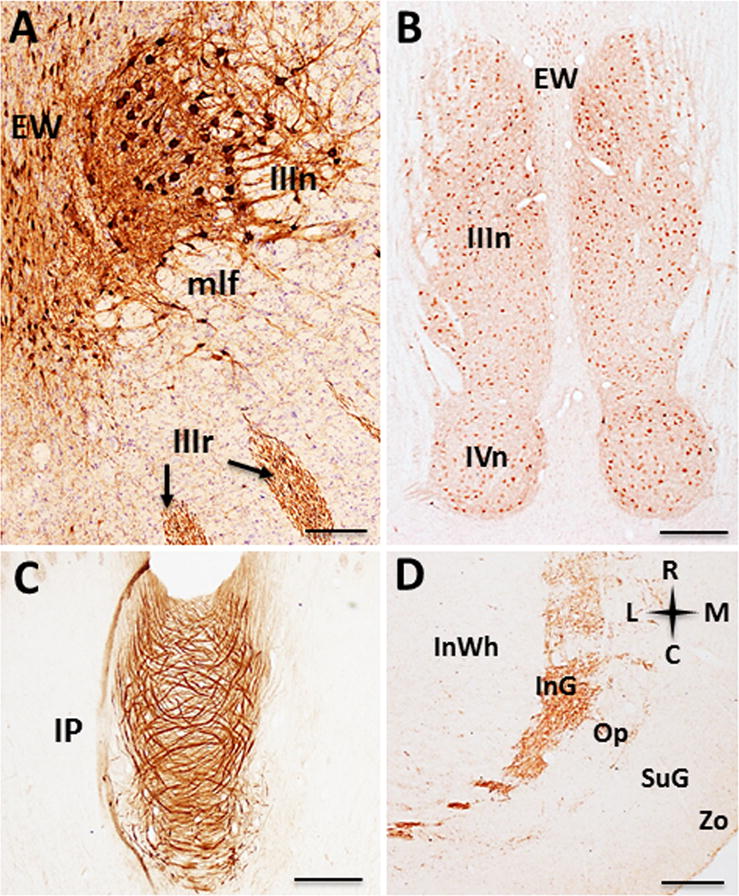
Photomicrographs of ChAT-ir profiles in the mesencephalon. Coronal section showing ChAT-ir neurons within the Edinger-Westphal (EW) and oculomotor nuclei and third cranial nerve rootlets (black arrows) (A). Low-power photomicrograph of a horizontal section showing the Edinger-Westphal, third (IIIn) and fourth (IVn) cranial nerve nuclei (B). Horizontal section showing swirling ChAT-ir fibers in the central portion of the interpeduncular nucleus (IP, C). Low-power photomicrograph (D) of ChAT-ir fibers within the superficial laminae of the superior colliculus. Scale: 250 μm A; 1000 μm B; 500 μm C, D. Anatomic orientation: R= rostral, C= caudal, L= lateral, M= medial.
Thalamus
Several thalamic nuclei displayed ChAT-ir fibers but not positive neurons (Fig. 6A, B). Rostrally, the medial and lateral portions of the anteroventral, and parateanial thalamic nuclei displayed intense ChAT-ir, while the central, periventricular and reticular thalamic nuclei displayed weak staining (Fig. 6B). The thalamic mediodorsal and laterodorsal nuclei were strongly ChAT-ir, followed by the ventrolateral nucleus (Fig. 6A). More caudally, ChAT-ir fibers were observed in rostral aspects of the anterior pretectal nucleus (Fig. 6G), but to a lesser degree more caudally at the level of the superior colliculus. ChAT-ir fibers were also observed in the lateral geniculate nucleus (Fig. 5A). The zona incerta contained small, spindle-shaped neurons. Ventromedial to the dorsolateral geniculate at the level of the medial habenula, a small group of faintly stained ChAT-ir neurons and fibers were observed in reticular thalamic territories.
Hypothalamus
Within the hypothalamus, weakly-stained small, oval shaped ChAT-ir neurons (mean diameter: 14 ± 1.13 μm) (Fig. 6E) were observed within the dorsal portion of the arcuate nucleus. A densely packed group of ChAT-ir neurons of similar shape and size with extensive processes were seen within the medial arcuate nucleus (mean diameter: 12 ± 0.72 μm) (Fig. 6F). ChAT-ir fibers were also seen coursing within the external aspect of the median eminence.
Mesencephalon
Small spindle-shaped ChAT-ir perikarya and fibers were seen in the Edinger-Westphal nucleus (mean diameter: 21 ± 1.84 μm) (Fig. 7A, B), forming a dense cluster running parallel to the midline, ending caudal to the superior colliculus. Laterally, large multipolar ChAT-ir somata were found in the oculomotor nucleus (mean diameter: 35 ± 2.87 μm) (Fig. 7A, B), giving rise to the III cranial nerve roots exiting at the level of the interpeduncular nucleus. ChAT-ir fibers, but not cells, were observed within the superficial layers of the superior colliculus in the intermediate gray, and intermediate optic layers (Fig. 7D).
Several ChAT-ir cell groups were observed within the tegmentum of the pontomesencephalon. ChAT-ir neurons in the pedunculopontine (Ch5) and laterodorsal tegmental (Ch6) nuclei were largely heteromorphic, ranging in appearance from pyramidal to fusiform to multipolar (Fig. 8A–C). ChAT positive neurons within Ch5 were slightly larger (mean diameter: 30 ± 2.70 μm) (Fig. 8B) and more spindle-shaped compared to Ch6 (mean diameter: 27 ± 1.90 μm) (Fig. 8C). A cluster of small oval shaped ChAT-ir perikarya were observed within the parabigeminal nucleus (Ch8) (mean diameter: 18 ± 1.35 μm) (Fig. 8A, D). A dense plexus of ChAT-ir fibers were found in close proximity to the parabigeminal nucleus (Fig. 8A, black arrows). Large ChAT-ir neurons (mean diameter: 38 ± 3.32 μm) and fibers were also observed in the trochlear nucleus (Fig. 7B).
Figure 8.
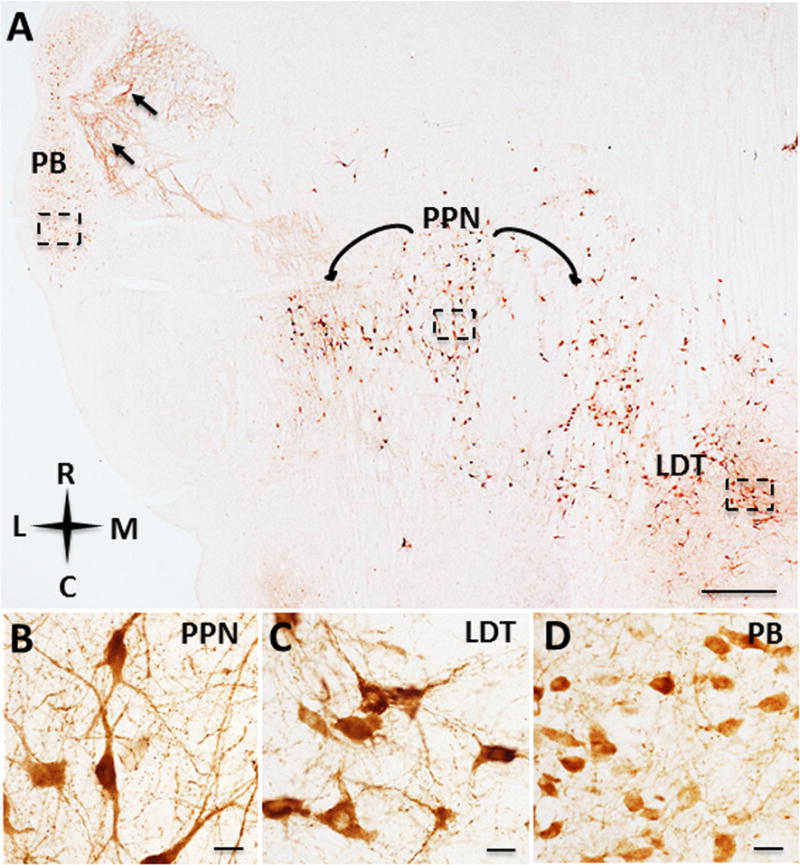
Photomicrographs of the pontomesencephalic cholinergic cell groups of Sus scrofa domesticus. ChAT-ir neurons and fibers (A, black arrows) of the parabigeminal (PB), pedunculopontine nucleus (PPN), and the laterodorsal tegmental nucleus (LDT). ChAT-ir neurons and fibers within the PPN (B), LDT (C) and PB (D). Scale: 1000 μm A; 25 μm B, C, D. Anatomic orientation: R= rostral, C= caudal, L= lateral, M= medial.
Discussion
The present findings are the first detailed description of cholinergic profiles within the brain of the female Goettingen miniature pig, Sus scrofa domesticus. ChAT-ir neurons and fibers were observed within the telencephalon, diencephalon, and mesencephalon similar to other mammalian species (see Table 2).
Table 2.
