Abstract
Herpes genitalis is caused by the herpes simplex virus type 1 or type 2 and can manifest as primary or recurrent infection. It is one of the most common sexually transmitted infections and due to associated physical and psychological morbidity it constitutes a considerable, often underestimated medical problem. In addition to providing the reader with basic knowledge of the pathogen and clinical presentation of herpes genitalis, this review article discusses important aspects of the laboratory diagnostics, antiviral therapy and prophylaxis. The article is aimed at all health-care workers managing patients with herpes genitalis and attempts to improve the often suboptimal counselling, targeted use of laboratory diagnostics, treatment and preventive measures provided to patients.
Key words: herpes simplex virus, herpes genitalis, laboratory diagnostics, antiviral therapy, prophylaxis
Zusammenfassung
Der Herpes genitalis wird durch Herpes-simplex-Virus Typ 1 oder Typ 2 hervorgerufen und kann sich als primäre und rekurrierende Infektion manifestieren. Die Erkrankung gehört zu den häufigsten sexuell erworbenen Infektionskrankheiten und stellt aufgrund ihrer physikalischen und psychosozialen Morbidität ein erhebliches und oft unterschätztes medizinisches Problem dar. In der vorliegenden Übersichtsarbeit werden neben medizinischen Grundkenntnissen zum Erreger des Herpes genitalis und der Erkrankung wesentliche Aspekte der Labordiagnostik, einschließlich antivirale Therapie und prophylaktische Möglichkeiten vermittelt. Der Beitrag wendet sich an alle Mitarbeiter im Gesundheitsdienst, die Patienten mit Herpes genitalis betreuen, und zielt darauf ab, die häufig unzureichende Beratung sowie Maßnahmen bez. einer gezielten Diagnostik, Therapie und Prävention bei den betroffenen Patienten zu verbessern.
Schlüsselwörter: Herpes-simplex-Virus, Herpes genitalis, Labordiagnostik, antivirale Therapie, Prophylaxe
Introduction
Herpes genitalis is among the most common sexually transmitted infections. It is caused by the herpes simplex virus type 2 (HSV-2) and also, increasingly, the herpes simplex virus type 1 (HSV-1). Both organisms are enveloped DNA viruses that are sensitive to disinfectants and environmental factors 1. Due to marked genetic homology between HSV-1 and HSV-2 numerous biological similarities and antigenic cross-reactions between the viruses exist. Type-specific epitopes include the viral glycoproteins (g) gG (HSV-1 and HSV-2) and gC (HSV-1) 2, 3.
The primary mode of transmission of both HSV-1 and HSV-2 is through direct contact. Initial infection with HSV-1 occurs most often during childhood following the disappearance of maternal antibodies during the first year of life. Current data on seroprevalence in Germany show a rise in anti-HSV-1 IgG to approx. 20 % by the age of 2–3 years, to 57 % in 10–12-year-olds, approx. 70 % in 16–18 year olds and around 80 % in adults aged 28–30 years 4. From the age of 40 years and onwards one can assume an HSV-1 seroprevalence of ≥ 85–90 % 5. Since HSV-2 is mainly transmitted through sexual intercourse infection rates only rise after puberty. In Germany the prevalence of anti-HSV-2 IgG antibodies rises from approx. 3 % in 10–15-year-olds to 7 % in the age group 16–18 years, to approx. 14 % in adults 4. Higher seroprevalences are found internationally among people who regularly change sexual partners and among homosexual men 6. Numerous studies have shown a significantly higher HSV-2 seroprevalence in women than in men 4, 5. As a possible reason for this it has been suggested that men have asymptomatic genital HSV-2 infections more often than women, resulting in higher virus transmission rates from men to women 7. Partial clinical cross-immunity exists between HSV-1 and HSV-2 and as a result, primary genital HSV-2 infection may be asymptomatic in patients with HSV-1 immunity and vice versa. A reduced HSV-1 seroprevalence among young people (adolescents) and adults may be associated with higher numbers of primary HSV-2 or HSV-1 infections due to oral sex. There is published evidence of this in the USA 8, 9 but not yet in Germany. Genital HSV-2 infection is associated with an increased risk of HIV infection 10.
Virus Transmission and Infection
Primary herpes genitalis
Primary genital infections with HSV-1 and HSV-2 are usually asymptomatic 11. The classical clinical features consist of macular or papular skin and mucous membrane lesions occurring approx. 4–7 days after sexual contact; these progress to vesicles, pustules and ulcers and can last for up to 3 weeks. 12. Typical symptoms also include pain, especially painful inflammatory swelling of the vulva in women, burning pain and dysuria. Lymphadenopathy, fever and cervicitis (in women)/proctitis (in men) are relatively common associated symptoms. Genital herpes may manifest atypically, particularly in the female genital tract, making the clinical diagnosis far more difficult. Signs of herpes lesions of the cervix are relatively common in the absence of symptoms, while urethral manifestations are often associated with severe micturition problems.
Recurrent herpes genitalis
Following the primary eruption the virus establishes lifelong latency in sensory neural ganglions 13; in the case of primary genital infection the sacral ganglions are mainly involved. From here the virus can reactivate, causing recurrent infection. Viral reactivation is common in the presence of immunogenetic predisposition, though reactivations decrease with increasing age. Numerous physiological and environmental factors such as fever, UV light, menstruation, stress or trauma can function as triggers 14, 15. Endogenous viral reactivations may manifest as recurrent herpes genitalis. Recurrences occur in almost every person suffering symptomatic primary herpes genitalis due to HSV-2, in a third of patients frequently (at least 6 times a year) 16. Recurrent genital HSV-1 infections occur over five times less commonly 17. Recurrences almost always initially present with prodromal symptoms such as neuralgic symptoms, dysaesthesia or lumbosacral dermatome pain 1–2 days before skin and mucosal lesions erupt 18. Compared to primary infection, symptoms of recurrence are much less severe and the clinical course shorter 19. In the experience of the reference laboratory for HSV and VSV of the Institute for Virology and Antiviral Therapy of the University Hospital of Jena, in its advisory capacity, frequent herpes genitalis recurrences particularly affect young women with a high burden of stress in the family and workplace. It must also be taken into account that recurrences themselves can cause high levels of emotional stress 20. Affected patients and their sexual partners therefore more commonly suffer significant psychosocial problems.