Summary of ChAT positive profiles across species
| Artiodactyla | Cetacea | Carnivora | Lagomorpha | Rodentia | Primates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Goettingen pig | Giraffe1 | Sheep2 | River hippopotamus3 |
Harbour porpoise4 |
Minke whale5 | Cat6–13 | Dog14–16 | Rabbit17–19 | CD-1 mouse20,21 | Rat22–40 | Guinea pig22,41,42 |
Strepsirrhines43 | Macaque44–53 | Marmoset54–56 | Baboon57 | Gorilla58–60 | Human61–71 | |
| S. s. domesticus | G. camelopardalis | O. aries | H. amphibius | P. phocoena | B. acutorostrata | F. cattus | C. familiaris | O. cuniculus | M. musculus | R. norvegicus | C. porcellus |
G.
demidoff P. potto L. catta |
M.
mullata M. nemestrina M. fascicularis M. fuscata |
C. jacchus | P. papio |
G.
gorilla G. g. gorilla G. b. beringei |
H. sapiens | |
| Cortex | + | N/A | − | N/A | N/A | N/A | − | N/A | + | + | − | − | − | − | − | − | − | −/+ |
| Tu | + | N/A | + | + | + | + | + | + | + | + | + | + | + | + | + | + | N/A | + |
| Str | + | + | + | + | + | N/A | + | + | + | + | + | + | + | + | + | + | N/A | + |
| LS | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + | N/A | N/A | + | N/A | N/A | N/A | − |
| MS (Ch1) | + | N/A | + | + | N/A | N/A | −/+ | + | + | + | + | + | + | + | + | + | N/A | + |
| VDB (Ch2) | + | N/A | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| HDB (Ch3) | + | N/A | + | + | + | + | + | + | + | + | + | + | + | + | + | + | N/A | + |
| NBM (Ch4) | + | + | + | + | + | + | + | + | + | + | + | + * | + | + | + | + | + | + |
| Hippocampus | + | N/A | N/A | N/A | N/A | N/A | − | N/A | + | + | + | N/A | − | − | N/A | − | N/A | + |
| Amygdala | − | N/A | N/A | N/A | N/A | N/A | + | N/A | − | N/A | − | N/A | − | − | N/A | N/A | N/A | − |
| MHb (Ch7) | + | + | N/A | N/A | + | N/A | + | N/A | N/A | + | + | + | + | + | + | − | N/A | + |
| LHb | − | N/A | N/A | N/A | N/A | N/A | + | N/A | − | N/A | − | N/A | N/A | N/A | N/A | N/A | N/A | − |
| Thalamus | − | N/A | N/A | +† | N/A | N/A | − | N/A | − | N/A | − | N/A | N/A | + ** | N/A | N/A | N/A | − |
| ZI | + | N/A | N/A | N/A | N/A | N/A | + | N/A | − | N/A | − | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| ArcD | + | +**** | N/A | +**** | +**** | +**** | N/A | N/A | N/A | N/A | −/+ | N/A | − | − *** | N/A | N/A | N/A | − *** |
| ArcM | + | +**** | N/A | +**** | +**** | +**** | N/A | N/A | N/A | N/A | −/+ | N/A | − | − *** | N/A | N/A | N/A | − *** |
| APT | − | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + | − | N/A | N/A | N/A | N/A | N/A | N/A |
| EW | + | + | N/A | + | + | + | + | N/A | + | N/A | + | + | + | + | N/A | + | N/A | + |
| IIIn | + | + | N/A | + | + | + | + | N/A | + | + | + | + | + | + | N/A | + | N/A | + |
| IVn | + | + | N/A | + | + | + | + | N/A | N/A | N/A | + | + | + | + | + | + | N/A | + |
| IP | − | − | N/A | N/A | N/A | N/A | − | N/A | − | − | − | − | N/A | − | N/A | N/A | N/A | N/A |
| SC | − | N/A | N/A | N/A | N/A | N/A | + | N/A | N/A | − | − /+ | + | − | N/A | N/A | N/A | N/A | N/A |
| PPT (Ch5) | + | + | N/A | + | + | + | + | + | N/A | + | + | + | + | + | + | + | N/A | + |
| LDT (Ch6) | + | + | N/A | + | + | + | + | + | N/A | + | + | + | + | + | + | + | N/A | + |
| PBN (Ch8) | + | + | N/A | + | + | + | + | N/A | N/A | + | + | + | + | N/A | N/A | N/A | N/A | + |
N/A= data not reported in cited references, + presence of ChAT-ir neurons, – absence of ChAT-ir neurons and −/+ = conflicting data.
guinea pig NBM/Ch4 region referred to as substantia innominata,
paracentral nucleus only thalamic subfield to contain ChAT-ir neurons,
arcuate nuclei referred to as infundibular nuclei33,
ChAT-ir neurons reported in the tuberal nuclei of the ventral hypothalamus which may correspond to arcuate nuclei1,3–5,
ChAT-ir neurons reported in intralaminar thalamic nuclei3. Cholinergic subfield nomenclature (Ch1-8) according to Mesulam and colleagues23. Tabular data was derived from the present and previous reports indicated by superscripted numbers:
Balaban, 2003;
Mizukawa et al., 1986;
Telencephalon
Cerebral Cortex
ACh is the major neuromodulatory neurotransmitter in the cortex and is thought to play an important role in learning, memory and attention (Callahan et al., 1993; Andrews et al., 1994; Muir et al., 1994; Hasselmo, 2006). The cortex of the Goettingen miniature pig displayed numerous ChAT-ir fibers similar to other mammals (e.g., humans, Mufson et al., 1989; Mesulam et al., 1992; nonhuman primates, Mrzljak and Goldman-Rakic, 1993; Mesulam, 2004; Raghanti et al., 2008; rodents, Ichikawa and Hirata, 1986; Lysakowski et al., 1986; Mechawar et al., 2000). Small ChAT positive neurons were seen within the cingulate, insular and piriform cortices as described in some rodent species (Eckenstein and Baughman, 1984; Mufson and Cunningham, 1988; Mechawar et al., 2000; Bhagwandin et al., 2006; Consonni et al., 2009) including murid rodents (Kruger et al., 2012) and the Hottentot golden mole (Calvey et al., 2013), but not in the BALB/c ByJ mouse (Kitt et al., 1994), guinea pig (Maley et al., 1988), monotremes (Manger et al., 2002) and most nonhuman primates (Mesulam et al., 1984; Everitt et al., 1988; Kordower et al., 1989). ChAT positive neurons in supragranular cortical layers (II–IV) have been reported in some rodent species (Eckenstein and Baughman, 1984; Mufson and Cunningham, 1988; Mechawar et al., 2000; Bhagwandin et al., 2006; Consonni et al., 2009), rabbit (Varga et al., 2003), feline (Avendaño et al., 1996), fetal Macaca mulatta (Hendry et al., 1987) and to a lesser degree in humans (Kasashima et al., 1999). Unlike the miniature pig, ChAT–ir neurons were found within the somatosensory cortex and to a lesser extent in transitional and limbic cortices in murine species and rabbits (Varga et al., 2003; Consonni et al., 2009). These cholinergic perikarya have been classified as interneurons, which contain calretinin and vasoactive intestinal peptide (VIP) and are involved in local cortical modulatory functions (von Endelhardt et al., 2007). Whether intrinsic cortical neurons in the miniature pig express calretinin and VIP, as well as their functional role remain unknown. In contrast to the rodent and pig cortex, a few studies have reported ChAT positive pyramidal neurons in layers III and V in the human motor and secondary sensory cortex (Kasashima et al., 1999; Benagiano et al., 2003). This species difference in the type of cortical neurons requires further investigation to verify the existence of cholinergic neurons in the human cortex. On the other hand, the pig cingulate, insular, and piriform cortices contained p75NTR-ir fibers similar to that seen in the rodent (Jaffar et al., 2001), monkey, and human (Mrzljak and Goldman-Rakic, 1993) cortex. Perikarya containing p75NTR have also been identified in somatosensory and motor cortices in the adult macaque (Miller, 2000) and in medial temporal cortex and amygdala in the aged human and Alzheimer diseased brain (Mufson and Kordower, 1992) but not in the rat (Lee et al., 1998) or mouse (Perez et al., 2007) suggesting species differences in the cortical p75NTR cellular phenotype.
Olfactory tubercle, Striatum and Basal Forebrain
The olfactory tubercle of the miniature pig contained ChAT-ir neurons and fibers as described in other mammals (McGeer et al., 1982; Armstrong et al., 1983; Wahle and Meyer, 1986; Woolf, 1991; Varga et al., 2003; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010; Pieters et al., 2010; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c). More caudally, large multipolar ChAT-ir neurons were located in the striatum of the miniature pig similar to other mammals (Sofroniew et al., 1982; Armstrong et al., 1983; Mesulam et al., 1984; Satoh and Fibiger, 1985; Mufson and Cunningham, 1988; Mufson et al., 1989; Woolf, 1991; Geula et al., 1993; Varga et al., 2003; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010; Pieters et al., 2010; Bajo et al., 2014; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c). The major source of cholinergic striatal innervation is the large aspiny interneurons that comprise 1% of striatal neurons (Woolf and Butcher, 1981; Graveland and DiFiglia, 1985), which regulate intrastriatal local circuit activity (Galarraga et al., 1999; Koós and Tepper, 2002). These cholinergic neurons are not p75NTR-ir (Sobreviela et al., 1994; Kordower et al., 1994, present study) and are related to the regulation of motoric function (Macintosh, 1941; Hebb and Silver, 1961; Woolf et al., 1984).
ChAT-ir neurons were observed in the MS/VDB (Ch1/Ch2) and HDB (Ch3). The MS/VDB complex projects to the hippocampus, entorhinal, cingulate and retrosplenial cortices in many mammals (Mesulam et al., 1983a; Irle and Markowitsch, 1984; Woolf et al., 1984; Amaral and Kurz, 1985), while the HDB projects to the olfactory bulb and visual cortex (Mesulam et al., 1983a; Woolf et al., 1984). The human HDB consists of a few cholinergic neurons (Mesulam et al., 1983a) suggesting a diminished functional role compared to other mammals. We also observed lightly immunoreactive cholinergic neurons within the lateral septum of the miniature pig, as reported in the rat (Kimura et al., 1990), raccoon (Brauer et al., 1999) and monkey (Kimura et al., 1990).
More caudally, Ch4 (NBM/SI) displayed ChAT-ir neurons extending from the crossing of the anterior commissure to the level of the lateral geniculate nucleus as well as a few scattered somata embedded within the internal capsule (termed interstitial cholinergic neurons), globus pallidus, and anterior regions of the amygdala; (Mesulam et al., 1983). These neurons share the magnocellular hyperchromic morphology observed in other mammals (Mesulam et al., 1983a, 1983b; Woolf, 1991; Varga et al., 2003). In rodents, and monotremes (the oldest mammalian group) a homologous NBM/SI cell group is found adjacent to the globus pallidus (Mesulam et al., 1983b; Manger et al., 2002) similar to other mammalian species (Woolf, 1991; Varga et al., 2003; Descarries et al., 2004; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010; Pieters et al., 2010; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c; see Table 2). Interestingly, the NBM/SI displays the greatest degree of subfield differentiation in monkeys, great apes (Benzing et al., 1993a) and humans (Gorry, 1963; Mesulam et al., 1983a; Saper and Chelimsky, 1984; Mesulam and Geula, 1988), compared to other mammals (Mesulam et al., 1983b) including the miniature pig (present findings). However, in sheep, the complexity of this region is intermediate between rodents and humans, four subsectors are found including the anterior, intermedioventral, intermediodorsal and posterior subfields but not an anterolateral or medial subfield (Ferreira et al., 2001). Thus Ch4 (NBM/SI) intricacy among species may be associated with a gain in neocortical chemoanatomical and functional complexity (Howells et al., 2010; Lui et al., 2011). In addition to innervating neocortical areas (Mesulam et al., 1983a), cholinergic Ch4 neurons also send a dense projection to the basal nucleus of the amygdala (Woolf, 1991). In this regard, extensive cholinergic innervation was seen in the basolateral and basomedial amygdaloid nuclei in the miniature pig similar to that observed in the rat, golden mole (Calvey et al., 2013), monkey (Woolf and Butcher, 1982; Amaral and Bassett, 1989) and human (Mufson et al., 1989; Benzing et al., 1993b). Although, only ChAT-ir fibers are found in the amygdala of most mammals including the minipig, a recent report revealed ChAT-ir neurons in the amygdala of the golden mole (Calvey et al., 2013).
We observed that the majority of neurons within the CBF of the miniature pig co-express the p75NTR similar to other mammals (Hefti et al., 1986; Mesulam et al., 1989; Mufson et al., 1989; Kordower et al., 1989; Higgins and Mufson, 1989; Hefti and Mash, 1989; Ferreira et al., 2001). The p75NTR receptor plays a key role in regulating the trophic status of CBF neurons (Kramer et al., 1999). Of interest was our observation that CBF neurons in the miniature pig were GAL immunonegative, while in rodents (Melander et al., 1985; Perez et al., 2001) and monkeys (Melander and Staines, 1986; Walker et al., 1989; Kordower and Mufson, 1990; Kordower et al., 1992) these neurons were GAL positive. However, GAL-ir fibers in the miniature pig were seen in close proximity to cholinergic neurons more similar to humans (Chan-Palay, 1988; Kordower and Mufson, 1990; Mufson et al., 1993) and great apes (Benzing et al., 1993a). We have suggested that the separation from intraneuronal GAL within cholinergic neurons to extra-neuronal innervation may relate to a greater control over cholinergic cellular activity in humans and great apes (Mufson et al., 2005; Counts et al., 2010).
Epithalamus, Thalamus, and Hypothalamus
In the epithalamus of the Goettingen miniature pig, ChAT-ir profiles were found in the medial habenular nucleus, fasciculus retroflexus and interpeduncular nucleus as described in other mammals (Woolf and Butcher, 1985; Mesulam et al., 1986; Mufson and Cunningham, 1988; Butcher et al., 1992; Oh et al., 1992; Manger et al., 2002; Kruger et al., 2012; Maseko et al., 2013). Tract tracing studies in mammals have shown that the medial habenula provides the main source of cholinergic innervation via the fasciculus retroflexus to the interpeduncular nucleus (Woolf and Butcher, 1985; Woolf et al., 1990; Woolf, 1991). The functional role of this dense cholinergic innervation remains to be investigated.
ChAT-ir neurons were virtually absent from the thalamic nuclei of the miniature pig (see Table 2), although they have been reported in the centromedian nucleus of the dorsal thalamus in the river hippopotamus (Dell et al., 2016a), an animal related to the pig, and in the paracentral thalamic nucleus of the macaque monkey (Rico and Cavada, 1998). Numerous cholinergic fibers were observed in the anteroventral, parateanial, mediodorsal, and laterodorsal thalamic nuclei as well as in the reticular nucleus in the miniature pig (present study) as reported previously in mammals (Hallanger et al., 1987; Levey et al., 1987; Schafer et al., 1998). In addition, ChAT positive fibers were found in the anterior pretectal nucleus, lateral geniculate nucleus, as well as in the subthalamic nucleus as previously described in the Goettingen minipig (Larsen et al., 2004). Neuroanatomical tract-tracing studies in the rat have demonstrated that the cholinergic neurons located in pedunculopontine tegmental nucleus innervate the thalamic anterior, laterodorsal, central medial, and mediodorsal thalamic nuclei as well as the lateral geniculate nucleus (Mufson et al., 1986; Hallanger et al., 1987; Fitzpatrick et al., 1988; Smith et al., 1988). Retrograde tracing studies have revealed that the cholinergic laterodorsal tegmental nucleus also projects to several thalamic nuclei and the pretectum (Satoh and Fibiger et al., 1986). Whether similar projections exist in the pig remains to be determined. The functional significance of these observations in the pig are not clear, but cholinergic pedunculopontine neurons have been suggested to play a role in regulating wakefulness and sleep-wake transitions (Brown et al., 2012), perhaps via its thalamic innervation.
Unlike the rat (Rao et al., 1987) and other vertebrates (Mason et al., 1983; Ekstrom, 1987; Tago et al., 1987), the hypothalamus of the miniature pig contained ChAT positive neurons only within the neurosecretory arcuate nucleus. Hypothalamic ChAT-ir fibers were observed coursing in the external portion of the median eminence in the minipig. Several mammals related to the miniature pig including the river hippopotamus (Dell et al., 2016a), the harbour porpoise (Dell et al., 2016b), and the minke whale (Dell et al., 2016c) display three separate ChAT positive hypothalamic cell groups located lateral, dorsal and ventral to the third ventricle. These cholinergic neuronal groups could be homologous to the arcuate nuclei in other mammals including the minipig (Table 2). Since arcuate neurons release hypophysiotropic hormones from terminals located within the median eminence via the hypophysial portal system into the anterior portion of the pituitary gland, it is likely that the cholinergic arcuate system regulates pituitary hormone release (Scott and Pepe, 1987). Although the physiological function of ACh in the neurosecretory system is unclear, perhaps it plays a role in the liberation of hormones acting as an autocrine signal (Wessler and Kirkpatrick, 2008).
Mesencephalon
ChAT-ir neurons were found within the Edinger-Westphal nucleus, the motor nuclei of the oculomotor (IIIn) and trochlear (IVn) cranial nerves. Cholinergic neurons of cranial nerves III, IV, and VI innervate the extraocular eye musculature and the levator palpebrae muscle (Warwick, 1953), while the Edinger-Westphal nucleus is considered the source of preganglionic input to the ciliary ganglion (Strassman et al., 1987). Cranial nerve motor neurons are some of the largest cholinergic cells in the brain and exhibit multiple thick processes in the rodent, feline, and primate brain (Strassman et al., 1987; Woolf, 1991), a pattern mirrored in the miniature pig. Cholinergic neurons in the motor cranial nerve nuclei are a persistent trait in mammalian vertebrates (Armstrong et al., 1983; Mesulam et al., 1983a; Vincent and Reiner, 1987; Everitt et al., 1988; Maley et al., 1988; Mufson and Cunningham, 1988; Kordower et al., 1989; Alonso and Amaral, 1995; Ichikawa et al., 1997; Varga et al., 2003; Maseko and Manger, 2007; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010, 2012; Pieters et al., 2010; Calvey et al., 2013; Maseko et al., 2013; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c) suggesting similar biological actions.
Similar to other mammalian-vertebrates (Armstrong et al., 1983; Mesulam et al., 1983b, 1984; Mufson et al., 1986; Mufson et al., 1988; Mesulam et al., 1989; Ichikawa and Hirata, 1990; Woolf et al., 1990; Lavoie and Parent, 1994; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010; Motts and Schofield, 2010; Pieters et al., 2010; Kruger et al., 2012; Maseko et al., 2013; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c), ChAT positive neurons were seen in the pedunculopontine (Ch5), and laterodorsal tegmental (Ch6) nuclei in the miniature pig, which provide cholinergic innervation to the thalamus (Hallanger et al., 1987; Woolf et al., 1990; Motts and Schofield, 2010). Like other mammals, porcine ChAT-ir perikarya within Ch5 and Ch6 are morphologically heterogeneous and relatively large, second only to neurons of the Ch4 complex (Mesulam et al., 1989). Cholinergic immunoreactive neurons within the pontomesencephalon subfields of other members of Artiodactyla display morphological differences compared to the miniature pig. For example, in the male giraffe, cholinergic neurons within the lateral tegmental nucleus (Ch6) are larger than those found in the pedunculopontine nucleus (Ch5) (Bux et al., 2010), which is opposite to what we observed for the minipig. In the river hippopotamus, Ch6 is separated into magnocellular and parvocellular subdivisions (Dell et al., 2016). Whereas, Ch5 and Ch6 are divided into parvocellular and magnocellular subdivisions in the rock hyrax (Gravett et al., 2009). By contrast, the cholinergic parabigeminal nucleus consists of small and oval-shaped perikarya (Mufson et al., 1986; Mufson et al., 1988; Mufson and Cunningham, 1988; Henderson, 1989; Woolf et al., 1990; Maseko and Manger, 2007; Maseko et al., 2007; Bhagwandin et al., 2008; Limacher et al., 2008; Gravett et al., 2009; Bux et al., 2010; Kruger et al., 2010; Pieters et al., 2010; Patzke et al., 2014; Calvey et al., 2015a, 2015b, 2016; Dell et al., 2010, 2016a, 2016b, 2016c), which project to the superficial layers of the superior colliculus (Beninato and Spencer, 1986; Mufson et al., 1986; Henderson, 1989). No cholinergic cells were observed in the superior colliculus of the miniature pig similar to other mammalian species (Woolf, 1991; Manger et al., 2002).