Asymptomatic genital viral shedding
In the majority of cases endogenous viral reactivation is characterised by asymptomatic genital viral shedding. Most commonly HSV-2 is shed by HSV-2 seropositive patients, and this is the case for almost anyone who is anti-HSV-2 IgG positive 21. In contrast, HSV-1 shedding is uncommon. These data allow the assumption, with a high level of certainty, that HSV-2 seropositive people should always be regarded as potential virus excretors.
Virus transmission
People with clinically manifest/apparent herpes genitalis and people who shed HSV asymptomatically can transmit the virus to their sexual partners. This almost always occurs via direct contact during sexual intercourse. In recent years an increased incidence of primary genital HSV-1 infection has been reported in the USA, particularly among adolescence and young adults 8, 9. This can most probably be ascribed to oral sex, which is more commonly practiced in this age group. Due to its low environmental stability HSV can only remain infectious for a period of days on moist surfaces 22. It can therefore be assumed with a high level of certainty that when normal hygiene (including bodily hygiene) is maintained, modes of transmission other than sexual intercourse do not play a significant role. Intrauterine and perinatal viral transmission are the exceptions. Both primary and recurrent HSV infection in pregnant women can result in intrauterine viral transmission and congenital HSV infection, although the incidence is low at just 5 % of all HSV infections in newborns 23. The clinical consequences of fetal infection described include abortion, stillbirth or other congenital manifestations usually including skin and eye lesions and/or neurological symptoms 24. The highest risk of fetal infection is during the first 20 weeks of pregnancy, and with primary maternal HSV-2 infection. Viral transmission to the child via the motherʼs genital tract during labour is regarded as the most common cause of neonatal HSV infection; of these infections 70–85 % are caused by HSV-2 25. The incidence in the USA is quoted at 5 to 31 per 100 000 live births with a worse prognosis for HSV-2 infection compared to HSV-1 23. There are no data available on the incidence of neonatal HSV infection in Germany. The highest risk is with perinatal maternal primary HSV infection, however most neonatal infections occur around the birth in the presence of asymptomatic genital tract viral shedding 23, 26. Disease manifests as localised infection of the skin, eyes and mucous membranes, CNS infection or disseminated systemic infection 27.
Laboratory Diagnostics
Sample submission
Herpes simplex virus-containing samples must be transported as UN 3373 category B, risk group 2 hazardous substances 22. The primary vessel containing the patient sample must be sent in a covering tube within a labelled transport container (cardboard box) with adsorbing material. In general samples can be sent at room temperature unless the material is being sent for virus isolation, in which case cooling is recommended.
Virus detection
The laboratory diagnosis of acute genital HSV infection or asymptomatic virus shedding is made via direct viral detection (Table 1). The method of choice is demonstration of viral genomes in skin or mucous membrane swabs using the polymerase chain reaction (PCR) 28. The content of vesicles provides the best swab material. The sample should be sent in physiological saline solution or viral transport medium. In the presence of complications involving other organs the examination of liquor, tissue samples, bronchoalveolar lavage, amniotic fluid, intraocular fluid, serum or EDTA blood should be considered. The PCR test should be able to differentiate between HSV-1 and HSV-2 29 and various in-house assays and commercially available kits are used 30. The sensitivity of PCR is quoted at ≥ 98 % independent of the qualitative or quantitative method used, the specificity at almost 100 % 30. Considering these data and the reduced stability of viral DNA when samples are stored for a few days at temperatures over 20 °C, a negative PCR by no means entirely excludes HSV infection. Current guidelines thus recommend starting antiviral treatment when typical herpes genitalis symptoms are present regardless of laboratory results 31, 32. Alternatively, acute genital HSV infection or asymptomatic viral shedding can be diagnosed by growing the virus in tissue cultures, whereby typing of viral isolates is performed by immunofluorescence using appropriate fluorescein labelled HSV serotype-specific monoclonal antibodies. Virus isolation is a sensitive method of detecting HSV since both HSV-1 and HSV-2 grow well in various cell types, such as diploid human embryonic fibroblasts or permanent Vero cells and HEp-2 cells. However in view of its higher sensitivity 33, PCR is rightly regarded as the gold standard of diagnosis in many laboratories. Virus isolation continues to be recommended as an alternative method for diagnosis of genital herpes 31. Direct HSV antigen detection using commercially available immunofluorescence tests is a commonly used and economic method of diagnosis that provides results within hours; sensitivity and specificity are however limited 34, 35. It must be remembered that direct viral detection does not differentiate between primary and recurrent infection or asymptomatic virus shedding.