Conclusions
The present study provides the first chemoanatomical distribution of cholinergic neurons and fibers in select regions of the Goettingen miniature pig brain. Cholinergic neurons were found in the cortex, hippocampus, striatum, MS, LS, VDB/HDB, NBM, medial habenula, pedunculopontine and laterodorsal tegmental nuclei, parabigeminal nucleus, and cranial nerve nuclei. In addition, cholinergic fibers displayed a laminar distribution in the cortex and hippocampus and were seen in the thalamus. The present observations provide detailed information showing that the organization of central cholinergic systems within the miniature pig brain are comparable to most other mammalian species (Table 2), suggesting that the miniature pig may be an appropriate model for the study of the central cholinergic system in various neurological disorders.
Acknowledgments
The authors thank Mr. M. Nadeem for technical assistance. We thank Dr. Jeffrey Kordower for providing the tissue used in this study.
Supported by: AG14499 and Barrow Neurological Institute Barrow and Beyond
Abbreviations
- 3V
third ventricle
- aca
anterior part of the anterior commissure
- Acb
accumbens nucleus
- APT
anterior pretectal nucleus
- ArcD
arcuate nucleus, dorsal part
- ArcM
arcuate nucleus, medial part
- AVL
lateral part of the anteroventral nucleus of the thalamus
- AVM
medial part of the anteroventral nucleus of the thalamus
- BLA
basolateral amygdaloid nucleus, anterior part
- BMA
basomedial amygdaloid nucleus, anterior part
- BST
bed nucleus of the stria terminalis
- CA1
field CA1 of hippocampus
- CA3
field CA3 of hippocampus
- cc
corpus callosum
- Cd
caudate
- IIIn
oculomotor nucleus (OCN)
- IIIr
third cranial nerve root
- IVn
trochlear nucleus
- IVr
fourth cranial nerve root
- cp
cerebral peduncle
- DG
dentate gyrus
- DLG
dorsal lateral geniculate nucleus
- DS
dorsal septum
- eml
external medullary lamina
- EW
Edinger-Westphal nucleus
- f
fornix
- fi
fimbria hippocampus
- fr
fasciculus retroflexus
- gcc
genu of the corpus callosum
- GP
globus pallidus
- GrDG
granular layer of the dentate gyrus
- HDB
horizontal limb of the diagonal band of Broca
- ic
internal capsule
- InG
intermediate gray layer of the superior colliculus
- InWh
intermediate white layer of the superior colliculus
- IP
interpeduncular nucleus
- LaDL
lateral amygdaloid nucleus, dorsolateral part
- lcj
islands of Calleja
- LD
laterodorsal thalamic nucleus
- LDT
laterodorsal tegmental nucleus
- LHb
lateral habenular nucleus
- LMol
lacunosum moleculare layer of the hippocampus
- lo
lateral olfactory tract
- LSD
dorsal part of the lateral septal nucleus
- LSV
ventral part of the lateral septal nucleus
- LV
lateral ventricle
- MD
mediodorsal thalamic nucleus
- MHb
medial habenular nucleus
- mlf
medial longitudinal fasciculus
- Mol
molecular layer of the dentate gyrus
- MS
medial septal nucleus
- mt
mammillothalamic tract
- NBM
nucleus basalis of Meynert
- Op
optic layer of the superior colliculus
- Or
oriens layer of the hippocampus
- ot
optic tract
- PB
parabigeminal nucleus
- Pi
piriform cortex
- Po
posterior thalamic nuclear group
- PoDG
polymorphic layer of the dentate gyrus
- PPN
pedunculopontine tegmental nucleus
- PT
parateanial thalamic nucleus
- Pu
putamen
- PVA
paraventricular thalamic nucleus
- Py
pyramidal cell layer of the hippocampus
- Rad
stratum radiatum of the hippocampus
- Re
reuniens thalamic nucleus
- Rt
reticular thalamic nucleus
- SC
superior colliculus
- SI
substantia innominata
- sm
stria medullaris of the thalamus
- Str
striatum
- SuG
superficial gray layer of the superior colliculus
- Tu
olfactory tubercle
- VDB
vertical limb of the diagonal band of Broca
- VL
ventrolateral thalamic nucleus
- VLG
ventral lateral geniculate
- VM
ventral posteromedial thalamic nucleus
- VP
ventral posterolateral thalamic nucleus
- ZI
zona incerta
- Zo
zonal layer of the superior colliculus
Footnotes
Conflict of Interest
The authors report no conflict of interest.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data. Study concept and design: E.J. Mufson, S.E. Perez and L. Mahady. Acquisition of data: L. Mahady, S.E. Perez, and E.J. Mufson. Analysis and interpretation of data: L. Mahady, S.E. Perez and E.J. Mufson. Drafting of the article: L. Mahady, S.E. Perez, and E.J. Mufson. Critical revision of the article for important intellectual content: L. Mahady, S.E. Perez, E.J. Mufson, D.F. Emerich, and L.U. Wahlberg.
References
- Alonso JR, Amaral DG. Cholinergic innervation of the primate hippocampal formation. I. Distribution of choline acetyltransferase immunoreactivity in the Macaca fascicularis and Macaca mulatta monkeys. J Comp Neurol. 1995;355(2):135–170. doi: 10.1002/cne.903550202. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281(3):337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Jansen JH, Linders S, Princen A. Effects of disrupting the cholinergic system on short-term spatial memory in rats. Psychopharmacology. 1994;115(4):485–494. doi: 10.1007/BF02245572. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216(1):53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav Neurosci. 2004;118(1):223–236. doi: 10.1037/0735-7044.118.1.223. [DOI] [PubMed] [Google Scholar]
- Avendaño C, Umbriaco D, Dykes RW, Descarries L. Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J Chem Neuroanat. 1996;11(2):113–130. doi: 10.1016/0891-0618(96)00132-9. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Leach ND, Cordery PM, Nodal FR, King AJ. The cholinergic basal forebrain in the ferret and its inputs to the auditory cortex. Eur J Neurosci. 2014;40(6):2922–2940. doi: 10.1111/ejn.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano V, Virgintino D, Flace P, Girolamo F, Errede M, Roncali L, Ambrosi G. Choline acetyltransferase-containing neurons in the human parietal neocortex. Eur J Histochem. 2003;47(3):253–256. doi: 10.4081/835. [DOI] [PubMed] [Google Scholar]
- Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol. 1986;253(4):525–538. doi: 10.1002/cne.902530409. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Kordower JH, Mufson EJ. Galanin immunoreactivity within the primate basal forebrain: evolutionary change between monkeys and apes. J Comp Neurol. 1993a;336(1):31–39. doi: 10.1002/cne.903360103. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Mufson EJ, Armstrong DM. Immunocytochemical distribution of peptidergic and cholinergic fibers in the human amygdala: their depletion in Alzheimer’s disease and morphologic alteration in non-demented elderly with numerous senile plaques. Brain Res. 1993b;625(1):125–138. doi: 10.1016/0006-8993(93)90145-d. [DOI] [PubMed] [Google Scholar]
- Bhagwandin A, Fuxe K, Manger PR. Choline acetyltransferase immunoreactive cortical interneurons do not occur in all rodents: a study of the phylogenetic occurrence of this neural characteristic. J Chem Neuroanat. 2006;32(4):208–216. doi: 10.1016/j.jchemneu.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bhagwandin A, Fuxe K, Bennett NC, Manger PR. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brains of two species of African mole-rat. J Chem Neuroanat. 2008;35(4):371–387. doi: 10.1016/j.jchemneu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Brauer K, Holzer M, Brückner G, Tremere L, Rasmusson DD, Poethke R, Arendt T, Härtig W. Two distinct populations of cholinergic neurons in the septum of raccoon (Procyon lotor): Evidence for a separate subset in the lateral septum. J Comp Neurol. 1999;412(1):112–122. doi: 10.1002/(sici)1096-9861(19990913)412:1<112::aid-cne8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Phys Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner G, Schober W, Härtig W, Ostermann-Latif C, Webster HH, Dykes RW, Rasmusson DD, Biesold D. The basal forebrain cholinergic system in the raccoon. J Chem Neuroanat. 1992;5(6):441–452. doi: 10.1016/0891-0618(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Butcher LL, Oh JD, Woolf NJ, Edwards RH, Roghani A. Organization of central cholinergic neurons revealed by combined in situ hybridization histochemistry and choline-O-acetyltransferase immunocytochemistry. Neurochem Int. 1992;21(3):429–445. doi: 10.1016/0197-0186(92)90195-w. [DOI] [PubMed] [Google Scholar]
- Bux F, Bhagwandin A, Fuxe K, Manger PR. Organization of cholinergic, putative catecholaminergic and serotonergic nuclei in the diencephalon, mibrain and pons of sub-adult male giraffes. J Chem Neuroanat. 2010;39(3):189–203. doi: 10.1016/j.jchemneu.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Kinsora JJ, Harbaugh RE, Reeder TM, Davis RE. Continuous ICV infusion of scopolamine impairs sustained attention of rhesus monkeys. Neurobiol Aging. 1993;14(2):147–151. doi: 10.1016/0197-4580(93)90090-x. [DOI] [PubMed] [Google Scholar]
- Calvey T, Patzke N, Kaswera C, Gilissen E, Bennett NC, Manger PR. Nuclear organisation of some immunohistochemically identifiable neural systems in three Afrotherian species-Potomogale velox, Amblysomus hottentotus and Petrodromus tetradactylus. J Chem Neuroanat. 2013;(1):50–51. 48–65. doi: 10.1016/j.jchemneu.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Calvey T, Patzke N, Kaswera-Kyamakya C, Gilissen E, Bertelsen MF, Pettigrew JD, Manger PR. Organization of cholinergic, catecholaminergic, serotonergic and orexinergic nuclei in three strepsirrhine primates: Galago demidoff, Perodicticus potto and Lemur catta. J Chem Neuroanat. 2015a;70:42–57. doi: 10.1016/j.jchemneu.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvey T, Alagaili A, Bertelsen M, Bhagwandin A, Pettigrew J, Manger P. Nuclear organization of some immunohistochemically identifiable neural systems in two species of the Euarchontoglires: A Lagomorph, Lepus capensis, and a Scandentia, Tupaia belangeri. J Chem Neuroanat. 2015b;70:1–19. doi: 10.1016/j.jchemneu.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Calvey T, Patzke N, Bennett N, Consolate K, Gilissen E, Alagaili A, Mohammed O, Pettigrew J, Manger P. Nuclear organisation of some immunohistochemically identifiable neural systems in five species of insectivore-Crocidura cyanea, Crocidura olivieri, Sylvisorex ollula, Paraechinus aethiopicus and Atelerix frontalis. J Chem Neuroanat. 2016;72:34–52. doi: 10.1016/j.jchemneu.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Neurons with galanin innervate cholinergic cells in the human basal forebrain and galanin and acetylcholine coexist. Brain Res Bull. 1988;21(3):465–472. doi: 10.1016/0361-9230(88)90160-8. [DOI] [PubMed] [Google Scholar]
- Chao LP, Kan KS, Hung FM. Immunohistochemical localization of choline acetyltransferase in rabbit forebrain. Brain Res. 1982;235(1):65–82. doi: 10.1016/0006-8993(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Consonni S, Leone S, Becchetti A, Amadeo A. Developmental and neurochemical features of cholinergic neurons in the murine cerebral cortex. BMC Neurosci. 2009;10:18. doi: 10.1186/1471-2202-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Villani L, Fasolo A, Franzoni MF, Gribaudo L, Oktedalen O, Fonnum F. Topography of cholinergic and substance P pathways in the habenulo-interpeduncular system of the rat. An immunocytochemical and microchemical approach. Neurosci. 1987;21(1):253–270. doi: 10.1016/0306-4522(87)90337-x. [DOI] [PubMed] [Google Scholar]
- Counts SE, Perez SE, Ginsberg SD, Mufson EJ. Neuroprotective role for galanin in Alzheimer’s disease. Exs. 2010;102:143–162. doi: 10.1007/978-3-0346-0228-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell LA, Kruger JL, Bhagwandin A, Jillani NE, Pettigrew JD, Manger PR. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic systems in the brains of two megachiropteran species. J Chem Neuroanat. 2010;40(2):177–195. doi: 10.1016/j.jchemneu.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Dell L, Patzke N, Spocter MA, Bertelsen MF, Siegel JM, Manger PR. Organization of the sleep-related neural systems in the brain of the river hippopotamus (Hippopotamus amphibius): A most unusual cetartiodactyl species. J Comp Neurol. 2016a;524(10):2036–58. doi: 10.1002/cne.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell L, Patzke N, Spocter MA, Siegel JM, Manger PR. Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena) J Comp Neurol. 2016b;524(10):1999–2017. doi: 10.1002/cne.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell L, Karlsson KÆ, Patzke N, Spocter MA, Siegel JM, Manger PR. Organization of the sleep-related neural systems in the brain of the minke whale (Balaenoptera acutorostrata) J Comp Neurol. 2016c;524(10):2018–35. doi: 10.1002/cne.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Krnjević Ki, Steriade M. Acetylcholine in the cerebral cortex. Amsterdam; Boston: Elsevier; 2004. p. xv.p. 327. [Google Scholar]
- Diez M, Danner S, Frey P, Sommer B, Staufenbiel M, Wiederhold K, Hokfelt Neuropeptide alterations in the hippocampal formation and cortex of transgenic mice overexpressing β-amyloid precursor protein (APP) with the Swedish double mutation (APP23) Neurobiol Dis. 2003;14(3):579–594. doi: 10.1016/j.nbd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dolezalova D, Hruska-Plochan M, Bjarkam CR, Sørensen JCH, Cunningham M, Weingarten D, Ciacci J, Juhas S, Juhasova J, Motlik J, Hefferan M, Hazel T, Johe K, Carromeu C, Muotri A, Bui J, Strnadel J, Marsala M. Pig models of neurodegenerative disorders: Utilization in cell replacement-based preclinical safety and efficacy studies. J Comp Neurol. 2014;522(12):2784–2801. doi: 10.1002/cne.23575. [DOI] [PubMed] [Google Scholar]
- Eckenstein F, Sofroniew M. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983;3(11):2286–2291. doi: 10.1523/JNEUROSCI.03-11-02286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenstein F, Baughman RW. Two types of cholinergic innervation in cortex, one co-localized with vasoactive intestinal polypeptide. Nature. 1984;309(5964):153–155. doi: 10.1038/309153a0. [DOI] [PubMed] [Google Scholar]
- Eckenstein FP, Baughman RW, Quinn J. An anatomical study of cholinergic innervation in rat cerebral cortex. Neurosci. 1988;25(2):457–474. doi: 10.1016/0306-4522(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Emson PC. Peptides as neurotransmitter candidates in the mammalian cns. Prog Neurobiol. 1979;13(1):61–116. [Google Scholar]
- Everitt BJ, Sirkiä TE, Roberts AC, Jones GH, Robbins TW. Distribution and some projections of cholinergic neurons in the brain of the common marmoset, Callithrix jacchus. J Comp Neurol. 1988;271(4):533–558. doi: 10.1002/cne.902710406. [DOI] [PubMed] [Google Scholar]
- Félix B, Léger M, Albe-Fessard D, Marcilloux J, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull. 49(1–2):1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Ferreira G, Meurisse M, Tillet Y, Lévy F. Distribution and co-localization of choline acetyltransferase and p75 neurotrophin receptors in the sheep basal forebrain: implications for the use of a specific cholinergic immunotoxin. Neurosci. 2001;104(2):419–439. doi: 10.1016/s0306-4522(01)00075-6. [DOI] [PubMed] [Google Scholar]
- Fisone G, Wu CF, Consolo S, Nordström O, Brynne N, Bartfai T, Melander T, Hökfelt T. Galanin inhibits acetylcholine release in the ventral hippocampus of the rat: histochemical, autoradiographic, in vivo, and in vitro studies. Proc Natl Acad Sci USA. 1987;84(20):7339–7343. doi: 10.1073/pnas.84.20.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Conley M, Luppino G, Matelli M, Diamond IT. Cholinergic projections from the midbrain reticular formation and the parabigeminal nucleus to the lateral geniculate nucleus in the tree shrew. J Comp Neurol. 1988;272(1):43–67. doi: 10.1002/cne.902720105. [DOI] [PubMed] [Google Scholar]
- Fjord-Larsen L, Kusk P, Tornoe J, Juliusson B, Torp M, Bjarkam CR, Nielsen MS, Handberg A, Sorensen JC, Wahlberg LU. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the Gottingen minipig basal forebrain. Mol Ther. 2010;18(12):2164–2172. doi: 10.1038/mt.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Schlander M, Léránth C. Cholinergic neurons in the hippocampus. A combined light- and electron-microscopic immunocytochemical study in the rat. Cell Tiss Res. 1986;246(2):293–301. doi: 10.1007/BF00215891. [DOI] [PubMed] [Google Scholar]
- Fu W, Le Maitre E, Fabre V, Bernard JF, David Xu ZQ, Hokfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518(17):3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- Galarraga E, Hernández-López S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci. 1999;19(9):3629–3638. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Tokuno H, Kuo CC. Developmentally transient expression of acetylcholinesterase within cortical pyramidal neurons of the rat brain. Brain Res Dev Brain Res. 1993;76(1):23–31. doi: 10.1016/0165-3806(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 1999;411(4):693–704. [PubMed] [Google Scholar]
- Glud AN, Hedegaard C, Nielsen MS, Søorensen JC, Bendixen C, Jensen PH, Mogensen PH, Larsen K, Bjarkam CR. Direct MRI-guided stereotaxic viral mediated gene transfer of alpha-synuclein in the Göttingen minipig CNS. Acta Neurobiol Exp. 2011;71(4):508–518. doi: 10.55782/ane-2011-1867. [DOI] [PubMed] [Google Scholar]
- Gorry JD. Studies on the comparative anatomy of the ganglion basale of Meynert. Acta Anat. 1963;55:51–104. doi: 10.1159/000142464. [DOI] [PubMed] [Google Scholar]
- Graveland GA, Difiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327(1–2):307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Gravett N, Bhagwandin A, Fuxe K, Manger PR. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the rock hyrax, Procavia capensis. J Chem Neuroanat. 2009;38(1):57–74. doi: 10.1016/j.jchemneu.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Luan Phan K, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Behav. 2005;81(3):575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262(1):105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hashikawa T. Regional and laminar distribution of choline acetyltransferase immunoreactivity in the cat superior colliculus. Neurosci Res. 1989;6(5):426–437. doi: 10.1016/0168-0102(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Hasselmo M, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo M. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb CO, Silver A. Gradient of cholinesterase activity and of choline acetylase activity in nerve fibres: gradient of choline acetylase activity. Nature. 1961;189(4759):123–125. doi: 10.1038/189123a0. [DOI] [PubMed] [Google Scholar]
- Heckers S, Geula C, Mesulam MM. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol. 1992;325(1):68–82. doi: 10.1002/cne.903250107. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Mash DC. Localization of nerve growth factor receptors in the normal human brain and in Alzheimer’s disease. Neurobiol Aging. 1989;10(1):75–87. doi: 10.1016/s0197-4580(89)80014-4. [DOI] [PubMed] [Google Scholar]
- Hendry SHC, Jones EG, Killackey HP, Chalupa LM. Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Dev Brain Res. 1987;465(1–2):313–317. doi: 10.1016/0165-3806(87)90252-5. [DOI] [PubMed] [Google Scholar]