Table 1 Methods to detect HSV and HSV-specific antibody.
| Principle | Method | Comments |
|---|---|---|
| Viral DNA detection | Polymerase chain reaction (PCR) |
|
| Virus isolation | Cultivation in cell cultures, detection/typing using monoclonal antibodies |
|
| Viral antibody detection | Immunofluorescence test with monoclonal antibodies |
|
| Detection of type-specific and non-type-specific antibodies | Enzyme-linked immunosorbent assay (ELISA) |
|
| Detection of non-type-specific antibodies | Indirect immunofluorescence antibody test (IFAT) |
|
| Detection of type-specific antibodies | Immunoblot |
|
Antibody detection
The detection of virus-specific antibodies for confirming HSV infection is widely used in clinical practice. One should however be aware of the limited value of serology results. HSV serology (Table 1) is mainly useful for confirming seroconversion following primary infection, through demonstration of IgG. This can be of particular value in the diagnosis of HSV-2 infections in the context of antenatal care. Confirming seroconversion is also possible by demonstrating type-specific IgG antibodies, and since HSV-1 and HSV-2 are so closely related this is only possible using ELISA/immunoblot on the basis of HSV-1 gG-1 or gC-1, and HSV-2 gG-2 2, 3. When interpreting results it is important to consider that partial cross-immunity exists between HSV-1 and HSV-2. The importance of HSV type-specific IgG is mostly that it allows rapid, reliable and economical identification of HSV-2 carriers and potential virus shedders 31, 36. Thus a patient in whom anti-HSV-2 IgG is detected can be considered a potential virus shedder and transmitter who may also suffer from anogenital HSV infection. If an initial serum sample is available from the early stage of a herpes genitalis infection, primary and recurrent infections can be differentiated from one another through the detection of virus type-specific DNA by PCR in combination with virus type-specific IgG 37. As an example, this means that when HSV-2 is detected on genital swab in a pregnant woman, primary genital herpes can be differentiated from a recurrence up to a few weeks before delivery using HSV type-specific IgG. This differentiation is of great significance, since the risk of severe neonatal HSV infection is many times higher following primary infection than with recurrent infection. Although HSV type-specific antibody tests have been commercially available for approx. two decades they are used seldom in Germany despite the above mentioned advantages, and most laboratories only offer non type-specific HSV antibody tests 38, 39. Avidity testing can also assist in differentiating between primary and recurrent infections although to date experience with this method is limited 40. Negative anti-HSV IgG excludes recurrent HSV infection. Table 2 provides a summary of virology and serology findings for the laboratory diagnosis of HSV infections with or without genital lesions and for asymptomatic viral shedding.
Table 2 HSV laboratory findings and correlating herpes genitalis symptoms.
| Clinical presentation | HSV serology | PCR | Interpretation/Infection status | |||
|---|---|---|---|---|---|---|
| HSV-1/2 IgG | HSV-1 IgG | HSV-2 IgG | HSV-1 | HSV-2 | ||
| Primary herpes genitalis | neg. | neg. | neg. | pos. | neg. | acute HSV-1 infection |
| pos. | neg. | pos. | pos. | neg. | acute HSV-1 infection, latent HSV-2 | |
| neg. | neg. | neg. | neg. | pos. | acute HSV-2 infection | |
| pos. | pos. | neg. | neg. | pos. | acute HSV-2 infection, latent HSV-1 | |
| Recurrent herpes genitalis | pos. | pos. | neg. | pos. | neg. | recurrent HSV-1 infection (recurrence) |
| pos. | pos. | pos. | pos. | neg. | recurrent HSV-1 infection, latent HSV-2 | |
| pos. | neg. | pos. | neg. | pos. | recurrent HSV-2 infection (recurrence) | |
| pos. | pos. | pos. | neg. | pos. | recurrent HSV-2 infection, latent HSV-1 | |
| No genital herpes lesions | neg. | neg. | neg. | neg. | neg. | seronegativity, susceptibility |
| pos. | pos. | neg. | neg. | neg. | previous HSV-1 infection (latent HSV-1) | |
| pos. | neg. | pos. | neg. | neg. | previous HSV-2 infection (latent HSV-2) | |
| pos. | pos. | pos. | neg. | neg. | previous HSV-1 and HSV-2 infection (latent HSV-1 and HSV-2) | |
| pos. | pos. | neg. | pos. | neg. | asymptomatic shedding of HSV-1, previous HSV-1 infection (latent HSV-1) | |
| pos. | neg. | pos. | neg. | pos. | asymptomatic shedding of HSV-2, previous HSV-2 infection (latent HSV-2) | |
| pos. | pos. | pos. | pos. | neg. | asymptomatic shedding of HSV-1, previous HSV-1 and HSV-2 infection (latent HSV-1 and HSV-2) | |
| pos. | pos. | pos. | neg. | pos. | asymptomatic shedding of HSV-2, previous HSV-1 and HSV-2 infection (latent HSV-1 and HSV-2) | |
The detection of anti-HSV IgM is of limited significance for early confirmation of acute HSV infection. False positive IgM results are possible due to cross-reactivity with other herpes viruses, e.g. the varicella-zoster virus. Confirmation of acute HSV infection is only possible using non type-specific HSV IgM tests that have high sensitivity and specificity 41. It must however be noted that the positive predictive value of anti-HSV IgM is low, and that it does not allow differentiation between primary and recurrent infection. Although IgM is usually positive following primary infection it can also be positive in the context of recurrence, independent of clinical symptoms. The rather unreliable measurement of HSV type-specific IgM antibodies should be avoided in clinical practice 41. Fig. 1 provides a summary of the recommended viral diagnostic algorithm for herpes genitalis.
Fig. 1.
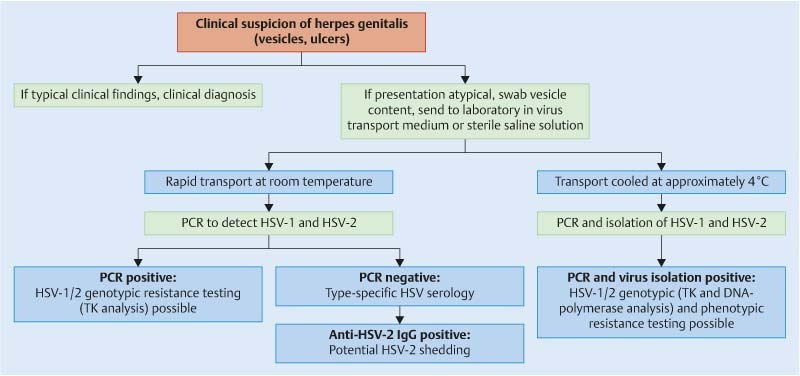
Recommended viral diagnostic algorithm for herpes genitalis. PCR – polymerase chain reaction, TK – thymidine kinase.