- Henderson Z. The cholinergic input to the superficial layers of the superior colliculus: an ultrastructural immunocytochemical study in the ferret. Brain Res. 1989;476(1):149–153. doi: 10.1016/0006-8993(89)91548-5. [DOI] [PubMed] [Google Scholar]
- Hersh LB, Coe B, Casey L. A fluorometric assay for choline acetyltransferase and its use in the purification of the enzyme from human placenta. J Neurochem. 1978;30(5):1077–1085. doi: 10.1111/j.1471-4159.1978.tb12401.x. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Mufson EJ. NGF receptor gene expression is decreased in the nucleus basalis in Alzheimer’s disease. Exp Neurol. 1989;106(3):222–236. doi: 10.1016/0014-4886(89)90155-6. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(8):1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Shimizu T. Organization of choline acetyltransferase-containing structures in the cranial nerve motor nuclei and spinal cord of the monkey. Brain Res. 1998;779(1–2):96–103. doi: 10.1016/s0006-8993(97)01090-1. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Ajiki K, Matsuura J, Misawa H. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: in situ hybridization histochemistry and immunohistochemistry. J Chem Neuroanat. 1997;13(1):23–39. doi: 10.1016/s0891-0618(97)00021-5. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Hirata Y. Organization of choline acetyltransferase-containing structures in the cranial nerve motor nuclei and lamina IX of the cervical spinal cord of the rat. J Hirnforschung. 1990;31(2):251–257. [PubMed] [Google Scholar]
- Ichikawa T, Hirata Y. Organization of choline acetyltransferase-containing structures in the forebrain of the rat. J Neurosci. 1986;6(1):281–292. doi: 10.1523/JNEUROSCI.06-01-00281.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Abrahamson EE, Isanski BA, Wuu J, Mufson EJ, DeKosky ST. Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch Neurol. 2007;64(9):1312–1317. doi: 10.1001/archneur.64.9.1312. [DOI] [PubMed] [Google Scholar]
- Irle E, Markowitsch HJ. Basal forebrain efferents reach the whole cerebral cortex of the cat. Brain Res Bull. 1984;12(5):493–512. doi: 10.1016/0361-9230(84)90165-5. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Tanaka D., Jr Cholinergic and non-cholinergic projections from the canine pontomesencephalic tegmentum (Ch5 area) to the caudal intralaminar thalamic nuclei. Experimental brain research. 1986;62(1):179–188. doi: 10.1007/BF00237414. [DOI] [PubMed] [Google Scholar]
- Jaffar S, Counts SE, Ma SY, Dadko E, Gordon MN, Morgan D, Mufson EJ. Neuropathology of mice carrying mutant APP(swe) and/or PS1(M146L) transgenes: alterations in the p75(NTR) cholinergic basal forebrain septohippocampal pathway. Exp Neurol. 2001;170(2):227–243. doi: 10.1006/exnr.2001.7710. [DOI] [PubMed] [Google Scholar]
- Jakobsen JE, Johansen MG, Schmidt M, Dagnæs-Hansen F, Dam K, Gunnarsson A, Liu Y, Kragh PM, Li R, Holm IE, Callesen H, Mikkelsen JG, Nielsen AL, Jørgensen AL. Generation of minipigs with targeted transgene insertion by recombinase-mediated cassette exchange (RMCE) and somatic cell nuclear transfer (SCNT) Trans Res. 2013;22(4):709–723. doi: 10.1007/s11248-012-9671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol. 1990;295(3):485–514. doi: 10.1002/cne.902950311. [DOI] [PubMed] [Google Scholar]
- Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol. 1987;261(1):15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- Kasa P. The cholinergic systems in brain and spinal cord. Prog Neurobiol. 1986;26(3):211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Kasashima S, Kawashima A, Muroishi Y, Futakuchi H, Nakanishi I, Oda Y. Neurons with choline acetyltransferase immunoreactivity and mRNA are present in the human cerebral cortex. Histochem Cell Biol. 1999;111(3):197–207. doi: 10.1007/s004180050349. [DOI] [PubMed] [Google Scholar]
- Kelley CM, Perez SE, Overk CR, Wynick D, Mufson EJ. Effect of neocortical and hippocampal amyloid deposition upon galaninergic and cholinergic neurites in AβPPswe/PS1ΔE9 mice. J Alz Dis. 2011;25(3):491–504. doi: 10.3233/JAD-2011-102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, McGeer PL, Peng F, McGeer EG. Choline acetyltransferase-containing neurons in rodent brain demonstrated by immunohistochemistry. Sci. 1980;208(4447):1057–1059. doi: 10.1126/science.6990490. [DOI] [PubMed] [Google Scholar]
- Kimura H, Tago H, Akiyama H, Hersh LB, Tooyama I, McGeer PL. Choline acetyltransferase immunopositive neurons in the lateral septum. Brain Res. 1990;533(1):165–170. doi: 10.1016/0006-8993(90)91812-u. [DOI] [PubMed] [Google Scholar]
- Kitt CA, Hohmann C, Coyle JT, Price DL. Cholinergic innervation of mouse forebrain structures. J Comp Neurol. 1994;341(1):117–129. doi: 10.1002/cne.903410110. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2011;221(2):430–442. doi: 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Kodama T, Honda Y. Acetylcholine releases of mesopontine PGO-on cells in the lateral geniculate nucleus in sleep-waking cycle and serotonergic regulation. Prog Neuropsychopharmacol Biol Psych. 1996;20(7):1213–1227. doi: 10.1016/s0278-5846(96)00107-8. [DOI] [PubMed] [Google Scholar]
- Köhn F, Sharifi AR, Simianer AH. Modeling the growth of the Goettingen minipig. J Animal Sci. 2007;85(1):84–92. doi: 10.2527/jas.2006-271. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22(2):529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, Gash DM. Telencephalic cholinergic system of the New World monkey (Cebus apella): morphological and cytoarchitectonic assessment and analysis of the projection to the amygdala. J Comp Neurol. 1989;279(4):528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Mufson EJ. Galanin-like immunoreactivity within the primate basal forebrain: differential staining patterns between humans and monkeys. J Comp Neurol. 1990;294(2):281–292. doi: 10.1002/cne.902940211. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Le HK, Mufson EJ. Galanin immunoreactivity in the primate central nervous system. J Comp Neurol. 1992;319(4):479–500. doi: 10.1002/cne.903190403. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek J, Mufson EJ. trk-immunoreactivity in the monkey central nervous system: forebrain. J Comp Neurol. 1994;349(1):20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bøgh IB, Holm IE, Jakobsen JE, Johansen MG, Purup S, Bolund L, Vajta G, Jørgensen AL. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer’s disease-causing dominant mutation APPsw. Trans Res. 2009;18(4):545–558. doi: 10.1007/s11248-009-9245-4. [DOI] [PubMed] [Google Scholar]
- Kramer BMR, Van der Zee CEEM, Hagg T. p75 nerve growth factor receptor is important for retrograde transport of neurotrophins in adult cholinergic basal forebrain neurons. Neurosci. 1999;94(4):1163–1172. doi: 10.1016/s0306-4522(99)00387-5. [DOI] [PubMed] [Google Scholar]
- Kruger JL, Dell LA, Bhagwandin A, Jillani NE, Pettigrew JD, Manger PR. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic systems in the brains of five microchiropteran species. J Chem Neuroanat. 2010;40(3):210–222. doi: 10.1016/j.jchemneu.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Kruger J, Patzke N, Fuxe K, Bennett NC, Manger PR. Nuclear organization of cholinergic, putative catecholaminergic, serotonergic and orexinergic systems in the brain of the African pygmy mouse (Mus minutoides): Organizational complexity is preserved in small brains. J Chem Neuroanat. 2012;44(1):45–56. doi: 10.1016/j.jchemneu.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Larsen M, Bjarkam CR, Østergaard K, West MJ, Sørensen JC. The anatomy of the porcine subthalamic nucleus evaluated with immunohistochemistry and design-based stereology. Anat Embryol. 2004;208(3):239–247. doi: 10.1007/s00429-004-0395-0. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol. 1994;344(2):210–231. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- Lee TH, Kato H, Pan LH, Ryu JH, Kogure K, Itoyama Y. Localization of nerve growth factor, trkA and P75 immunoreactivity in the hippocampal formation and basal forebrain of adult rats. Neurosci. 1998;83(2):335–349. doi: 10.1016/s0306-4522(97)00346-1. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hallanger AE, Wainer BH. Choline acetyltransferase immunoreactivity in the rat thalamus. J Comp Neurol. 1987;257(3):317–332. doi: 10.1002/cne.902570302. [DOI] [PubMed] [Google Scholar]
- Limacher A, Bhagwandin A, Fuxe K, Manger PR. Nuclear organization and morphology of cholinergic, putative catecholaminergic and serotonergic neurons in the brain of the Cape porcupine (Hystrix africaeaustralis): Increased brain size does not lead to increased organizational complexity. J Chem Neuroanat. 2008;36(1):33–52. doi: 10.1016/j.jchemneu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Liu AK, Chang RC, Pearce RK, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015;129(4):527–540. doi: 10.1007/s00401-015-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146(1):18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Wainer BH, Rye DB, Bruce G, Hersh LB. Cholinergic innervation displays strikingly different laminar preferences in several cortical areas. Neurosci Letters. 1986;64(1):102–108. doi: 10.1016/0304-3940(86)90671-3. [DOI] [PubMed] [Google Scholar]
- Macintosh FC. The distribution of acetylcholine in the peripheral and the central nervous system. J Physiol. 1941;99(4):436–442. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Fine A, Ridley RM. The distribution of p75 neurotrophin receptor-immunoreactive cells in the forebrain of the common marmoset (Callithrix jacchus) Brain Res Bull. 1997;43(2):197–208. doi: 10.1016/s0361-9230(96)00441-8. [DOI] [PubMed] [Google Scholar]
- Maley BE, Frick ML, Levey AI, Wainer BH, Elde RP. Immunohistochemistry of choline acetyltransferase in the guinea pig brain. Neurosci Lett. 1988;84(2):137–142. doi: 10.1016/0304-3940(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, German DC. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neurosci. 1999;89(3):759–770. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- Manger PR, Fahringer HM, Pettigrew JD, Siegel JM. The distribution and morphological characteristics of cholinergic cells in the brain of monotremes as revealed by ChAT immunohistochemistry. Brain Behav Evolut. 2002;60(5):275–297. doi: 10.1159/000067195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseko BC, Manger PR. Distribution and morphology of cholinergic, catecholaminergic and serotonergic neurons in the brain of Schreiber’s long-fingered bat, Miniopterus schreibersii. J Chem Neuroanat. 2007;34(3–4):80–94. doi: 10.1016/j.jchemneu.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Maseko BC, Patzke N, Fuxe K, Manger PR. Architectural organization of the african elephant diencephalon and brainstem. Brain Behav Evol. 2013;82(2):83–128. doi: 10.1159/000352004. [DOI] [PubMed] [Google Scholar]
- Mason WT, Ho YW, Eckenstein F, Hatton GI. Mapping of cholinergic neurons associated with rat supraoptic nucleus: Combined immunocytochemical and histochemical identification. Brain Res Bull. 1983;11(5):617–626. doi: 10.1016/0361-9230(83)90133-8. [DOI] [PubMed] [Google Scholar]
- Matusica D, Skeldal S, Sykes AM, Palstra N, Sharma A, Coulson EJ. An intracellular domain fragment of the p75 neurotrophin receptor (p75(NTR)) enhances tropomyosin receptor kinase A (TrkA) receptor function. J Biol Chem. 2013;288(16):11144–11154. doi: 10.1074/jbc.M112.436469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507(3):1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kimura H, McGeer EG, Peng JH. Cholinergic systems in the CNS. In: Bradford HF, editor. Neurotransmitter Interactions and Compartmentation. 1st. New York: Plenum Press; 1982. pp. 253–289. [Google Scholar]
- McKeon-O’Malley C, Siwek D, Lamoureux JA, Williams CL, Kowall NW. Prenatal choline deficiency decreases the cross-sectional area of cholinergic neurons in the medial septal nucleus. Brain Res. 2003;977(2):278–283. doi: 10.1016/s0006-8993(03)02599-x. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Cozzari C, Descarries L. Cholinergic innervation in adult rat cerebral cortex: a quantitative immunocytochemical description. J Comp Neurol. 2000;428(2):305–318. doi: 10.1002/1096-9861(20001211)428:2<305::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Melander T, Staines WA, Hökfelt T, Rökaeus Å, Eckenstein F, Salvaterra PM, Wainer BH. Galanin-like immunoreactivity in cholinergic neurons of the septum-basal forebrain complex projecting to the hippocampus of the rat. Brain Res. 1985;360(1–2):130–138. doi: 10.1016/0006-8993(85)91228-4. [DOI] [PubMed] [Google Scholar]
- Melander T, Staines WA. A galanin-like peptide coexists in putative cholinergic somata of the septum-basal forebrain complex and in acetylcholinesterase-containing fibers and varicosities within the hippocampus in the owl monkey (aotus trivirgatus) Neurosci Lett. 1986;68(1):17–22. doi: 10.1016/0304-3940(86)90222-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neurosci. 1983b;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neurosci. 1984;12(3):669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH. Three-dimensional representation and cortical projection topography of the nucleus basalis (Ch4) in the macaque: concurrent demonstration of choline acetyltransferase and retrograde transport with a stabilized tetramethylbenzidine method for horseradish peroxidase. Brain Res. 1986;367(1–2):301–308. doi: 10.1016/0006-8993(86)91607-0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275(2):216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283(4):611–633. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992;318(3):316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn Mem. 2004;11(1):43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Møller A, Jensen LH, Pedersen A, Harajehi JB, Pakkenberg H. MPTP-induced Parkinsonism in minipigs: A behavioral, biochemical, and histological study. Neurotoxicol Teratol. 1999;21(2):169–175. doi: 10.1016/s0892-0362(98)00037-3. [DOI] [PubMed] [Google Scholar]
- Miller MW. Expression of nerve growth factor and its receptors in the somatosensory-motor cortex of macaca nemestrina. J Neurocytol. 2000;29(7):453–469. doi: 10.1023/a:1007207527842. [DOI] [PubMed] [Google Scholar]
- Milner AD, Lines CR, Migdal B. Visual orientation and detection following lesions of the superior colliculus in rats. Exp Brain Res. 1984;56(1):106–114. doi: 10.1007/BF00237446. [DOI] [PubMed] [Google Scholar]
- Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neurosci. 2008;154(1):186–195. doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Sources of cholinergic input to the inferior colliculus. Neurosci. 2009;160(1):103–114. doi: 10.1016/j.neuroscience.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Cholinergic and non-cholinergic projections from the pedunculopontine and laterodorsal tegmental nuclei to the medial geniculate body in Guinea pigs. Front Neuroanat. 2010;4:137. doi: 10.3389/fnana.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Goldman-Rakic PS. Low-affinity nerve growth factor receptor (p75NGFR)- and choline acetyltransferase (ChAT)-immunoreactive axons in the cerebral cortex and hippocampus of adult macaque monkeys and humans. Cereb Cortex. 1993;3(2):133–147. doi: 10.1093/cercor/3.2.133. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Martin TL, Mash DC, Wainer BH, Mesulam MM. Cholinergic projections from the parabigeminal nucleus (Ch8) to the superior colliculus in the mouse: a combined analysis of horseradish peroxidase transport and choline acetyltransferase immunohistochemistry. Brain Res. 1986;370(1):144–148. doi: 10.1016/0006-8993(86)91114-5. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cunningham MG. Observations on choline acetyltransferase containing structures in the CD-1 mouse brain. Neurosci Lett. 1988;84(1):7–12. doi: 10.1016/0304-3940(88)90328-x. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mash DC, Hersh LB. Neurofibrillary tangles in cholinergic pedunculopontine neurons in Alzheimer’s disease. Ann Neurol. 1988;24(5):623–629. doi: 10.1002/ana.410240506. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Bothwell M, Hersh LB, Kordower JH. Nerve growth factor receptor immunoreactive profiles in the normal, aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285(2):196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Presley LN, Kordower JH. Nerve growth factor receptor immunoreactivity within the nucleus basalis (Ch4) in Parkinson’s disease: reduced cell numbers and co-localization with cholinergic neurons. Brain Res. 1991;539(1):19–30. doi: 10.1016/0006-8993(91)90682-l. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cochran E, Benzing W, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging, Alzheimer’s disease and Down’s syndrome. Dementia. 1993;4(5):237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Perez SE, Binder L. Galanin plasticity in the cholinergic basal forebrain in Alzheimer’s disease and transgenic mice. Neuropeptides. 2005;39(3):233–237. doi: 10.1016/j.npep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14(4):2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata S, Obata J, Das A, Gilbert CD. Molecular correlates of topographic reorganization in primary visual cortex following retinal lesions. Cereb Cortex. 1999;9(3):238–248. doi: 10.1093/cercor/9.3.238. [DOI] [PubMed] [Google Scholar]
- Oh JD, Woolf NJ, Roghani A, Edwards RH, Butcher LL. Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase mRNA. Neurosci. 1992;47(4):807–822. doi: 10.1016/0306-4522(92)90031-v. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Perez SE, Lagalwar S, Vana L, Binder L, Mufson EJ. Staging of Alzheimer’s disease pathology in triple transgenic mice: a light and electron microscopic analysis. Int J Alz Dis. 2010;2010 doi: 10.4061/2010/780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke N, Bertelsen MF, Fuxe K, Manger PR. Nuclear organization of cholinergic, catecholaminergic, serotonergic and orexinergic systems in the brain of the Tasmanian devil (Sarcophilus harrisii) J Chem Neuroanat. 2014;61–62:94–106. doi: 10.1016/j.jchemneu.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Chemoarchitectonic atlas of the rat forebrain. San Diego, Calif.: Academic Press; 1999. p. xix.p. 248. [Google Scholar]
- Perez SE, Yáñez J, Marín O, Anadón R, González A, Rodríguez-Moldes I. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the brain of the adult trout and tract-tracing observations on the connections of the nuclei of the isthmus. J Comp Neurol. 2000;428(3):450–474. doi: 10.1002/1096-9861(20001218)428:3<450::aid-cne5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434(2):158–185. doi: 10.1002/cne.1171. [DOI] [PubMed] [Google Scholar]