Antiviral Therapy
Standard treatment
Table 3 gives an overview of antiviral drugs and dosages for the treatment of herpes genitalis according to current guidelines 31, 32, 42, 43. Standard first-line drugs include acyclovir, valacyclovir and famciclovir. The specific antiviral action of these acyclic nucleoside analogues 44 is based on their phosphorylation to monophosphate form by thymidine kinase (TK), the key enzyme of HSV-1 and HSV-2, with subsequent phosphorylation via di- to triphosphate form by cellular enzymes. The triphosphate nucleoside analogues inhibit and fixate the viral DNA polymerase by being incorporated into the growing DNA chain as “false” enzyme substrates. In the case of acyclovir/valacyclovir this leads to chain termination, since hydroxyl groups in the 3′ position, which are essential for further linkage, are missing. Famciclovir may be incorporated into the growing DNA chain.
Table 3 Antiviral treatment of herpes genitalis.
| Condition/type of treatment | Acyclovir | Valacyclovir | Famciclovir | Foscarnet |
|---|---|---|---|---|
| 1 Acyclovir is not licensed for use during pregnancy (off-label use). Use should be avoided particularly before the 15th week of gestation. 2 Mild presentations can also be treated topically with acyclovir or foscarnet. This is not adequate during pregnancy. 3 In immunosuppressed patients (e.g. HIV) higher doses, longer treatment periods and acyclovir i. v. may be necessary. 4 According to a Cochrane study 45 viral suppression/prophylaxis may already be indicated after at least 4 recurrences per year. 5 Longer than 6 months in certain settings (e.g. HIV). Prerequisite for viral suppression/prophylaxis: monthly renal and liver laboratory parameters. 6 Alternative: Cidofovir (5 mg/kg i. v. 1× weekly, later 2× weekly, off-label use); 1 % foscarnet cream or 1 % cidofovir gel topically. | ||||
| Primary herpes genitalis | 3 × 400 mg p. o. daily, 7–10 days | 2 × 500 mg p. o. daily, 7–10 days | 3 × 250 mg p. o. daily, 7–10 days | |
| 5 × 200 mg p. o. daily, 7–10 days | ||||
| Severe primary herpes genitalis | 3 × 5 mg/kg i. v. daily, 5–7 days, 10 days for immunosuppressed | |||
| Primary herpes genitalis during pregnancy | 5 × 200 mg p. o. daily, 10 days | |||
| Recurrent herpes genitalis (< 5–6 episodes per year)2,3 | 2 × 800 mg p. o. daily, 5 days | 2 × 500 mg p. o. daily, 3 days | 2 × 125 mg p. o. daily, 5 days | |
| 3 × 400 mg p. o. daily, 5 days | 1 × 1 000 mg p. o. daily, 5 days | 2 × 1 000 mg p. o. daily, 1 day | ||
| 3 × 800 mg p. o. daily, 2 days | ||||
| Recurrent herpes genitalis during pregnancy | 3 × 400 mg p. o. daily, 10 days; preventive: from 36th gestational week to delivery | 2 × 250 mg p. o. daily, 3 days; preventive: 36th gestational week to delivery | ||
| Preventive treatment before pregnancy | 2 × 400 mg p. o. daily, max. 6 months5 | |||
| Prophylaxis/viral suppression (≥ 5–6 episodes per year)3,4 | 2 × 400 mg p. o. daily, max. 6 months5 | 1 × 500 mg p. o. daily, max. 6 months5 | 2 × 250 mg p. o. daily, max. 6 months5 | |
| 4 × 200 mg p. o. daily, max. 6 months5 | ||||
| Recurrent herpes genitalis with acyclovir resistance | 3 × 40 (− 80) mg/kg i. v. daily, until clinical improvement (max. 20 days)6 | |||
Acyclovir is the first choice therapeutic agent for HSV infections, including herpes genitalis. However bioavailability is only 15–30 % with oral administration. Infections of the skin and mucous membranes including herpes genitalis are treated orally in immune competent people. Severe HSV infections, particularly in immunodeficient patients, should be treated with intravenous (i. v.) acyclovir. Acyclovir dosage for the treatment of herpes genitalis is dependant on infection status, immune competence and whether or not the patient is pregnant. If recurrences occur at a rate of over four 45 to six episodes annually 31, 32, 42, 43 long-term treatment to suppress the virus (prophylaxis) should be considered. The benefits of prophylaxis have been proven particularly during pregnancy 46. Topical acyclovir is only recommended for herpes labialis, herpes keratoconjunctivitis and mildly symptomatic herpes genitalis. Officially acyclovir is not licensed for use in pregnancy, though administration should be avoided particularly before the 15th week of gestation 22. Results from a pregnancy register published by an acyclovir producer and the Centers for Disease Control and Prevention (USA) 47 as well as results of a retrospective cohort study in Denmark 48 have shown, however, that oral and topical acyclovir do not appear to increase the rate of congenital anomalies. Since these data are not sufficient for general authorisation, particularly in early pregnancy, pregnant patients must be informed about the limited evidence on use in pregnancy (off-label use). Occasional central nervous system side effects have been described following i. v. acyclovir administration and oral acyclovir may produce gastrointestinal side effects. Nephrotoxic substances should not be administrated concurrently and both renal and liver laboratory parameters should be monitored.