- Perez SE, Dar S, Ikonomovic MD, DeKosky ST, Mufson EJ. Cholinergic forebrain degeneration in the APPswe/PS1DeltaE9 transgenic mouse. Neurobiol Dis. 2007;28(1):3–15. doi: 10.1016/j.nbd.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla) J Comp Neurol. 2013;521(18):4318–4338. doi: 10.1002/cne.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Sherwood CC, Cranfield MR, Erwin JM, Mudakikwa A, Hof PR, Mufson EJ. Early Alzheimer’s disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei) Neurobiol Aging. 2016;39:195–201. doi: 10.1016/j.neurobiolaging.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters RP, Gravett N, Fuxe K, Manger PR. Nuclear organization of cholinergic, putative catecholaminergic and serotonergic nuclei in the brain of the eastern rock elephant shrew, Elephantulus myurus. J Chem Neuroanat. 2010;39(3):175–188. doi: 10.1016/j.jchemneu.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Simic G, Watson S, Stimpson CD, Hof PR, Sherwood CC. Comparative analysis of the nucleus basalis of Meynert among primates. Neurosci. 2011;184:1–15. doi: 10.1016/j.neuroscience.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cholinergic innervation of the frontal cortex: Differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol. 2008;506(3):409–424. doi: 10.1002/cne.21546. [DOI] [PubMed] [Google Scholar]
- Ransmayr G, Cervera P, Hirsch E, Ruberg M, Hersh LB, Duyckaerts C, Hauw JJ, Delumeau C, Agid Y. Choline acetyltransferase-like immunoreactivity in the hippocampal formation of control subjects and patients with Alzheimer’s disease. Neurosci. 1989;32(3):701–714. doi: 10.1016/0306-4522(89)90291-1. [DOI] [PubMed] [Google Scholar]
- Rao ZR, Yamano M, Wanaka A, Tatehata T, Shiosaka S, Tohyama M. Distribution of cholinergic neurons and fibers in the hypothalamus of the rat using choline acetyltransferase as a marker. Neurosci. 1987;20(3):923–934. doi: 10.1016/0306-4522(87)90253-3. [DOI] [PubMed] [Google Scholar]
- Rico B, Cavada C. A population of cholinergic neurons is present in the macaque monkey thalamus. European J Neurosci. 1998;10(7):2346–2352. doi: 10.1046/j.1460-9568.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259(4):483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neurosci. 1984;13(3):627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222(3):313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chelimsky TC. A cytoarchitectonic and histochemical study of nucleus basalis and associated cell groups in the normal human brain. Neurosci. 1984;13(4):1023–1037. doi: 10.1016/0306-4522(84)90286-0. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Distribution of central cholinergic neurons in the baboon (Papio papio). I. General morphology. J Comp Neurol. 1985;236(2):197–214. doi: 10.1002/cne.902360205. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253(3):277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res. 2000;115(2):117–141. doi: 10.1016/s0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neurosci. 1998;84(2):361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Schnurr B, Spatz WB, Illing RB. Similarities and differences between cholinergic systems in the superior colliculus of guinea pig and rat. Exp Brain Res. 1992;90(2):291. doi: 10.1007/BF00227240. [DOI] [PubMed] [Google Scholar]
- Scott DE, Pepe GJ. The fetal baboon median eminence as a circumventricular organ: I. Transmission electron microscopy. Brain Res Bull. 1987;19(1):87–94. doi: 10.1016/0361-9230(87)90170-5. [DOI] [PubMed] [Google Scholar]
- Schuleri KH, Boyle Andrew J, Centola Marco, Amado Luciano C, Evers Robert, Zimmet Jeffrey M, Evers Kristine S, et al. The adult gottingen minipig as a model for chronic heart failure after myocardial infarction: Focus on cardiovascular imaging and regenerative therapies. Comp Med. 2008;58(6):568–579. [PMC free article] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC. Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep. 1988;11(1):1–16. doi: 10.1093/sleep/11.1.1. [DOI] [PubMed] [Google Scholar]
- Simianer H, Köhn F. Genetic management of the Göttingen Minipig population. J Pharmacol Toxicol. 2010;62(3):221–226. doi: 10.1016/j.vascn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D, Deschenes M, Parent A, Steriade M. Cholinergic and non-cholinergic projections from the upper brainstem core to the visual thalamus in the cat. Expl Brain Res. 1988;70(1):166–180. doi: 10.1007/BF00271858. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350(4):587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Eckenstein F, Thoenen H, Cuello AC. Topography of choline acetyltransferase-containing neurons in the forebrain of the rat. Neurosci Lett. 1982;33(1):7–12. doi: 10.1016/0304-3940(82)90121-5. [DOI] [PubMed] [Google Scholar]
- Søndergaard LV, Ladewig J, Dagnæs-Hansen F, Herskin MS, Holm IE. Object recognition as a measure of memory in 1–2 years old transgenic minipigs carrying the APPsw mutation for Alzheimer’s disease. Trans Res. 2012;21(6):1341. doi: 10.1007/s11248-012-9620-4. [DOI] [PubMed] [Google Scholar]
- St-Jacques R, Gorczyca W, Mohr G, Schipper HM. Mapping of the basal forebrain cholinergic system of the dog: a choline acetyltransferase immunohistochemical study. The Journal of comparative neurology. 1996;366(4):717–725. doi: 10.1002/(SICI)1096-9861(19960318)366:4<717::AID-CNE10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Strassman A, Mason P, Eckenstein F, Baugman RW, Macieweitz R. Choline acetyltransferase immunocytochemistry of Edinger-Westphal and ciliary ganglion afferent neurons in the cat. Brain Res. 1987;423(1–2):293–304. doi: 10.1016/0006-8993(87)90852-3. [DOI] [PubMed] [Google Scholar]
- Tafti M, Nishino S, Liao W, Dement WC, Mignot E. Mesopontine organization of cholinergic and catecholaminergic cell groups in the normal and narcoleptic dog. The Journal of comparative neurology. 1997;379(2):185–197. doi: 10.1002/(sici)1096-9861(19970310)379:2<185::aid-cne2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tago H, McGeer PL, Bruce G, Hersh LB. Distribution of choline acetyltransferase-containing neurons of the hypothalamus. Brain Res. 1987;415(1):49–62. doi: 10.1016/0006-8993(87)90268-x. [DOI] [PubMed] [Google Scholar]
- Tago H, McGeer PL, McGeer EG, Akiyama H, Hersh LB. Distribution of choline acetyltransferase immunopositive structures in the rat brainstem. Brain Res. 1989;495(2):271–297. doi: 10.1016/0006-8993(89)90221-7. [DOI] [PubMed] [Google Scholar]
- Tan MM, Harvey AR. The cholinergic innervation of normal and transplanted superior colliculus in the rat: an immunohistochemical study. Neurosci. 1989;32(2):511–520. doi: 10.1016/0306-4522(89)90098-5. [DOI] [PubMed] [Google Scholar]
- Theodorsson E, Rugarn O. Radioimmunoassay for rat galanin: immunochemical and chromatographic characterization of immunoreactivity in tissue extracts. Scand J Clin Lab Invest. 2000;60(5):411–418. doi: 10.1080/003655100750019323. [DOI] [PubMed] [Google Scholar]
- Tinner B, Fuxe K, Kohler C, Hersh L, Andersson K, Jansson A, Goldstein M, Agnati LF. Evidence for the existence of a population of arcuate neurons costoring choline acetyltransferase and tyrosine hydroxylase immunoreactivities in the male rat. Neurosci Lett. 1989;99(1–2):44–49. doi: 10.1016/0304-3940(89)90262-0. [DOI] [PubMed] [Google Scholar]
- Varga C, Härtig W, Grosche J, Keijser J, Luiten PG, Seeger J, Brauer K, Harkany T. Rabbit forebrain cholinergic system: morphological characterization of nuclei and distribution of cholinergic terminals in the cerebral cortex and hippocampus. J Comp Neurol. 2003;460(4):597–611. doi: 10.1002/cne.10673. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Reiner PB. The immunohistochemical localization of choline acetyltransferase in the cat brain. Brain Res Bull. 1987;18(3):371–415. doi: 10.1016/0361-9230(87)90015-3. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27(21):5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle P, Meyer G. The olfactory tubercle of the cat. II. Immunohistochemical compartmentation. Exp Brain Res. 1986;62(3):528–540. doi: 10.1007/BF00236031. [DOI] [PubMed] [Google Scholar]
- Walker LC, Koliatsos VE, Kitt CA, Richardson RT, Rokaeus A, Price DL. Peptidergic neurons in the basal forebrain magnocellular complex of the rhesus monkey. J Comp Neurol. 1989;280(2):272–282. doi: 10.1002/cne.902800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick R. Representation of the extraocular muscles in the oculomotor nuclei of the monkey. J Comp Neurol. 1953;98(3):449–504. doi: 10.1002/cne.900980305. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1981;7(5):487–507. doi: 10.1016/0361-9230(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis. Brain Res Bull. 1982;8(6):751–763. doi: 10.1016/0361-9230(82)90102-2. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in rat brain: I. Projections to the limbic telencephalon. Brain Res Bull. 1984;13(6):751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: II. Projections to the interpeduncular nucleus. Brain Res Bull. 1985;14(1):63–83. doi: 10.1016/0361-9230(85)90178-9. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Harrison JB, Buchwald JS. Cholinergic neurons of the feline pontomesencephalon. II. Ascending anatomical projections. Brain Res. 1990;520(1–2):55–72. doi: 10.1016/0006-8993(90)91691-9. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37(6):475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Wu CK, Hersh LB, Geula C. Cyto- and chemoarchitecture of basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus) Exp Neurol. 2000;165(2):306–326. doi: 10.1006/exnr.2000.7468. [DOI] [PubMed] [Google Scholar]