Valacyclovir is a prodrug (an L-valyl ester) of acyclovir suitable for oral administration. After ingestion it is converted to acyclovir by the hepatic enzyme valacyclovir hydrolase. Oral valacyclovir has a bioavailability of 54 %, achieving active ingredient concentrations three to four times higher than oral acyclovir. This allows increased dose intervals and is associated with better compliance. Valacyclovir is also a standard treatment for herpes genitalis in immunocompetent patients and studies have shown its efficacy for viral suppression and prevention of recurrent herpes genitalis 46. Valacyclovir is not licensed for antiviral treatment in children and adolescents since its efficacy and safety profiles have not yet been adequately studied in this population. This applies to pregnancy too as there is also little data on its safety in this context 48. Possible side effects are similar to those of acyclovir.
Famciclovir is the inactive diacetyl ester prodrug of the only topically effective acyclic nucleotide analogue penciclovir, which arises after cleavage of two ester groups in the small bowel and liver. The bioavailability of famciclovir is 77 % after oral application. Famciclovir is also regarded as one of the standard therapeutic agents for herpes genitalis, along with acyclovir and valacyclovir, and is also not licensed for use in children and adolescents, immunosuppressed patients under the age of 25 years or in pregnancy. It should therefore not be used as the treatment of choice in pregnancy 49. In rare cases famciclovir can cause headaches, nausea and confusion.
Alternative treatment options
Following prolonged use of acyclovir/valacyclovir resistance, including cross-resistance to famciclovir, may develop in up to 5 % of immunosuppressed or HIV positive patients 50, 51. In such cases the pyrophosphate analogue foscarnet (Table 3) is recommended as alternative treatment 35. Phenotypic and/or genotypic resistance testing should be performed in this situation. Foscarnet inhibits the viral DNA polymerase of numerous DNA and RNA viruses by suppression of pyrophosphate exchange. Since this substance does not need to be metabolised in order to inhibit viral replication it is also effective against TK-negative HSV strains, which are resistant to nucleoside analogues. Important side effects include renal dysfunction and toxin-induced ulcers of the urogenital mucous membranes. Since the 3 times daily i. v. application of foscarnet requires obligatory hospital admission off-label use of cidofovir i. v. once to twice weekly provides an alternative for acyclovir resistant cases (Table 3). Cidofovir is exclusively licensed for treatment of cytomegalovirus retinitis in the context of HIV, however it is also effective against HSV. Marked nephrotoxicity and a lack of clinical experience with its use in treating HSV infections are significant barriers to the use of cidofovir in clinical practice. In view of the often atypical presentation of genital herpes systemic treatment with foscarnet (or cidofovir) is generally required. Important contraindications include renal dysfunction, hypersensitivity, pregnancy and breastfeeding. When side effects or patient contraindications preclude using systemic foscarnet (or cidofovir) in a patient with acyclovir resistant herpes genitalis, topical application of 1 % foscarnet cream or 1 % cidofovir gel may provide an effective alternative 52.
The helicase blockers, a new class of drug that is currently still in clinical development and testing, may improve the antiviral treatment of herpes genitalis significantly in future. Thus far effective inhibition of HSV replication has been demonstrated in cell cultures, animal models and initial clinical studies without evidence of major side effects. It is thought that helicase blockers bind to the helicase-primase complex, a protein component essential for virus replication, effectively inhibiting DNA synthesis and thus viral replication. Indeed, better results have been achieved in vitro and in vivo than for acyclovir and valacyclovir. For oral pritelivir (BAY 57-1293, AIC316) a significant reduction of clinical symptoms and HSV-2 shedding was shown in herpes genitalis patients 53. Helicase blockers have also been shown to be effective against acyclovir resistant HSV strains 54.
Resistance testing
Clinical treatment failure is defined as lack of response to antiviral treatment (usually acyclovir/valacyclovir) within 10 days. In such cases infection with a resistant strain of virus should be suspected 55 and phenotypic and/or genotypic resistance testing performed. These specialised investigations are performed routinely at the HSV and VZV consulting laboratory. In the presence of acyclovir/valacyclovir resistance, which is almost always associated with cross-resistance to famciclovir, alternative treatment with foscarnet is indicated 56. Phenotypic resistance tests in particular are time consuming, requiring at least 7–10 days, so that when severe resistance is present clinically appropriate changes to treatment should not be delayed until results become available.
In the published literature plaque reduction/cytopathic effect inhibiting tests, dye uptake assays and DNA hybridisation assays have been described, plaque reduction being the most commonly used method 56. The sensitivity of HSV to virostatic agents can be measured on the basis of inhibition of morphologically induced, virus-specific cell changes; so-called cytopathic effects. By testing the antiviral agent in a geometric dilution series in descending order the agentʼs mean inhibitory concentration (IC50), which results in 50 % inhibition of viral replication, is calculated. Largely due to the biological disturbance variable “cell culture” there is currently no internationally standardised cut-off defining resistance. It is therefore necessary to run a control using a TK-positive reference strain along with each test. Most commonly and reliably resistance to nucleoside analogues and cidofovir is assumed when the IC50 of the tested HSV strain is 3 to 5-times higher than that of the sensitive control strain 57, 58. For foscarnet a set cut-off of 330 µM has proven useful 59. The main advantage of phenotyping is that the interpretation of results is unequivocal, which is why this method continues to be regarded as the gold standard for HSV resistance testing. Disadvantages include the fact that it is time consuming, the high cost of materials needed for isolation and testing of HSV strains in cell cultures, and the lack of standardisation. Phenotypic resistance testing is practically only possible when vesicle or respiratory tract swabs are available from which HSV can be easily isolated. In the majority of cases attempts to isolate the virus from liquor, blood or eye samples are unsuccessful.
Genotypic resistance testing usually involves amplification and sequencing of the TK and DNA polymerase genes 56. The data are then compared to a sensitive reference strain from the gene bank (e.g. HSV-1 strain 17 accession no. X14112, HSV-2 strain HG52 accession no. Z86099). Findings suggestive of resistance include frameshift mutations, extra stop codons and non-synonymous nucleotide substitutions in conserved and functionally important gene regions. Interpretation of amino acid substitutions outside of active or conserved gene regions requires access to a data bank in which all resistance mutations described in the literature are pooled 56. HSV-1 and HSV-2 resistance to acyclovir/valacyclovir/famciclovir is almost always associated with non-synonymous mutations of the TK gene and only rarely the DNA polymerase gene, while resistance to foscarnet/cidofovir is exclusively associated with mutations to the DNA polymerase gene. Matching of resistance pheno- and genotypes of HSV isolates is the most reliable and practical method available for confirming the resistance associations of new, as yet unknown amino acid substitutions. The essential advantage of genotyping is that direct testing of patient samples is performed, making virus isolation in cell culture unnecessary. Depending on virus load results may be available within two days, which is of immense importance for clinical decision-making.
Prophylaxis
Vaccination
The question of possible immunisation is often raised not only by doctors but also by many patients affected by herpes genitalis. To date, however, there is no licensed vaccine against herpes genitalis, though research has been ongoing for a number of decades. “Therapeutic” vaccines are differentiated from “prophylactic” vaccines depending on their modes of action. Whereas therapeutic vaccines aim to prevent recurrent HSV infections and asymptomatic viral shedding in people with latent HSV infection, prophylactic vaccines are intended to prevent primary infection and subsequent virus latency. Here HSV-2 has been the main focus of research. A large number of vaccines have been tested in vaginal animal models (mouse and guinea pig) 60, though currently vaccines based on recombinant viral proteins appear to be the most promising 61. Randomised placebo-controlled trials of adjuvant HSV-2 protein subunit vaccines 62, 63 and live attenuated HSV-2 deletion mutants 64, 65 in humans have not produced results convincing enough to justify the licensing of a vaccine. Although these vaccines are not associated with a risk of vaccine virus infection and latency, there have been significant difficulties in meaningfully reducing the number of recurrences and preventing primary genital infection with both HSV-1 and HSV-2. There is hope in the knowledge that protection from primary infection is chiefly mediated by virus-specific antibodies, while cell-mediated immunity is of greatest importance in the prevention of recurrence 60, 66. Thus vaccine-induced cellular immunity must be stronger than that following natural HSV infection.
Other methods
Sound, comprehensive partnership counselling is an essential component of the medical management of herpes genitalis patients. For this, HSV type-specific serology is an important tool as it allows identification of the HSV-2 carrier. If no HSV-2-specific antibodies can be detected in the partner of an HSV-2 seropositive person the couple should be advised to use condoms 67. If herpes genitalis symptoms are present sexual intercourse should be discouraged 68. Since these measures are particularly important for the prevention of viral transmission during pregnancy both partners should be informed about their HSV serostatus and the possible consequences of viral transmission, both with symptomatic herpes genitalis and asymptomatic viral shedding. Psychotherapy can help reduce the number of herpes genitalis recurrences in women with high levels of emotional stress.
In future microbicides in the form of gels, creams or lotions may be an option for the prevention of herpes virus transmission via sexual intercourse. The best studied substance for this method of herpes genitalis prevention is tenofovir, a nucleoside analogue reverse transcriptase inhibitor licensed for treatment of HIV infection. Studies have shown that vaginal application of tenofovir gel 12 hours before sexual intercourse prevents HSV-2 infection in HSV-2-negative women 69 but does not prevent asymptomatic viral shedding or genital symptoms in women with known herpes genitalis 70. VivaGel® is another promising microbicide that inhibits HSV replication following vaginal application. This product contains the nanotechnologically manufactured dendrimer SPL7013 as its active substance 71. However, at best these microbicides will constitute supplementary options for future herpes genitalis prevention.
Practical Conclusions
The medical management of patients with herpes genitalis is often unsatisfactory. It can be significantly improved through competent patient counselling and correct implementation of existing methods of diagnosis, treatment and prevention. Nevertheless currently available antiviral treatment and prophylaxis still has shortcomings, especially in the management of frequent recurrences. A new drug class – the helicase blockers – and the development of effective vaccines are expected to improve the situation significantly in future.
Footnotes
Conflict of Interest None.
Supporting Information
References
- 1.King A M Adams M J Carstens E B Lefcowitz E JHrsg.Virus Taxonomy. Ninth Report of the International Committee on Taxonomy of Viruses San Diego, CA: Elsevier Academic Press; 201299–124. [Google Scholar]
- 2.Bergström T, Trybala E. Antigenic differences between HSV-1 and HSV-2 glycoproteins and their importance for type-specific serology. Intervirology. 1996;39:176–184. doi: 10.1159/000150493. [DOI] [PubMed] [Google Scholar]
- 3.Scheper T, Saschenbrecker S, Steinhagen K. et al. The glycoproteins C and G are equivalent target antigens for the determination of herpes simplex virus type 1-specific antibodies. J Virol Methods. 2010;166:42–47. doi: 10.1016/j.jviromet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Sauerbrei A, Schmitt S, Scheper T. et al. Seroprevalence of herpes simplex virus type 1 and type 2 in Thuringia, Germany, 1999 to 2006. Euro Surveill. 2011:pii:20005. [PubMed] [Google Scholar]
- 5.Wutzler P, Doerr H W, Färber I. et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations-relevance for the incidence of genital herpes. J Med Virol. 2000;61:201–207. doi: 10.1002/(sici)1096-9071(200006)61:2<201::aid-jmv5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Smith J S, Robinson N J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186 01:S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 7.Langenberg A G, Corey L, Ashley R L. et al. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chirion HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 8.Roberts C M, Pfister J R, Spear S J. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 9.Peña K C, Adelson M E, Mordechai E. et al. Genital herpes simplex virus type 1 in women: detection in cervicovaginal specimens from gynecological practices in the United States. J Clin Microbiol. 2010;48:150–153. doi: 10.1128/JCM.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman E E, Weiss H A, Glynn J R. et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein D I, Bellamy A R, Hook E W 3rd. et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L, Adams H G, Brown Z A. et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 13.Stevens J G, Cook M L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 14.Perna J J, Mannix M L, Rooney J F. et al. Reactivation of latent herpes simplex virus infection by ultraviolet light: a human model. J Am Acad Dermatol. 1987;17:473–478. doi: 10.1016/s0190-9622(87)70232-1. [DOI] [PubMed] [Google Scholar]
- 15.Rand K H, Hoon E F, Massey J K. et al. Daily stress and recurrence of genital herpes. Arch Intern Med. 1990;150:1889–1893. [PubMed] [Google Scholar]
- 16.Bennedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Engelberg R, Carrell D, Krantz E. et al. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis. 2003;30:174–177. doi: 10.1097/00007435-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 19.Whitley R J, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 20.Catotti D N, Clarke P, Catoe K E. Herpes revisited. Sex Transm Dis. 1993;20:77–80. [PubMed] [Google Scholar]
- 21.Hofstetter A M, Rosenthal L, Stanberry L R. Current thinking on herpes genitalis. Curr Opin Infect Dis. 2014;27:75–83. doi: 10.1097/QCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 22.Sauerbrei A. Berlin, Heidelberg: Springer; 2014. Herpes-simplex-Virusinfektionen; pp. 145–157. [Google Scholar]
- 23.Anzivino E, Fioriti D, Mischitelli M. et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6:40. doi: 10.1186/1743-422X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy – current concepts of prevention, diagnosis and therapy. Part 1: Herpes simplex virus infections. Med Microbiol Immunol. 2007;196:89–94. doi: 10.1007/s00430-006-0031-0. [DOI] [PubMed] [Google Scholar]
- 25.Rudnick C M, Hoekzema G S. Neonatal herpes simplex virus infections. Am Fam Physician. 2002;6:1138–1142. [PubMed] [Google Scholar]
- 26.Corey L, Wald A. Maternal and neonatal HSV infections. N Engl J Med. 2009;361:1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl S. Neonatal herpes simplex virus infection. Clin Perinatol. 1997;24:129–150. [PubMed] [Google Scholar]
- 28.Deutsche STI-Gesellschaft S1-Leitlinie STI/STD – Beratung, Diagnostik und TherapieOnline:http://www.awmf.org/uploads/tx_szleitlinien/059-006l_S1_STI_STD-Beratung_2015-07.pdflast access: 22.06.2016
- 29.Sauerbrei A, Eichhorn U, Hottenrott G. et al. Virological diagnosis of herpes simplex encephalitis. J Clin Virol. 2000;17:31–36. doi: 10.1016/s1386-6532(00)00069-x. [DOI] [PubMed] [Google Scholar]
- 30.LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83. doi: 10.1186/1743-422X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Workowski K A, Bolan G A. Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 32.Patel R, Alderson S, Geretti A. et al. European guideline for the management of genital herpes, 2010. Int J STD AIDS. 2011;22:1–10. doi: 10.1258/ijsa.2010.010278. [DOI] [PubMed] [Google Scholar]
- 33.Wald A, Huang M L, Carrell D. et al. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surface: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 34.Sauerbrei A, Eichhorn U, Schacke M. et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31–36. doi: 10.1016/s1386-6532(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 35.Wagenlehner F M, Brockmeyer N H, Discher T. et al. The presentation, diagnosis and treatment of sexually transmitted infections. Dtsch Arztebl Int. 2016;113:11–23. doi: 10.3238/arztebl.2016.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swiss Herpes Management Forum . Swiss recommendations for the management of genital herpes and herpes simplex virus infection of the neonate. Swiss Med Wkly. 2004;134:205–214. doi: 10.4414/smw.2004.10572. [DOI] [PubMed] [Google Scholar]
- 37.Brown Z A. Case study: type-specific HSV serology and the correct diagnosis of first-episode genital herpes during pregnancy. Herpes. 2002;9:24–26. [PubMed] [Google Scholar]
- 38.Zahariadis G, Severini A. Evaluation of a novel serology algorithm to detect herpes simplex virus 1 or 2 antibodies. Sex Transm Dis. 2010;37:696–699. doi: 10.1097/OLQ.0b013e3181e2cdab. [DOI] [PubMed] [Google Scholar]
- 39.Bentley J, Neubauer A, Sauerbrei A. Value of herpes simplex virus type-specific serology: a case report. J Clin Virol. 2012;54:269–271. doi: 10.1016/j.jcv.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Hashido M, Inouye S, Kawana T. Differentiation of primary genital herpes infections by a herpes simplex virus-specific immunoglobulin G avidity assay. J Clin Microbiol. 1997;35:1766–1768. doi: 10.1128/jcm.35.7.1766-1768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liermann K, Schäfler A, Henke A. et al. Evaluation of commercial HSV IgG and IgM enzyme immunoassays. J Virol Methods. 2014;199:29–34. doi: 10.1016/j.jviromet.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Public Health Agency of Canada Canadian guidelines on sexually transmitted infections. Section 5 – Management and treatment of specific infections. Genital herpes simplex virus (HSV) infectionsOnline:http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/section-5-4-eng.phplast access: 11.08.2016
- 43.German STI-Society STI-Treatment Pocket GuideOnline:http://dstig.de/images/DSTIG-Flyer/Leitfaden/sti-treatment%2520pocket%2520guide_2014.2015_web.pdflast access: 22.06.2016
- 44.De Clercq E. Selective anti-herpesvirus agents. Antivir Chem Chemother. 2013;23:93–101. doi: 10.3851/IMP2533. [DOI] [PubMed] [Google Scholar]
- 45.Le Cleach L, Trinquart L, Do G. et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients (review) Cochrane Database Syst Rev. 2014;(8):CD009036. doi: 10.1002/14651858.CD009036.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollier L M, Wendel D. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal herpes. Cochrane Database Syst Rev. 2008;(1):CD004946. doi: 10.1002/14651858.CD004946.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Stone K M, Reiff-Eldridge R, White A D. et al. Pregnancy outcomes following systemic prenatal acyclovir exposure: conclusions from the international acyclovir pregnancy registry, 1984-1999. Birth Defects Res Clin Mol Teratol. 2004;70:201–207. doi: 10.1002/bdra.20013. [DOI] [PubMed] [Google Scholar]
- 48.Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304:859–866. doi: 10.1001/jama.2010.1206. [DOI] [PubMed] [Google Scholar]
- 49.Kang S H, Chua-Gocheco A, Einarson A. Safety of antiviral medication for the treatment of herpes during pregnancy. Can Fam Physician. 2011;57:427–428. [PMC free article] [PubMed] [Google Scholar]
- 50.Danve-Szatanek C, Aymard M, Thouvenot D. et al. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol. 2004;42:242–249. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes M, Shaik N S, Graber J M. et al. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163:76–80. doi: 10.1001/archinte.163.1.76. [DOI] [PubMed] [Google Scholar]
- 52.Patel R, Green J, Clarke E. et al. 2014 UK national guideline for the management of anogenital herpes. Int J STD AIDS. 2015;26:763–776. doi: 10.1177/0956462415580512. [DOI] [PubMed] [Google Scholar]
- 53.Wald A, Corey L, Timmler B. et al. Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med. 2014;370:201–210. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- 54.Collot M, Rouard C, Brunet C. et al. High conservation of herpes simplex virus UL5/UL52 helicase-primase complex in the era of new antiviral therapies. Antiviral Res. 2016;128:1–6. doi: 10.1016/j.antiviral.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Balfour H H jr., Benson C, Braun J. et al. Management of acyclovir-resistant herpes simplex and varicella-zoster virus infections. J Acquir Immune Defic Syndr. 1994;7:254–260. [PubMed] [Google Scholar]
- 56.Sauerbrei A, Bohn-Wippert K, Kaspar M. et al. Database on natural polymorphisms and resistance-related non-synonymous mutations in thymidine kinase and DNA polymerase genes of herpes simplex virus type 1 and 2. J Antimicrob Chemother. 2016;71:6–16. doi: 10.1093/jac/dkv285. [DOI] [PubMed] [Google Scholar]
- 57.Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26:29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt S, Bohn-Wippert K, Zell R. et al. Sequence analysis of herpes simplex virus type 1 thymidine kinase and DNA polymerase genes from over 300 clinical isolates from 1973–2014 finds novel mutations which may be relevant for development of antiviral resistance. Antimicrob Agents Chemother. 2015;59:4938–4945. doi: 10.1128/AAC.00977-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safrin S, Crumpacker C, Chatis P. et al. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. N Engl J Med. 1991;325:551–555. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- 60.Awasthi S, Friedman M. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol. 2014;6:6–12. doi: 10.1016/j.coviro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Odegard J M, Flynn P A, Campbell D J. et al. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine. 2016;34:101–109. doi: 10.1016/j.vaccine.2015.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straus S E, Corey L, Burke R L. et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet. 1994;343:1460–1463. doi: 10.1016/s0140-6736(94)92581-x. [DOI] [PubMed] [Google Scholar]
- 63.Straus S E, Wald A, Kost R K. et al. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoprotein D and B. results of a placebo-controlled vaccine trial. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 64.Casanova G, Cancela R, Alonzo L. et al. A double-blind study of the efficacy and safety of the ICP10deltaPK vaccine against recurrent genital HSV-2 infections. Cutis. 2002;70:235–239. [PubMed] [Google Scholar]
- 65.deBruyn G, Vargas-Cortez M, Warren T. et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine. 2006;24:914–920. doi: 10.1016/j.vaccine.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 66.Halford W P, Geltz J, Messer R J. et al. Antibodies are required for complete vaccine-induced protection against herpes simplex virus 2. PLoS One. 2015;10:e0145228. doi: 10.1371/journal.pone.0145228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanaway J D, Wald A, Martin E T. et al. Case-crossover analysis of condom use and herpes simplex virus type 2 acquisition. Sex Transm Dis. 2012;39:388–393. doi: 10.1097/OLQ.0b013e318248aa8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottlieb S L, Douglas J M jr., Foster M. et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counselling. J Infect Dis. 2004;15:1059–1067. doi: 10.1086/423323. [DOI] [PubMed] [Google Scholar]
- 69.Abdool Karim S S, Abdool Karim Q, Kharsany A B. et al. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. N Engl J Med. 2015;373:530–539. doi: 10.1056/NEJMoa1410649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bender Ignacio R A, Perti T, Magaret A S. et al. Oral and vaginal tenofovir for genital herpes simplex virus type 2 shedding in immunocompetent women: a double-blind, randomized, cross-over trial. J Infect Dis. 2015;212:1949–1956. doi: 10.1093/infdis/jiv317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Price C F, Tyssen D, Sonza S. et al. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6:e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


